Abstract
Acute coronary syndromes can give rise to myocardial injury-infarction (MI), which in turn promulgates a series of cellular and extracellular events that result in left ventricular (LV) dilation and dysfunction. Localized strategies focused upon interrupting this inexorable process include delivery of bioactive molecules and stem cell derivatives. These localized treatment strategies are often delivered in a biomaterial complex in order to facilitate elution of the bioactive molecules or stem cell engraftment. However, these biomaterials can impart significant and independent effects upon the MI remodeling process. In addition, significant changes in local cell and interstitial biology within the targeted MI region can occur following injection of certain biomaterials, which may hold important considerations when using these materials as matrices for adjuvant drug/cell therapies.
Introduction
While considerable advancements have been made in terms of achieving early reperfusion with an acute coronary syndrome, myocardial injury still commonly occurs, giving rise to a cascade of adverse biological events and can culminate in a myocardial infarction (MI) and progressive left ventricular (LV) remodeling (Roger et al., 2011; Weir et al., 2006; Pfeffer et al., 1990; Frangogiannis, 2006; Ertl et al., 2005; Frantz et al., 2009; Frangogiannis, 2008). This process, generically termed post-MI remodeling, is a significant contributory factor to the development and progression to heart failure. Specifically, changes in the extracellular matrix (ECM) occur within the MI region, causing structural instability, progressive thinning, and stress upon the adjacent viable myocardium (infarct expansion). Thus, the result is progressive changes in LV geometry and function. Accordingly, the development of strategies that interrupt post-MI remodeling with particular emphasis on the infarct expansion process would hold great clinical significance.
The canonical wound healing process, generally applicable with respect to an MI, occurs in three overlapping phases: inflammation, proliferation, and maturation (Frangogiannis, 2006; Ertl et al., 2005; Frantz et al., 2009; Frangogiannis, 2008). The first phase is the acute period in which reactive oxygen species, bioactive signaling molecules, and peptides released from the local environment cause inflammatory cell recruitment and invasion. The second phase is heralded by proliferation and transdifferentiation of myocardial fibroblasts into myofibroblasts (myoFBs). (Frangogiannis et al., 2000; Darby et al., 1990; Widgerow, 2011; Souders et al., 2009; Porter et al., 2009; Baum et al., 2011). Further, released matrix structural proteins, such as fibrillar collagens, as well as matrix-integrin-cytoskeletal interactions facilitate contraction of the wound. The third phase of the wound healing process normally results in complete contraction of the wound, apoptosis of the myoFBs, and the formation of a relatively acellular scar. However, in the context of post-MI remodeling, this canonical set of events does not necessarily occur. Rather, there is a continued proliferation and transdifferentiation of myoFBs within the MI and border regions, accompanied by a shift in matrix proliferative/degradative pathways within these transformed cells (Dixon et al., 2011; Spinale, 2007; Spinale et al., 2013). Specifically, the relative expression of the ECM proteolytic enzymes, the matrix metalloproteinases (MMPs), is amplified and can increase proteolysis of the newly synthesized and immature collagen fibrils within the MI. As a consequence, the continued proliferation and altered expression of the myoFBs within the MI region may actually contribute to matrix instability and infarct expansion, ultimately leading to adverse LV remodeling. While the basis for the persistence of ECM proteolysis and infarct expansion is likely to be multifactorial, biomechanical factors expectedly play a significant role (Wall et al., 2006; Gorman et al., 2010; Wenk et al., 2011; Macarthur et al., 2013; Dang et al., 2005; Guccione et al., 2001; Wang et al., 2003; Poobalarahi et al., 2006; Santiago et al., 2010; Tomasek et al., 2002). Specifically, as a function of MI wall thinning, increased radial wall stress and local strain patterns occur, which is further compounded by dyskinesis of the affected region. Through ex-vivo and in-vitro studies (Poobalarahi et al., 2006; Santiago et al., 2010; Tomasek et al., 2002), changes in stress and strain patterns have been demonstrated to alter myoFB phenotype and MMP expression. Thus, strategies focused upon altering these biophysical stimuli following MI holds potential in terms of interrupting cellular and proteolytic pathways, which promulgate adverse remodeling. One such strategy is through the use of localized injections of a biomaterial directly into or nearby the MI region and thereby altering local stress-strain patterns. Further, this would in turn interrupt/retard the progressive LV remodeling, which invariably occurs following MI (Wall et al., 2006; Gorman et al., 2010; Wenk et al., 2011; Macarthur et al., 2013; Ifkovits et al., 2010; Iwakura et al., 2003; Kraehenbuehl et al., 2011; Tous et al., 2012; Wu et al., 2011; Garbern et al., 2011; Chen et al., 2010; Dobner et al., 2009; Ryan et al., 2009; Morita et al., 2011; Singelyn et al., 2012; Purcell et al., 2012; Dixon et al., 2011; Ruvinov et al., 2011; Yu et al., 2009; Mukherjee et al., 2008; Solis et al., 2010; Rane et al., 2011; Daneviz et al., 2010; Nakamuta et al., 2009; Hao et al., 2007; Fujimoto et al., 2009; Tous et al., 2011; Okada et al., 2010; Landa et al., 2008; Leor et al., 2009; Kofidis et al., 2004; Dai et al., 2005; Christman et al., 2004; Christman et al., 2006).
The purpose of this review is not to provide a comprehensive overview of the structural characteristics and composition of different biomaterials that have been utilized in the context of post-MI remodeling, as this has been the subject of several reviews (Gorman et al., 2010; Christman et al., 2006; Jain et al., 2008; Sisco et al., 2008; Wilson et al., 2009). Rather, the purpose of this review is to place these findings in a more physiological context in terms of translational findings and potential relevance to clinical applications. The review will first examine the consequences of direct injections of relatively non-durable materials, which will be defined as those materials with approximately 50% or more degradation/absorption within 30 days, and that of more durable, non-degradable materials. While these classifications are an oversimplification and are arguably arbitrary, it will provide a framework for identifying some functional consequences of these approaches in terms of post-MI remodeling. The focus will then shift to the specific effects of these biomaterials upon changes in critical determinants of post-MI remodeling, such as myoFBs and MMP profiles. Finally, the relevance of these findings in terms of next steps in translational/clinical applications of biomaterial injections and post-MI remodeling will be briefly discussed.
Non-Durable Biomaterials and Post-MI Remodeling
In terms of degradable/non-durable materials for myocardial injections, these can take the form of modifications of naturally occurring substances, such as fibrin, chitosan, or decellularized collagen matrix (Singelyn et al., 2012; Okada et al., 2010; Kofidis et al., 2004; Dai et al., 2005). Regarding synthesized compounds, the most commonly used are with methacrylates, acrylamides, or poly(ethylene glycol; PEG) based materials that undergo gelation at body temperature (Ifkovits et al., 2010; Iwakura et al., 2003; Kraehenbuehl et al., 2011; Tous et al., 2012; Wu et al., 2010; Garbern et al., 2011; Ryan et al., 2009; Fujimoto et al., 2009; Tous et al., 2011). These materials are considered injectable as they can be formulated in a viscous gel that will allow for loading into syringes and injections with relevant caliber needles. Very often, these materials will require an activation substance; for example, fibrin injections will require co-administration of thrombin and calcium. In this case, the mixture must be rapidly delivered upon mixing or can be delivered by a dual barrel syringe system. The synthetic materials have several advantages in that these are more “tunable” in terms of gelation times, stiffness modulus, and degradation characteristics (Ifkovits et al., Tous et al., 2012; Fujimoto et al., 2009; Tous et al., 2011; Jain et al., 2008).
Effects on LV Remodeling
The use of both natural and synthesized degradable materials has been evaluated in both rodent and large animal models of MI. Those studies that have incorporated indices of LV function and/or geometry in regards to primary response variables are summarized in Table 1. The predominant experimental design is to inject these biomaterials at the time of the initial coronary occlusion. However, a number of studies have delayed the time of injection in order to allow for the formation of a well formed MI region (Wu et al., 2011; Singelyn et al., 2012; Landa et al., 2008; Dai et al., 2005). Irrespective of the timing of the injections, the overarching findings from these studies was that when a biomaterial was injected within the MI region, an increase in LV wall thickness of the affected region was realized with a subsequent reduction in LV dilation. Since LV dilation is one of the critical determinants with respect to clinical outcomes, a relative reduction in the rate and extent of LV dilation with this localized injection approach is highly relevant. What is most likely the underlying mechanism for this effect is the local increase in MI thickness, which in turn will alter the stress and strain upon this region (Wall et al., 2006; Gorman et al., 2011; Wenk et al., 2011; Macarthur et al., 2013). This localized increase in LV wall thickness will also reduce circumferential radial wall stress patterns, which would have particularly relevant effects in the post-MI context. First, reduced LV wall stress will reduce myocardial oxygen demand, and thereby reduce metabolic demands upon viable myocytes along the border region of the MI. This would likely contribute to a reduction in infarct expansion and improved LV performance. Secondly, reduced LV wall stress will effectively reduce LV afterload. Since LV afterload is inversely proportional to systolic performance, the relative reduction in LV wall stress achieved by biomaterial injection in the post MI period would in turn potentially increase indices of LV pump function. Indeed, in those studies where this physiological variable was measured, the relative decline in LV pump function was attenuated when compared to untreated, time matched MI values. While one fundamental premise of all of these compounds is to provide a relatively non-compressive material within the MI region after injection, a number of biological effects relevant to post-MI remodeling may also occur, which includes angiogenesis, alterations in the immune/inflammatory response, and the homing/engraftment of both endogenous as well as exogenously delivered stem cells (Kraehenbuehl et al., 2011; Chen et al., 2010; Kofidis et al., 2004; Christman et al., 2004; Christman et al., 2006; Jain et al., 2008; Sisco et al., 2008; Wilson et al., 2009).
Table 1.
Non-Durable Injectable Biomaterials: Effects of Post MI Remodeling with Biomaterial Alone
| Material | MI Model | LV Remodeling Response Variable | Reference |
|---|---|---|---|
| Small Intestinal Submucosa Gel | Mice | ↑ LVWTh; ↓ EDV | Okada 2010 |
| Poly(NIPAAm-co-PAA-co-BA) | Rat | ↑ LVWTh; ↑ LVFx | Garbern 2011 |
| Polyethylene Glycol Hydrogel (MMP crosslinker) | Rat | ↑ LVFx; ↓ EDV | Kraehenbuehl 2011 |
| Aliphatic Polyester Hydrogel | Rat | ↑ LVWTh; ↑ LVFx; ↓ EDV | Wu 2011 |
| Decellularized Ventricular Extracellular Matrix derived Hydrogel | Rat | ↑ LVFx; ↓ EDV | Singelyn 2012 |
| Poly(NIPAAm-co-AAc-co-HEMAPTMC) Hydrogel | Rat | ↑ LVWTh; ↑ LVFx; ↓ EDV | Fujimoto 2008 |
| Matrigel | Rat | ↑ LVWTh; ↑ LVFx | Kofidis 2004 |
| Fibrin | Rat | ↑ LVWTh; ↓ EDV | Danoviz 2010, Nakamuta 2009 |
| Fibrin Glue | Rat | ↑ LVWTh | Christman 2004 |
| Collagen | Rat | ↑ LVWTh; ↑ LVFx | Dai 2005 |
| Hydroxy-ethyl Methylacrylated Hyaluronic Acid (HeMA-HA) | Sheep | ↑ LVWTh; ↑ LVFx; ↓ EDV | Tous 2011 |
Non-Durable defined as ≥50% biomaterial degraded within 30 days post-injection.
↑: LVWTh: Increased LV Wall Thickness
↑: LVFx: Increased LV Pump Function
↓: EDV: Reduced LV Dilation
→: No Significant Change from Control
In terms of the effects of these biomaterials on LV function and geometry, the type of material (natural versus synthetic) and the bulk amount of the material injected are likely important considerations. Dai et al injected a purified bovine collagen containing predominantly type I collagen into rats at 7 days post-MI (Dai et al., 2005). These investigators reported an increase in relative LV wall thickness within the MI region and improved regional wall motion up to 6 weeks following injection. Using a decellularized porcine ECM injected into rats at 2 weeks post-MI, Singelyn and colleagues reported reduced post-MI dilation as assessed by magnetic resonance imaging (Singelyn et al., 2012). Using a porcine small intestine submucosa preparation injected into immunodeficient mice (Okada et al., 2010), a reduction in LV dilation was observed at 6 weeks post-MI. This study also identified that the injection of this preparation was associated with increased growth factor signaling. Using an ECM material derived from a sarcoma cell line (Matrigel) (Kofidis et al., 2004), Kofidis et al reported a modest effect of this material in a rat MI model. Specifically, while this study focused primarily on the use of this ECM material as a substrate for embryonic stem cell delivery, injection of Matrigel alone augmented LV posterior wall thickness when compared to MI only values. Thus, an important consideration that will be a theme throughout this review is that the biomaterial alone can impart significant and independent effects upon the post-MI remodeling process.
In contrast to decellularized or synthesized ECM, synthetic biomaterials with somewhat longer durability and perhaps less direct biological/immune effects have been the focus of intense investigation in terms of post-MI remodeling (Kraehenbuehl et al., 2011; Wu et al., 2010; Garbern et al., 2011; Ryan et al., 2009; Fujimoto et al., 2009; Tous et al., 2011). Using hyaluronic acid based hydrogels, it has been demonstrated that alterations in the formulation can yield relative changes in degradation times (Tous et al., 2011). Several past studies suggest that the biophysical characteristics of the hydrogel regarding stiffness and durability are important determinants in terms of the effects on post-MI remodeling (Ifkovits et al., 2010; Tous et al., 2011). A clear advantage of these synthesized materials is that the biophysical properties can be regulated. For example, bioactive molecules can be captured within these biomaterials, and following injection, in-vivo degradation will result in release of these molecules (Wu et al., 2010; Garbern et al., 2011). Using a poly-acrylamide based hydrogel, Garbern et al mixed a basic fibroblast growth factor in the biomaterial for subsequent injection in a rat MI model (Garbern et al., 2011). Both the hydrogel alone and the containing growth factor increased LV posterior wall thickness at the site of the MI, but only the growth factor group resulted in increased capillary density.
Durable Biomaterials and Post-MI Remodeling
One of the more common substances used in this particular category is alginate, which can be modified in terms of cell binding characteristics (Ruvinov et al., 2011; Yu et al., 2009; Mukherjee et al., 2008; Hao et al., 2007). Arguably, alginate and covalently modified alginate degrades over time, but the retention time is quite long (months). Hydroxyapatite based materials (i.e. dermal filler) have also been utilized post-MI and degrade over time, but can be quite slow (months), and therefore can also be classified in this durable category (Wenk et al., 2011; Ryan et al., 2009; Dixon et al., 2011). In addition, poly-ethylene glycol based polymers have been formulated with very long retention characteristics and are used in the post-MI context (Solis et al., 2010; Rane et al., 2011).
Effects on LV Remodeling
The generalized effects of these biomaterials in terms of post-MI remodeling are summarized in Table 2. In the majority of studies, a significant and persistent increase in LV posterior wall (MI region) thickness was achieved following the targeted injection, which was accompanied by a slowing of post-MI dilation. For example, a fibrin-alginate composite was injected into pigs at 7-days post-MI and resulted in a significant reduction in the rate of infarct expansion over a one month follow-up period (Mukherjee et al., 2008). For this approach, a dual barrel syringe system was utilized in order to allow for polymerization of the alginate immediately following injection (Figure 1). Using a hydroxyapatite material (Radiesse) injected into the MI region of sheep, a significant reduction in LV dilation and regional wall stress was reported for up to 8 weeks post-MI (Dixon et al., 2011). Using a hydrogel formulation loaded with increasing concentrations of poly(lactide-co-glycolide) microspheres, the concept that more durable biomaterials causes “tissue bulking”, which has been utilized in other applications, may hold relevance in terms of post-MI remodeling (Tous et al., 2012). Utilizing either alginate or hydroxyapatite, large islands of injected material surrounded by a chronic inflammatory response could be readily detected within the injected region a month following injection (Ryan et al., 2009; Dixon et al., 2011; Mukherjee et al., 2008). However, the finding that a long lasting, durable material favorably alters the course of post-MI remodeling is not uniform. Specifically, when using a polyethylene glycol gel injected into the MI region of rats, there were no detectable changes in key variables of the post-MI remodeling process (Rane et al., 2011).
Table 2.
Durable Injectable Biomaterials: Effects of Post MI Remodeling with Biomaterial Alone
| Material | MI Model | LV Remodeling Response Variable | Reference |
|---|---|---|---|
| Alginate Hydrogel | Rat | ↑ LVWTh; ↑ LVFx; ↓ EDV | Yu 2009 |
| Affinity-binding Alginate | Rat | ↑ LVWTh | Ruvinov 2011 |
| Alginate Hydrogel | Rat | → | Hao 2007 |
| Poly(ethylene glycol) gel | Rat | ↑ LVWTh; ↑ LVFx; ↓ EDV | Dobner 2009, Rane 2011 |
| Calcium-Crosslinked Alginate | Rat | ↑ LVWTh; ↓ EDV | Landa 2008 |
| Fibrin Alginate Composite | Pig | ↑ LVWTh; ↑ LVFx; ↓ EDV | Mukherjee 2008 |
| Calcium-Crosslinked Alginate | Pig | ↑ LVWTh; ↓ EDV | Leor 2009 |
| Calcium Hydroxyapatite | Sheep | ↑ LVWTh; ↑ LVFx; ↓ EDV | Dixon 2011,Morita 2011,Ryan 2009 |
| Polyvinyl-alcohol Hydrogel | Sheep | ↑ EDV | Solis 2010 |
| HeMA-HA+poly(lactide-co-glycolide) | Sheep | ↑ LVWTh; ↓ EDV | Toues 2012 |
| Methacrylated Hyaluronic Acid (MeHA) | Sheep | ↑ LVWTh; ↑ LVFx; ↓ EDV | Ifkovits 2010 |
Durable defined as ≤50% biomaterial degraded at 30 days post-injection
↑: LVWTh: Increased LV Wall Thickness
↑: LVFx: Increased LV Pump Function
↓: EDV: Reduced LV Dilation
→: No Significant Change from Control
Figure 1.
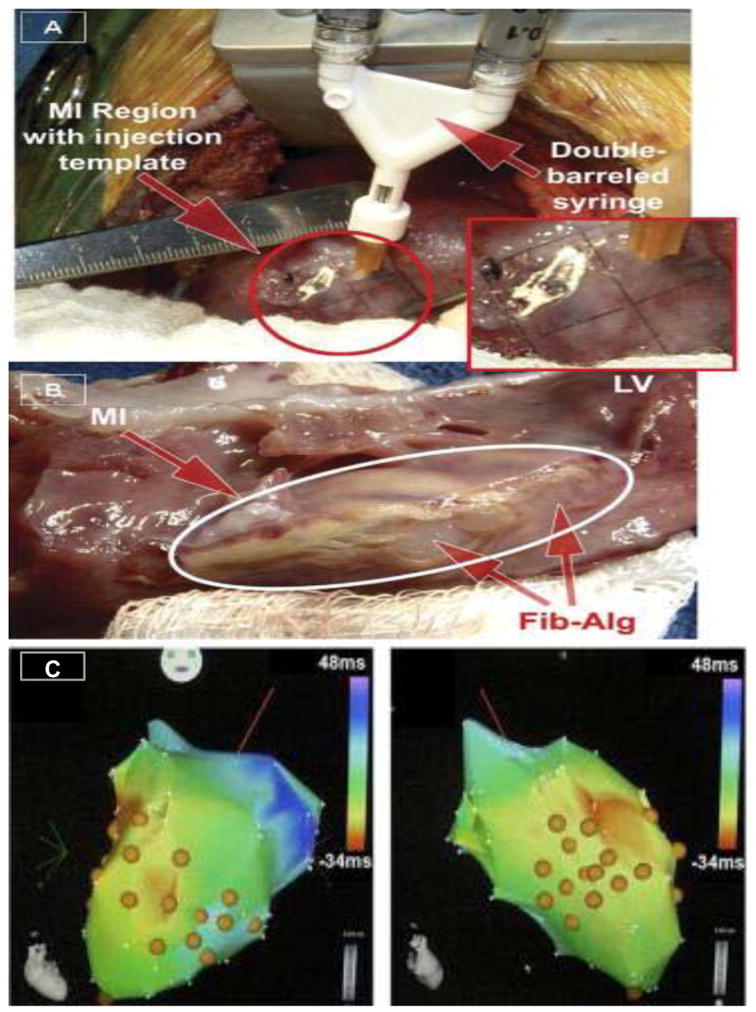
(A) Using a double barrel syringe containing fibrin and alginate composites, targeted injections were performed at uniform positions and depth within the MI region. (Inset) Uniform injections were achieved through the use of a template temporarily sutured to the MI region. (B) At 3 weeks post injection, clear regions of the alginate injections could be visualized within the MI region (arrows). Reproduced from Mukherjee et al., 2008. (C) Porcine decellularized ECM based hydrogel was injected through a percutaneous transcardial delivery approach (MyoStar) and visual guidance of the injections with unipolar electromechanical mapping (NOGA). These are representative images of NOGA maps where the orange spheres indicate sites of ECM hydrogel injections. Thus, catheter based methods to target specific regions of the myocardium for the delivery of biomaterials has moved to the translational research stage. Figure modified from Singelyn et al., 2012, with permission from the American College of Cardiology.
Nanoparticles
Nanoparticle emulsions and composites are likely to be an area of research growth and potential in terms of targeting the MI region, and in general, would be considered a durable or non-degradable entity. The term nanoparticle is used to describe materials that measure between 1 and 100 nm in at least one dimension. These materials are being developed for diagnostic/cellular/molecular imaging applications (Jain et al., 2008), but certain nanoparticles hold potential in terms of altering the local cellular/extracellular environment and therefore relevance to post-MI remodeling. Gold nanomaterials are particularly attractive due to the ease of synthesis and surface modification, low toxicity, and optical properties. Studies using polyelectrolyte-coated gold nanorods demonstrated that the presence of these nanoparticles inhibited cardiac fibroblast contraction of 3-dimensional collagen matrices without compromising cell viability (Sisco et al., 2008; Wilson et al., 2009). These studies demonstrated that the presence of polyelectrolyte-coated nanorods altered the kinetics of collagen assembly in addition to the mechanical properties of the collagen-nanorod composites (Wilson et al., 2009), which may in part account for the lack of fibroblast transformation and reduced contraction observed (Sisco et al., 2008). In addition, the use of the glycosaminoglycans, such as chondroitin sulfate, to coat gold nanoparticles revealed that immobilization of these biomolecules on gold nanorods altered fibroblast phenotype and function in-vitro (Wilson et al., 2009). Due to the fact that these nanoparticle-based materials may hold advantages in terms of immune response and adjunctive use with stem cells, the use of these materials in the context of post-MI remodeling is likely to be an area of significant research development (Dvir et al., 2011; Giraud et al., 2012).
Injected Biomaterials and the Extracellular Matrix
With either a prolonged period of ischemia followed by reperfusion or that of permanent coronary occlusion, the ubiquitous consequence is the formation of collagenous scar within the MI region. While overall collagen content increases to a 10-fold magnitude within the MI region compared to normal myocardium, this does not imply structural stability. Specifically, continuous turnover of the fibrillar ECM component and alterations in collagen stability (i.e. cross-linking) within the MI region can contribute to reduced resistance to deformation with increased intracavitary pressures generated during the cardiac cycle (Dixon et al., 2011; Spinale et al., 2007; Wall et al., 2006; Gorman et al., 2010; Wenk et al., 2011; Dang et al., 2005; Guccione et al., 2001). Despite the importance of the ECM in terms of post-MI remodeling and the likely effects biomaterial injections may have on this entity, quantitative measures of collagen content within the MI region following injections of a biomaterial has not been uniformly performed. With injection of a porcine submucosal preparation, the investigators reported a trend toward reduced overall collagen content within the MI region (Okada et al., 2010). On the other hand, following alginate injections, quantitative measures of total content of fibrillar collagen appear to be unchanged, but importantly appear to cause increased cross-linking (Ruvinov et al., 2011; Mukherjee et al., 2008). This would suggest that targeted injections of alginate based biomaterials may increase the stability as well as the stiffness characteristics of the ECM. Using a hydroxyapatite based biomaterial in a sheep post-MI model (Dixon et al., 2011), there was no change in the mRNA expression of collagen type I, but an increase in relative mRNA expression of collagen type III occurred. Thus, the injection of biomaterials within the MI region may not only alter post-translational processes within the ECM (i.e. cross-linking) but transcriptional processes as well. Since the primary cell type for fibrillar collagen expression is the fibroblast, studies have examined this cell type within the MI region following biomaterial injections with particular attention to the myoFB (Rane et al., 2011; Fujimoto et al., 2009; Leor et al., 2009). The most common approach in terms of identifying the transformation of fibroblasts to the myoFB phenotype is through localization and identification of the smooth muscle contractile protein, alpha smooth muscle actin (SMA) (Frangogiannis et al., 2000; Darby et al., 1990; Widgerow, 2011; Souders et al., 2009; Porter et al., 2009; Baum et al., 2011). Following alginate injections into the MI region, significantly increased SMA positive myofibroblasts have been reported (Fujimoto et al., 2009; Leor et al., 2009), which would suggest that increased proliferation and/or transdifferentiation of this fibroblast phenotype occurs with biomaterial injections. The transdifferentiation to a myoFB phenotype is likely influenced by local biophysical stimuli, whereby changes in strain patterns can directly influence fibroblast phenotype (Poobalarahi et al., 2006; Santiago et al., 2010). In an in-vitro study, culturing fibroblasts on a hydroxyapatite based biomaterial induced the expression of proteins consistent with a myoFB phenotype (Dixon et al., 2011). However, using a polyethylene glycol based biomaterial, increased SMA positive myofibroblast density was not observed (Rane et al., 2011). The use of SMA positive fibroblasts as the sole approach to define myoFBs may be an incomplete approach (Frangogiannis et al., 2000; Baum et al., 2011). Furthermore, in terms of biomaterial injections, a number of past studies have utilized SMA staining to identify angiogenesis, i.e. vascular smooth muscle cells (Kraehenbuehl et al., 2011; Tous et al., 2012; Wu et al., 2011; Garbern et al., 2011; Ruvinov et al., 2011; Yu et al., 2009; Hao et al., 2007). As such, it may be difficult to differentiate in a quantitative manner vascular smooth muscle cell and myoFB density and distribution within the MI region. Nevertheless, it appears that the injection of biomaterials within the MI region can likely influence the phenotype and biological behavior of the critical cell type, which regulates the ECM – the fibroblast. In that regard, determinants of ECM degradation, MMPs, appear also to be influenced by biomaterial injections following MI (Dixon et al., 2011; Mukherjee et al., 2008). For example, in a study by Dixon et al., hydroxyapatite injections into a sheep model of MI altered certain MMP profiles, which included a 6-fold reduction in the interstitial collagenase MMP-13 as well as a normalization of MMP-7 (Dixon et al., 2011). The changes in these MMP types may be of particular importance, as MMP-13 can cause significant ECM proteolysis, and MMP-7 is associated with inflammatory cell mediated ECM degradation (Spinale, 2007; Spinale et al., 2013). Taken together, it appears that the injection of biomaterials of both non-durable and durable types can significantly influence the local ECM architecture and thereby influence the post-MI remodeling process.
Future Directions and Clinical Applications
Biomaterials as a Platform for Drug/Cell Based Therapies
Both non-durable and durable biomaterials have been utilized in conjunction with pharmacological agents/bioactive molecules and with a stem cell type expanded population of cells (Iwakura et al., 2003; Kraehenbuehl et al., 2011; Wu et al., 2010; Garbern et al., 2011; Chen et al., 2010; Purcell et al., 2012; Ruvinov et al., 2011; Yu et al., 2008; Danoviz et al., 2010; Nakamuta et al., 2009; Hao et al., 2007; Okada et al., 2010; Landa et al., 2008; Kofidis et al., 2004; Christman et al., 2004). Some of the more common bioactive molecules utilized in this context are growth factors, such as fibroblast growth factor (Iwakura et al., 2003; Garbern et al., 2011; Okada et al., 2010). For example, injection of a gelatin hydrogel that was mixed with 100 ug of fibroblast growth factor reduced LV dilation in rats post-MI (Iwakura et al., 2003). Using a polyacrylamide based hydrogel containing 2.5 ug of fibroblast growth factor, targeted injections within the MI region in rats improved LV pump function (Garbern et al., 2011). Using a modified alginate preparation containing vascular endothelial growth factor, Hao et al. demonstrated a relative improvement in LV function and angiogenesis in a post-MI rat model (Hao et al., 2007). While this is most certainly a highly relevant use of these biomaterials, consideration of the independent effects of the underlying material/matrix being utilized would be appropriate. Without assessing this independent treatment effect, it is difficult to assess the overall efficacy and drug/cell based influences. For example, in animal studies as well as initial clinical trials, biomaterials containing stem cell preparations have been utilized for targeted myocardial injections but did not consider the potential effects of the biomaterial alone (Iwakura et al., 2003; Chen et al., 2010; Landa et al., 2008; Chachques et al., 2008; Chachques et al., 2007). This holds particular relevance as it has been demonstrated that hydrogel constructs containing hyaluronic acid can influence the viability and chemotaxis of bone marrow derived stem cells (Purcell et al., 2012). Representative results from this past study shown in Figure 2 underscore the importance of considering the underlying independent effects of the biomaterial when used in conjunction with stem cells for post-MI injection studies. In the clinical feasibility study of injection of an ECM biomaterial seeded with bone marrow cells (MAGNUM trial) (Chachques et al., 2008), relative MI thickness was augmented up to one year post-injection (Chachquest et al., 2007). However, it remains unclear from these initial clinical studies what effects injection of the processed ECM biomaterial alone may have imparted in terms of endogenous bone marrow cell homing and viability. It will be important for future studies, which utilize a biomaterial as a means for bioactive molecule elution or for stem cell scaffolds, that the independent effects of the biomaterial alone be considered in the experimental design.
Figure 2.
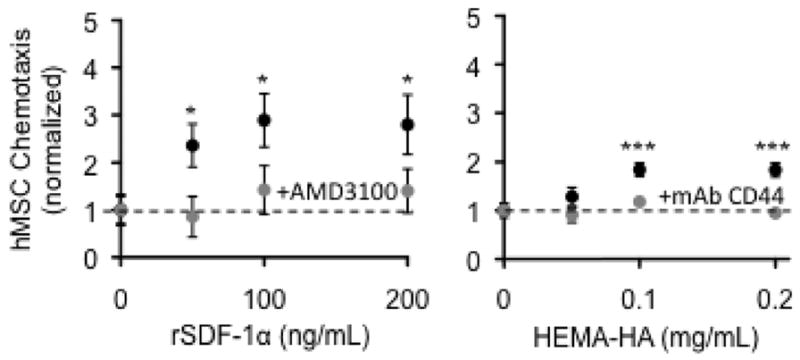
Human mesenchymal stem cell chemotaxis to recombinant SDF-1 alpha (left) and to a synthesized hyaluronic acid macromer (Hema-HA) (right). This response was blocked when either a CXCR4 antagonist (AMD3100) or a monoclonal antibody to CD44 was included to block the response to SDF-1a or HA, respectively. These findings underscore the importance of considering the effects of the injected biomaterial alone when evaluating the effects of stem cell treatment in the context of MI. Data reproduced from Purcell et al., 2012.
The concept of modifying the biomaterial in terms of degradation rates and thereby altering the retention and/or release of bioactive molecules is likely to be an area of active research. For example, alginate based biomaterials that tend to be long lasting (i.e. more durable) have been modified to degrade in a much faster rate than conventional alginate hydrogels (Landa et al., 2008). The concept that MMP activation is a ubiquitous event in the post-MI remodeling process has led to the development of “MMP sensitive” biomaterials (Kraehenbuehl et al., 2011; Seliktar et al., 2004; Kim et al., 2010). Specifically, the degradation rate of these biomaterials is determined by local MMP activity within the targeted injection region and in turn will release bioactive molecules into the interstitial space. However, direct application of this modified biomaterial in the context of post-MI remodeling remains an area for future investigation.
Biomaterial Delivery Methods
The use of biomaterial injection strategies have advanced to relevant preclinical models using direct myocardial injection techniques (Ifkovits et al., 2010; Tous et al., 2012; Ryan et al., 2009; Morita et al., 2011; Dixon et al., 2011; Solis et al., 2010; Tous et al., 2011; Mukherjee et al., 2008; Leor et al., 2009), and indeed the Magnum trial has demonstrated clinical feasibility in targeted myocardial injections during the conduct of coronary revascularization surgery (Chachques et al., 2008); the advantage of this approach is several. First, clinical trials can be initiated whereby the targeted injections are considered adjunctive to the primary cardiac procedure and thereby allow for reasonably structured study designs. Second, targeted myocardial injections allow for precise control in terms of area and depth of the biomaterial injections whereby the targeted MI region can be precisely visualized. Third, these injections can be performed under carefully controlled conditions, such as cardioplegic arrest, thereby allowing the injections to proceed in a quiescent and bloodless field. These are important considerations when the safety and proof of concept trials are the studies initially performed with biomaterial injections and are likely to be of limited sample size. Indeed, the use of an alginate hydrogel has been advanced to clinical trials in terms of safety and feasibility (AUGMENT HF), whereby targeted myocardial injections will be performed using conventional cardiac surgery techniques (Lee et al., 2012; LoneStar Heart Inc., 2011). It is likely that this clinical study will be one of several in the near future that utilize a particular biomaterial for direct myocardial injections in order to interrupt adverse LV remodeling.
As these direct myocardial injection methods of different biomaterials advance to safety and proof of concept in man, a transition to a less invasive approach is likely to occur in parallel fashion. For example, direct visualization of the myocardium using modified thoracoscopy and video-microscopy will allow for small incisions and yet precise localization of the myocardial target for injection (Papiashvilli et al., 2011; Hatam et al., 2010; Jaroszewski et al., 2009). The ultimate goal will be development of strategies, which would allow for the delivery of the biomaterial through a catheter based approach. There are several obstacles that must be overcome in this regard. Firstly, precise localization of the MI region by a catheter based method must be achieved. Secondly, biomaterials will need to be formulated that will allow for injection of these materials to proceed unimpeded despite catheter distance and dead space. Localization of the MI region using a percutaneous mapping approach has been developed utilizing non-fluoroscopic electromechanical mapping (NOGA) (Vale et al., 1999; Vale et al., 2001). The system is designed so that it can integrate intracardiac electrograms at multiple endocardial locations to create an electroanatomical map that generates a geometric map of the LV. Ischemic myocardium is detected by calculating localized fractional shortening from endocardial potentials and thereby provides a target for precise endocardial injections (Vale et al., 2001). Using this approach, Singelyn and colleagues demonstrated that decellularized porcine ECM could be successfully injected in a targeted and controlled manner in porcine hearts using NOGA (Figure 1C) (Singelyn et al., 2012). Other investigators have demonstrated that modified alginate based biomaterials can be developed that are amenable to catheter based injection techniques (Landa et al., 2008; Leor et al., 2009).
Biomaterial Injections Post-MI; Considerations and Obstacles
One of the potentially confounding issues regarding biomaterials is that of immunogenicity, which can be provoked due to several factors including the mechanical injection procedure itself, the polymerization process following injection, the inherent immune response to a foreign material, and finally in terms of non-durable biomaterials, potential immune response to the degradation of the materials. In regards to using decellularized ECM or harvested tissue composites, past studies have often been performed in immune deficient rodent systems (Okada et al., 2010; Yeh et al., 2010), and therefore the extent of the inherent immune response could not be evaluated. With respect to non-durable biomaterials, these biomaterials likely undergo rapid degradation following injection and can potentially provoke a significant immune response. Thus, a contributory underlying mechanism for the effects observed in past studies using highly degradable biomaterials (Dai et al., 2005; Kofidis at al., 2004) may be due to altering endogenous biological signals within the MI region, and in turn, modifying the post-MI remodeling process. While potentially less “immunogenic” than decellularized ECM materials, synthesized materials such as hydrogels do appear to evoke a localized inflammatory response, primarily around the injection site (Tous et al., 2011). Using a pH responsive hydrogel, an intense inflammatory cell reaction was reported, as evidenced by CD45 positive cells within the MI region (Garbern et al., 2011). In terms of more durable materials, it is also likely that an immune response may be an important consideration, but the immunogenicity appears to be much more muted than that of the non-durable biomaterials. Nevertheless, a chronic inflammatory response localized to the site of biomaterial injection has been observed using more durable constructs (Ryan et al., 2009; Dixon et al., 2011; Mukherjee et al., 2008). In the post-MI context, a significant and robust induction of inflammatory response invariably occurs. Thus, whether and to what degree the inflammatory response specifically and independently induced by these degradable hydrogels is a relevant factor in altering the course of post-MI remodeling remains to be established. For example, it may be that one of the important features of biomaterial injections is the induction of a specific pattern of inflammatory signaling cascade. Indeed, cytokine augmentation of an injected biomaterial used in a synthetic patch post-MI enhanced LV function in a rat model (Kang et al., 2012). On the other hand, it may be possible to attenuate the immune response of the biomaterial through concomitant local release of anti-inflammatory molecules (Webber et al., 2012; Holladay et al., 2012). In this case, the broad spectrum anti-inflammatory dexamethasone has been incorporated into a nanofiber-gel construct and shown to reduce localized inflammation following injection in mice (Webber et al., 2012). Thus, similar to other properties of these biomaterials, it is likely that the local inflammatory response to these injected biomaterials may be “tunable”.
In addition to addressing the potential benefits and drawbacks of inducing a local inflammatory response, there are other issues regarding biomaterial injections in the post-MI context that must also be recognized and addressed. For example, there are inherent risks involved in localized targeted injection/application in the MI region. These include the induction of arrhythmias and off target effects. In terms of arrhythmogenesis, this can occur during the injection process as well as in the post delivery period. As these biomaterials incorporate into the MI region, there is the potential for increasing the heterogeneity of the conduction pathways, which in turn may cause re-entry pathways and arrhythmias. In terms of off target injection effects, injection of the material into the LV chamber rather than the MI region can result in a potential thromboembolic event. As the use of these biomaterials progress to more translationally relevant model systems and early clinical applications, these potential adverse effects will require careful monitoring.
Summary
In summary, targeted myocardial injection of biomaterials holds significant promise in terms of interrupting post-MI remodeling in and of itself as well as a means for drug delivery and scaffolding for cell based therapies. Future research that more carefully addresses the unique and independent biophysical properties of these biomaterials as well as the direct effects upon biological processes within the evolving MI is necessary. The precise therapeutic role that biomaterials may play in adverse LV remodeling in terms of a stand-alone treatment or as an adjunctive therapy remains to be established.
Acknowledgments
This work was supported by the National Institute of Health grants HL057952, HL089944, HL095608, HL111090, and a Merit Award from the Veterans’ Affairs Health Administration. The authors wish to acknowledge Craig P. Novack for his assistance during manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol. 2011 Apr;57(4):376–9. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chachques JC, Trainini JC, Lago N, et al. Myocardial Assistance by Grafting a New Bioartificial Upgraded Myocardium (MAGNUM trial): clinical feasibility study. Ann Thorac Surg. 2008 Mar;85(3):901–8. doi: 10.1016/j.athoracsur.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 3.Chachques JC, Trainini JC, Lago N, et al. Myocardial assistance by grafting a new bioartificial upgraded myocardium (MAGNUM clinical trial): one year follow-up. Cell Transplant. 2007;16(9):927–34. doi: 10.3727/096368907783338217. [DOI] [PubMed] [Google Scholar]
- 4.Chen QZ, Ishii H, Thouas GA, et al. An elastomeric patch derived from poly(glycerol sebacate) for delivery of embryonic stem cells to the heart. Biomaterials. 2010 May;31(14):3885–93. doi: 10.1016/j.biomaterials.2010.01.108. Epub 2010 Feb 11. [DOI] [PubMed] [Google Scholar]
- 5.Christman KL, Fok HH, Sievers RE, et al. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004 Mar-Apr;10(3–4):403–9. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 6.Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2006 Sep 5;48(5):907–13. doi: 10.1016/j.jacc.2006.06.005. Epub 2006 Aug 17. [DOI] [PubMed] [Google Scholar]
- 7.Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005 Aug;46(4):714–9. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 8.Dang AB, Guccione JM, Mishell JM, et al. Akinetic myocardial infarcts must contain contracting myocytes: finite-element model study. Am J Physiol Heart Circ Physiol. 2005 Apr;288(4):H1844–50. doi: 10.1152/ajpheart.00961.2003. Epub 2004 Dec 16. [DOI] [PubMed] [Google Scholar]
- 9.Danoviz ME, Nakamuta JS, Marques FL, et al. Rat adipose tissue-derived stem cells transplantation attenuates cardiac dysfunction post infarction and biopolymers enhance cell retention. PLoS One. 2010 Aug 10;5(8):e12077. doi: 10.1371/journal.pone.0012077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990 Jul;63(1):21–9. [PubMed] [Google Scholar]
- 11.Dixon JA, Gorman RC, Stroud RE, et al. Targeted regional injection of biocomposite microspheres alters post-myocardial infarction remodeling and matrix proteolytic pathways. Circulation. 2011 Sep 13;124(11 Suppl):S35–45. doi: 10.1161/CIRCULATIONAHA.111.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon JA, Spinale FG. Myocardial remodeling: cellular and extracellular events and targets. Annu Rev Physiol. 2011;73:47–68. doi: 10.1146/annurev-physiol-012110-142230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobner S, Bezuidenhout D, Govender P, et al. A synthetic non-degradable polyethylene glycol hydrogel retards adverse post-infarct left ventricular remodeling. J Card Fail. 2009 Sep;15(7):629–36. doi: 10.1016/j.cardfail.2009.03.003. Epub 2009 May 7. [DOI] [PubMed] [Google Scholar]
- 14.Dvir T, Bauer M, Schroeder A, et al. Nanoparticles targeting the infarcted heart. Nano Lett. 2011 Oct 12;11(10):4411–4. doi: 10.1021/nl2025882. Epub 2011 Sep 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ertl G, Frantz S. Healing after myocardial infarction. Cardiovasc Res. 2005;66(1):22–32. doi: 10.1016/j.cardiores.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb) Cardiovasc Res. 2000 Oct;48(1):89–100. doi: 10.1016/s0008-6363(00)00158-9. [DOI] [PubMed] [Google Scholar]
- 17.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008 Aug;58(2):88–111. doi: 10.1016/j.phrs.2008.06.007. Epub 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frangogiannis NG. The mechanistic basis of infarct healing. Antioxidants & Redox Signaling. 2006;8:1907–1939. doi: 10.1089/ars.2006.8.1907. [DOI] [PubMed] [Google Scholar]
- 19.Frantz S, Bauersachs J, Ertl G. Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc Res. 2009 Feb 15;81(3):474–81. doi: 10.1093/cvr/cvn292. Epub 2008 Oct 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujimoto KL, Ma Z, Nelson DM, et al. Synthesis, characterization and therapeutic efficacy of a biodegradable, thermoresponsive hydrogel designed for application in chronic infarcted myocardium. Biomaterials. 2009;30(26):4357–68. doi: 10.1016/j.biomaterials.2009.04.055. Epub 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garbern JC, Minami E, Stayton PS, et al. Delivery of basic fibroblast growth factor with a pH-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials. 2011 Mar;32(9):2407–16. doi: 10.1016/j.biomaterials.2010.11.075. Epub 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giraud MN, Guex AG, Tevaearai HT. Cell therapies for heart function recovery: focus on myocardial tissue engineering and nanotechnologies. Cardiol Res Pract. 2012 doi: 10.1155/2012/971614. Epub 2012 Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorman RC, Jackson BM, Burdick JA, et al. Infarct restraint to limit adverse ventricular remodeling. J Cardiovasc Transl Res. 2011;4(1):73–81. doi: 10.1007/s12265-010-9244-0. Epub 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guccione JM, Moonly SM, Moustakidis P, et al. Mechanism underlying mechanical dysfunction in the border zone of left ventricular aneurysm: a finite element model study. Ann Thorac Surg. 2001 Feb;71(2):654–62. doi: 10.1016/s0003-4975(00)02338-9. [DOI] [PubMed] [Google Scholar]
- 25.Hao X, Silva EA, Månsson-Broberg A, et al. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc Res. 2007 Jul 1;75(1):178–85. doi: 10.1016/j.cardiores.2007.03.028. Epub 2007 Apr 6. [DOI] [PubMed] [Google Scholar]
- 26.Hatam N, Amerini AL, Steiner F, et al. Video-assisted pericardioscopic surgery: refinement of a new technique for implanting epimyocardial pacemaker leads. Eur J Cardiothorac Surg. 2011 Mar;39(3):335–41. doi: 10.1016/j.ejcts.2010.06.016. Epub 2010. [DOI] [PubMed] [Google Scholar]
- 27.Holladay CA, Duffy AM, Chen X, Sefton MV, O’Brien TD, Pandit AS. Recovery of cardiac function mediated by MSC and interleukin-10 plasmid functionalised scaffold. Biomaterials. 2012 Feb;33(5):1303–14. doi: 10.1016/j.biomaterials.2011.10.019. Epub 2011 Nov 10. [DOI] [PubMed] [Google Scholar]
- 28.Ifkovits JL, Tous E, Minakawa M, et al. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc Natl Acad Sci U S A. 2010 Jun 22;107(25):11507–12. doi: 10.1073/pnas.1004097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwakura A, Fujita M, Kataoka K, et al. Intramyocardial sustained delivery of basic fibroblast growth factor improves angiogenesis and ventricular function in a rat infarct model. Heart Vessels. 2003 May;18(2):93–9. doi: 10.1007/s10380-002-0686-5. [DOI] [PubMed] [Google Scholar]
- 30.Jain PK, Huang X, El-Sayed IH, et al. Nobel metals on the Nanoscale: optical and photothermal properties and some application in imaging, sensing, biology and medicine. Acc Chem Res. 2008;41:1578–1586. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- 31.Jaroszewski DE, Altemose GT, Scott LR, et al. Nontraditional surgical approaches for implantation of pacemaker and cardioverter defibrillator systems in patients with limited venous access. Ann Thorac Surg. 2009 Jul;88(1):112–6. doi: 10.1016/j.athoracsur.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Kang K, Sun L, Xiao Y, Li SH, Wu J, Guo J, Jiang SL, Yang L, Yau TM, Weisel RD, Radisic M, Li RK. Aged human cells rejuvenated by cytokine enhancement of biomaterials for surgical ventricular restoration. J Am Coll Cardiol. 2012 Nov 20;60(21):2237–49. doi: 10.1016/j.jacc.2012.08.985. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Kim IS, Cho TH, et al. In vivo evaluation of MMP sensitive high-molecular weight HA-based hydrogels for bone tissue engineering. J Biomed Mater Res A. 2010;95(3):673–81. doi: 10.1002/jbm.a.32884. [DOI] [PubMed] [Google Scholar]
- 34.Kofidis T, de Bruin JL, Hoyt GL, et al. Injectable bioartificial myocardial tissue for large-scale intramural cell transfer and functional recovery of injured heart muscle. J Thorac Cardiovasc Surg. 2004 Oct;128(4):571–8. doi: 10.1016/j.jtcvs.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Kraehenbuehl TP, Ferreira LS, Hayward AM, et al. Human embryonic stem cell-derived microvascular grafts for cardiac tissue preservation after myocardial infarction. Biomaterials. 2011 Feb;32(4):1102–9. doi: 10.1016/j.biomaterials.2010.10.005. Epub 2010 Oct 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landa N, Miller L, Feinberg MS, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008 Mar 18;117(11):1388–96. doi: 10.1161/CIRCULATIONAHA.107.727420. Epub 2008 Mar 3. [DOI] [PubMed] [Google Scholar]
- 37.Lee RJ, Hinson A, Helgerson S, et al. Polymer-based restoration of left ventricular mechanics. Cell Transplant. 2012 Mar 28; doi: 10.3727/096368911X637461. [DOI] [PubMed] [Google Scholar]
- 38.Leor J, Tuvia S, Guetta V, et al. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J Am Coll Cardiol. 2009 Sep 8;54(11):1014–23. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 39.LoneStar Heart, Inc. A Randomized, Controlled Study to Evaluate Algisyl-LVR™ as a Method of Left Ventricular Augmentation for Heart Failure (AUGMENT-HF) ClinicalTrials.gov. 2011–2012. Available from: http://clinicaltrials.gov/ct2/show/NCT01311791.
- 40.Macarthur JW, Jr, Trubelja A, Shudo Y, et al. Mathematically engineered stromal cell-derived factor-13 stem cell cytokine analog enhances mechanical properties of infarcted myocardium. J Thorac Cardiovasc Surg. 2013 Jan;145(1):278–84. doi: 10.1016/j.jtcvs.2012.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morita M, Eckert CE, Matsuzaki K, et al. Modification of infarct material properties limits adverse ventricular remodeling. Ann Thorac Surg. 2011 Aug;92(2):617–24. doi: 10.1016/j.athoracsur.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukherjee R, Zavadzkas JA, Saunders SM, et al. Targeted myocardial microinjections of a biocomposite material reduces infarct expansion in pigs. Ann Thorac Surg. 2008 Oct;86(4):1268–76. doi: 10.1016/j.athoracsur.2008.04.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamuta JS, Danoviz ME, Marques FL, et al. Cell therapy attenuates cardiac dysfunction post myocardial infarction: effect of timing, routes of injection and a fibrin scaffold. PLoS One. 2009 Jun 23;4(6):e6005. doi: 10.1371/journal.pone.0006005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okada M, Payne TR, Oshima H, et al. Differential efficacy of gels derived from small intestinal submucosa as an injectable biomaterial for myocardial infarct repair. Biomaterials. 2010 Oct;31(30):7678–83. doi: 10.1016/j.biomaterials.2010.06.056. Epub 2010. [DOI] [PubMed] [Google Scholar]
- 45.Papiashvilli M, Haitov Z, Fuchs T, et al. Left ventricular epicardial lead implantation for resynchronisation therapy using a video-assisted thoracoscopic approach. Heart Lung Circ. 2011 Apr;20(4):220–2. doi: 10.1016/j.hlc.2010.11.003. Epub 2010 Dec 10. [DOI] [PubMed] [Google Scholar]
- 46.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81(4):1161–72. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 47.Poobalarahi F, Baicu CF, Bradshaw AD. Cardiac myofibroblasts differentiated in 3D culture exhibit distinct changes in collagen I production, processing, and matrix deposition. Am J Physiol Heart Circ Physiol. 2006;291(6):H2924–32. doi: 10.1152/ajpheart.00153.2006. [DOI] [PubMed] [Google Scholar]
- 48.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009 Aug;123(2):255–78. doi: 10.1016/j.pharmthera.2009.05.002. Epub 2009 May 19. [DOI] [PubMed] [Google Scholar]
- 49.Purcell BP, Elser JA, Mu A, et al. Synergistic effects of SDF-13 chemokine and hyaluronic acid release from degradable hydrogels on directing bone marrow derived cell homing to the myocardium. Biomaterials. 2012 Nov;33(31):7849–57. doi: 10.1016/j.biomaterials.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rane AA, Chuang JS, Shah A, et al. Increased infarct wall thickness by a bio-inert material is insufficient to prevent negative left ventricular remodeling after myocardial infarction. PLoS One. 2011;6(6):e21571. doi: 10.1371/journal.pone.0021571. Epub 2011 Jun 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics-- 2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruvinov E, Leor J, Cohen S. The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials. 2011 Jan;32(2):565–78. doi: 10.1016/j.biomaterials.2010.08.097. [DOI] [PubMed] [Google Scholar]
- 53.Ryan LP, Matsuzaki K, Noma M, et al. Dermal filler injection: a novel approach for limiting infarct expansion. Ann Thorac Surg. 2009 Jan;87(1):148–55. doi: 10.1016/j.athoracsur.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santiago JJ, Dangerfield AL, Rattan SG, et al. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: Expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev Dyn. 2010;239:1573–1584. doi: 10.1002/dvdy.22280. [DOI] [PubMed] [Google Scholar]
- 55.Seliktar D, Zisch AH, Lutolf MP, et al. MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. J Biomed Mater Res A. 2004 Mar 15;68(4):704–16. doi: 10.1002/jbm.a.20091. [DOI] [PubMed] [Google Scholar]
- 56.Singelyn JM, Sundaramurthy P, Johnson TD, et al. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J Am Coll Cardiol. 2012 Feb 21;59(8):751–63. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sisco PN, Wilson CG, Mironova E, et al. 2008 The effect of gold nanorods on cell-mediated collagen remodeling. NanoLetters. 8:3409–3412. doi: 10.1021/nl802142h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Solis J, Levine RA, Johnson B, et al. Polymer injection therapy to reverse remodel the papillary muscles: efficacy in reducing mitral regurgitation in a chronic ischemic model. Circ Cardiovasc Interv. 2010 Oct;3(5):499–505. doi: 10.1161/CIRCINTERVENTIONS.109.850255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009 Dec 4;105(12):1164–76. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spinale FG, Janicki JS, Zile MR. Invited Review: Membrane associated matrix proteolysis and heart failure. Circ Res. 2013 Jan 4;112(1):195–208. doi: 10.1161/CIRCRESAHA.112.266882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spinale FG. Matrix remodeling and the matrix metalloproteinases: Influence on cardiac form and function. Physiol Rev. 2007 Oct;87(4):1285–342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 62.Tomasek JJ, Gabbiani G, Hinz B, et al. Myofibroblasts and mechanoregulation of connective tissue remodeling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 63.Tous E, Ifkovits JL, Koomalsingh KJ, et al. Influence of injectable hyaluronic acid hydrogel degradation behavior on infarction-induced ventricular remodeling. Biomacromolecules. 2011 Nov 14;12(11):4127–35. doi: 10.1021/bm201198x. Epub 2011 Oct 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tous E, Weber HM, Lee MH, et al. Tunable hydrogel-microsphere composites that modulate local inflammation and collagen bulking. Acta Biomater. 2012 Sep;8(9):3218–27. doi: 10.1016/j.actbio.2012.05.027. Epub 2012 May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vale PR, Losordo DW, Milliken CE, et al. Randomized, single-blind, placebo-controlled pilot study of catheter-based myocardial gene transfer for therapeutic angiogenesis using left ventricular electromechanical mapping in patients with chronic myocardial ischemia. Circulation. 2001;103:2138–43. doi: 10.1161/01.cir.103.17.2138. [DOI] [PubMed] [Google Scholar]
- 66.Vale PR, Losordo DW, Tkebuchava T, et al. Catheter-based myocardial gene transfer utilizing nonfluoroscopic electromechanical left ventricular mapping. J Am Coll Cardiol. 1999;34:246–54. doi: 10.1016/s0735-1097(99)00143-6. [DOI] [PubMed] [Google Scholar]
- 67.Wall ST, Walker JC, Healy KE, et al. Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation. 2006 Dec 12;114(24):2627–35. doi: 10.1161/CIRCULATIONAHA.106.657270. Epub 2006 Nov 27. [DOI] [PubMed] [Google Scholar]
- 68.Wang J, Chen H, Seth A, et al. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2003;285:H1871–H1881. doi: 10.1152/ajpheart.00387.2003. [DOI] [PubMed] [Google Scholar]
- 69.Webber MJ, Matson JB, Tamboli VK, Stupp SI. Controlled release of dexamethasone from peptide nanofiber gels to modulate inflammatory response. Biomaterials. 2012 Oct;33(28):6823–32. doi: 10.1016/j.biomaterials.2012.06.003. Epub 2012 Jun 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weir RA, McMurray JJ, Velazquez EJ. Epidemiology of heart failure and left ventricular systolic dysfunction after acute myocardial infarction: prevalence, clinical characteristics, and prognostic importance. Am J Cardiol. 2006 May 22;97(10A):13F–25F. doi: 10.1016/j.amjcard.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 71.Wenk JF, Eslami P, Zhang Z, et al. A novel method for quantifying the in-vivo mechanical effect of material injected into a myocardial infarction. Ann Thorac Surg. 2011 Sep;92(3):935–41. doi: 10.1016/j.athoracsur.2011.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Widgerow AD. Cellular/extracellular matrix cross-talk in scar evolution and control. Wound Repair Regen. 2011 Mar-Apr;19(2):117–33. doi: 10.1111/j.1524-475X.2010.00662.x. [DOI] [PubMed] [Google Scholar]
- 73.Wilson CG, Sisco PN, Gadala-Maria FA, et al. Polyelectrolyte-coated gold nanorods and their interactions with type I collagen. Biomaterials. 2009;30:5639–5648. doi: 10.1016/j.biomaterials.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilson CG, Sisco PN, Goldsmith EC, et al. Glycosaminoglycan-functionalized gold nanorods: interaction with cardiac cells and type I collagen. J Mat Chem. 2009;19:6332–6340. [Google Scholar]
- 75.Wu J, Zeng F, Huang XP, et al. Infarct stabilization and cardiac repair with a VEGF-conjugated, injectable hydrogel. Biomaterials. 2011 Jan;32(2):579–86. doi: 10.1016/j.biomaterials.2010.08.098. [DOI] [PubMed] [Google Scholar]
- 76.Yeh YC, Lee WY, Yu CL, Hwang SM, Chung MF, Hsu LW, Chang Y, Lin WW, Tsai MS, Wei HJ, Sung HW. Cardiac repair with injectable cell sheet fragments of human amniotic fluid stem cells in an immune-suppressed rat model. Biomaterials. 2010 Sep;31(25):6444–53. doi: 10.1016/j.biomaterials.2010.04.069. [DOI] [PubMed] [Google Scholar]
- 77.Yu J, Gu Y, Du KT, et al. The effect of injected RGD modified alginate on angiogenesis and left ventricular function in a chronic rat infarct model. Biomaterials. 2009 Feb;30(5):751–6. doi: 10.1016/j.biomaterials.2008.09.059. Epub 2008 Nov 17. [DOI] [PubMed] [Google Scholar]


