Abstract
Fibroblast clusters have been observed in tissues under a variety of circumstances: in fibrosis and scar, in the formation of hair follicle dermal papilla, and as part of the general process of mesenchymal condensation that takes place during development. Cell clustering has been shown to depend on features of the extracellular matrix, growth factor environment, and mechanisms to stabilize cell-cell interactions. In vitro studies have shown that increasing the potential for cell-cell adhesion relative to cell-substrate adhesion promotes cell clustering. Experimental models to study fibroblast clustering have utilized centrifugation, hanging drops, and substrata with poorly adhesive, soft and mechanically unstable properties. In this review, we summarize work on a new, highly tractable, cell clustering research model in which human fibroblasts are incubated on the surfaces of collagen matrices. Fibroblast clustering occurs under procontractile growth factor conditions (e.g., serum or the serum lipid agonist lysophosphatidic acid) but not under promigratory growth factor conditions (e.g., platelet-derived growth factor) and can be reversed by switching growth factor environments. Cell contraction plays a dual role in clustering to bring cells closer together and to stimulate cells to organize fibronectin into a fibrillar matrix. Binding of fibroblasts to a shared fibronectin fibrillar matrix stabilizes clusters, and fragmentation of the fibrillar matrix occurs when growth factor conditions are switched to promote cell dispersal.
Keywords: Cell clustering, cell aggregation, cell contraction, cell migration, fibronectin, adherens junctions, 3D-collagen matrix, tissue morphogenesis
Introduction
Composition, organization and physiological function of multicellular organisms depend in part on the presence of connective tissues, which are observed as early in Metazoan evolution as Porifera [1]. Connective tissues exhibit remarkable diversity ranging from blood to fibrous connective tissue to cartilage to bone. In fibrous connective tissue, cells interact with a non-cellular component called extracellular matrix (ECM), composed of glycoproteins, proteoglycans, and glycosaminoglycans. ECM exhibits a dual function in fibrous connective tissue as structural organizer and as physiological regulator of cell behavior including diverse properties such as proliferation, differentiation, and migration. Cell-ECM interactions are active and iterative in the sense that cellular responses to ECM result in chemical and physical remodeling of the matrix, which in turn influences subsequent cell behavior [2–4].
Type I collagen is the major ECM component of fibrous connective tissue. Type I collagen matrices containing fibroblasts and other cell types have been used as an in vitro model of connective tissue to learn about cell physiology and biomechanics in a 3D tissue-like environment [5–10]. Recently, we and others began to study fibroblasts cultured on the surfaces of 3D collagen matrices as a new platform to investigate morphogenic cell clustering and dispersion [11–13].
Diverse terms have been used to describe multicellular aggregates including cell clusters, spheroids, and microtissues. Given their stability, cell clusters can in a sense be treated as biomaterials [14] and used to produce more complex higher order structures such as tissue cubes and tubes incorporating homotypic and heterotypic cellular interactions [14–17]. In this review, we will summarize briefly the phenomenon of cell clustering and then describe in more detail findings regarding the molecular mechanism of clustering by fibroblasts.
Different Types of Cell Clusters
Cell clustering phenomena first were studied to better understand tissue organization. Dissociated cells from early chick embryos were shown to re-associate into tissue-specific arrangements [18], giving rise to the hypothesis of tissue morphogenesis based on differential cell-cell adhesion [19]. Cell-cell adhesive interactions mediated by adherens junctions were believed to provide the specificity underlying the differential adhesion hypothesis [20, 21].
During embryonic development, cell clustering known as mesenchymal condensation represents a pivotal developmental stage that precedes tissue-specific differential gene expression [22–26]. Directional cell migration and cell adhesion both influence cluster formation [22]. For instance, undifferentiated mesenchymal cells can be stimulated to migrate to the site of skeletal formation by release of chemotactic factors from the overlaying epithelium [26]. Mesenchymal cells form adherens junctions with N-cadherins [27] and cell-cell interactions also are stabilized by fibronectin (FN) and integrins [28, 29]. After mesenchymal cells aggregate, cartilage and bone formation begin [23].
Skin appendage formation represents another important example of mesenchymal condensation [30]. In the case of hair follicles, the cluster of specialized fibroblasts known as the dermal papilla provides the inductive signals that drive skin epidermal cells to undergo differentiation into hair follicle cells [31–33]. 3D organization of dermal papilla cells is believed to be essential for hair follicle induction, and expression of markers associated with induction occurs preferentially in cells cultured on poorly adhesive substrates under conditions in which the cells cluster [34]. On conventional surfaces to which cells attach well, they do not cluster and expression of dermal papilla specific markers is lost [35, 36]. Fibronectin also is believed to be important for dermal papilla organization based on its localization in vivo [37] and in vitro [38], and addition of exogenous FN promotes cell clustering and formation of dermal papilla structures [39].
Another type of cell clustering, distinct from mesenchymal condensation, occurs during wound repair. During repair, fibroblasts are recruited to the wound site from three different sources: the local fibroblast population, local epithelial-mesenchymal transition, and extravasation of circulating fibrocytes [40–42] These cells differentiate into myofibroblasts and organize into a network interconnected by adherens junctions and stabilized by extracellular matrix-integrin interactions [43–46]. Contraction of the myofibroblast network contributes to wound closure and can be a major cause of scarring [47–50]. Nodules of highly contractile, aggregated fibroblasts occur in hypertrophic scars [51–53]. In idiopathic pulmonary fibrosis, clusters of myofibroblasts are known as fibroblastic foci [54–57]. Fibroblast spheroids formed in vitro have been reported to produce and release proinflammatory mediators [58].
Solid tumors historically were described as three-dimensional cell aggregates [59]. However, unlike the clustering phenomena discussed so far, unregulated cell proliferation is responsible for primary tumor formation [60], and it is the loss of normal cell-cell interactions that results in tumor cell invasiveness into surrounding tissues [61, 62]. In the case of ovarian tumors, spheroids of tumor cells can be released from the primary tumor and attach at secondary sites where they are invasive [63, 64].
Mechanisms of Fibroblast Clustering
Cell clustering has been shown to depend on features of the extracellular matrix, growth factor environment, and mechanisms to stabilize cell-cell interactions. In general, conditions that increase the possibility for cell-cell adhesion relative to cell-substrate adhesion promote cell clustering. Approaches to influence this balance include cell growth on poorly adhesive substrata, cell centrifugation, and hanging drop culture [16, 21, 35, 58, 65–67]. Formation of cell clusters on soft polyacrylamide gels [68, 69] was found to be relate to the ability of cells to displace the gel substrate sufficiently so as pull themselves closer together. Cell clustering on mechanically unstable substrata [70] was suggested to depend on cell-mediated detachment of the ECM coating the matrix in which the cells subsequently became enmeshed.
Our laboratory has been particularly interested in the behavior of fibroblasts interacting with 3D collagen matrices. Since 3D collagen matrices are softer (<0.1 kPa) [71, 72] than the softest polyacrylamide substrates (> 500 kPa) [68, 69], we anticipated that fibroblasts cultured on the surfaces of soft collagen matrices also would undergo clustering. In addition, however, we wanted to learn if cell-clustering depended on the growth factor environment. That is, fibroblasts interacting with collagen matrices exhibit markedly different behavior under what we refer to as promigratory and procontractile conditions. Promigratory conditions occur in the presence of growth factors (e.g., platelet-derived growth factor, PDGF) that activate Rac and stimulate protrusion of fibroblast dendritic extensions; whereas procontractile conditions occur in the presence of growth factors (e.g., serum, or lysophosphatidic acid, LPA) that activate Rho and stimulate retraction of dendritic cell extensions [4]. Most previous cell clustering experiments such as those on soft polyacrylamide substrates had been carried out in medium containing serum, i.e., procontractile.
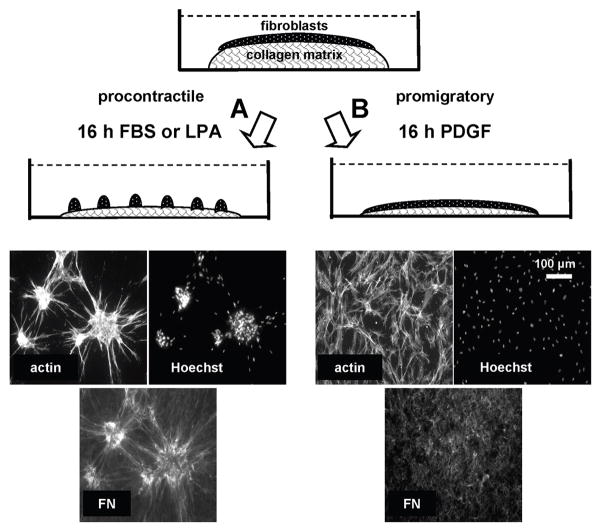
We found that fibroblasts cultured on 1 mg/ml 3D collagen matrices exhibited cell clustering according to the growth factor environment and collagen matrix density [11, 13]. Figure 1 illustrates some key features of our findings. After overnight incubation of human fibroblasts on collagen matrices in fetal bovine serum (FBS)-containing medium (procontractile conditions), cells were well spread (actin) and organized into clusters (Hoechst nuclear stain). However, if the incubations were carried out in PDGF-containing medium (promigratory conditions), then cells were well spread but remained dispersed. In either case, collagen matrix remodeling occurred, which resulted in a marked decrease in matrix height (>70%) as described previously [77]. Increasing the collagen concentration from 1 to 4 mg/ml, which increases substrate stiffness and also decreases spacing between potential collagen adhesion sites [72], resulted in decreased cell clustering [11],.
Figure 1.
Model to study fibroblast clustering and dispersion.
Human fibroblasts cultured on 3D collagen matrices form clusters under procontractile growth factor conditions (A) but migrate as individuals without clustering under promigratory growth conditions (B). Contraction plays a dual role in cell clustering, bringing cells closer together and stimulating fibronectin fibrillar matrix organization. See text for other details.
Time-lapse microscopy showed that cell-cell encounters occurred continuously under promigratory conditions. Therefore, collagen matrix remodeling and cell-cell encounters were not sufficient for cell clustering to occur. The results suggested that procontractile conditions were necessary for cell interactions that stabilized cell clusters, and these morphogenic changes were reversible. That is, switching from procontractile to promigratory conditions resulted in cluster dispersion as cell migrated away, whereas switching from promigratory to procontractile conditions resulted in cell clustering [11].
Fibroblast adherens junctions mediated by N-cadherin play diverse roles in fibrous connective tissues [78]. As already discussed, a variety of evidence has implicated adherens junctions in cell clustering. Adherens junctions can be observed along the boundaries of corneal fibroblasts that develop cell-cell interactions when cultured on fibrin matrices or fibrin-coated 2D substrates [79]. Transformed fibroblasts expressing low levels of cadherins were shown to form loose rather than compact clusters [80]. Similarly, we observed that oncogenic Ras transformed fibroblasts lost their ability to form adherens junction [76] and were unable to form cell clusters on collagen matrices [11]. Therefore, we anticipated that adherens junctions might be required for stabilizing cell-cell interactions necessary for fibroblast cluster formation. However, while siRNA silencing of N-cadherin in human fibroblasts interfered with the ability of cells to form adherens junctions, the cells still were able to form cell clusters (unpublished observation).
In addition to adherens junctions, various studies have shown that fibronectin and its receptor integrin α5β1 play a role in formation and compaction of cell clusters [21, 28, 58, 65, 66]. Moreover, FN-null mouse embryo fibroblasts cultured on collagen matrices can utilize exogenously added FN to form a fibrillar FN matrix in which cells spread and proliferate [12]. Also, exogenous FN also can promote compaction of cell spheroids [21] and formation of dermal papilla [39]. Conversely, interfering with FN matrix organization using a 70kDa FN fragment that blocks fibril formation was observed to prevent cell clustering [12, 65]. Therefore, we tested the possibility that organization of FN into a fibrillar matrix also played a role in human fibroblast clustering on collagen matrices [13].
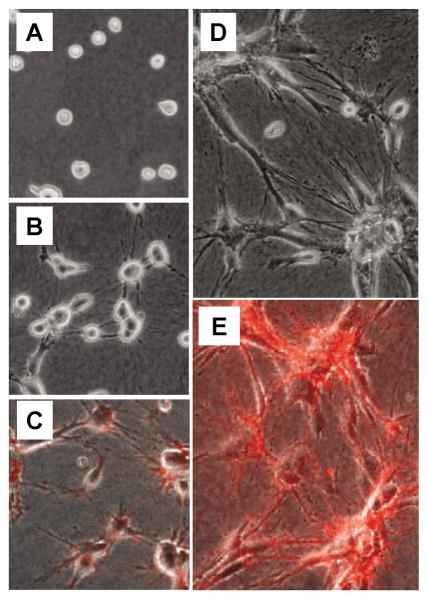
As shown in Figure 1, fibronectin matrix assembly occurred under procontractile conditions that resulted in cell clustering but not under promigratory conditions. Selected frames from time-lapse videos shown in Figure 2 model the events that occur during cell clustering under procontractile conditions. After initial cell attachment to the collagen matrix (Figure 2A), cell spreading begins. As cells attempt to spread, they reorganize and align the collagen matrix resulting in the appearance of tension lines between cells, and the cells pull closer to each other (Figure 2B). At this time, organization of fibronectin into a fibrillar matrix (detected using rhodamine-labeled fibronectin) can be observed along cell extensions and associated with small cell clusters (Figure 2C).
Figure 2.
Stages in fibroblast clustering.
(A) Initial cell attachment to the collagen matrix. (B) As fibroblasts begin to spread, they reorganize and align the collagen matrix resulting in the appearance of tension lines in the collagen between cells, and the cells pull closer to each other. (C) Fibronectin can be seen to organize along cell extensions and associate with small cell clusters. (D) By 6–8 hours, most cells have spread in elongated morphology and formation of large cell clusters becomes evident. (E) At this time, an extensive fibrillar matrix can be observed associated with the cell clusters. Images A, C, and E were selected from Supplemental Video #2 [11]. Images B and D were selected from Supplemental Video #2 [13]. See text for other details.
Initial cell spreading establishes preferential paths of future cell protrusion [81, 82]. The resulting positive feedback promotes alignment of cell extensions and radial organization of collagen fibrils under tension. After several hours, most cells have spread in elongated morphology and formation of cell clusters becomes evident (Figure 2D). The extensive fibrillar FN matrix associated with cell clusters (Figure 2E) appears by confocal microscopy to be localized primarily beneath and along their margins [13].
The dynamics of cell clustering and FN matrix organization suggested that clustering requires cells to bind to a shared FN matrix. Consistent with this idea, blocking FN fibrillar matrix formation by inhibiting Rho kinase and myosin II activity [83, 84] prevented cell clustering. Also, blocking FN using antibodies or inhibiting or interfering with expression of integrin α5β1 receptors prevented FN matrix formation and cell clustering [13]. Therefore, cell contraction was required not only to bring cells closer together [68, 69], but also to organize FN into a fibrillar matrix. Cell-cell encounters and collagen matrix remodeling that occur under promigratory conditions are not sufficient to promote cell clustering.
Dispersion of Cell Clusters
Epithelial cell morphogenesis provided the major initial focus of studies on mechanisms of cell clustering. Loss of epithelial morphogenic stability as a result of malignant transformation has provided the predominant focus of studies on cell dispersion. Factors contributing to the invasiveness of epithelial cells into the surrounding stromal connective tissue (epithelial-mesenchymal transition), include loss of normal cadherin-mediate cell-cell adhesions, changes in integrins, and upregulation of matrix metalloproteinases [60, 61, 85–88]
Relatively little is known about reversibility of mesenchymal cell clusters. However, the model system described in Figure 1 offers unique opportunities to study this phenomenon. The observation that fragmentation of FN matrix occurs during disruption of cell clusters and cell dispersion suggests that the dispersal process requires more than just PDGF-activation of cell migration. In 2D culture, fibronectin matrix can retard cell migration, which can be reversed by matrix metalloproteinase-dependent degradation of the FN matrix [89]. In preliminary experiments we found that broad spectrum metalloproteinase inhibitors GM6001 and BB-94 added to pre-clustered fibroblasts on collagen matrices blocked subsequent fibroblast dispersion and FN matrix fragmentation stimulated by switching to promigratory conditions. Cell clusters and their fibronectin matrix remain intact.
Future perspectives.
Future work regarding cell clustering on 3D collagen matrices can be understood from three different perspectives: mechanism, physiological consequences, and potential usefulness as a research model. In terms of mechanism, formation of cell clusters appears to be a consequence of the changing balance between cell-cell and cell-substrate adhesive properties and migratory conditions. Much remains to be learned about the effects of the growth factor environment, stabilization of cell-cell interactions, and factors controlling cluster dispersion. In terms of physiological consequences, fibroblast clustering results in a potentially important ‘gain in function” such as occurs when cell differentiation follows mesenchymal stem cell condensation and hair follicle development follows dermal papilla formation. The physiological consequences also can be potentially negative such as production and release of proinflammatory mediators following formation of fibroblast spheroids. Fibroblast clustering also might be viewed as analogous to formation of the interconnected myofibroblast network responsible for stromal fibrosis. In this case, understanding the failure of oncogenic Ras transformed fibroblasts to undergo clustering might provide insights into the independence of invasive, tumor-derived mesenchymal cells from the interconnected myofibroblast network of the tumor stroma. Finally, several features make fibroblast clustering on collagen matrices a highly tractable research model for investigating the mechanism and physiological consequences of clustering including the ease of measuring clustering and modifying experimental clustering conditions and the possibility to study cluster dispersion. Research on the fibroblast-collagen clustering model offers a new approach to understanding differential fibroblast morphogenic responses in relationship to substratum biochemistry and biomechanics and should provide new insights into tissue organization and advance the field of tissue engineering.
Highlights.
Fibroblasts clusters occur under diverse physiological and pathological conditions.
Cluster formation depends interplay between ECM and the growth factor environment.
Soft substrates and procontractile growth factors promote fibroblast clustering.
Clustering depends on organization of a fibronectin fibrillar matrix scaffold.
Fragmentation of the FN fibrillar matrix occurs during cell dispersal from clusters.
Acknowledgments
Thanks to Chin-Han Ho and Zhenan Liu for helpful comments. Research in our laboratory is supported by NIH grant GM031321. This work was performed in laboratories constructed with support from NIH grant C06-RR30414.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dewel RA. Colonial origin for Emetazoa: major morphological transitions and the origin of bilaterian complexity. J Morphol. 2000;243:35–74. doi: 10.1002/(SICI)1097-4687(200001)243:1<35::AID-JMOR3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 2.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–22. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 2009;28:167–76. doi: 10.1007/s10555-008-9178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grinnell F, Petroll WM. Cell motility and mechanics in three-dimensional collagen matrices. Annu Rev Cell Dev Biol. 2010;26:335–61. doi: 10.1146/annurev.cellbio.042308.113318. [DOI] [PubMed] [Google Scholar]
- 5.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–37. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proceedings of the National Academy of Sciences U S A. 1979;76:1274–8. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrlich HP. Wound closure: evidence of cooperation between fibroblasts and collagen matrix. Eye (Lond) 1988;2(Pt 2):149–57. doi: 10.1038/eye.1988.28. [DOI] [PubMed] [Google Scholar]
- 8.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–4. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–10. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Davis GE, Senger DR. Extracellular matrix mediates a molecular balance between vascular morphogenesis and regression. Curr Opin Hematol. 2008;15:197–203. doi: 10.1097/MOH.0b013e3282fcc321. [DOI] [PubMed] [Google Scholar]
- 11.Rhee S, Ho CH, Grinnell F. Promigratory and procontractile growth factor environments differentially regulate cell morphogenesis. Exp Cell Res. 2010;316:232–244. doi: 10.1016/j.yexcr.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sevilla CA, Dalecki D, Hocking DC. Extracellular matrix fibronectin stimulates the self-assembly of microtissues on native collagen gels. Tissue Eng Part A. 2010;16:3805–19. doi: 10.1089/ten.tea.2010.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.da Rocha-Azevedo B, Ho CH, Grinnell F. Fibroblast cluster formation on 3D collagen matrices requires cell contraction dependent fibronectin matrix organization. Exp Cell Res. 2013;319:546–555. doi: 10.1016/j.yexcr.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30:2164–74. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez-Pomares JM, Mironov V, Guadix JA, Macias D, Markwald RR, Munoz-Chapuli R. In vitro self-assembly of proepicardial cell aggregates: an embryonic vasculogenic model for vascular tissue engineering. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:700–13. doi: 10.1002/ar.a.20338. [DOI] [PubMed] [Google Scholar]
- 16.Kelm JM, Moritz W, Schmidt D, Hoerstrup SP, Fussenegger M. In vitro vascularization of human connective microtissues. Methods Mol Med. 2007;140:153–66. doi: 10.1007/978-1-59745-443-8_9. [DOI] [PubMed] [Google Scholar]
- 17.Imani R, Hojjati Emami S, Fakhrzadeh H, Baheiraei N, Sharifi AM. Optimization and comparison of two different 3D culture methods to prepare cell aggregates as a bioink for organ printing. Biocell. 2012;36:37–45. [PubMed] [Google Scholar]
- 18.Moscona A, Moscona H. The dissociation and aggregation of cells from organ rudiments of the early chick embryo. J Anat. 1952;86:287–301. [PMC free article] [PubMed] [Google Scholar]
- 19.Steinberg MS. On the mechanism of tissue reconstruction by dissociated cells. I. Population kinetics, differential adhesiveness. and the absence of directed migration. Proc Natl Acad Sci U S A. 1962;48:1577–82. doi: 10.1073/pnas.48.9.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duguay D, Foty RA, Steinberg MS. Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants. Dev Biol. 2003;253:309–23. doi: 10.1016/s0012-1606(02)00016-7. [DOI] [PubMed] [Google Scholar]
- 21.Robinson EE, Zazzali KM, Corbett SA, Foty RA. Alpha5beta1 integrin mediates strong tissue cohesion. J Cell Sci. 2003;116:377–86. doi: 10.1242/jcs.00231. [DOI] [PubMed] [Google Scholar]
- 22.Hall BK, Miyake T. Divide, accumulate, differentiate: cell condensation in skeletal development revisited. Int J Dev Biol. 1995;39:881–93. [PubMed] [Google Scholar]
- 23.Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 2000;22:138–47. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Tickle C. Making digit patterns in the vertebrate limb. Nat Rev Mol Cell Biol. 2006;7:45–53. doi: 10.1038/nrm1830. [DOI] [PubMed] [Google Scholar]
- 25.Newman SA, Bhat R. Activator-inhibitor dynamics of vertebrate limb pattern formation. Birth Defects Res C Embryo Today. 2007;81:305–19. doi: 10.1002/bdrc.20112. [DOI] [PubMed] [Google Scholar]
- 26.Mammoto T, Mammoto A, Torisawa YS, Tat T, Gibbs A, Derda R, Mannix R, de Bruijn M, Yung CW, Huh D, Ingber DE. Mechanochemical control of mesenchymal condensation and embryonic tooth organ formation. Dev Cell. 2011;21:758–69. doi: 10.1016/j.devcel.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delise AM, Tuan RS. Analysis of N-cadherin function in limb mesenchymal chondrogenesis in vitro. Dev Dyn. 2002;225:195–204. doi: 10.1002/dvdy.10151. [DOI] [PubMed] [Google Scholar]
- 28.Dessau W, von der Mark H, von der Mark K, Fischer S. Changes in the patterns of collagens and fibronectin during limb-bud chondrogenesis. J Embryol Exp Morphol. 1980;57:51–60. [PubMed] [Google Scholar]
- 29.Frenz DA, Jaikaria NS, Newman SA. The mechanism of precartilage mesenchymal condensation: a major role for interaction of the cell surface with the amino-terminal heparin-binding domain of fibronectin. Dev Biol. 1989;136:97–103. doi: 10.1016/0012-1606(89)90133-4. [DOI] [PubMed] [Google Scholar]
- 30.Widelitz RB, Chuong CM. Early events in skin appendage formation: induction of epithelial placodes and condensation of dermal mesenchyme. J Investig Dermatol Symp Proc. 1999;4:302–6. doi: 10.1038/sj.jidsp.5640234. [DOI] [PubMed] [Google Scholar]
- 31.Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–25. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 32.Stenn KS, Cotsarelis G. Bioengineering the hair follicle: fringe benefits of stem cell technology. Curr Opin Biotechnol. 2005;16:493–7. doi: 10.1016/j.copbio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Driskell RR, Clavel C, Rendl M, Watt FM. Hair follicle dermal papilla cells at a glance. J Cell Sci. 2011;124:1179–82. doi: 10.1242/jcs.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang BM, Kwack MH, Kim MK, Kim JC, Sung YK. Sphere formation increases the ability of cultured human dermal papilla cells to induce hair follicles from mouse epidermal cells in a reconstitution assay. J Invest Dermatol. 2012;132:237–9. doi: 10.1038/jid.2011.250. [DOI] [PubMed] [Google Scholar]
- 35.Young TH, Lee CY, Chiu HC, Hsu CJ, Lin SJ. Self-assembly of dermal papilla cells into inductive spheroidal microtissues on poly(ethylene-co-vinyl alcohol) membranes for hair follicle regeneration. Biomaterials. 2008;29:3521–30. doi: 10.1016/j.biomaterials.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Higgins CA, Richardson GD, Ferdinando D, Westgate GE, Jahoda CA. Modelling the hair follicle dermal papilla using spheroid cell cultures. Exp Dermatol. 2010;19:546–8. doi: 10.1111/j.1600-0625.2009.01007.x. [DOI] [PubMed] [Google Scholar]
- 37.Jahoda CA, Mauger A, Bard S, Sengel P. Changes in fibronectin, laminin and type IV collagen distribution relate to basement membrane restructuring during the rat vibrissa follicle hair growth cycle. J Anat. 1992;181(Pt 1):47–60. [PMC free article] [PubMed] [Google Scholar]
- 38.Pflieger D, Chabane S, Gaillard O, Bernard BA, Ducoroy P, Rossier J, Vinh J. Comparative proteomic analysis of extracellular matrix proteins secreted by two types of skin fibroblasts. Proteomics. 2006;6:5868–79. doi: 10.1002/pmic.200402040. [DOI] [PubMed] [Google Scholar]
- 39.Young TH, Tu HR, Chan CC, Huang YC, Yen MH, Cheng NC, Chiu HC, Lin SJ. The enhancement of dermal papilla cell aggregation by extracellular matrix proteins through effects on cell-substratum adhesivity and cell motility. Biomaterials. 2009;30:5031–40. doi: 10.1016/j.biomaterials.2009.05.065. [DOI] [PubMed] [Google Scholar]
- 40.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grieb G, Steffens G, Pallua N, Bernhagen J, Bucala R. Circulating fibrocytes--biology and mechanisms in wound healing and scar formation. Int Rev Cell Mol Biol. 2011;291:1–19. doi: 10.1016/B978-0-12-386035-4.00001-X. [DOI] [PubMed] [Google Scholar]
- 42.Phan SH. Genesis of the myofibroblast in lung injury and fibrosis. Proc Am Thorac Soc. 2012;9:148–52. doi: 10.1513/pats.201201-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singer II, Kawka DW, Kazazis DM, Clark RA. In vivo co-distribution of fibronectin and actin fibers in granulation tissue: immunofluorescence and electron microscope studies of the fibronexus at the myofibroblast surface. J Cell Biol. 1984;98:2091–106. doi: 10.1083/jcb.98.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petroll WM, Cavanagh HD, Jester JV. Assessment of stress fiber orientation during healing of radial keratotomy wounds using confocal microscopy. Scanning. 1998;20:74–82. doi: 10.1002/sca.1998.4950200202. [DOI] [PubMed] [Google Scholar]
- 45.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 46.Hinz B, Gabbiani G. Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodeling. Thromb Haemost. 2003;90:993–1002. doi: 10.1160/TH03-05-0328. [DOI] [PubMed] [Google Scholar]
- 47.Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999;18:311–56. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- 48.Abraham DJ, Eckes B, Rajkumar V, Krieg T. New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma. Curr Rheumatol Rep. 2007;9:136–43. doi: 10.1007/s11926-007-0008-z. [DOI] [PubMed] [Google Scholar]
- 49.Hinz B. The myofibroblast: Paradigm for a mechanically active cell. J Biomech. 2010;43:146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 50.Sarrazy V, Billet F, Micallef L, Coulomb B, Desmouliere A. Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair Regen. 2011;19(Suppl 1):s10–5. doi: 10.1111/j.1524-475X.2011.00708.x. [DOI] [PubMed] [Google Scholar]
- 51.Kischer CW, Hendrix MJ. Fibronectin (FN) in hypertrophic scars and keloids. Cell Tissue Res. 1983;231:29–37. doi: 10.1007/BF00215771. [DOI] [PubMed] [Google Scholar]
- 52.Ehrlich HP, Desmouliere A, Diegelmann RF, Cohen IK, Compton CC, Garner WL, Kapanci Y, Gabbiani G. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol. 1994;145:105–13. [PMC free article] [PubMed] [Google Scholar]
- 53.Tuan TL, Nichter LS. The molecular basis of keloid and hypertrophic scar formation. Mol Med Today. 1998;4:19–24. doi: 10.1016/S1357-4310(97)80541-2. [DOI] [PubMed] [Google Scholar]
- 54.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991;88:6642–6. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuhn C, McDonald JA. The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol. 1991;138:1257–65. [PMC free article] [PubMed] [Google Scholar]
- 56.Kapanci Y, Desmouliere A, Pache JC, Redard M, Gabbiani G. Cytoskeletal protein modulation in pulmonary alveolar myofibroblasts during idiopathic pulmonary fibrosis. Possible role of transforming growth factor beta and tumor necrosis factor alpha. Am J Respir Crit Care Med. 1995;152:2163–9. doi: 10.1164/ajrccm.152.6.8520791. [DOI] [PubMed] [Google Scholar]
- 57.Hardie WD, Glasser SW, Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol. 2009;175:3–16. doi: 10.2353/ajpath.2009.081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salmenpera P, Kankuri E, Bizik J, Siren V, Virtanen I, Takahashi S, Leiss M, Fassler R, Vaheri A. Formation and activation of fibroblast spheroids depend on fibronectin-integrin interaction. Exp Cell Res. 2008;314:3444–52. doi: 10.1016/j.yexcr.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 59.Pulvertaft RJ, Weiss L. The identification of living malignant cells in exudates. J Clin Pathol. 1957;10:390–3. doi: 10.1136/jcp.10.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 61.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 63.Shield K, Ackland ML, Ahmed N, Rice GE. Multicellular spheroids in ovarian cancer metastases: Biology and pathology. Gynecol Oncol. 2009;113:143–8. doi: 10.1016/j.ygyno.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 64.Iwanicki MP, Davidowitz RA, Ng MR, Besser A, Muranen T, Merritt M, Danuser G, Ince TA, Brugge JS. Ovarian cancer spheroids use myosin-generated force to clear the mesothelium. Cancer Discov. 2011;1:144–57. doi: 10.1158/2159-8274.CD-11-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robinson EE, Foty RA, Corbett SA. Fibronectin matrix assembly regulates alpha5beta1-mediated cell cohesion. Mol Biol Cell. 2004;15:973–81. doi: 10.1091/mbc.E03-07-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caicedo-Carvajal CE, Shinbrot T, Foty RA. Alpha5beta1 integrin-fibronectin interactions specify liquid to solid phase transition of 3D cellular aggregates. PLoS One. 2010;5:e11830. doi: 10.1371/journal.pone.0011830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bao B, Jiang J, Yanase T, Nishi Y, Morgan JR. Connexon-mediated cell adhesion drives microtissue self-assembly. Faseb J. 2011;25:255–64. doi: 10.1096/fj.10-155291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo WH, Frey MT, Burnham NA, Wang YL. Substrate rigidity regulates the formation and maintenance of tissues. Biophys J. 2006;90:2213–20. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reinhart-King CA, Dembo M, Hammer DA. Cell-cell mechanical communication through compliant substrates. Biophys J. 2008;95:6044–51. doi: 10.1529/biophysj.107.127662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kihara T, Imamura Y, Takemura Y, Mizuno K, Adachi E, Hayashi T. Intercellular accumulation of type V collagen fibrils in accordance with cell aggregation. J Biochem. 2008;144:625–33. doi: 10.1093/jb/mvn109. [DOI] [PubMed] [Google Scholar]
- 71.Barocas VH, Moon AG, Tranquillo RT. The fibroblast-populated collagen microsphere assay of cell traction force--Part 2: Measurement of the cell traction parameter. J Biomech Eng. 1995;117:161–70. doi: 10.1115/1.2795998. [DOI] [PubMed] [Google Scholar]
- 72.Miron-Mendoza M, Seemann J, Grinnell F. The differential regulation of cell motile activity through matrix stiffness and porosity in three dimensional collagen matrices. Biomaterials. 2010;31:6425–35. doi: 10.1016/j.biomaterials.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao Z, Sasaoka T, Fujimori T, Oya T, Ishii Y, Sabit H, Kawaguchi M, Kurotaki Y, Naito M, Wada T, Ishizawa S, Kobayashi M, Nabeshima Y, Sasahara M. Deletion of the PDGFR-beta gene affects key fibroblast functions important for wound healing. J Biol Chem. 2005;280:9375–89. doi: 10.1074/jbc.M413081200. [DOI] [PubMed] [Google Scholar]
- 74.Li W, Fan J, Chen M, Guan S, Sawcer D, Bokoch GM, Woodley DT. Mechanism of human dermal fibroblast migration driven by type I collagen and platelet-derived growth factor-BB. Mol Biol Cell. 2004;15:294–309. doi: 10.1091/mbc.E03-05-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang H, Rhee S, Ho CH, Grinnell F. Distinguishing fibroblast promigratory and procontractile growth factor environments in 3D collagen matrices. FASEB J. 2008;22:2151–2160. doi: 10.1096/fj.07-097014. [DOI] [PubMed] [Google Scholar]
- 76.Menezes GC, Miron-Mendoza M, Ho CH, Jiang H, Grinnell F. Oncogenic Ras-transformed human fibroblasts exhibit differential changes in contraction and migration in 3D collagen matrices. Exp Cell Res. 2008;314:3081–91. doi: 10.1016/j.yexcr.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guidry C, Grinnell F. Studies on the mechanism of hydrated collagen gel reorganization by human skin fibroblasts. J Cell Sci. 1985;79:67–81. doi: 10.1242/jcs.79.1.67. [DOI] [PubMed] [Google Scholar]
- 78.El Sayegh TY, Kapus A, McCulloch CA. Beyond the epithelium: cadherin function in fibrous connective tissues. FEBS Lett. 2007;581:167–74. doi: 10.1016/j.febslet.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 79.Miron-Mendoza M, Lin X, Ma L, Ririe P, Petroll WM. Individual versus collective fibroblast spreading and migration: regulation by matrix composition in 3D culture. Exp Eye Res. 2012;99:36–44. doi: 10.1016/j.exer.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsuyoshi N, Hamaguchi M, Taniguchi S, Nagafuchi A, Tsukita S, Takeichi M. Cadherin-mediated cell-cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts. J Cell Biol. 1992;118:703–14. doi: 10.1083/jcb.118.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Friedl P, Maaser K, Klein CE, Niggemann B, Krohne G, Zanker KS. Migration of highly aggressive MV3 melanoma cells in 3-dimensional collagen lattices results in local matrix reorganization and shedding of alpha2 and beta1 integrins and CD44. Cancer Res. 1997;57:2061–70. [PubMed] [Google Scholar]
- 82.Dickinson RB, Guido S, Tranquillo RT. Biased cell migration of fibroblasts exhibiting contact guidance in oriented collagen gels. Ann Biomed Eng. 1994;22:342–56. doi: 10.1007/BF02368241. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Q, Magnusson MK, Mosher DF. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through rho-dependent actin stress fiber formation and cell contraction. Mol Biol Cell. 1997;8:1415–25. doi: 10.1091/mbc.8.8.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trusolino L, Comoglio PM. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer. 2002;2:289–300. doi: 10.1038/nrc779. [DOI] [PubMed] [Google Scholar]
- 86.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 87.Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010;20:161–8. doi: 10.1016/j.semcancer.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martins VL, Caley M, O’Toole EA. Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res. 2012 doi: 10.1007/s00441-012-1410-z. [DOI] [PubMed] [Google Scholar]
- 89.Takino T, Nagao R, Manabe R, Domoto T, Sekiguchi K, Sato H. Membrane-type 1 matrix metalloproteinase regulates fibronectin assembly to promote cell motility. FEBS Lett. 2011;585:3378–84. doi: 10.1016/j.febslet.2011.09.039. [DOI] [PubMed] [Google Scholar]