Abstract
The prevalence of obesity and related morbidities such as nonalcoholic fatty liver disease (NAFLD) is high among adolescents. Current treatment recommendations for NAFLD focus on lifestyle optimization via nutrition and exercise. After encouraging exercise, many adolescents choose to participate in organized sports, which may lead to use of illicit substances such as anabolic androgenic steroids (AAS) to boost athletic performance. Approximately 3,000,000 individuals use non-therapeutic AAS at supra-physiologic doses in the United States.1 In 2012, 5.9% of adolescent boys reported steroid use in the previous year.2 We anticipate adolescents with pre-existing liver disease are at increased risk for AAS induced hepatotoxicity. We present such a case with IRB approval and written individual patient consent.
Keywords: obesity, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, hepatotoxicity, drug-induced liver disease, anabolic androgenic steroids, liver biopsy, Food and Drug Administration
Patient Case
An 18 year-old male with history of nonalcoholic steatohepatitis (NASH), obesity (BMI 36.5 Kg/m2), and hypertension presented with 3 days of scleral icterus, intermittent emesis and difficulty concentrating and staying awake.
The patient was diagnosed with NASH five years earlier - his liver biopsy demonstrated diffuse moderately severe macrovesicular steatosis, mild portal inflammation and mild portal fibrosis with focal areas of bridging fibrosis. [Figure 1] After lifestyle counseling, the patient lost 30 pounds and played football and basketball in high school. Liver chemistry improved substantially but did not reach normal. Upon current presentation, physical exam demonstrated BMI 34kg/m2, scleral icterus, jaundice to the knees and hepatomegaly to 3 centimeters below the costal margin with mild tenderness to palpation. Labs were notable for cholestasis, and elevated aminotransferases: AST 163 U/L, ALT 229 U/L, GGT 65 U/L, total bilirubin 11 mg/dL and direct bilirubin 10 mg/dL. Coagulation studies were normal. An abdominal ultrasound demonstrated thickening of the gallbladder wall without gallstones. Further evaluation was negative including: acute and autoimmune hepatitis panels, toxicology screen for drugs and alcohol, and viral serologies (HIV, EBV, CMV, adenovirus, enterovirus). Therapy was initiated with ursodeoxycholic acid and lactulose.
Figure 1.
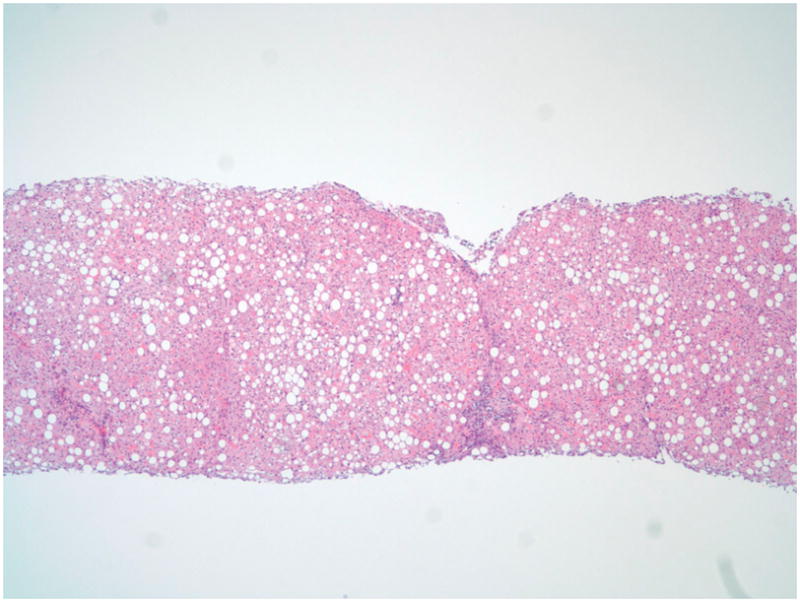
Hepatic parenchyma with panacinar large and small droplet steatosis and focal expansion of portal tracts with mixed chronic inflammation (H&E, 4x)
The patient revealed he took Beastrdrol, an AAS, for 2 weeks prior to symptom onset to increase muscle mass. He took 40 mg daily – the distributor’s recommended dose. He was not taking other medications nor was he consuming alcohol. Despite formally discontinuing AAS use, eight days after presentation direct bilirubin increased to 20.9. A liver biopsy was performed and demonstrated cholestatic hepatitis with hepatocellular and intracanilicular cholestasis, portal and lobular inflammation, fibrous expansion of the portal triads and an inflammatory infiltrate with frequent eosinophils and neutrophils suggestive of immunoallergic drug-induced hepatotoxicity.
Bilirubin levels peaked one month after symptom onset (total bilirubin 38.5, direct 32.6). Cholestyramine was added to the treatment regimen. The most debilitating symptom for this patient was severe pruritus, impairing school performance and sleep. He was treated with hydroxyzine and rifampin. Symptoms finally abated after 3 months.
Discussion
Case reports of AAS induced liver toxicity in adults indicate that most cases will resolve within a few months after discontinuation of AAS.3,4 Our patient’s liver histology was similar to previous reports of AAS abuse induced hepatic injury: cholestatic jaundice with intrahepatic cholestasis.3,5 This is the first case report of AAS induced liver toxicity in an adolescent with NASH. We speculate that pre-existing NAFLD may make adolescents more vulnerable to hepatotoxicity from AAS. Although the exact mechanism for AAS-induced hepatotoxicity is not well-defined in the literature, data from mouse models suggest the mechanism is immunoallergenic resulting from hypersensitivity6. From a pathological standpoint, the fatty liver may be more vulnerable to injury since it has impaired microciruclation, Kupffer cell dysfunction, increased adhesion of leukocytes, impaired mitochondrial function, and ATP depletion.7 Given the prevalence of NAFLD and the illicit use of AAS, there is a need for enhanced diagnosis, education, and awareness.
In the United States, AAS have been listed as Schedule III controlled substances under the Controlled Substances Act since 1990. Possession of AAS without a prescription is a federal crime punishable by up to one year in prison; unlawful distribution or possession with intent to distribute AAS is punishable by up to ten years in prison.8 The legal use of AAS is limited to physician prescription for indications such as replacement of male sex steroids in men with androgen deficiency and counteracting catabolic states such as trauma or HIV wasting.1 Despite these laws, AAS can be purchased online without a prescription. When new AAS are marketed online, there is a natural lag before they are recognized by the FDA and appropriately regulated or banned. Thus, our patient was able to purchase Beastdrol, an AAS, online for $52.99. Regardless of laws and warnings, unrestricted internet access promotes an increased risk for use in adolescents who can purchase AAS without the knowledge of parents or physicians.
In addition to legal risks, AAS abuse poses numerous well-documented health risks: liver disease, hypertension, thrombosis, arrhythmia, acne, hypotrophic hypogonadism, oligospermia, irregular menses, premature epiphyseal closure, and psychiatric dysfunction.1 Prevalence data are lacking due to few empirical studies and speculative data. In addition, minimal literature exists regarding the risk of liver disease among adolescents using AAS.
One factor that may increase the risk for liver toxicity in adolescent males using AAS is that within the pediatric population, adolescent boys have the highest prevalence of NAFLD. However, due to a combination of a lack of specific symptoms and inadequate awareness, most cases remain undiagnosed. Because of their large body habitus, many adolescent boys with NAFLD are encouraged to play football and to proactively get even bigger. The intersection of the risk factors for NAFLD (adolescence, male gender, high BMI) and AAS use may contribute to an increase of cases such as the one presented.
The novelty of this report of AAS induced liver toxicity in an adolescent with pre-existing NASH emphasizes the need for clinicians to have an awareness of this possibility. We now routinely counsel patients with NAFLD to avoid AAS. Moreover, we ask about AAS usage when assessing new onset liver disease in adolescents. To prevent further cases of liver toxicity in adolescent patients with NAFLD, it is of utmost importance to broaden an awareness and understanding among physicians, parents, and children of the dangers of steroid use.
Figure 2.
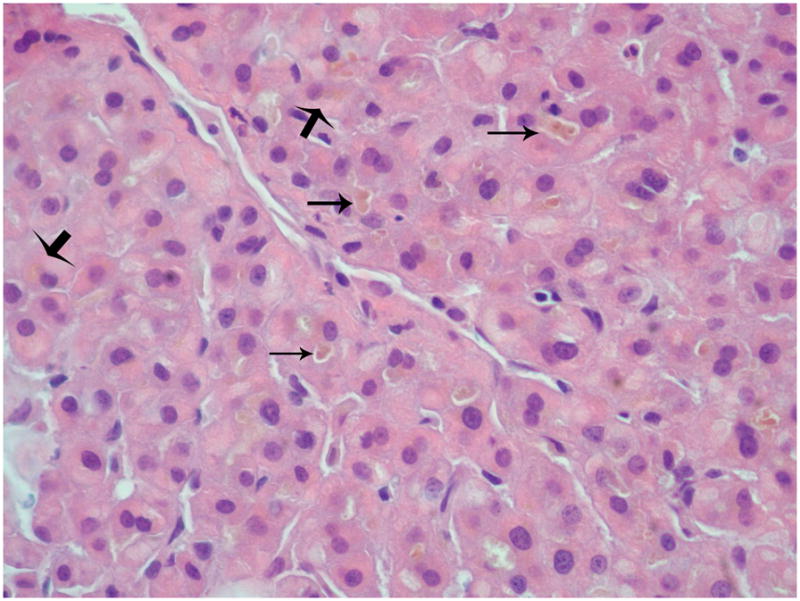
Hepatic parenchyma with hepatocellular and canalicular cholestasis with bile plugs (H&E, 40x). Thin arrows: hepatocellular cholestasis; Thick arrows: canalicular cholestasis
Table 1.
Trend in Laboratory Values
| Initial Diagnosis | 1 Year pre-AAS | AAS Presentation | 1 month post-AAS | 3 months post-AAS | |
|---|---|---|---|---|---|
| AST, U/L | 43 | 31 | 163 | 111 | 48 |
| ALT, U/L | 59 | 57 | 229 | 72 | 57 |
| GGT, U/L | 40 | 34 | 65 | 57 | 27 |
| Alkaline Phosphatase, U/L | 358 | 69 | 94 | 218 | 71 |
| Total Bilirubin, mg/dL | 0.2 | 0.5 | 11.4 | 28.0 | 1.1 |
| Direct Bilirubin, mg/dL | <0.1 | <0.1 | 10.4 | 26.5 | 0.5 |
Acknowledgments
Funding: This work was supported in part by DK088925-02S1. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ALT
alanine aminotransferase
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- AAS
anabolic androgenic steroids
- BMI
body mass index
- HIV
human immunodeficiency virus
- EBV
Epstein-Barr virus
- CMV
cytomegalovirus
- FDA
federal drug administration
References
- 1.Evans NA. Current concepts in anabolic-androgenic steroids. Am J Sports Med. 2004;32:534–42. doi: 10.1177/0363546503262202. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg ME, Wall M, Neumark-Sztainer D. Muscle-Enhancing Behaviors Among Adolescent Girls and Boys. Pediatrics. 2012;130:1019–36. doi: 10.1542/peds.2012-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez-Osorio M, Duarte-Rojo A, Martinez-Benitez B, et al. Anabolic Androgenic Steroids and Liver Injury. Liver International. 2008;2:278–82. doi: 10.1111/j.1478-3231.2007.01579.x. [DOI] [PubMed] [Google Scholar]
- 4.Stimac D, Millic S, Dintinjana RD, et al. Androgenic/anabolic steroid-induced toxic hepatitis. J Clin Gastroenterol. 2002;35:350–52. doi: 10.1097/00004836-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Hall RC, Hall RC. Abuse of supraphysiologic doses of anabolic steroids. South Med J. 2005;98:550–55. doi: 10.1097/01.SMJ.0000157531.04472.B2. [DOI] [PubMed] [Google Scholar]
- 6.Boada LD, Zumbado M, Torres S, et al. Evaluation of acute and chronic hepatotoxic effects exerted by anabolic-androgenic steroid stanozol in adult male rats. Archives of Toxicology. 1999;73:465–72. doi: 10.1007/s002040050636. [DOI] [PubMed] [Google Scholar]
- 7.Varela AT, Rolo AP, Palmeira CM. Fatty liver and ischemia/reperfusion: are there drugs able to mitigate injury? Curr Med Chem. 2011;18:4987–5002. doi: 10.2174/092986711797535164. [DOI] [PubMed] [Google Scholar]
- 8.US Department of Justice, Drug Enforcement Administration; Jan 3, 2007. [Accessed June 11, 2012]. Title 21 United States Code (USC) Controlled Substances Act, 841-Prohibited Acts A, 844-Penalty for Simple Possession. web site. Available at: http://www.deadiversion.usdoj.gov/21cfr/21usc/index.html. [Google Scholar]


