Abstract
Streptozotocin (STZ) is a selective pancreatic β cell toxin used to generate experimental hyperglycemia in rodent models. Several laboratory animal protocols suggest that STZ be administered to fasted rodents to minimize competition between STZ and glucose for low affinity GLUT2 transporters on β cells. However, whether the diabetogenic effects of multiple low dose (MLD)-STZ administration are enhanced by fasting has not been addressed. Given that repeated bouts of fasting can cause undue metabolic stress in mice, we compared the efficacy of MLD-STZ injections (50 mg/kg body weight daily for 5 days) to induce experimental hyperglycemia in both NOD/SCID/γchainnull and C57BL/6J mice that were either ad libitum fed (STZ-Fed) or that had been fasted for 6 h (STZ-Fasted) prior to the time of STZ administration. Both STZ-Fed and STZ-Fasted mice had significantly worse glucose tolerance than vehicle-treated control mice 10 days after initiation of the MLD-STZ regimen. In C57BL/6J mice, fasting glucose levels, serum insulin levels, β cell mass, and glucose disposal during intraperitoneal glucose tolerance tests (IPGTTs) were indistinguishable between STZ-Fed and STZ-Fasted mice 20 days after MLD-STZ. The glucose intolerant phenotypes persisted for 20 weeks thereafter, irrespective of whether C57BL/6J mice were fed or fasted at the time of STZ injections. However, STZ-Fasted C57BL/6J mice experienced significant weight loss during the repeated bouts of fasting/re-feeding that were required to complete the MLD-STZ protocol. In summary, induction of experimental hyperglycemia can be achieved using the MLD-STZ protocol without repeated bouts of fasting, which have the potential to cause metabolic stress in laboratory mice.
Keywords: streptozotocin, fasting, diabetes, animal protocol refinement
Streptozotocin (STZ) is a broad spectrum antibiotic with unique toxic selectivity for the β cells in the pancreatic islets of Langerhans. Clinically, STZ has been used in the oncology field for the treatment of meta-static insulinomas.1–3 Aside from this clinical application, STZ has been used experimentally since the early 1960s in diabetes research as a tool to induce hyperglycemia in laboratory rodents via selective β cell destruction. STZ-induced β cell death has been attributed to different mechanisms.4–6 STZ contains a nitroso moiety, and once inside β cells, STZ can act as a nitric oxide (NO) donor to create reactive oxygen species that induce β cell death.7 STZ has also been shown to increase O-linked protein glycosylation in rat islets by inhibiting O-GlcNAc-selective N-acetyl-beta-D-glucosaminidase.8 Structurally, STZ contains a DNA alkylating moiety conjugated to the two carbon position of glucose, and the cytotoxic effects of STZ on β cells have also been attributed to its ability to alkylate DNA. However, DNA alkylation per se may not be primarily responsible for β cell destruction. Rather, STZ-induced DNA damage triggers poly-ADP-ribosylation, a process that significantly depletes cellular NAD+ levels and adenosine triphosphate (ATP) stores resulting in β cell death and necrosis.5
When delivered by intraperitoneal injection as a single large dose (180–190 mg/kg body weight), STZ causes massive β cell necrosis within 2–3 days after administration. This bolus method of STZ administration is typically employed to achieve complete ablation of recipient β cells before islet transplantation.9,10 One moderate dose of STZ (150 mg/kg body weight) in conjunction with high fat feeding can also accelerate β cell endoplasmic reticulum (ER) stress and has recently been used to model advanced Type 2 diabetes in mice.11 By contrast, multiple low dose (MLD)-STZ injections (e.g. 35–55 mg/kg body weight/day for 4–5 consecutive days) are often used to model T cell dependent autoimmune destruction of pancreatic β cells.10 Compared with a single large ablative dose that induces rapid β cell necrosis, MLD-STZ induces subtoxic effects on β cells, which in most mouse strains is accompanied by acute insulitis.12,13 Although it can be used as a model for insulin-dependent diabetes mellitus (IDDM), the MLD-STZ model does not fully mimic the autoimmune responses associated with Type 1 diabetes, as MLD-STZ induced β cell destruction and hyperglycemia does not require functional T and B cells in mice.14,15
Uptake of STZ into rodent β cells is mediated by GLUT2 transporters, which are highly expressed on β cells in mice and rats. Notably, protocols from the National Institutes of Health (NIH) Diabetic Complications Consortium suggest that mice be fasted for at least 4–6 h prior to MLD-STZ administration.16 This recommendation likely stems from concerns that the STZ will compete with postprandial glucose for GLUT2-mediated uptake into the β cell, which seems logical when one considers that GLUT2 has a low affinity (high Km) for glucose, and glucose transport into β cells via GLUT2 is thought to occur mostly at high blood glucose concentrations during postprandial periods.17,18 Fasting for 6–16 h before MLD-STZ does have diabetogenic effects in both mice and rats.19,20 However, to our knowledge, whether the efficacy of MLD-STZ to induce hyperglycemia is truly enhanced by fasting, or conversely impaired during feeding, has never been addressed before in a mouse model. This is an important consideration given that laboratory mice have also been reported to lose 5–16% of their body weight during fasting,21 suggesting that repeated rounds of food restriction may be a source of considerable stress in experimental mice.
Given this concern and the lack of literature dealing with this aspect of experimental design, we tested whether MLD-STZ has differential effects on the induction of experimental hyperglycemia when administered to fed or fasted mice. Our results show that in two inbred strains of laboratory mice commonly used in diabetes research (NOD/SCID/γchainnull and C57BL/6J mice), MLD-STZ was equally efficacious at inducing glucose intolerance and β cell loss irrespective of whether male mice were fed ad libitum or fasted for 6 h at the time of STZ injections. However, in C57BL/6J mice, 6 h of food withdrawal prior to STZ treatments led to significant cumulative weight loss. Based on these results, we conclude that fasting mice prior to STZ administration is not required for induction of hyperglycemia. Moreover, our findings raise the possibility that repeated rounds of fasting/re-feeding required for MLD-STZ protocols may unnecessarily increase metabolic stress in laboratory rodents. This is an important consideration when refining experimental animal protocols to minimize animal discomfort and stress.
Animals
Immunodeficient male NOD/SCID/γchainnull (NOD–SCID) mice (20 weeks of age) were obtained from a breeding colony at the Indiana University (IU) School of Medicine Laboratory Animal Research Center (LARC) maintained by the IU In Vivo Therapeutic Core within the Simon Cancer Center (Indianapolis, IN, USA).22 Male C57BL/6J mice (8 weeks of age) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The IU School of Medicine Institutional Animal Care and Use Committee approved all mice protocols according to the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines. Mice were fed a sterilized rodent diet (Harlan Teklad LM-485, #7912; Harlan Teklad, Madison, WI, USA). Mice were housed at a density of no more than four per cage in ventilated cages with free access to food and water at a controlled temperature of 23° C with 12 h light–dark cycles (lights on at 07:00 h; lights off at 19:00 h). Normally, the cages were lined with corncob bedding, but the mice were transferred to cages lined with pelleted paper bedding for MLD-STZ injections and while fasting for intraperitoneal glucose tolerance tests (IPGTTs; described below). The mice were anesthesized by intraperitoneal administration of ketamine (60 mg/kg) and xylazine (5 mg/kg) and euthanized by bilateral thoracotomy before tissues were harvested. GraphPad StatMate (GraphPad Software, Inc, San Diego, CA, USA) was used for sample size power calculations based on area under the curve analysis (AUC) during IPGTTs between STZ- and non-STZ-injected mice.
Materials and methods
STZ was purchased from Sigma-Aldrich (St Louis, MO, USA). The drug was solubilized in phosphate buffered saline (PBS; pH 7.4) at a final concentration of 2.92 mg/mL immediately before use and injected intraperitoneally within 5 min of preparation. MLD-STZ (50 mg/kg body weight) was administered daily at 16:00 h for five consecutive days. At the time of STZ injection each day, the mice were either ad libitum fed (herein referred to as STZ-Fed mice) or fasted for 6 h (10:00–16:00 h) (herein referred to as STZ-Fasted mice) with free access to water. Four NOD/SCID mice per experimental group were injected with STZ; one mouse in the NOD–SCID STZ-Fed group died prior to the initial analysis at day 10. Sixteen C57BL/6J mice per experimental group were injected with STZ, and measurable blood glucose data from all the STZ-injected C57BL/6J mice were included for analysis.
Age-matched control mice were fasted for 6 h (Control-Fasted) or ad libitum fed (Control-Fed) before being injected with a PBS vehicle control. C57BL/6J mice were weighed at 10:00 h and again at 16:00 h to compare changes in body weight between treatment groups (STZ-Fed, STZ-Fasted, Control-Fed, and Control-Fasted) during the five-day MLD-STZ treatment protocol and body weights were recorded weekly (at 10:00 h) thereafter.
For IPGTTs, mice were fasted for 6 h (10:00–16:00 h). Glucose (2 g/kg body weight) was injected intraperitoneally and blood glucose concentrations (mg/dL) before (0 min) and 10, 20, 30, 60, 90, and 120 min after intraperitoneal glucose injection were determined from tail vein bleeds using an AlphaTRAK glucometer (Abbott Laboratories, Abbott Park, IL, USA). To examine in vivo glucose stimulated insulin secretion (GSIS), mice were fasted for 6 h and glucose (2 g/kg body weight) was injected intraperitoneally; serum was collected from tail vein bleeds 0, 5, and 10 min after the glucose injections and stored at –80° C before analysis. Serum was collected from ad libitum fed mice at 10:00 h to determine random fed plasma insulin concentrations. Plasma insulin concentrations were measured using an ultra-sensitive mouse insulin ELISA (Crystal Chem, Downers Grove, IL, USA).
Following euthanasia, pancreata were dissected, weighed, and placed in a Z-Fix buffered zinc formalin fixative (Anatech, Battle Creek, MI, USA) for 24 h. Pancreata were paraffin-embedded and sectioned longitudinally at 5 μm intervals. Three sections spaced 25 μm apart from three mice in each experimental group were immunostained for insulin and counterstained with hematoxylin as previously described.23 A Zeiss Z1 inverted microscope equipped with an Orca ER CCD camera (Hamamatsu Photonics, Hamamatsu City, Japan) was used to acquire digital images of the entire stained pancreatic section. The β cell area was calculated using AxioVision software (Carl Zeiss Inc, Thornwood, NY, USA), and the average relative β cell area for each animal was multiplied by pancreatic weight to estimate β cell mass.
Differences between groups were examined for significance using one-way or two-way analysis of variance (ANOVA) as appropriate followed by the Tukey–Kramer post hoc test using GraphPad Prism statistics software (GraphPad Software, Inc). Data are expressed as means ± SEM. P values <0.05 were considered significant.
Results
MLD-STZ is equally diabetogenic in immunodeficient mice whether administered in the fed or fasted state
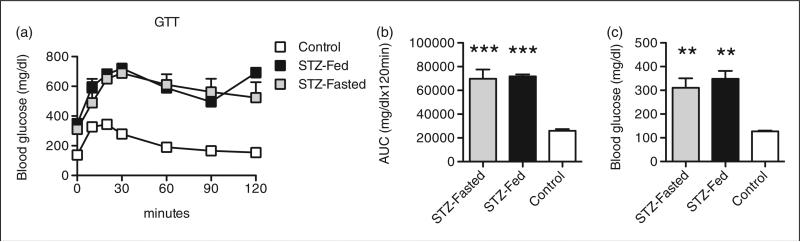
The diabetogenic effects of MLD-STZ are reportedly magnified in immunocompromised mice.14,15,24 Therefore, the effects of MLD-STZ (50 mg STZ/kg body weight/day for 5 consecutive days) were examined in immunodeficient male NOD/SCID/γchainnull mice that were either ad libitum fed (STZ-Fed mice) or that had been fasted for 6 h (10:00–16:00 h; STZ-Fasted) at the time of daily STZ injections. Mice were subjected to IPGTTs 10 days later to assess glucose homeostasis. Both groups of STZ-treated mice had significantly higher glucose excursions during IPGTTs compared with PBS-injected ad libitum fed NOD/SCID/γchainnull control mice (Figure 1a). The AUC analysis of blood glucose levels during IPGTTs in both groups of STZ-treated mice was significantly larger than in the PBS-injected controls, but, importantly, there was no difference in glucose tolerance between STZ-Fed and STZ-Fasted NOD/SCID/γchainnull mice (Figure 1b). Although STZ was sufficient to induce hyperglycemia within 10 days in both groups of STZ-treated NOD/SCID/γchainnull mice, fasting prior to STZ injections did not alter the development of hyperglycemia in this immunodeficient model as fasting glucose levels were comparable between STZ-Fed and STZ-Fasted mice (Figure 1c). NOD/SCID/γchainnull mice rapidly became ill following STZ administration, preventing continued longitudinal assessment of the effects of MLD-STZ in this strain. However, these data indicate that MLD-STZ was equally efficacious at inducing a severe hyperglycemic phenotype in male immunodeficient mice irrespective of whether the mice were ad libitum fed or fasted before consecutive STZ injections.
Figure 1.
Multiple low dose streptozotocin (MLD-STZ) is equally diabetogenic in immunodeficient mice whether administered in the fed or fasted state. NOD/SCID/γchainnull mice were either ad libitum fed (STZ-Fed) or fasted (6 h; STZ-Fasted) at the time of MLD-STZ injections (50 mg STZ/kg body weight/day for 5 consecutive days). (a) Results of intraperitoneal glucose tolerance tests (IPGTTs) performed on treated mice 10 days after MLD-STZ. IPGTTs were performed as described in the Materials and methods section. (b) Area under curve (AUC) analyses for IPGTTs. (c) Fasting (6 h) blood glucose concentrations in treated mice. Results are expressed as the means ± SEM, n = 3–5 mice per group. **P < 0.01, ***P < 0.001 compared with phosphate buffered saline (PBS)-treated NOD/SCID/γchainnull mice that were ad libitum fed (Control) at the time of injection.
Fasting does not alter STZ-induced hyperglycemia in C57BL/6J mice
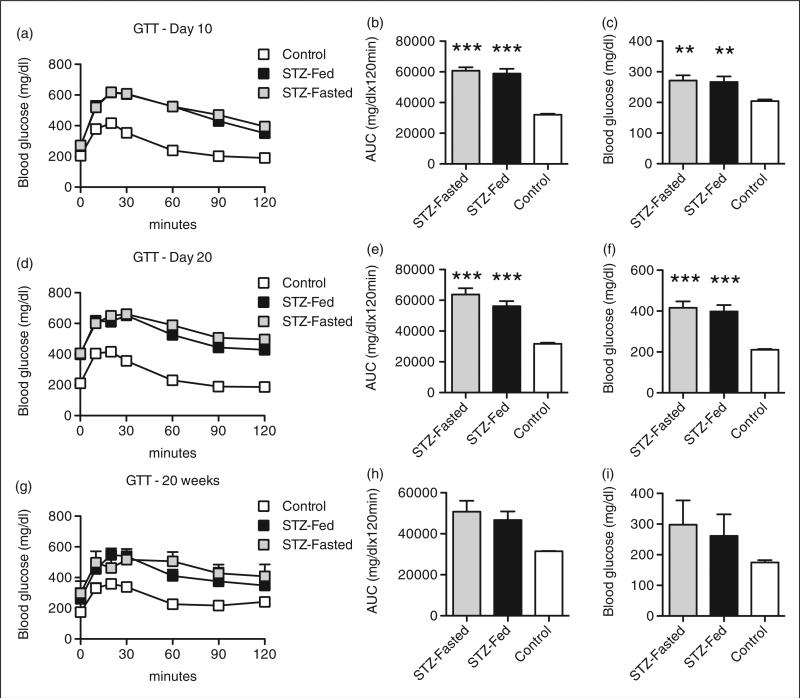
C57BL/6J mice are a commonly used inbred strain in the diabetes literature and many knockout and transgenic mouse models are generated and described on congenic C57BL/6 backgrounds. Thus, ad libitum fed and fasted (6 h) male C57BL/6J (8 weeks of age) mice were treated with MLD-STZ (50 mg/kg body weight/day) for five consecutive days. STZ-treated C57BL/6J mice had impaired glucose tolerance compared with PBS-treated control mice 10 days after MLD-STZ (Figure 2a). However, AUC analysis of blood glucose levels during IPGTTs showed that glucose intolerance was comparable in the STZ-Fed and STZ-Fasted mice (Figure 2b). Both groups of STZ-treated mice were frankly hyperglycemic at this early time point (Figure 2c). IPGTTs performed 20 days after MLD-STZ revealed that STZ-induced glucose intolerance persisted in both STZ-Fed and STZ-Fasted C57BL/6J mice, but again there were no differences between the two groups (Figures 2d and e). Hyperglycemia was detectable 20 days after MLD-STZ injections in both STZ-Fed and STZ-Fasted mice, with 87% (14 out of 16) of the STZ-Fasted mice and 93% (15 out of 16) of the STZ-Fed mice demonstrating fasting blood glucose values greater than 250 mg/dL. In aggregate, fasting at the time of MLD-STZ did not further exacerbate STZ-induced hyperglycemia (Figure 2f). STZ-induced glucose intolerance and hyperglycemia tended to be worse in STZ-treated mice when these parameters were re-evaluated 20 weeks after STZ injections, but glycemia was more variable at this later time point in both the STZ-Fed and STZ-Fasted groups and statistically not different to the PBS-Fed mice (Figures 2g–i). Importantly, AUC analyses of GTT data from STZ-Fed and STZ-Fasted mice were essentially indistinguishable at this later time point. Collectively, these data indicate that the development of MLD-STZ-induced glucose intolerance and hyperglycemia is similar regardless of whether C57BL/6J mice are ad libitum fed or fasted before consecutive STZ injections.
Figure 2.
Fasting does not alter streptozotocin (STZ)-induced hyperglycemia in C57BL/6J mice. Male C57BL/6J mice were either ad libitum fed (STZ-Fed) or fasted (6 h; STZ-Fasted) at the time of multiple low dose (MLD)-STZ injections (50 mg STZ/kg body weight/day for 5 consecutive days). (a) Results of intraperitoneal glucose tolerance tests (IPGTTs) performed on treated mice 10 days after MLD-STZ. (b) Area under curve (AUC) analyses for IPGTTs. (c) Fasting (6 h) blood glucose concentrations in treated mice 10 days after MLD-STZ. (d) Results of IPGTTs performed on treated mice 20 days after MLD-STZ. (e) AUC analyses for IPGTTs. (f) Fasting (6 h) blood glucose concentrations in treated mice 20 days after MLD-STZ. (g) Results of IPGTTs performed on treated mice 20 weeks after MLD-STZ. (h) AUC analyses for IPGTTs. (i) Fasting (6 h) blood glucose concentrations in treated mice 20 days after MLD-STZ. Results are expressed as the means ± SEM. (a–f) n = 15–25 mice per group. (g–i) n = 4–5 mice per group. **P < 0.01, ***P < 0.001 compared with Control mice.
β cell function is impaired and β cell mass is decreased to a similar extent in STZ-Fed and STZ-Fasted C57BL/6J mice
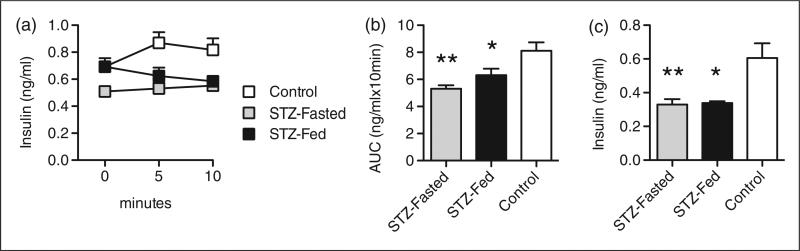
To assess whether fasting mice during MLD-STZ differentially alters β cell function compared with administering STZ to ad libitum fed mice, in vivo GSIS experiments were performed in STZ-Fed and STZ-Fasted C57BL/6J mice 10 days after MLD-STZ injections. As expected, serum insulin levels in the control mice (PBS-treated) increased transiently in response to an intraperitoneal glucose challenge (2 g/kg body weight) (Figure 3a). By contrast, an intraperitoneal glucose challenge failed to result in an increase in serum insulin levels in either the STZ-Fed or STZ-Fasted mice (Figure 3a). The AUC analysis of insulin secretion over 10 min was significantly decreased in the STZ-Fed and STZ-Fasted C57BL/6J mice compared with the PBS-Fed controls, and no difference was observed between the two STZ-treated groups (Figure 3b). Likewise, random fed insulin levels 25 days after MLD-STZ were measured and found to be identically decreased in both STZ-treated groups compared with the PBS-treated control mice (Figure 3c). These data reveal that MLD-STZ is able to induce β cell dysfunction to a similar extent in C57BL/6J mice that are ad libitum fed or fasted before consecutive STZ injections.
Figure 3.
β cell function is impaired in streptozotocin (STZ)-Fed and STZ-Fasted C57BL/6J mice. (a) In vivo glucose stimulated insulin secretion (GSIS) in treated C57BL/6J mice 10 days after multiple low dose (MLD)-STZ. (b) Corresponding area under curve (AUC) analyses for GSIS. (c) Random fed serum insulin levels (ng/mL) in treated C57BL/6J mice 25 days after MLD-STZ. Results are expressed as the means ± SEM. n = 5 mice per group. *P < 0.05, **P < 0.01 compared with Control mice that were ad libitum fed at the time of injection.
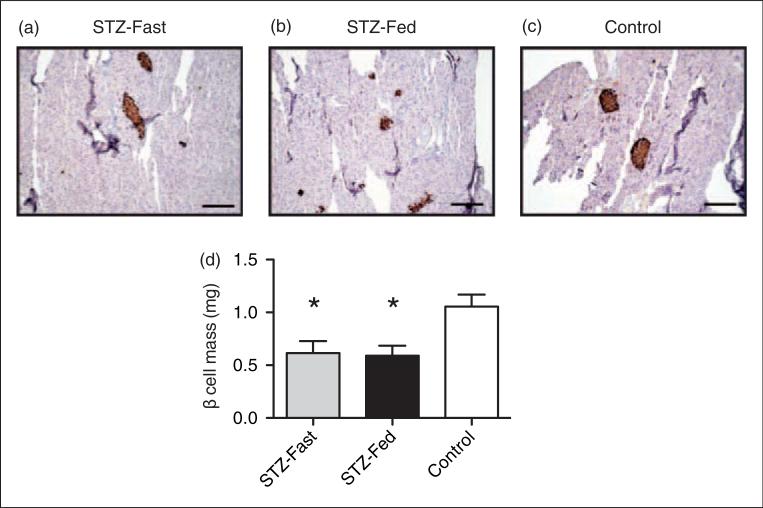
Islet area and β cell mass were also measured in the STZ-Fed and STZ-Fasted C57BL/6J mice and compared with the PBS-Fed mice. Both the STZ-Fed and STZ-Fasted mice had smaller islets than the control mice (Figures 4a–c). Morphometric analysis showed a 50% reduction in β cell mass in both groups of STZ-treated mice compared with the controls, and again no difference was observed between the STZ-Fasted and STZ-Fed mice (Figure 4d). This suggests that reduced β cell mass in response to MLD-STZ occurs independently of whether the drug is administered under fed or fasting conditions.
Figure 4.
β cell mass is decreased to a similar extent in streptozotocin (STZ)-Fed and STZ-Fasted C57BL/6J mice. Pancreata from treated C57BL/6J mice were harvested 25 days after multiple low dose (MLD)-STZ injections and sectioned. Sections were stained for insulin and counterstained with hemotoxylin. (a–c) Representative pancreatic sections at 10× magnification are shown from each treatment group. (d) Average β cell mass in treated mice. Results are expressed as the means ± SEM. n = 4 mice per group. *P < 0.05 compared with Control mice that were ad libitum fed (phosphate buffered saline (PBS)-Fed) at the time of injection.
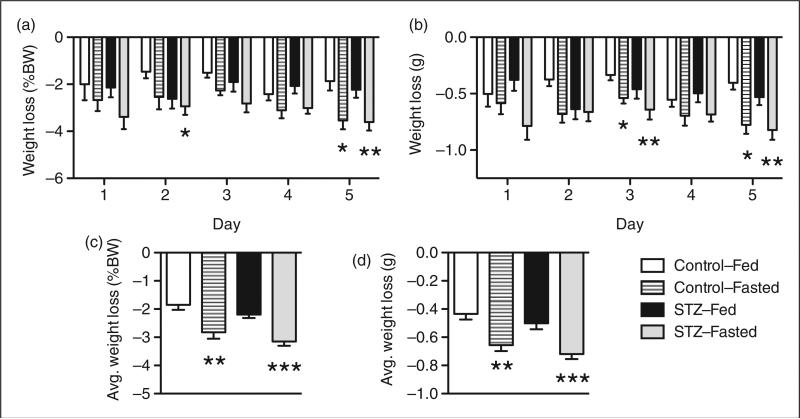
Repeated bouts of fasting result in weight loss in C57BL/6J mice
Laboratory mice have been reported to lose up to 5% of their body weight during a 6 h fast and nearly 16% of their body weight during a 16 h fast.21 The STZ-Fed and STZ-Fasted C57BL/6J mice were weighed daily the morning before (10:00 h) and again at the time of the STZ or PBS injections (16:00 h) for five consecutive days to monitor changes in body weight during the treatment protocol. In parallel, two control groups of mice were either fed (Control-Fed) or fasted (Control-Fasted) prior to PBS injections. When comparing the absolute and relative (% body weight) changes in body weight across the four treatment groups, the fasted mice tended to lose more body weight each day than the ad libitum fed mice (Figures 5a and b). During the five bouts of fasting/re-feeding, both the Control-Fasted and STZ-Fasted C57BL/6J mice experienced significant weight loss compared with the Control-Fed and STZ-Fed groups (Figure 5c and d). Over the five-day treatment period, the Control-Fasted and STZ-Fasted mice lost an average of 2.8% (2.825 ± 0.38%) and 3.2% (3.153 ± 0.37%) of their initial body weight each day, whereas the Control-Fed and STZ-Fed mice lost an average of 1.9% (1.850 ± 0.37%) and 2.2% (2.195 ± 0.38%) of their initial body weight. These data suggest that repeated bouts of fasting are a source of metabolic stress in C57BL/6J mice.
Figure 5.
Repeated bouts of fasting results in weight loss in C57BL/6J mice. Male C57BL/6J mice were weighed at the start (10:00 h) and end (16:00 h) of the fasting period daily for five days. (a) Relative (% body weight) and (b) absolute (g) daily weight loss in C57BL/6J mice during multiple low dose streptozotocin (MLD-STZ) injection protocol. (c) Average relative (% body weight) and (d) absolute (g) daily weight loss for each treatment group calculated from the average weight lost during days 1–5 of MLD-STZ injections. Values were expressed as % body weight and absolute values in grams. Results are expressed as the means ± SEM. n = 15–16 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001 compared with Control mice that were ad libitum fed (Control-Fed) at the time of injection.
Discussion
The main focus of our study was to provide objective data on whether midday fasting (6 h) is required for MLD-STZ to induce hyperglycemia in two strains of laboratory mice commonly used in the diabetes literature. We found that fasting NOD/SCID/γchainnull and C57BL/6J mice for 6 h before MLD-STZ injections did not enhance the effects of STZ when compared directly with ad libitum fed mice that received MLD-STZ at the same time. In our study, MLD-STZ was equally efficacious at inducing glucose intolerance, hyperglycemia, β cell dysfunction, and β cell loss irrespective of whether male mice were ad libitum fed or fasted for 6 h at the time of STZ injections. Notably, repeated bouts of fasting combined with STZ had detectable consequences on body weight regulation in C57BL/6J mice, as fasted mice treated with STZ lost on average 3.2% of their starting body weight each day of a 6 h fast.
The NIH Diabetic Complications Consortium suggests that mice be fasted for at least 4–6 h prior to MLD-STZ administration.16 Others have reported fasting laboratory rodents for durations ranging from 4–16 h before administering MLD-STZ to induce experimental hyperglycemia.19,20 Clearly, fasted rodents respond to MLD-STZ; however, whether fasting is advantageous or necessary for the diabetogenic effects of MLD-STZ is less clear. The rationale for fasting mice before administering MLD-STZ is also debatable. While it is conceivable that STZ may compete with glucose for uptake into β cells via low affinity GLUT2 transporters during postprandial periods, there are little published data to support this concern. In fact, intravenous infusion of D-glucose did not prevent β cell damage when a single dose of STZ (60 mg/kg body weight) was given to rats that had been fasted for 24 h25 By contrast, two non-metabolizable forms of glucose (3-O-methyl-D-glucose (3-OMG) and 2-deoxy-D-glucose) were able to partially protect against STZ-induced hyperglycemia in the same experimental setting when administered immediately prior to STZ.25 In a follow-up study, 3-OMG administered immediately prior to MLD-STZ also delayed insulitis and diabetes by two weeks in outbred CD-1 mice.13 These data suggest that β cell glucose metabolism, not GLUT2-mediated uptake, is the limiting factor for MLD-STZ efficacy. In agreement with this, our experimental findings indicate that repeated rounds of fasting reported in some MLD-STZ protocols do not actually improve or enhance the efficacy of MLD-STZ to induce a hyperglycemic phenotype in inbred mouse strains.
To fast or not to fast experimental mice during MLD-STZ injection protocols is an important question that should be carefully considered when refining experimental protocols in an effort to minimize animal discomfort and stress. Mice have very high metabolic rates and, although they are primarily nocturnal eaters, laboratory mice can consume up to 30% of their food during the light cycle.21 This indicates that mice in the laboratory setting with free access to food and water are never truly fasting, and are therefore likely not conditioned for the metabolic stressors of acute daytime food restriction. In support of this idea, previous reports show that laboratory mice can lose 5–16% of their body weight during prolonged fasting performed as part of physiological assessments.21 Fasting-induced weight loss is primarily thought to be due to reductions in fat and muscle tissue mass, but fasting also reduces liver glycogen stores.21 While fasting-induced weight loss in C57BL/6J mice was somewhat smaller in our study, our data demonstrate that repeated cycles of fasting combined with STZ can negatively impact body weight regulation in mice without improving the effects of MLD-STZ. While it is admittedly difficult to assess stress in rodents undergoing standard laboratory procedures, collectively these findings predict that repeated rounds of food restriction during MLD-STZ protocols could be a source of undesirable metabolic stress in experimental cohorts of mice. This is an important consideration, given that C57BL/6J mice are a commonly used inbred strain and many knockout and transgenic mouse models are generated on congenic C57BL/6 backgrounds. As our data indicate that ad libitum fed mice respond equally well to MLD-STZ compared with mice that are fasted for 6 h before MLD-STZ, we conclude that fasting mice before MLD-STZ injections can justifiably be considered for omission from animal protocols. We acknowledge that the most consistent differences between experimental groups were observed at 10 and 20 days, and that metabolic data 20 weeks after MLD-STZ was more variable. The lack of statistical significance between groups at 20 weeks may indicate that our study was underpowered to detect differences at later time points, which should have bearing on sample size selection for extended longitudinal studies.
The effects of STZ can often be heterogeneous among various mouse models and additional factors also need to be considered in preparing animal protocols. Certainly, male mice are more susceptible to MLD-STZ than females of the same strain.26 In general, the diabetogenic effects of STZ are magnified in many immunodeficient strains.14,15,24 In our present study, older NOD/SCID/γchainnull mice required euthanasia within two weeks of MLD-STZ, suggesting that lower doses or even fewer doses are needed for longitudinal studies in this immunodeficient strain. By contrast, MLD-STZ induced a very durable phenotype in C57BL/6J mice, and these mice could be studied five months after STZ injections. Together, these data point to the presence of important genetic modifiers that may limit or promote the toxic effects of MLD-STZ in divergent inbred strains.
In addition to the fasting/fed status and genetic background of experimental mice, multiple factors may influence the response to MLD-STZ, including STZ preparation and handling, the time of day that mice are injected, and the site or route of injection. With regard to these factors, we dissolved STZ immediately before injection, maintained it at room temperature, and injected all mice within 5 min of preparation. Many protocols indicate that STZ should only be dissolved in a low pH citrate buffer. However, other groups, including our own, cite good diabetogenic results when preparing STZ in a neutral pH buffer (e.g. in PBS).10,27 There is, however, a time-dependent decrease in the stability of STZ in a neutral pH buffer,27 so the time between mixing and injection for each treated mouse should be minimized. A previous study showed a circadian rhythm for STZ response, with the largest induction of experimental diabetes observed when STZ was delivered at 16:00 h,28 which is the time that we performed our MLD-STZ injections. Finally, intravenous and intraperitoneal injections of STZ have shown similar diabetogenic results, as seen in the literature.10,26 Administration of STZ via intraperitoneal injection is technically easier and can be done with high reproducibility by most trained laboratory staff.
In summary, our results objectively show that multiple low doses of STZ are equally effective in inducing experimental hyperglycemia whether administered under fed or fasted conditions. We conclude that fasting prior to administration of multiple low doses of STZ is not required, nor is it beneficial, for inducing glucose intolerance, hyperglycemia, β cell dysfunction, and β cell loss. Furthermore, fasting may add unnecessary steps to experimental designs and unnecessary metabolic stress to experimental mice.
Acknowledgments
Funding
This work was supported by NIH grants F32 DK091976 (to DLM), T32 DK065549 (to EKS), T32 HL079995 (to TK) and DCC Pilot and Feasibility Award, K08 DK080225, R03 DK 089147, and R01 DK093954 (to CEM), a Clarian/Indiana University Health Values Research Award (to CEM), and a grant from Sigma Beta Sorority, Inc (to CEM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of this manuscript.
References
- 1.Brentjens R, Saltz L. Islet cell tumors of the pancreas: the medical oncologist's perspective. Surg Clin North Am. 2001;81:527–542. doi: 10.1016/s0039-6109(05)70141-9. [DOI] [PubMed] [Google Scholar]
- 2.Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin–doxorubicin, streptozocin–fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519–523. doi: 10.1056/NEJM199202203260804. [DOI] [PubMed] [Google Scholar]
- 3.Dominguez S, Denys A, Madeira I, et al. Hepatic arterial chemoembolization with streptozotocin in patients with metastatic digestive endocrine tumours. Eur J Gastroenterol Hepatol. 2000;12:151–157. doi: 10.1097/00042737-200012020-00004. [DOI] [PubMed] [Google Scholar]
- 4.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 5.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiological research/Academia Scientiarum Bohemoslovaca (Physiol Res) 2001;50:537–546. [PubMed] [Google Scholar]
- 6.Konrad RJ, Kudlow JE. The role of O-linked protein glycosylation in beta-cell dysfunction. Int J Mol Med. 2002;10:535–539. [PubMed] [Google Scholar]
- 7.Kaneto H, Fujii J, Seo HG, et al. Apoptotic cell death triggered by nitric oxide in pancreatic beta-cells. Diabetes. 1995;44:733–738. doi: 10.2337/diab.44.7.733. [DOI] [PubMed] [Google Scholar]
- 8.Konrad RJ, Mikolaenko I, Tolar JF, Liu K, Kudlow JE. The potential mechanism of the diabetogenic action of streptozotocin: inhibition of pancreatic beta-cell O-GlcNAc-selective N-acetyl-beta-D-glucosaminidase. Biochem J. 2001;356(Pt 1):31–41. doi: 10.1042/0264-6021:3560031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levitt HE, Cyphert TJ, Pascoe JL, et al. Glucose stimulates human beta cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia. 2011;54:572–582. doi: 10.1007/s00125-010-1919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deeds MC, Anderson JM, Armstrong AS, et al. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab Anim. 2011;45:131–140. doi: 10.1258/la.2010.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976;193:415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- 13.Rossini AA, Williams RM, Appel MC, Like AA. Complete protection from low-dose streptozotocin-induced diabetes in mice. Nature. 1978;276:182–184. doi: 10.1038/276182a0. [DOI] [PubMed] [Google Scholar]
- 14.Gerling IC, Friedman H, Greiner DL, Shultz LD, Leiter EH. Multiple low-dose streptozocin-induced diabetes in NOD-scid/scid mice in the absence of functional lymphocytes. Diabetes. 1994;43:433–440. doi: 10.2337/diab.43.3.433. [DOI] [PubMed] [Google Scholar]
- 15.Reddy S, Wu D, Elliott RB. Low dose streptozotocin causes diabetes in severe combined immunodeficient (SCID) mice without immune cell infiltration of the pancreatic islets. Autoimmunity. 1995;20:83–92. doi: 10.3109/08916939509001931. [DOI] [PubMed] [Google Scholar]
- 16.Diabetic Complications Consortium NDC http://www.diacomp.org/shared/protocols.aspx.
- 17.Schnedl WJ, Ferber S, Johnson JH, Newgard CB. STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes. 1994;43:1326–1333. doi: 10.2337/diab.43.11.1326. [DOI] [PubMed] [Google Scholar]
- 18.Olson AL, Pessin JE. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu Rev Nutr. 1996;16:235–56. doi: 10.1146/annurev.nu.16.070196.001315. [DOI] [PubMed] [Google Scholar]
- 19.Masson E, Koren S, Razik F, et al. High beta-cell mass prevents streptozotocin-induced diabetes in thioredoxin-interacting protein-deficient mice. Am J Physiol Endocrinol Metab. 2009;296:E1251–1261. doi: 10.1152/ajpendo.90619.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Pan Y, Zhang QY, Wang FM, Kong LD. Quercetin and allopurinol ameliorate kidney injury in STZ-treated rats with regulation of renal NLRP3 inflammasome activation and lipid accumulation. PLoS One. 2012;7:e38285. doi: 10.1371/journal.pone.0038285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemiceuglycemic clamps in the conscious mouse. Diabetes. 2006;55:390–397. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- 22.Cai S, Wang H, Bailey B, et al. Humanized bone marrow mouse model as a preclinical tool to assess therapy-mediated hematotoxicity. Clin Cancer Res (official journal of the American Association for Cancer Research) 2011;17:2195–2206. doi: 10.1158/1078-0432.CCR-10-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans-Molina C, Robbins RD, Kono T, et al. Peroxisome proliferator-activated receptor gamma activation restores islet function in diabetic mice through reduction of endoplasmic reticulum stress and maintenance of euchromatin structure. Mol Cell Biol. 2009;29:2053–2067. doi: 10.1128/MCB.01179-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy S, Chang M, Robinson E. Young NOD mice show increased diabetes sensitivity to low doses of streptozotocin. Ann NY Acad Sci. 2006;1079:109–113. doi: 10.1196/annals.1375.015. [DOI] [PubMed] [Google Scholar]
- 25.Ganda OP, Rossini AA, Like AA. Studies on streptozotocin diabetes. Diabetes. 1976;25:595–603. doi: 10.2337/diab.25.7.595. [DOI] [PubMed] [Google Scholar]
- 26.Leiter EH. Multiple low-dose streptozotocin-induced hyperglycemia and insulitis in C57BL mice: influence of inbred background, sex, and thymus. Proc Nat Acad Sci USA. 1982;79:630–634. doi: 10.1073/pnas.79.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JY, Kim MJ, Moon CK, Chung JH. Degradation products of streptozotocin do not induce hyperglycemia in rats. Biochem Pharmacol. 1993;46:2111–2113. doi: 10.1016/0006-2952(93)90657-i. [DOI] [PubMed] [Google Scholar]
- 28.Candela S, Hernandez RE, Gagliardino JJ. Circadian variation of the streptozotocin-diabetogenic effect in mice. Experientia. 1979;35:1256–1257. doi: 10.1007/BF01963323. [DOI] [PubMed] [Google Scholar]