Abstract
Aging impairs arterial function through oxidative stress and diminished nitric oxide (NO) bioavailability. Life-long caloric restriction (CR) reduces oxidative stress, but its impact on arterial aging is incompletely understood. We tested the hypothesis that life-long CR attenuates key features of arterial aging. Blood pressure, pulse wave velocity (PWV) (arterial stiffness), carotid artery wall thickness and endothelium-dependent dilation (EDD) (endothelial function) were assessed in young (Y: 5–7 mo), old ad libitum (Old AL: 30–31 mo.) and life-long 40% CR old (30–31 mo.) B6D2F1 mice. Blood pressure was elevated with aging (P<0.05) and was blunted by CR (P<0.05 vs. Old AL). PWV was 27% greater in old vs. young AL fed mice (P<0.05), and CR prevented this increase (P<0.05 vs. Old AL). Carotid wall thickness was greater with age (P<0.05), and CR reduced this by 30%. CR effects were associated with amelioration of age-related changes in aortic collagen and elastin. Nitrotyrosine, a marker of cellular oxidative stress, and superoxide production was greater in old AL vs. young (P<0.05) and CR attenuated this increase. Carotid artery EDD was impaired with age (P<0.05); CR prevented this by enhancing NO and reducing superoxide-dependent suppression of EDD (Both P<0.05 vs. Old AL). This was associated with a smaller age-related increase in NADPH oxidase activity and p67 expression, with increases in superoxide dismutase (SOD), total SOD and catalase activities (All P<0.05 Old CR vs. Old AL). Lastly, CR normalized age-related changes in the critical nutrient sensing pathways SIRT-1 and mTOR (P<0.05 vs. Old AL). Our findings demonstrate that CR is an effective strategy for attenuation of arterial aging.
Keywords: Aging, Arteries, Oxidative Stress, Calorie Restriction
Introduction
Older age is a major risk factor for development of cardiovascular disease (CVD) (Lakatta 2003). Aging appears to exert its pathological influence primarily via adverse functional and structural effects on arteries, including the large elastic arteries (aorta and carotid arteries) (Lakatta 2003). In particular, aging is associated with elevated systolic blood pressure, large elastic artery stiffening, arterial wall hypertrophy and impaired endothelial function in the absence of clinical disease (Tanaka et al. 2000; Lakatta 2003; Seals et al. 2011), all of which are predictors of incident CV events and key features of a phenotype coined “arterial aging” (Lakatta 2003; Widlansky et al. 2003; Mitchell et al. 2010). The mechanisms of arterial aging are incompletely understood, but reduced nitric oxide (NO) bioavailability secondary to enhanced arterial oxidative stress is believed to play an important role (Taddei et al. 2001; Spier et al. 2004; Brandes et al. 2005; Seals et al. 2011).
Age-related arterial oxidative stress is associated with increased production of reactive oxygen species (ROS), including superoxide anion (Sun et al. 2004; Rippe et al. 2010). This is, in turn, at least partly mediated by increases in expression and activity of the oxidant enzyme NADPH oxidase (Hamilton et al. 2002; Donato et al. 2007a; Durrant et al. 2009), in the face of either lower or unchanged antioxidant enzymes (Donato et al. 2007a; Durrant et al. 2009; Trott et al. 2009). Reduced NO bioavailability with aging is caused primarily by superoxide reacting with NO to form peroxynitrite, leading to elevated nitration of tyrosine residues on arterial proteins (nitrotyrosine) (Donato et al. 2007a; Durrant et al. 2009; Lesniewski et al. 2009). Collectively, such oxidative stress-driven biochemical events lead to arterial dysfunction (Moncada et al. 1991; Soucy et al. 2006; Seals et al. 2011; Fleenor et al. 2012).
Life-long caloric restriction (CR), defined as a reduction in energy intake (typically 40% less than ad libitum) without malnutrition, is associated with enhanced physiological function in advanced age in several mammalian species (Rattus norvegicus, Mus musculus, and Macaca mulatta) (Weindruch & Sohal 1997; Masoro 2005). The beneficial physiological effects of calorie restriction are thought to be mediated through nutrient-sensing pathways such as SIRT-1 and mTOR (Fontana et al. 2010). Importantly, life-long caloric restriction is associated with dramatically attenuated development of CVD in non-human primates (Colman et al. 2009). We and others have shown that life-long caloric restriction provides cardioprotection during ischemia (Edwards et al. 2010) and improves endothelial vasodilatory function (Csiszar et al. 2009), respectively, in old rodents. However, despite these promising observations regarding potential benefits on cardiovascular aging, effects of life-long caloric restriction on the interactions between blood pressure, large artery function wall thickness, and nitric oxide bioavailability are unknown.
Utilizing our well-established mouse model of age-related arterial dysfunction (Donato et al. 2009; Durrant et al. 2009; Lesniewski et al. 2009; Fleenor et al. 2010; Lesniewski et al. 2011a), we hypothesized that life-long caloric restriction would prevent vascular aging and associated age-related increases in blood pressure. We further postulated that this protective effect would be associated with greater NO bioavailability and reduced superoxide-related oxidative stress. The latter, would, in turn, be associated with suppression of age-related increases in NADPH oxidase expression, along with enhanced arterial SOD. Finally, we hypothesized that arterial expression and activity of two key nutrient-sensing pathways, SIRT-1 and mTOR, would be preserved with life-long caloric restriction.
Results
Animal Characteristics
Body mass and soleus muscle mass were not different with aging, but were lower in the old CR vs. AL mice (P<0.05, Table 1). Heart mass was greater in the old AL vs. young AL and old CR mice (P<0.05), but heart mass normalized to total body mass actually was greatest in the old CR group (P<0.05). Soleus muscle mass was lower in the old CR mice vs. other two groups (P<0.05), but greater in the CR animals when normalized to body mass (P<0.05). Old AL animals had greater absolute and relative (to body mass) epididymal white adipose tissue mass vs. young AL mice (P<0.05). Old CR mice had lower absolute epididymal adipose tissue mass vs. the other two groups, and lower relative adipose mass vs. old AL controls (Table 1). Total plasma cholesterol was not affected by aging or life-long CR (Table 1). In contrast, plasma triglycerides were greater with age in the AL animals, but markedly lower in the old CR group (Table 1). Spontaneous cage activity was not different with aging in ad libitum fed mice (P>0.05), but was greater in old CR vs. young and old AL animals (both P<0.05, Table 1). Soleus muscle citrate synthase activity was reduced with aging in the ad libitum fed animals (P<0.05), but was not different in old CR mice compared with the other 2 groups (both P>0.05) (Table 1).
Table 1.
Animal Characteristics
| YOUNG | OLD AL | OLD CR | |
|---|---|---|---|
| Age, mo | 6.1 ± 0.5 | 30.2 ± 0.4 * | 30.6 ± 0.7 * |
| Food intake, g/day | 4.8 ± 0.1 | 4.1 ± 0.1* | 3.2 ± 0.0 *† |
| Body mass, g | 42.6 ± 1.2 | 40.5 ± 1.3 | 27.5 ± 0.06 *† |
| Heart, mg | 223 ± 7 | 258 ± 7 * | 214 ± 13 † |
| Heart:BW, g/g x 100 | 0.55 ± 0.02 | 0.67 ± 0.03 * | 0.80 ± 0.06 *† |
| Soleus, mg | 11 ± 1 | 13 ± 2 | 10 ± 1† |
| Soleus:BW, g/g x 100 | 0.05 ± 0.00 | 0.06 ± 0.00 | 0.07 ± 0.00 * |
| WAT, mg | 684 ± 37 | 972 ± 108 * | 346 ± 33 *† |
| WAT:BW, g/g x 100 | 1.58 ± 0.08 | 2.26 ± 0.21 * | 1.27 ± 0.13 † |
| Total cholesterol, mg/dl | 70 ± 11 | 72 ± 13 | 70 ± 10 |
| Triglycerides, mg/dl | 67 ± 12 | 130 ± 20 * | 15 ± 4 *† |
| Total daily activity, beam breaks / day |
866 ± 79 | 915 ± 92 | 1215 ± 83 *† |
| Soleus citrate synthase activity, mMol * µg protein−1 * min−1 |
4472 ± 665 | 2960 ± 417 * | 3338 ± 772 |
Data are mean±SE. AL, ad libitum; CR, caloric restricted; BW, mass to body weight ratio; WAT, epididymal white adipose tissue.
P<0.05 vs. YOUNG;
P<0.05 vs. OLD AL.
Arterial Blood Pressure and Large Elastic Artery Stiffness and Wall Thickness
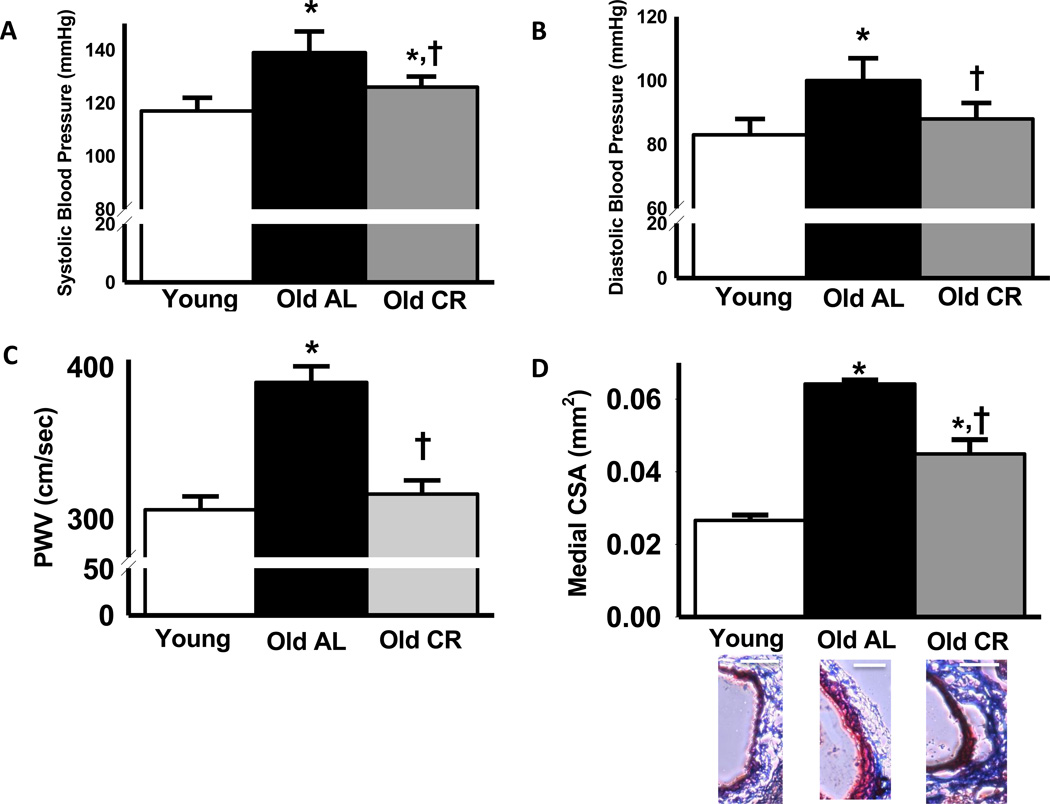
Systolic and diastolic blood pressure increased by ~20 mmHg with age in AL mice (P<0.05), which was largely prevented by life-long CR (Figure 1 A & B). Heart rate did not differ significantly among groups (Young: 699 ± 10, Old AL: 714 ± 18, Old CR: 690 ± 21 beats/min; all P>0.05). Aortic pulse wave velocity (PWV) was 27% higher in old compared with young AL mice (P<0.05), and this age-related increase was completely prevented by life-long CR (Figure 1 C). Carotid artery medial wall cross-sectional area increased markedly with age in AL mice (P<0.05), and this wall thickening was attenuated by ~50% with aging in mice undergoing life-long CR (Figure 1 D).
Figure 1.
Systolic (A) and diastolic (B) blood pressure (A&B: n = 9–11 per group), aortic pulse wave velocity (PWV) (C) and carotid medial artery cross sectional area (CSA; D) in young, old ad libitum (AL) and old life-long caloric restricted (CR) B6D2F1 mice (C&D: n = 7–8 per group). Representative images of trichrome stained carotid artery wall thickness are provided below the summary graph. White Scale bars are equal to 100 microns. Values are means ± s.e.m. *P<0.05 vs. Young. † P<0.05 vs. Old AL
Large Elastic Artery Structural Components
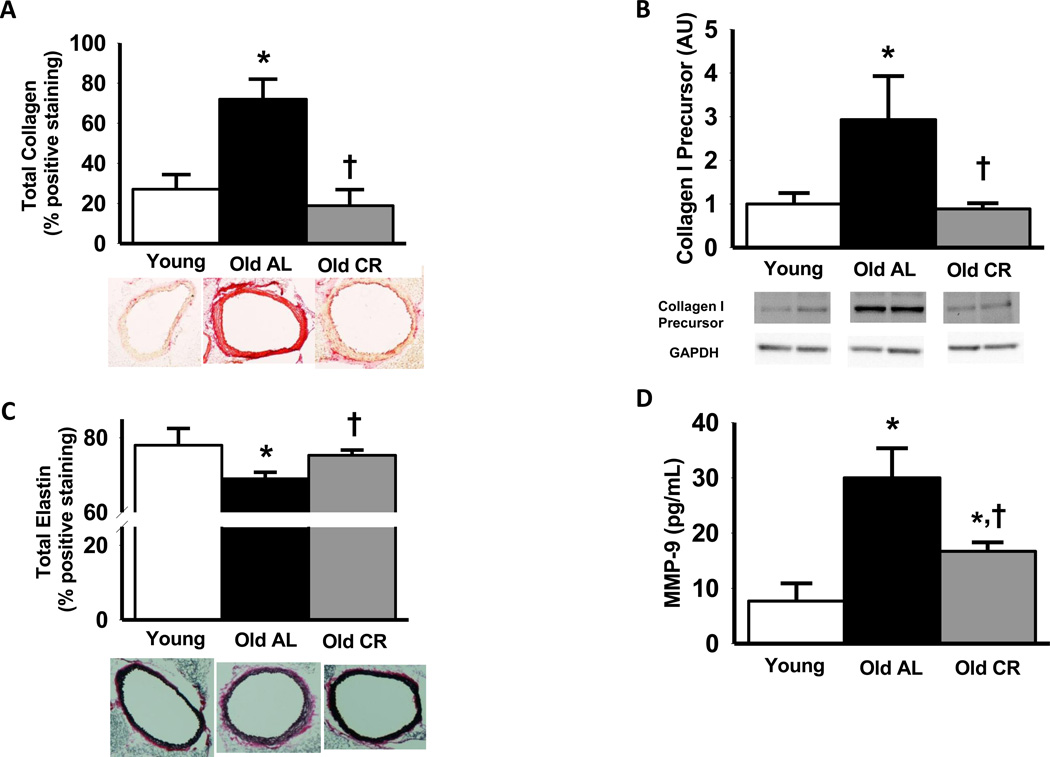
Aging resulted in a 167% increase in total aortic collagen content in AL mice (P<0.05), whereas this increase was entirely prevented in old CR animals (Figure 2 A). Results were similar for the medial (Young: 18 ± 5, Old AL: 75 ± 12, Old CR: 25 ± 7, % total positive area) and adventitial (Young: 39 ± 10, Old AL: 73 ± 8, Old CR: 21 ± 6, % total positive area) layers. Protein expression of precursor collagen I, a key modulator of total arterial collagen, was elevated by 190% in old AL compared with young AL controls (Figure 2 B), and this was completely prevented by life-long CR (Figure 2 B).
Figure 2.
Total thoracic aortic collagen (A), precursor collagen I (B), elastin (C) and matrix metalloproteinase 9 (MMP-9; D) content, in young, old ad libitum (AL) and old life-long caloric restricted (CR) B6D2F1 mice (n = 6–10 per group). Representative images for collagen and elastin content in thoracic aorta are provided below the summary graphs. Collagen I is expressed relative to GAPDH to account for differences in protein loading and presented normalized to the Young mean. Representative blots shown below the summary graph. Values are means ± s.e.m. *P<0.05 vs. Young. † P<0.05 vs. Old AL
Total aortic elastin content declined modestly with aging in the AL mice, but was preserved in life-long CR mice (Figure 2 C). These differences were the same as those in the media alone as elastin is highly localized in the medial layer (see representative images Figure 2 C). Aging resulted in a 290% increase in aortic matrix metalloproteinase −9, a protease that breaks down elastin, and the majority of this increase was prevented by life-long CR (Figure 2 D).
Large Elastic Artery Endothelial Function, NO Bioavailability and eNOS
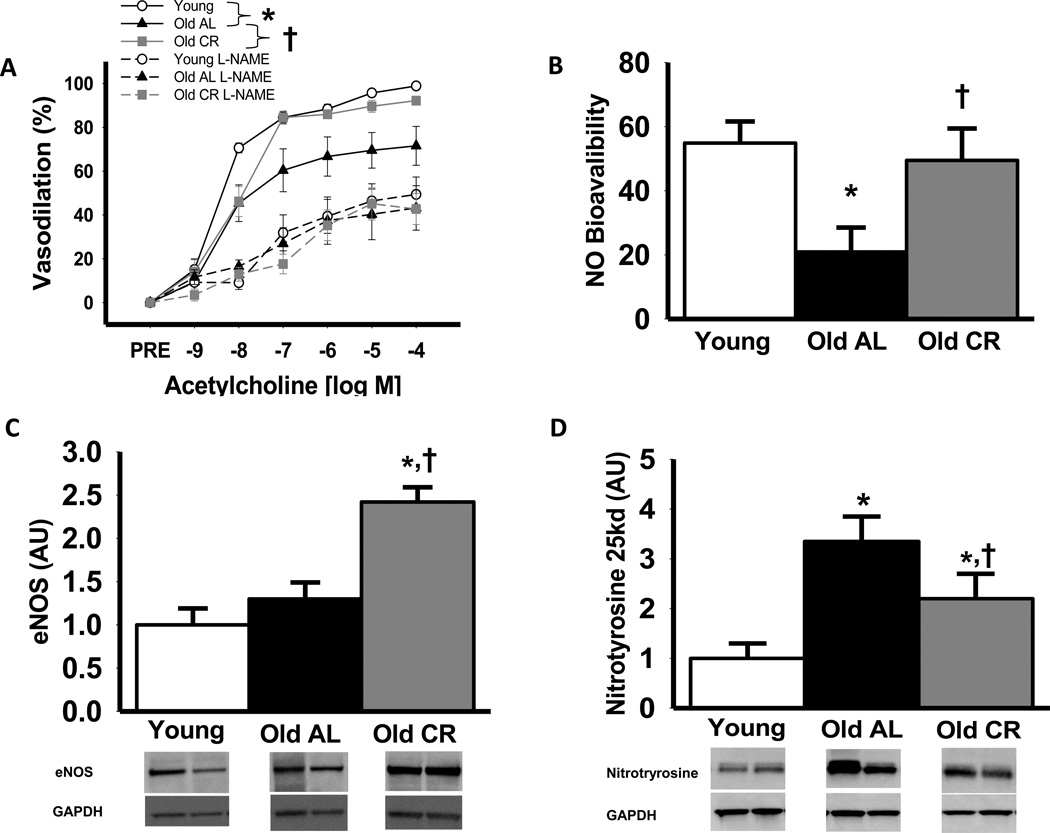
Endothelium-dependent dilation (EDD) to ACh was impaired in isolated carotid arteries of old compared with young AL mice (maximal and dose response, P<0.05; Fig 3 A), whereas sensitivity to ACh (IC50) was not different (P>0.05). Life-long CR prevented the age-related reduction in EDD for the entire dose response, although maximal dilation to ACh was slightly lower in the old CR vs. young AL mice (P<0.05). Incubation with NG-nitro-L-arginine methyl ester (L-NAME) reduced maximal EDD in the carotid arteries of all groups (All P<0.05 vs. ACh alone; Figure 3 A) and abolished group differences (All P>0.05; Figure 3 A). Life-long CR restored the age-related reduction in the NO component of EDD in old mice to that of young animals (Figure 3 B). Aortic eNOS protein expression was not different in young and old AL mice (P>0.05; Figure 3 C), whereas life-long caloric restriction increased eNOS protein by 85% compared with AL mice (P<0.05, Figure 3 C). There were no differences in carotid artery dilation (All P>0.60) or sensitivity (All P>0.4) to the endothelium-independent dilator sodium nitroprusside (SNP) among the groups (maximal dilation to SNP, Young: 98 ± 2, Old AL: 99 ± 1, Old CR: 99 ± 1 % dilation).
Figure 3.
Vasodilation of carotid arteries to acetylcholine (ACh) alone or after pre-treatment with the NOS inhibitor, L-NAME (A), nitric oxide (NO) component of Ach-mediated vasodilation (NO bioavailability) in carotid arteries; NO bioavailability (%) = Maximum DilatationACh+L−NAME – Maximum DilatationACh (B) (A&B: n = 8–12 per group), aortic eNOS content (C) and nitrotyrosine abundance, (D) an oxidative stress marker, in young, old ad libitum (AL) and old life-long caloric restricted (CR) B6D2F1 mice; (C&D: n = 6–9 per group) eNOS and nitrotyrosine are expressed relative to GAPDH to account for differences in protein loading and presented normalized to the Young mean. Representative blots shown below the summary graph (C&D). Values are means ± s.e.m. *P<0.05 vs. Young. † P<0.05 vs. Old AL
Arterial Oxidative Stress and Superoxide Suppression of EDD and NO Bioavailability
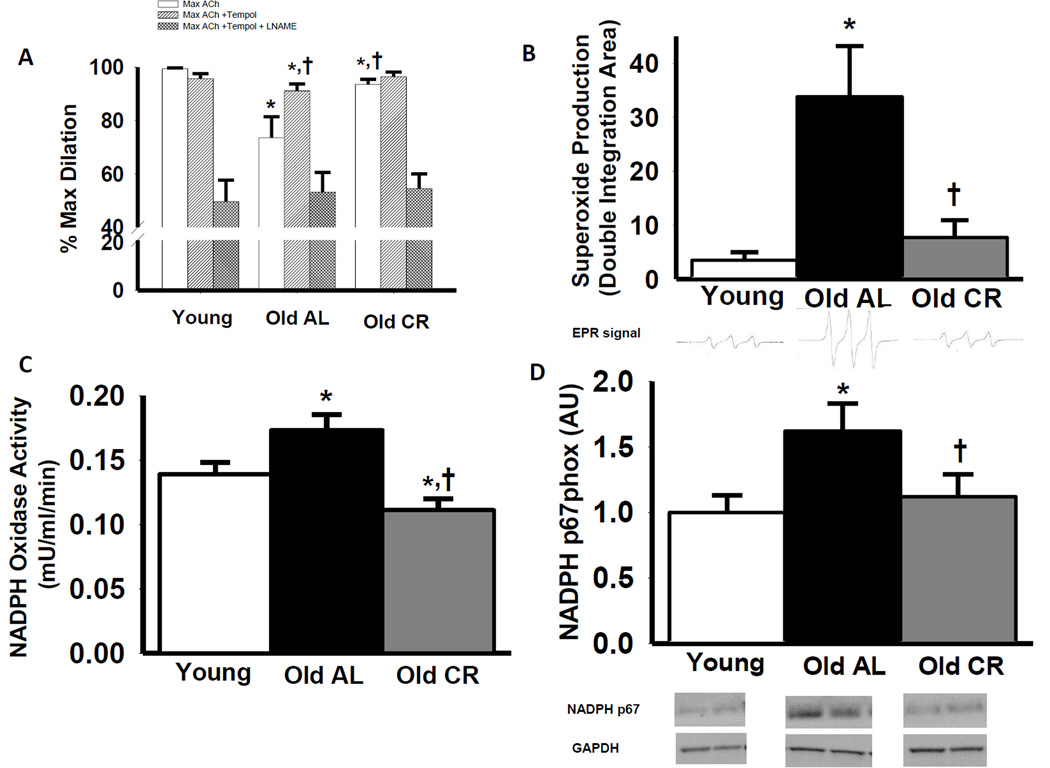
Aortas from old AL mice had greater nitrotyrosine content, a cellular marker of oxidative stress, compared with young AL (P<0.05), which was blunted by life-long CR (25kd band, Figure 3 D; 55kd band Supplemental Figure 1 C). Incubation with the superoxide scavenger, TEMPOL, restored carotid artery EDD (P<0.05) in old AL mice, but had no effect in young AL or old CR mice (Figure 4 A). TEMPOL administration selectively increased EDD in carotid arteries from old AL mice in a NO-dependent manner (P<0.05), whereas TEMPOL did not alter EDD or NO mediated EDD in young AL or old CR mice (P>0.05; Figure 4 A; Supplemental Figure 1 A & B). Superoxide production was 10-fold higher in aortic segments from old compared with young AL mice (P<0.05), whereas old CR animals had values similar to the young AL group (Figure 4 B; P>0.05)
Figure 4.
Maximal vasodilation of carotid arteries to acetylcholine (ACh) alone or to ACh after pre-treatment with TEMPOL in the absence or presence of the NOS inhibitor, L-NAME (A), superoxide production (assessed by electron paramagnetic resonance spectroscopy (B), aortic NADPH oxidase activity (C) and aortic NADPH oxidase p67 (NADPH p67 phox) subunit (D) protein expression in in young, old ad libitum (AL) and old life-long caloric restricted (CR) B6D2F1 mice (n = 6–17 per group). NADPH p67 is expressed relative to GAPDH to account for differences in protein loading and presented normalized to the Young mean. Representative electron paramagnetic resonance of CMH shown below summary graph (B) Representative blots shown below the summary graph (C&D). Values are means ± s.e.m. * P<0.05 vs. Young ACh alone, † P<0.05 vs. Old ACh alone
Enzymatic Oxidant and Antioxidant Expression and Activity
Old AL mice demonstrated greater aortic NADPH oxidase activity and expression of the p67 subunit of NADPH oxidase compared with young AL animals (Figure 4 C and D, P<0.05) and these age-associated changes were prevented by CR (Figure 4 C and D, P<0.05).
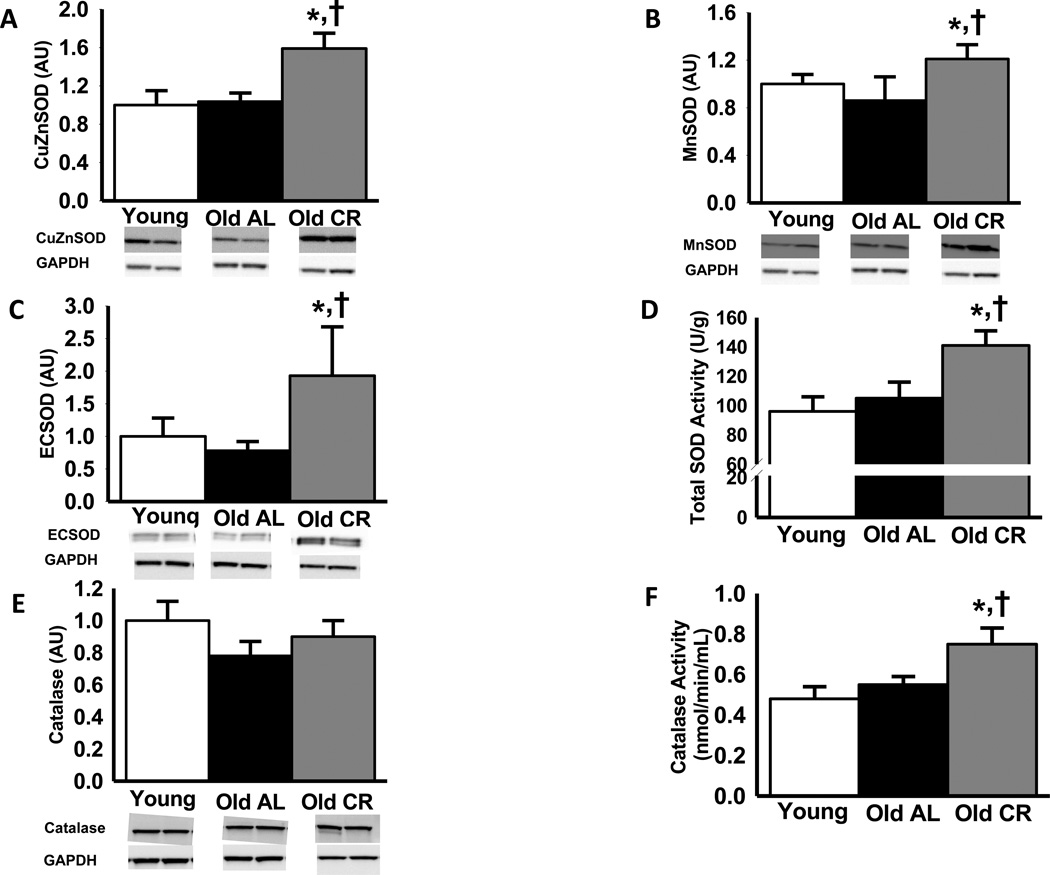
Protein expression of CuZn, Mn and EC SOD isoforms, and catalase, as well as total SOD and catalyze enzyme activities did not differ in the young and old AL mice (P>0.05, Figure 5). Life-long CR induced increases (P<0.05) in CuZnSOD, MnSOD and ECSOD expression, and increased total SOD and catalase enzyme activities compared with the AL groups.
Figure 5.
Aortic protein expression of the copper zinc (CuZn) (A), manganese (Mn) (B) and extra cellular (EC) (C) isoforms of superoxide dismutase (SOD), total aortic SOD activity (D), aortic catalase protein expression (E) and catalase activity (F) from young and old ad libitum (AL) and old life-long caloric restricted (CR) B6D2F1 mice (n = 7–10 per group). Protein expression is expressed relative to GAPDH to account for differences in protein loading and shown normalized to the Young mean. Representative blots shown below the summary graph. Values are means ± s.e.m. *P<0.05 vs. Young. † P<0.05 vs. Old AL
Arterial SIRT-1 and mTOR
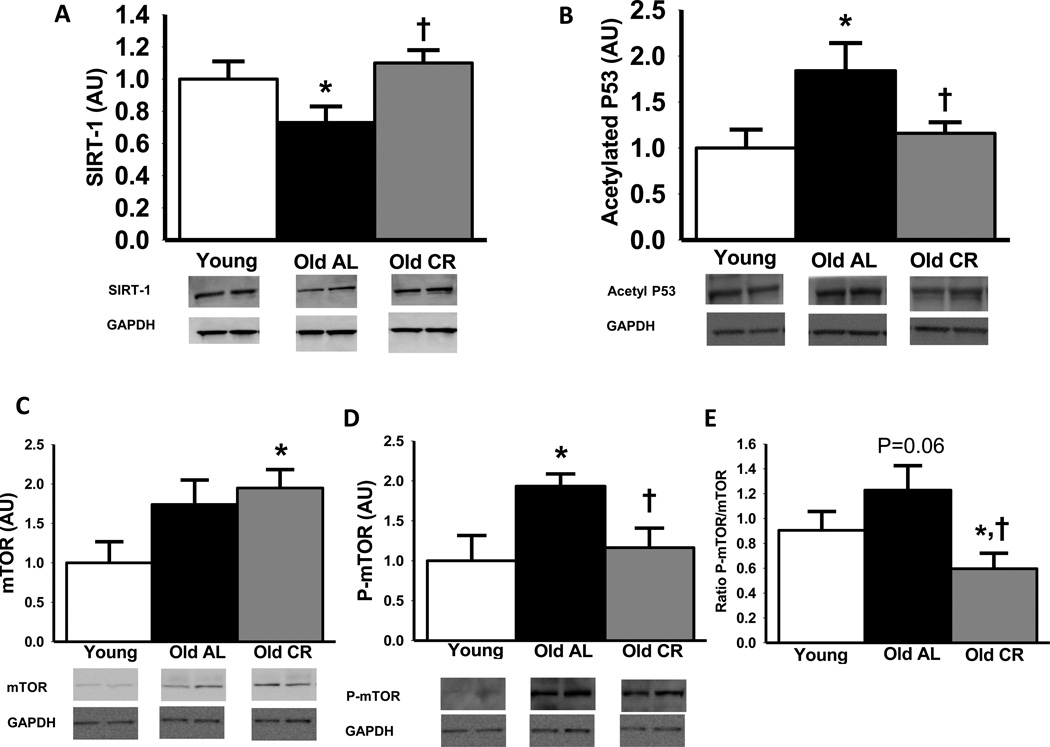
Aortic SIRT-1 protein was reduced with aging in AL mice (P<0.05), but expression was completely preserved in life-long CR mice (Figure 6 A). The activity of the SIRT-1 enzyme, a protein deacetylase, was assessed by the amount of acetylated P53 (note that changes are opposite in direction to activity). Aortic expression of acetylated P53 was increased (SIRT-1 activity was decreased) with aging in AL mice (P<0.05; Figure 6 B), and this was prevented by life-long CR.
Figure 6.
Aortic sirtuin (SIRT)-1 (A), acetylated P53 (B), total mammalian target of rapamycin (mTOR; C), Ser2448- phosphorylated mTOR (D) and ratio of total to phosphorylated mTOR (E) protein expression from young and old ad libitum (AL) and old life-long caloric restricted (CR) B6D2F1 mice (n = 6–11 per group). Protein expression is expressed relative to GAPDH to account for differences in protein loading and shown normalized to the Young mean. Representative blots shown below the summary graph. Values are means ± s.e.m. *P<0.05 vs. Young. † P<0.05 vs. Old AL
Old AL mice tended to have higher total aortic mTOR protein expression (P=0.10; Figure 6 C) and old CR mice had a similar age-related increase, reaching significance compared with young AL mice (P<0.05). In contrast, phosphorylated mTOR (serine 2448) was increased by 93% with aging AL mice (P<0.05; Figure 6 D) and this was prevented by life-long CR. The ratio of phosphorylated to total mTOR, used as a measure of mTOR activity, tended to be greater with aging in AL mice (P=0.06; Figure 6 E), whereas life-long CR was associated with markedly lower aortic mTOR activation compared with old AL mice, and somewhat lower than young AL animals (Both P<0.05).
DISCUSSION
Our results demonstrate that life-long CR can prevent or significantly lessen multiple adverse features of arterial aging as well as age-related increases in arterial blood pressure. Our findings also provide broad insight into the mechanisms underlying the vasoprotective effects of life-long CR, which include preservation of major structural proteins (collagen and elastin), NO bioavailability, superoxide production, and expression and activity of the oxidant enzyme NADPH oxidase at young adult-like levels, as well as enhancement of key antioxidant enzymes. Finally, our data show that arterial aging is associated with potentially deleterious changes in expression and/or activity of the key cellular nutrient sensing pathways SIRT-1 and mTOR, effects that are ameliorated by life-long CR.
Arterial Blood Pressure, Large Elastic Artery Stiffness, and Wall Thickening
Our results demonstrate for the first time in combination that life-long CR largely prevents age-related increases in systolic and diastolic arterial blood pressure, completely prevents aortic stiffening and significantly reduces large elastic artery wall hypertrophy. These findings are biomedically important in that these characteristics of cardiovascular aging change subclinically with aging in humans and are independent predictors of future CV events and clinical CVD (Lakatta & Levy 2003). Our findings are consistent with previous reports from both intervention and cross-sectional studies in humans that shorter-term energy-restriction and reduced body weight are associated with lower blood pressure and carotid wall thickness (Walford et al. 1992; Fontana et al. 2004). In contrast, the recent CR clinical trial (CALERIE) has not shown significant changes in blood pressure after 6 months of 25% CR in non-obese human subjects (Lefevre et al. 2009). Our results extend these prior observations by showing that life-long energy restriction can partially or totally prevent the increases in stiffening of large elastic arteries that occurs with adult aging.
We cannot discern the temporal sequence of these cardiovascular-protective effects of life-long CR in the present study. It is believed that large elastic artery stiffening contributes to age-associated increases in systolic blood pressure, which, in turn, stimulates compensatory wall thickening to normalize wall stress (Najjar et al. 2005). However, these interactions are complex and many other factors (structural, autonomic, hemodynamic, hormonal, etc.) are involved in determining arterial pressure and properties of large elastic arteries with aging. Such influences likely explain the variance in the relative changes observed among these outcomes in response to life-long CR in our study.
Structural Proteins
Large elastic artery stiffening with aging is thought to be mediated primarily by changes in the major structural proteins involving increases in collagen and fragmentation of elastin (Fornieri et al. 1992; Nosaka et al. 2003; Wang et al. 2007; Fleenor et al. 2010). In the present investigation, the absence of aortic stiffening with life-long CR was associated with a lack of accumulation of collagen and maintenance of elastin in the aorta, as reported previously (Fornieri et al. 1999). Our findings further suggest that the inhibition of collagen accumulation by life-long CR may be mediated, at least in part, by suppression of collagen production, as indicated by the absence of increases in precursor collagen I in our old CR mice. Moreover, the preservation of aortic elastin with life-long CR in the present study was associated with inhibition of age-related increases in MMP-9, an elastin-degrading enzyme, suggesting the possible importance of CR-induced suppression of that arterial elastase. Thus, our findings suggest that CR likely prevents age-related increases in aortic stiffness by suppressing collagen production and elastin degradation.
NO-Dependent Endothelial Function
Endothelial dysfunction develops with aging as indicated by impaired EDD in response to chemical (ACh) and mechanical (flow) stimulation as a result of reduced NO bioavailability (Celermajer et al. 1992; Taddei et al. 2001; Spier et al. 2004; Lesniewski et al. 2009). The cause and effect relation between reduced NO biovailability and the major constitutive NO producing enzyme, eNOS, is less clear, as protein expression and activation of eNOS has been reported to be augmented, attenuated and similar eNOS in arteries from rodents and endothelial cells from humans (Cernadas et al. 1998; van der Loo et al. 2000; Sun et al. 2004; Donato et al. 2009; Durrant et al. 2009; Trott et al. 2009).
It has been reported that life-long CR is associated with preserved EDD to ACh in the rat aorta (Csiszar et al. 2009). Our results extend these findings by showing that the preservation of large artery endothelial function in old CR mice is mediated by maintained NO bioavailability, and that these events are associated with increases in the expression of the eNOS , consistent with the effects of other vasoprotective lifestyle interventions in aged animals such as exercise training (Spier et al. 2004; Durrant et al. 2009). Our observations regarding NO likely have important clinical implications given that NO bioavailability is a critical component of a healthy endothelial phenotype (Forstermann & Munzel 2006). Moreover, NO is anti-proliferative (Garg & Hassid 1989) and modulates large elastic artery stiffness (Fitch et al. 2001) and structural proteins (Myers & Tanner 1998), suggesting a possible role for this mechanism in several of the anti-vascular aging effects of life-long CR observed in the present study.
Endothelial Function and Oxidative Stress
Endothelial dysfunction with aging is mediated by excessive superoxide-dependent oxidative stress (van der Loo et al. 2000; Taddei et al. 2001; Csiszar et al. 2002; Donato et al. 2007a). Consistent with this, acute administration of superoxide anion scavengers such as ascorbic acid or TEMPOL improve EDD and NO bioavailability with aging (Taddei et al. 2001; Sun et al. 2004; Durrant et al. 2009). A previous study in aortic rings demonstrated that TEMPOL restores EDD in old AL, but not life-long CR rats (Csiszar et al. 2009). Here we extend these findings by showing that life-long CR preserves NO-mediated large artery endothelial function with aging by suppressing superoxide-associated vascular oxidative stress. The latter is supported by several lines of evidence, including functional data using TEMPOL to scavenge superoxide and L-NAME to inhibit NO production, and biochemical analyses showing that aortic nitrotyrosine, a marker of cellular oxidative stress, and superoxide production, measured by the gold standard approach of electron paramagnetic resonance (EPR) spectroscopy, were markedly lower in old CR compared with AL animals, and similar to young AL mice.
Our results also provide novel insight into the cellular mechanisms that may contribute to life-long CR inhibition of oxidative stress with arterial aging. In the present study, CR increased protein expression and activity of SOD, a critical enzymatic antioxidant that protects NO as it diffuses from the endothelium to vascular smooth muscle cells (Jung et al. 2003). Our results are consistent with the effects of life-long CR on SOD expression in other tissue types such as skeletal muscle (Sreekumar et al. 2002). We also show here for the first time that life-long CR results in increased aortic activity of catalase, a key antioxidant enzyme that removes hydrogen peroxide, without an increase in protein expression, indicating a post-translational modification of the enzyme resulting in enhanced activity. This is similar to a previous report of increased catalase activity with CR non-vascular tissues (Mote et al. 1991). In addition to boosting antioxidant enzymes, our data indicate that life-long CR inhibits aortic expression of the major superoxide-producing oxidant enzyme in arteries, NADPH oxidase. Activation of this enzyme is broadly implicated in arterial aging (Hamilton et al. 2002; Durrant et al. 2009). Together, our findings suggest that both enhanced antioxidant enzymes and inhibition of NADPH oxidase may be involved in the suppression of superoxide-related oxidation stress by life-long CR.
Nutrient Sensing Pathways
The longevity and physiological benefits of life-long CR have been linked to alterations in nutrient sensing pathways, particularly SIRT-1 (Cohen et al. 2004; Dali-Youcef et al. 2007) and mTOR (mammalian target of rapamycin)(Fontana et al. 2010). These pathways are activated (SIRT-1) or inhibited (mTOR) during periods of reduced energy availability and such changes are associated with reduced oxidative stress in several tissues (Lindsley & Rutter 2004; Feng et al. 2005; Csiszar et al. 2009). The present data are the first to demonstrate in arteries that life-long CR prevents potentially adverse age-related changes in protein expression and/or the signaling state of these pathways. Specifically, life-long CR preserved aortic SIRT-1 expression and activity (measured via acetylated P53) with aging. Previously, we reported age-related reductions in SIRT-1 protein expression in mouse aorta and human endothelial cells (Donato et al. 2011), and others have documented the importance of SIRT-1 expression related to endothelial function in healthy arteries (Mattagajasingh et al. 2007; Tajbakhsh & Sokoya 2012). In an earlier study, we demonstrated that short-term CR could restore arterial SIRT-1 protein concentrations in old mice (Rippe et al. 2010). Preservation of aortic SIRT-1 mRNA expression was not observed with life-long CR in a rat model of aging (Csiszar et al. 2009), but the differences with our studies may be due to assessment of SIRT mRNA compared with protein and deacetylase activity, or the rodent species used.
In the present investigation, we also found that aortic mTOR signaling (phosphorylation at serine 2448) was increased with aging in AL mice, but maintained at young adult levels with life-long CR, similar to previous findings in non-vascular tissues (Wu et al. 2009). Together, our findings suggest that CR may preserve the function of important signaling pathways that regulate cellular energy status with aging in arteries, laying the foundation for future mechanistic studies on this topic.
Short-term vs. Long-term CR
Results of a previous study from our laboratory suggest that many of the effects of life-long CR on vascular endothelial function, NO and oxidative stress also may be conferred by shorter-term CR (Rippe et al. 2010). In this earlier investigation, we reported that 8 weeks of ~30% CR with attendant acute weight loss restored NO-mediated EDD in carotid arteries of old B6D2F1 mice, and that this was associated with reductions in arterial oxidative stress and NADPH oxidase protein expression, as well as selective increases in antioxidant enzymes. Several other vascular features of arterial aging assessed in the present investigation, including arterial blood pressure, stiffness, wall hypertrophy and structural proteins were not assessed in this earlier study on short-term CR. Moreover, findings regarding the cardiovascular benefits of shorter-term energy intake restriction in humans have been inconsistent (Fontana et al. 2004; Fontana et al. 2007; Lefevre et al. 2009). As such, further investigation of the cardiovascular-protective effects of short- vs. long-term CR will be required to gain more definitive insight.
Limitations
In the present study, we sought to gain initial insight into mechanisms by which life-long CR may preserve arterial function with aging. We focused on selective mechanisms relating to structural proteins, NO, oxidative stress and nutrient sensing pathways. Several other mechanisms not studied here may contribute to reduced oxidative stress with life-long CR in old mice including reductions in mitochondrial superoxide production, enhanced eNOS coupling and/or increases in other antioxidant or stress resistance pathways. Moreover, our findings related to the important nutrient sensing pathways of SIRT-1 and mTOR and vascular protection with aging by life-long CR are associative. As such, pharmacological and/or genetic approaches will be required to provide direct cause and effect evidence among these events. Finally, it is possible that some of the beneficial effects of life-long CR on aging arteries may be due to, at least in part, to increases in spontaneous activity, which has been observed in other rodent models of life-long CR. This cannot be determined experimentally because increased activity is a fundamental part of the CR phenotype. Moreover, it is possible that such increases in spontaneous activity could have induced an “exercise training effect”. If so, this could, in turn, explain the preservation of vascular function with aging in CR animals because models of aerobic exercise training such as treadmill running (Donato et al. 2005; Donato et al. 2007b) and voluntary wheel running (Durrant et al. 2009) have previously been shown to reverse some expressions of vascular dysfunction in old rodents. However, such exercise training studies consistently showed an increase citrate synthase activity in skeletal muscle in the exercising animals, which is a well established marker of the exercise training effect. In the present study, CR was not associated with increases in citrate synthase. Thus, the preservation of arterial structure and function with aging in the CR animals cannot be attributed to an exercise training effect per se.
Perspectives and Conclusions
With the changing demographics of aging leading to unprecedented numbers of older adults, it is imperative that we establish the efficacy of strategies to delay, slow and even prevent the development of arterial dysfunction and CVD with aging. There is accumulating evidence that energy restriction is an effective lifestyle intervention for preserving many aspects of physiological function with aging. In the present study, we extend insight into the physiological benefits of life-long CR by providing the first direct evidence that CR partly or completely prevents large elastic artery stiffening, wall hypertrophy, endothelial dysfunction, reductions in NO bioavailability and increases in arterial blood pressure with aging in mice. We also provide novel and comprehensive mechanistic insight into the role of suppression of oxidative stress in mediating these favorable effects of life-long CR in the prevention of arterial aging, and advance the possible involvement of two key nutrient sensing pathways, SIRT-1 and mTOR, in the vasoprotection conferred by long-term energy restriction.
Methods
Animals
Young (Y, 5–7 months) and old ad libitum (Old AL, 30–31 months) and old life-long calorically restricted (Old CR, 30–31 months) male B6D2F1 mice were obtained from the National Institute on Aging rodent colony. In NIH life-long CR mice, calorie restriction is initiated at 14 weeks of age at ~10% below ad libitum, increased to ~25% restriction at 15 weeks and to ~40% restriction at 16 weeks, and maintained throughout the life of the animal. All mice (n=43 University of Colorado; n=61 University of Utah) were housed for at least 2–3 months in an animal care facility at the University of Colorado at Boulder or the University of Utah on a 12:12 light:dark cycle at 24 ° C and fed the appropriate NIH-31(Y or Old AL) or NIH-31 fortified diet (Old CR). See supplemental material (Supplemental Figure 2) for full nutritional breakdown of these diets or access the NIA link (http://www.nia.nih.gov/sites/default/files/nia_dietspdf_0.pdf). All animal procedures conformed to the Guide to the Care and Use of Laboratory Animals (version 8, revised 2011) and were approved by the University of Colorado and Utah Animal Care and Use Committee.
Arterial Blood Pressure
Blood pressure was assessed non-invasively in the conscious state by determining the tail blood volume with a volume pressure recording (VPR) sensor and an occlusion tail-cuff (CODA System, Kent Scientific, Torrington, CT) as previously described in detail (Feng et al. 2008; Rateri et al. 2011). This method has been validated versus arterial catheter blood pressure (Feng et al. 2008). Blood pressure and heart rate recordings were made in a quiet and warm (24 °C) environment. Mice were placed in restrainers on a heating unit and given 15–20 minutes to acclimate and reach a steady tail skin temperature of (30–35 °C). Each session consisted of 5–10 acclimatization measures and documentation of stable values, followed by 20 experimental measures. Measures with aberrant movement/behavior or inadequate tail volume/flow values were excluded and remaining values used to calculate mean values for each animal. This method is quite reproducible in our laboratories as the coefficient of variation for this method is 5% for systolic and 7% diastolic blood pressure (n=10) (Seals, unpublished).
Arterial Stiffness
Several (2–3) weeks before euthanasia, aortic pulse wave velocity (PWV) was measured as described previously (Reddy et al. 2005; Fleenor et al. 2012). Briefly, mice were anesthetized under 2% isoflurane in a closed chamber anesthesia machine (V3000PK, Parkland Scientific, Coral Springs, FL) for ~1–3 min. Anesthesia was maintained using a nose-cone and mice were secured in a supine position on a heating board (~35 °C) to maintain body temperature. Velocities were measured with 20-MHz Doppler probes (Indus Instruments, Webster, TX) at the transverse aortic arch and ~ 4 cm distal at the abdominal aorta simultaneously and collected using WinDAQ Pro+ software (DataQ Instruments, Akron, OH). After velocities were collected, a precise measure of the distance between the probes was obtained using a scientific caliper and recorded. Absolute pulse arrival times were indicated by the sharp upstroke, or foot, of each velocity waveform analyzed with WinDAQ Waveform Browser (DataQ Instruments, Akron, OH). Aortic PWV is then calculated as the quotient of the separation distance and difference in absolute arrival times.
Endothelial Function: Modulation by Superoxide and NO
Measurements of endothelial dependent dilation (EDD) and endothelial independent dilation (EID) in isolated carotid arteries studied ex vivo were performed using a method previously described in detail (Donato et al. 2009; Lesniewski et al. 2009; Donato et al. 2011). The carotid arteries were used as a large elastic artery for these measurements because we have previously established age-associated impairments in oxidative stress-associated NO-mediated endothelial dysfunction in this model (Durrant et al. 2009; Lesniewski et al. 2009; Fleenor et al. 2012). Moreover, use of the carotid arteries for this functional measurement allowed biochemical analyses, which require large tissue volumes, to be performed on the aortic samples, thus significantly reducing the number of animals required to complete the overall assessments of vascular function and structure.
Briefly, mice were euthanized by exsanguination via cardiac puncture while under isoflurane anesthesia. Both the right and left carotid arteries were excised and placed in myograph chambers (DMT Inc.) perfused by physiological saline solution (PSS) that contained 145.0 mM NaCl, 4.7 mM KCl, 2.0 mM CaCl2, 1.17 mM MgSO4, 1.2 mM NaH2PO4, 5.0 mM glucose, 2.0 mM pyruvate, 0.02 mM EDTA, 3.0 mM MOPS buffer and 1 g/100 ml BSA, pH 7.4 at 37 °C, cannulated onto glass micropipettes and secured with nylon (11-0) suture. Once cannulated, both carotid arteries were warmed to 37 °C and pressurized and allowed to equilibrate for ~1 hour. All arteries were submaximally preconstricted with phenylephrine (2 µM) and increases in luminal diameter in response to increasing concentrations of the endothelium-dependent dilator, acetylcholine (ACh:1×10−9 to 1×10−4 M) and endothelium-independent dilator, sodium nitroprusside (SNP: 1×10−10 to 1×10−4 M) were determined. Responses to ACh were repeated in the presence of the NO synthase inhibitor, NG-nitro-L-arginine methyl ester (L-NAME, 0.1mM, 30-minute incubation) to determine the contribution of NO. To determine superoxide (oxidative stress) suppression of EDD, measurements were repeated in the contra-lateral vessel following a 60-minute incubation in the presence of the superoxide scavenger, TEMPOL (1mM) (Zhang et al. 2003; Qamirani et al. 2005; Didion et al. 2006). NO bioavailability was determined from the maximal EDD in the absence or presence of L-NAME in each vessel according to the following formula: NO bioavailability (%) = Max Dilation Ach+L-NAME – Max Dilation Ach.
Arterial Wall Thickness and Histochemistry
Carotid arteries and thoracic aortas were saved in optimal cutting temperature (OCT) solution, frozen and sliced into 8-micron sections. Each mouse carotid artery or aorta had 3 to 4 sections per slide, which were averaged. Slides were batched to have equal numbers of each group and stained for wall thickness via Masson TriChrome (carotid), collagen via Picosirius red (aorta) and elastin via Verhoff’s Van Gieson (aorta), as previously described (Fleenor et al. 2010; Raub et al. 2010). All area (total, medial layer and adventitial layers) and color threshold quantification was performed with ImageJ software (NIH, Bethesda, MD, USA). For measures of medial cross-sectional area (wall thickness) trichrome stained carotid arteries, the lumen border and the outer medial border were traced in ImageJ and internal areas measured. These areas were used to calculate medial cross sectional area and were calculated as the outer media border area minus the lumen area. Green channel images from a RGB stack were used for collagen (Aikawa et al. 1998; Fleenor et al. 2010) and the 8-bit grey scale was used for elastin analyses (Raub et al. 2010). The same color threshold was applied to all collagen and elastin samples. Content was calculated as the percentage of the selected area (arterial wall) with positive color.
Arterial Protein Expression and Enzyme Activities
To obtain sufficient tissue to perform measurements of protein expression and enzyme activity, the thoracic aorta was excised, cleared of surrounding tissues while maintained in ice-cold (4 ° C) PSS (similar composition as above, but at pH of 7.4 at 4°C), and then frozen in liquid nitrogen. Whole artery lysates were prepared as previously described (Donato et al. 2011; Lesniewski et al. 2011a). Briefly, 15 µg of protein with 2 mol/L dithiothreitol was loaded into polyacrylamide gels, separated by electrophoresis and transferred onto a nitrocellulose membrane. The membrane was treated with 5% nonfat dry milk in TBS with 0.05% Tween (TBST) overnight at 4°C. Membranes were then washed with TBST and incubated overnight at 4°C in primary antibody. Extracellular SOD (ECSOD)(1:500, Sigma-Aldrich, St. Louis, MO) endothelial NO synthase (eNOS) (1:1000, BD Biosciences, San Jose, CA), catalase (1:250, Abcam, Cambridge MA), collagen I precursor (1:1000, Millipore, Billerica, MA), copper zinc (CuZn) SOD (1:2000, Stressgen, Ann Arbor, MI), manganese (Mn) SOD (1:2000, Stressgen, Ann Arbor, MI), mTOR and phosphorylated mTOR serine 2448 (1:1000, Cell Signaling, Danvers, MA), actelyated p53 (1:500, Cell Signaling, Danvers, MA), p67phox-NADPH oxidase (p67, 1:1000, BD Biosciences, San Jose, CA), SIRT-1 (1:1000, Abcam, Cambridge, MA), and nitrotyrosine (1:1000, Abcam, Cambridge, MA), expression were measured by standard western blotting techniques using an HRP-conjugated secondary antibody (Jackson Immunological, West Grove, PA) and Supersignal ECL (Pierce, Rockford, IL). Bands were visualized using a digital acquisition system (ChemiDoc-It, UVP, Upland, CA) and quantified using ImageJ software (NIH, Bethesda, MD). To account for differences in protein loading, expression is presented normalized to GAPDH (1:1000, Cell Signaling, Danvers, MA) expression. Data are then normalized to the mean of the young control group.
NADPH oxidase activitiy was determined in aortic lysates (10 µg protein) using the Amplex Red Xanthine/Xanthine Oxidase Assay kit (Invitrogen, Carlsbad, CA), according to manufacturer instructions with NADPH (200 µmol/L/reaction) as the reaction substrate, as previously described (Durrant et al. 2009).
Total SOD and catalase activity were determined in aortic lysates (1 µg protein) using SOD and Catalase Activity Assay kits (Cayman Chemical, Ann Arbor, MI) according to manufacturer instructions as previously described (Durrant et al. 2009). MMP-9 was measured in aortic lysates (10 µg protein) by ELISA (Thermo / Fisher Scientific Waltham, MA) as previously described (Lesniewski et al. 2011b).
Arterial Superoxide Production
Production of superoxide was measured by EPR spectrometry using the spin probe 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH, Alexis Biochemicals). CMH was prepared in ice-cold deoxygenated Krebs-HEPES buffer (mmol L−1: NaCl, 99.01, KCl 4.69, CaCl2 2.50, MgSO4 1.20, K2HPO4 1.03, NaHCO3 25.0, glucose 11.10, Na-HEPES 20.00; pH 7.4) containing 0.1 mmol L−1 diethylenetriamine-penta-acetic acid, 5 µmol L−1 sodium diethyldithiocarbamate and pretreated with Chelex (Sigma) to minimize auto-oxidation of the spin probe. Three-millimeter aortic rings were washed once in PSS and again in modified Krebs-HEPES buffer. Rings were then incubated for 60 min at 37°C in 200 µL Krebs-HEPES buffer containing 0.5 mmol L−1 CMH and analyzed immediately on an EMX Plus EPR spectrometer (Bruker, Rheinstetten, Germany). Instrument settings were: microwave frequency 9.83 Ghz, centerfield 3480 G, sweep 80 G, modulation amplitude 3.3 G, microwave power 40 mW, microwave attenuation 7, and receiver gain 30. A total of six sweeps were conducted lasting 8.7 seconds per sweep. The running average of the six sweeps was collected with the double integration (area under and over the baseline) of the triplet used to display the magnitude of the signal. The magnitude of this signal directly relates to the amount of superoxide that has been trapped by the CMH.
Cage Activity
Mice were individually housed in clear Plexiglass cages and studied for a period of 72 hours using the Comprehensive Laboratory Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH). Animals were acclimatized to the chambers for 5 days prior to data collection and maintained at approximately 24 ° C with a 12-hour light/dark cycle. Food and water were freely available for ad libitum animals and CR animals were fed at the same time (9 A.M. MST as was standard in the control cages). Mouse movements in the X, Y, and Z planes were monitored by laser beam interruption. The sum of the movement in these planes was summed over each 72-hour period then averaged divided by 3 for average activity per 24 hours.
Citrate Synthase Activity
Soleus citrate synthase activity was assessed as described previously (Tweedie et al. 2011). Briefly, frozen soleus samples were lysed in homogenization buffer (1 mM EDTA and 50 mM Triethanolamine in dH2O) with a handheld homogenizer. Soleus lystate protein concentration was assessed by Pierce BCA Assay and aliquots of lysate were brought up to standardized protein concentrations with homogenization buffer. Citrate synthase activity was determined in triplicate in a 96 well plate. The reaction mix consisted of 10 uL standardized protein lysate, 7 uL Oxaloacetic acid (2 mM) and 200 uL of reaction buffer (Tris Buffer, dH2O, 200 uM Acetyl CoA, 200 uM DTNB, and 10% Triton X). Plates were read at 412 Absorbance over 5 minutes to determine citrate synthase activity and expressed as mM/ug protein lysate /min.
Statistics
For animal and vessel characteristics and protein expression / activity, group differences were determined by one-way analysis of variance (ANOVA). For all dose responses, group differences were determined by repeated measures ANOVA. An LSD post hoc test for equal variances was used for preplanned comparisons where appropriate. Data are presented as mean ± SEM. Significance was set at P< 0.05.
Supplementary Material
Supplemental Figure 1. Vasodilation of carotid arteries to acetylcholine (ACh) alone or after pre-treatment with TEMPOL (A), vasodilation to ACh after TEMPOL pretreatment in the absence or presence of the NOS inhibitor, L-NAME (B) (A&B: n = 8–10 per group) and aortic nitrotyrosine, an oxidative stress marker, abundance (C) in young, old ad libitum (AL) and old life-long caloric restricted (CR) B6D2F1 mice; (n = 6–8 per group) Nitrotyrosine is expressed relative to GAPDH to account for differences in protein loading and presented normalized to the Young mean. Representative blots shown below the summary graph (C&D). Values are means ± s.e.m. *P<0.05 vs. Young. † P<0.05 vs. Old AL
Acknowledgements
The authors would like to acknowledge the support of multiple NIH awards HL107120, AG013038, AG033196, AG033755, AG000279, AG040297, AG029337.
Footnotes
Author Contributions
A.J.D., L.A.L., and D.R.S. contributed to the conception and design, analysis and interpretation of data, and drafting and revision of the article, and provided final approval of the version to be published. All authors contributed to the collection and interpretation of data and the revision of the manuscript, and provided final approval of the version to be published. All experiments were carried out at the University of Colorado at Boulder and the University of Utah.
Conflict of Interest / Disclosures
We have no disclosures or conflict of interest
References
- Aikawa M, Rabkin E, Okada Y, Voglic SJ, Clinton SK, Brinckerhoff CE, Sukhova GK, Libby P. Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content of rabbit atheroma: a potential mechanism of lesion stabilization. Circulation. 1998;97:2433–2444. doi: 10.1161/01.cir.97.24.2433. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- Cernadas MR, Sanchez de Miguel L, Garcia-Duran M, Gonzalez-Fernandez F, Millas I, Monton M, Rodrigo J, Rico L, Fernandez P, de Frutos T, Rodriguez-Feo JA, Guerra J, Caramelo C, Casado S, Lopez F. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res. 1998;83:279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: the 'magnificent seven', function, metabolism and longevity. Ann Med. 2007;39:335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- Didion SP, Kinzenbaw DA, Schrader LI, Faraci FM. Heterozygous CuZn superoxide dismutase deficiency produces a vascular phenotype with aging. Hypertension. 2006;48:1072–1079. doi: 10.1161/01.HYP.0000247302.20559.3a. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007a;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H425–H432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Lesniewski LA, Delp MD. The effects of aging and exercise training on endothelin-1 vasoconstrictor responses in rat skeletal muscle arterioles. Cardiovasc Res. 2005;66:393–401. doi: 10.1016/j.cardiores.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Lesniewski LA, Delp MD. Ageing and exercise training alter adrenergic vasomotor responses of rat skeletal muscle arterioles. J Physiol. 2007b;579:115–125. doi: 10.1113/jphysiol.2006.120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol. 2011;589:4545–4554. doi: 10.1113/jphysiol.2011.211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AG, Donato AJ, Lesniewski LA, Gioscia RA, Seals DR, Moore RL. Life-long caloric restriction elicits pronounced protection of the aged myocardium: a role for AMPK. Mech Ageing Dev. 2010;131:739–742. doi: 10.1016/j.mad.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M, Whitesall S, Zhang Y, Beibel M, D'Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens. 2008;21:1288–1291. doi: 10.1038/ajh.2008.301. [DOI] [PubMed] [Google Scholar]
- Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci U S A. 2005;102:8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch RM, Vergona R, Sullivan ME, Wang YX. Nitric oxide synthase inhibition increases aortic stiffness measured by pulse wave velocity in rats. Cardiovasc Res. 2001;51:351–358. doi: 10.1016/s0008-6363(01)00299-1. [DOI] [PubMed] [Google Scholar]
- Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol. 2010;588:3971–3982. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell. 2012;11:269–276. doi: 10.1111/j.1474-9726.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JO. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab. 2007;293:E197–E202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- Fornieri C, Quaglino D, Jr, Mori G. Role of the extracellular matrix in age-related modifications of the rat aorta. Ultrastructural, morphometric, and enzymatic evaluations. Arterioscler Thromb. 1992;12:1008–1016. doi: 10.1161/01.atv.12.9.1008. [DOI] [PubMed] [Google Scholar]
- Fornieri C, Taparelli F, Quaglino D, Jr, Contri MB, Davidson JM, Algeri S, Ronchetti IP. The effect of caloric restriction on the aortic tissue of aging rats. Connect Tissue Res. 1999;40:131–143. doi: 10.3109/03008209909029109. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, Al-Benna S, Berg G, Dominiczak AF. NAD(P)H oxidase inhibition improves endothelial function in rat and human blood vessels. Hypertension. 2002;40:755–762. doi: 10.1161/01.hyp.0000037063.90643.0b. [DOI] [PubMed] [Google Scholar]
- Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res. 2003;93:622–629. doi: 10.1161/01.RES.0000092140.81594.A8. [DOI] [PubMed] [Google Scholar]
- Lakatta E, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises: Part III: Cellular and Molecular Clues to Heart and Arterial Aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- Lefevre M, Redman LM, Heilbronn LK, Smith JV, Martin CK, Rood JC, Greenway FL, Williamson DA, Smith SR, Ravussin E. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis. 2009;203:206–213. doi: 10.1016/j.atherosclerosis.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 Mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci. 2009;64:9–20. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate Treatment Improves Age-Associated Vascular Endothelial Dysfunction: Potential Role of Nuclear Factor {kappa}B and Forkhead Box O Phosphorylation. J Gerontol A Biol Sci Med Sci. 2011a;66:409–418. doi: 10.1093/gerona/glq233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ, Seals DR. Aerobic Exercise Reverses Arterial Inflammation with Aging in Mice. Am J Physiol Heart Circ Physiol. 2011b doi: 10.1152/ajpheart.01276.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley JE, Rutter J. Nutrient sensing and metabolic decisions. Comp Biochem Physiol B Biochem Mol Biol. 2004;139:543–559. doi: 10.1016/j.cbpc.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Mote PL, Grizzle JM, Walford RL, Spindler SR. Influence of age and caloric restriction on expression of hepatic genes for xenobiotic and oxygen metabolizing enzymes in the mouse. J Gerontol. 1991;46:B95–B100. doi: 10.1093/geronj/46.3.b95. [DOI] [PubMed] [Google Scholar]
- Myers PR, Tanner MA. Vascular endothelial cell regulation of extracellular matrix collagen: role of nitric oxide. Arterioscler Thromb Vasc Biol. 1998;18:717–722. doi: 10.1161/01.atv.18.5.717. [DOI] [PubMed] [Google Scholar]
- Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- Nosaka T, Tanaka H, Watanabe I, Sato M, Matsuda M. Influence of regular exercise on age-related changes in arterial elasticity: mechanistic insights from wall compositions in rat aorta. Can J Appl Physiol. 2003;28:204–212. doi: 10.1139/h03-016. [DOI] [PubMed] [Google Scholar]
- Qamirani E, Ren Y, Kuo L, Hein TW. C-reactive protein inhibits endothelium-dependent NO-mediated dilation in coronary arterioles by activating p38 kinase and NAD(P)H oxidase. Arterioscler Thromb Vasc Biol. 2005;25:995–1001. doi: 10.1161/01.ATV.0000159890.10526.1e. [DOI] [PubMed] [Google Scholar]
- Rateri DL, Moorleghen JJ, Balakrishnan A, Owens AP, 3rd, Howatt DA, Subramanian V, Poduri A, Charnigo R, Cassis LA, Daugherty A. Endothelial cell-specific deficiency of Ang II type 1a receptors attenuates Ang II-induced ascending aortic aneurysms in LDL receptor−/− mice. Circ Res. 2011;108:574–581. doi: 10.1161/CIRCRESAHA.110.222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raub CB, Mahon S, Narula N, Tromberg BJ, Brenner M, George SC. Linking optics and mechanics in an in vivo model of airway fibrosis and epithelial injury. J Biomed Opt. 2010;15:015004. doi: 10.1117/1.3322296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AK, Jones AD, Martono C, Caro WA, Madala S, Hartley CJ. Pulsed Doppler signal processing for use in mice: design and evaluation. IEEE Trans Biomed Eng. 2005;52:1764–1770. doi: 10.1109/tbme.2005.855710. [DOI] [PubMed] [Google Scholar]
- Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA, Berkowitz DE. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol. 2006;101:1751–1759. doi: 10.1152/japplphysiol.00138.2006. [DOI] [PubMed] [Google Scholar]
- Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–958. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar R, Unnikrishnan J, Fu A, Nygren J, Short KR, Schimke J, Barazzoni R, Nair KS. Effects of caloric restriction on mitochondrial function and gene transcripts in rat muscle. Am J Physiol Endocrinol Metab. 2002;283:E38–E43. doi: 10.1152/ajpendo.00387.2001. [DOI] [PubMed] [Google Scholar]
- Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol. 2004;286:H2249–H2256. doi: 10.1152/ajpheart.00854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh N, Sokoya EM. Regulation of cerebral vascular function by sirtuin 1. Microcirculation. 2012;19:336–342. doi: 10.1111/j.1549-8719.2012.00167.x. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, Habitual Exercise, and Dynamic Arterial Compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- Trott DW, Gunduz F, Laughlin MH, Woodman CR. Exercise training reverses age-related decrements in endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol. 2009;106:1925–1934. doi: 10.1152/japplphysiol.91232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie C, Romestaing C, Burelle Y, Safdar A, Tarnopolsky MA, Seadon S, Britton SL, Koch LG, Hepple RT. Lower oxidative DNA damage despite greater ROS production in muscles from rats selectively bred for high running capacity. American journal of physiology. Regulatory, integrative and comparative physiology. 2011;300:R544–R553. doi: 10.1152/ajpregu.00250.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walford RL, Harris SB, Gunion MW. The calorically restricted low-fat nutrient-dense diet in Biosphere 2 significantly lowers blood glucose, total leukocyte count, cholesterol, and blood pressure in humans. Proc Natl Acad Sci U S A. 1992;89:11533–11537. doi: 10.1073/pnas.89.23.11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007;50:219–227. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J. Am. Coll. Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- Wu P, Jiang C, Shen Q, Hu Y. Systematic gene expression profile of hypothalamus in calorie-restricted mice implicates the involvement of mTOR signaling in neuroprotective activity. Mech Ageing Dev. 2009;130:602–610. doi: 10.1016/j.mad.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hein TW, Wang W, Kuo L. Divergent roles of angiotensin II AT1 and AT2 receptors in modulating coronary microvascular function. Circ Res. 2003;92:322–329. doi: 10.1161/01.res.0000056759.53828.2c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Vasodilation of carotid arteries to acetylcholine (ACh) alone or after pre-treatment with TEMPOL (A), vasodilation to ACh after TEMPOL pretreatment in the absence or presence of the NOS inhibitor, L-NAME (B) (A&B: n = 8–10 per group) and aortic nitrotyrosine, an oxidative stress marker, abundance (C) in young, old ad libitum (AL) and old life-long caloric restricted (CR) B6D2F1 mice; (n = 6–8 per group) Nitrotyrosine is expressed relative to GAPDH to account for differences in protein loading and presented normalized to the Young mean. Representative blots shown below the summary graph (C&D). Values are means ± s.e.m. *P<0.05 vs. Young. † P<0.05 vs. Old AL