Abstract
Genes and environmental conditions interact in the development of cognitive capacities and each plays an important role in neuropsychiatric disorders such as attention deficit/hyperactivity disorder (ADHD) and schizophrenia. Multiple studies have indicated that the gene for the SNARE protein SNAP-25 is a candidate susceptibility gene for ADHD, as well as schizophrenia, while maternal smoking is a candidate environmental risk factor for ADHD. We utilized mice heterozygous for a Snap25 null allele and deficient in SNAP-25 expression to model genetic effects in combination with prenatal exposure to nicotine to explore genetic and environmental interactions in synaptic plasticity and behavior. We show that SNAP-25 deficient mice exposed to prenatal nicotine exhibit hyperactivity and deficits in social interaction. Using a high frequency stimulus electrophysiological paradigm for long-term depression (LTD) induction, we examined the roles of dopaminergic D2 receptors (D2Rs) and cannabinoid CB1 receptors (CB1Rs), both critical for LTD induction in the striatum. We found that prenatal exposure to nicotine in Snap25 heterozygote null mice produced a deficit in the D2R-dependent induction of LTD, although CB1R regulation of plasticity was not impaired. We also show that prenatal nicotine exposure altered the affinity and/or receptor coupling of D2Rs, but not the number of these receptors in heterozygote null Snap25 mutants. These results refine the observations made in the coloboma mouse mutant, a proposed mouse model of ADHD, and illustrate how gene × environmental influences can interact to perturb neural functions that regulate behavior.
Keywords: SNAP-25, Prenatal nicotine exposure, Dopaminergic D2 receptors (D2Rs), Cannabinoid CB1 receptors (CB1Rs), Short-term depression (STD), Long-term depression (LTD)
1. Introduction
The interplay between genetic and environmental factors is likely to play a significant role in the development of cognitive capacities and in neuropsychiatric disorders (Wermter et al., 2010). Attention deficit/hyperactivity disorder (ADHD) is a common, albeit heterogeneous, neuropsychiatric disorder with a prevalence of greater than 5% worldwide (Faraone et al., 2003; Polanczyk et al., 2007), which begins in childhood and often extends into adulthood (Biederman et al., 2011). ADHD is characterized by its debilitating social and behavioral symptoms of excessive inattention, hyperactivity, and impulsivity (American Psychiatric Association, DSM IV-TR, 2000). Genetic association studies have demonstrated that ADHD has strong heritability, although it appears to have a complex multigenic etiology (Faraone et al., 2005; Faraone and Mick, 2010) consistent with small effects due to multiple genes. Furthermore, there is evidence that environmental toxins, in particular prenatal exposure to tobacco smoke and postnatal lead exposure, are significant risk factors for ADHD (Braun et al., 2006; Linnet et al., 2003). To date, direct evidence for interaction of genes and environment underlying ADHD, which could potentially promote distinct endophenotypes, has not been clearly established.
The therapeutic response to psychostimulant treatment has implicated the involvement of dopamine and norepinephrine systems, as well as deficits in cortico-striatal circuitry and executive function in ADHD (Swanson et al., 2007). Recent imaging studies further suggest the contribution of a wider range of neural networks to the diversity of ADHD symptoms (Castellanos and Proal, 2012). Based on the evidence for alterations in dopaminergic neurotransmitter systems, a number of genes that encode proteins involved in dopamine transmission have been examined in genetic studies and shown to be associated with ADHD (Faraone and Mick, 2010). Among these identified candidates is the gene SNAP25 that encodes the presynaptic protein SNAP-25, which is a critical component of the SNARE protein-protein complex responsible for action-potential triggered synaptic release of neurotransmitters (Rizo and Sudhof, 2012; Washbourne et al., 2002). Because of the critical role of SNAP-25 in the vesicular exocytosis required for synaptic transmission (Jahn and Scheller, 2006), the gene SNAP25 could play a part in several heritable neurocognitive and behavioral abnormalities (Corradini et al., 2012), for example, SNAP25 has been proposed as a candidate gene for schizophrenia based upon genome wide analysis (Lewis et al., 2003).
Animal models have been valuable in the characterization of mechanisms that may underlie human neuropsychiatric disorders. The coloboma mouse mutant, heterozygous for a ~2 cM deletion of chromosome 2 (Cm) that encompasses the gene encoding SNAP-25, is one of several experimental constructs that meet the criteria of a valid animal model of ADHD (Fan et al., 2012; Wilson, 2000). This SNAP-25 deficient mouse, which exhibits a 50% reduction of SNAP-25 expression (Hess et al., 1992), displays certain hallmarks of ADHD including hyperactivity that is ameliorated by the psychostimulant amphetamine (Hess et al., 1996) as well as inattention and impulsivity (Bruno et al., 2007). Moreover, the hyperkinesis and amphetamine responsiveness of these mutants have been shown to be mediated through D2 dopamine receptors (Fan and Hess, 2007; Fan et al., 2010). Interestingly, the robust hyperactive behavior of this mutant appeared not to be recapitulated in heterozygote Snap25 null mutants (Washbourne et al., 2002), although it was recently reported that these mice do display more subtle behavioral deficits and a susceptibility to seizures (Corradini et al., 2012). The hypothesis that alterations in SNAP-25 expression or function increase susceptibility to genetic, environmental, or age-related factors to promote neural dysfunctions that lead to neurocognitive disorders is underscored by the finding that prenatal stress both enhances sensorimotor gating defects and reveals depression-like behavior in mice bearing a Snap25 gain of function mutation (Bdr) (Oliver and Davies, 2009).
In order to refine the SNAP-25 deficient coloboma model and test for gene × environmental (G × E) interactions that may represent ADHD-like phenotypes and underlying mechanisms, we evaluated adolescent (PN35 – PN50) Snap25 null mutant heterozygotes (Snap25+/−) and control littermates (Snap25+/+) (Washbourne et al., 2002) using a previously characterized prenatal nicotine exposure paradigm (Paz et al., 2007). We show that SNAP-25 deficient mice exposed to nicotine throughout gestation and early perinatal development show elevated hyperactivity similar to that observed in coloboma mice. In addition, we observe deficits in social interactions, which may have relevance for other neuropsychiatric disorders such as schizophrenia as has been reported recently for other mouse mutants (Oliver and Davies, 2009; Oliver and Davies, 2012; Pletnikov et al., 2008). Importantly, we also show that the prenatal exposure of Snap25 heterozygotes to nicotine results in deficits in long-term depression (LTD), a measure of synaptic plasticity of cortico-striatal circuits (Surmeier et al., 2007). Furthermore, these changes in synaptic plasticity are paralleled by changes in affinity of D2 receptors, key components of the pathway responsible for cortico-striatal LTD (Lovinger, 2010; Surmeier et al., 2007). These results set the stage to begin to dissect the interplay between specific neural circuits, alterations in gene expression, and environmental effects that collectively influence specific behaviors and potentially contribute to neuropsychiatric disorders.
2. Results
2.1 Prenatal nicotine exposure paradigm
To examine the effect of prenatal exposure to nicotine, we employed the previously described model of nicotine administration via drinking water (Paz et al., 2007). In this paradigm, breeding pairs were provided either with drinking water containing nicotine and saccharine (0.05 mg/ml and 0.6 mg/ml, respectively; PNE) or saccharine alone (Sac) ad libitum prior to and throughout gestation. To avoid potential effects due to an interaction between nicotine and a SNAP-25 deficient intrauterine environment, we used wild type females and heterozygote Snap25 null males as breeding pairs. Following birth, the nicotine/saccharine content of the drinking water was gradually tapered down over one week to standard drinking water to limit any possible effects on prenatal/perinatal brain development. Wild type (WT) and heterozygous (HET) male offspring were evaluated between 35-50 days old (average ~PN 40 for each group, see Table 1) to model an adolescent stage of brain maturation. The four G × E experimental groups comprised of these cohorts were designated WT/Sac, WT/PNE, HET/Sac, and HET/PNE to represent genotype and treatment, respectively. Previously it was found that there was no difference in the litter size or birth weight of pups of either the PNE or control Sac treated dams (Paz et al., 2007). Consistent with this report, we found no significant differences among the different groups in weights of the mice at PN40 (Table 1).
Table 1. Average age and weight of mice used for electrophysiology and binding studies.
Table shows ages and weights for males used from electrophysiology and binding studies (n=24- 31 animals). Table shows ages and weights for females used in addition to males (n=10 animals) only for [3H]-Quinpirole D2R agonist saturation binding experiments (Table 3; see Experimental procedures). Values shown are mean ± SEM. No significant differences were found between ages used or weights of mice in experiments.
| Group | Age (PN day) | Weight (g) | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| WT/Sac | 40.2 ± 0.7 | 37.8 ± 0.4 | 20.5 ± 0.3 | 17.0 ± 0.3 |
| WT/PNE | 38.8 ± 0.6 | 39.1 ± 0.4 | 20.8 ± 0.3 | 18.2 ± 0.5 |
| HET/Sac | 39.6 ± 1.0 | 38.0 ± 0.4 | 19.9 ± 0.4 | 16.5 ± 0.3 |
| HET/PNE | 39.8 ± 0.5 | 39.5 ± 0.6 | 20.6 ± 0.3 | 17.1 ± 0.3 |
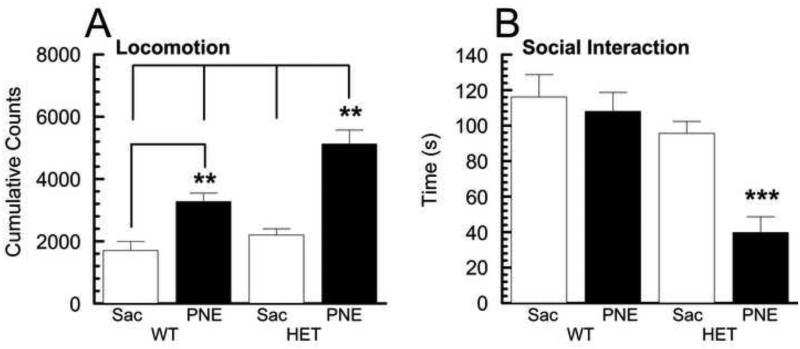
2.2 Increased locomotor activity and decreased social interaction in HET/PNE group due to HET genotype and PNE treatment and their interactions
As an initial behavioral evaluation, we assessed the four G × E experimental groups for spontaneous locomotor activity in a novel environment and determined their social interaction phenotype (Fig. 1A, B). For these behaviors, the mice were tested during their nocturnal active phase. Cumulative data collected for the locomotor activity over 180 minutes is shown in Fig. 1A. A two-way ANOVA of the data obtained from the two genotype (HET v WT) × two prenatal treatment (PNE v Sac) group comparisons revealed a significant effect of genotype (F(1,14) = 14.11 ,p < 0.01), treatment (F(1,14)=51.76, p <0.001), as well as a significant interaction of genotype × treatment (F(1,14)=4.70, p <0.05). Post-hoc tests showed further that the activity of the HET/PNE group was significantly greater (~1.5 − 3.5 fold) than the other groups (Fig. 1A; p<0.01) indicating increased locomotor activity among the HET mice exposed to prenatal nicotine. Importantly, we did not observe significantly increased activity of the HET control group compared to WT mice, consistent with our initial report on the behavior of these mice (Washbourne et al., 2002). However, in agreement with earlier findings (Paz et al., 2007), PNE treatment did result in greater activity of wild type offspring (WT/PNE group) compared to saccharine-treated wild type controls (WT/Sac group; ~2.2 fold, p <0.01). Nevertheless, the significant ~3.5 fold increase of spontaneous activity observed in HET/PNE groups compared to WT/Sac groups was more comparable to the hyperactivity of coloboma mutants, which was 3-4 fold greater compared to their control wild type littermates when tested over a similar time course (Hess et al., 1996).
Fig. 1. Locomotor activity and social interaction in the four G × E groups.
A Locomotor activity of animals from the four G × E experimental groups were monitored in individual photocell activity cages. Values shown (mean ± SEM, n = 4 – 5 animals) represent beam breaks recorded over a 180 minute period. Post-hoc revealed HET/PNE group had significantly greater activity than other groups (p < 0.01) and WT/PNE significantly greater than the WT/Sac control group (p < 0.01). B Social interaction of animals from the four G × E groups was monitored individually. Values shown (mean ± SEM, n = 7 – 8 animals) represent time spent interacting with novel mouse. Post-hoc tests for social interaction revealed HET/PNE group interacted significantly less frequently than other groups (p < 0.001).
To examine a more complex behavior, we subjected the four groups of mice to a social interaction test in which the mice were assessed for the amount of time spent with an unfamiliar mouse of the same sex and age (Fig. 1B). A two-way ANOVA revealed a significant effect of genotype (F(1,26)=20.80, p < 0.001), treatment (F(1,26)=10.82, p <0.01), and a significant interaction of genotype × treatment (F(1,26)=6.02, p <0.05). Post-hoc tests demonstrated further that the social interaction of the HET/PNE group was significantly less (~0.3 − 0.4 fold) than the other groups (Fig. 1B; p<0.001) indicating reduced social interaction among the HET mice exposed to prenatal nicotine.
When taken together, these results provide evidence that prenatal exposure to nicotine has a pronounced effect on the behavior of Snap25 heterozygote null mutant mice that is greater than its effect on wild type littermates, consistent with the idea that a SNAP-25 deficiency during brain development confers a vulnerability to the behavioral consequences of in utero nicotine exposure.
2.3 D2R-dependent induction of long-term synaptic depression (LTD) in the striatum is affected by HET genotype and PNE treatment and their interactions
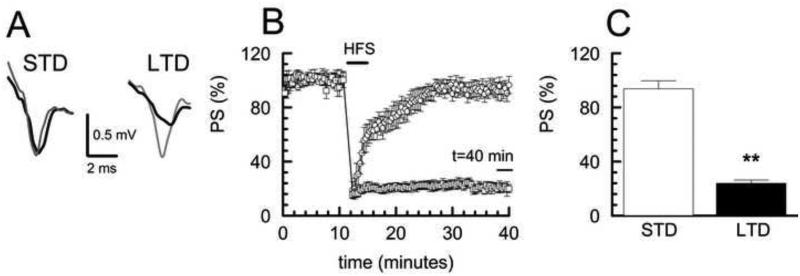
Because of the critical role played by the dorsal striatum in controlling the execution of motor functions, as well as the integration and decision-making processes that further elucidate these behaviors (Balleine et al., 2007), we next investigated synaptic plasticity in the corticostriatal synaptic field of the G × E experimental groups. To first characterize use-dependent plasticity in these preparations, we used a high frequency stimulus (HFS) paradigm to elicit synaptic depression of cortical inputs measured by population spike (PS) amplitude recordings in the dorsal striatum from coronal slices of the WT/Sac control group. We found that following the HFS stimulus, all slices exhibited an initial depression in the PS to ~20% of baseline (posttetanic depression, PTD). For some slices, the PS returned to near baseline within a few minutes, while for other slices the PS remained depressed for the full 30 minute recording period (Fig. 2B). We used a cluster analysis (see Experimental procedures) to make an unbiased differentiation of the two apparent populations of responses that were then defined operationally as short-term depression (STD) and long-term depression (LTD) based on the percent of fEPSP recovery at 30 minutes. Fig. 2A shows representative PS records from slices of the designated STD and LTD clusters that were taken during baseline recording and then 30 minutes after HFS. Fig. 2C shows summary data of the percent PS recovery following HFS for the two populations of responses defined by cluster analysis with ~40% (6/15) of the slices in the LTD cluster and the remaining ~60% (9/15) of the slices in the STD cluster. The observation that the HFS paradigm can result in STD, as well as LTD, has been reported previously (Lovinger et al., 1993; Sung et al., 2001) and suggests that the LTD may evolve from STD.
Fig. 2. Cluster analysis determination of STD and LTD populations in WT/Sac control group.
A Representative population spike (PS) traces of STD and LTD for baseline (gray) and 30 minutes after HFS (black). B Long term synaptic plasticity in WT/Sac control group was induced with HFS (4 × 100 Hz, 1 s) producing either LTD (<30% recovery at 40 min, □) or STD (>70% recovery at 40 min, ○) as determined by cluster analysis. Plot shows time course of PS amplitude normalized to initial 10 minute baseline following HFS. STD samples n = 9 slices; LTD samples n= 6 slices; values shown as mean ± SEM. C Comparison of percent recovery of PS at 30 minutes after HFS for LTD and STD clusters showed significance between STD and LTD, (p<0.01).
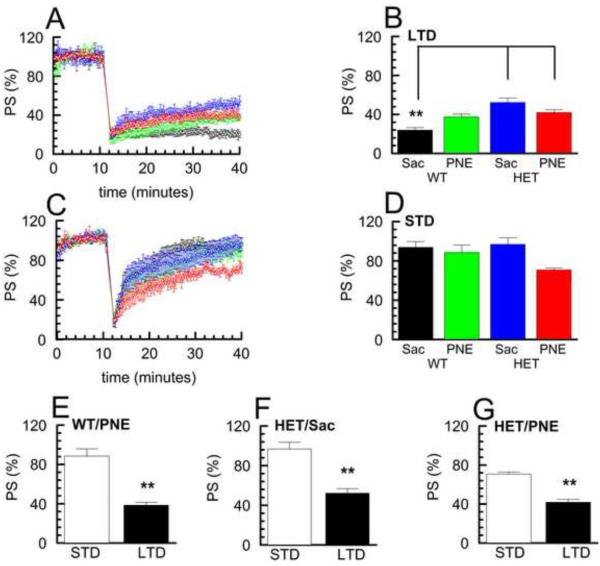
We next assessed whether the induction of these two forms of synaptic plasticity differed in the remaining experimental groups (WT/PNE, HET/Sac, and HET/PNE). HFS in slices prepared from each of these three groups produced responses that were qualitatively similar to those seen in the WT/Sac control group and could be resolved by cluster analysis into LTD (Fig. 3A) or STD (Fig. 3C) populations that were significantly different from each other (Fig. 3 E, F & G, p < 0.01). Interestingly, comparison of the relative level of LTD to that of STD obtained for each group (% PSstd / % PSltd) revealed that the difference between STD and LTD in the HET/PNE group was less (~1.7 fold; Fig. 3G) than that found for the other groups (WT/Sac ~3.9 fold, Fig. 2C; WT/PNE ~2.4 fold, Fig. 3E; HET/Sac ~1.9 fold; Fig. 3F). Additionally, a larger percentage of slices in the HET/PNE group underwent LTD rather than STD (~62%, 8/13) than in the other groups (WT/Sac ~40%, 6/15; WT/PNE ~40%, 6/15; HET/Sac ~43%; 6/14). These results suggested that there may be a selective alteration in the mechanisms responsible for eliciting either an STD or LTD response in the HET/PNE group. Indeed, a two-way ANOVA comparing the levels of LTD revealed a significant effect of genotype (F(1,22)=23.81, p < 0.001), as well as a significant interaction of genotype × treatment (F(1,22)=12.42, p < 0.01), but not of treatment alone (F(1,22)=0.20, p=0.66). A post-hoc test of the comparisons between LTD populations showed that HET/PNE and HET/Sac group exhibited less robust LTD (~1.7 – 2.2 fold larger PS after 30 minutes) compared to the WT/Sac control group (Fig. 3A, B; p<0.01) indicating reduced LTD in the HET mice regardless of prenatal exposure. The WT/PNE group also approached significance in exhibited less robust LTD comparison to the WT/Sac group (p=0.057). In contrast, two-way ANOVA of the levels of STD (% PS recovered after 30 minutes) revealed a significant effect of treatment (F(1,27)=5.16, p < 0.05), but not genotype (F(1,27)=1.13, p = 0.30), or genotype × treatment (F(1,27)=2.26, p = 0.14). While the STD obtained from slices of the HET/PNE group showed less recovery to baseline, ~0.7 − 0.8 fold ompared to the other groups, this decrease was not significant after post-hoc analysis (Fig. 3C, D).
Fig. 3. Comparison of STD and LTD in the four G × E groups.
A, C Time course of PS amplitude normalized to initial 10 minute baseline following HFS in LTD (A) and STD (C) populations of the four G × E experimental groups, (mean ± SEM, STD n = 5- 9 slices; LTD n= 6- 8 slices) (WT/Sac, black ○; WT/PNE, green □; HET/Sac, blue △; HET/PNE, red ◇). B, D Percent recovery of PS at 30 minutes following HFS in LTD (B) and STD (D) clusters shown in A and C for the four G × E experimental groups. Post-hoc tests for LTD group revealed that WT/Sac group showed significantly greater maintained depression than HET/Sac and HET/PNE groups (p < 0.01). E - G Comparison of percent recovery of PS 30 minutes after HFS for STD and LTD clusters in WT/PNE, HET/Sac and HET/PNE groups (p<0.01).
We assessed whether there were any differences in the stimulus intensity necessary to evoke similar PS amplitudes in the four groups to determine if there were differences in intrinsic excitability between groups. Comparisons were made of PS amplitudes measured at half maximum stimulus intensity for each group. The values found for each group were WT/Sac 1.096 ± 0.136; WT/PNE 1.044 ± 0.145; HET/Sac 1.102 ± 0.093; HET/PNE 1.132 ± 0.109 (magnitude (mV/mA) ± SE; n–7 slices per group). A two-way ANOVA comparing the response between these groups showed no significant effects for genotype (F(1,24)= 0.15, p =0.71), treatment (F(1,24)=0.08, p =0.93), or interaction of genotype × treatment (F(1,24)=0.11, p =0.74).
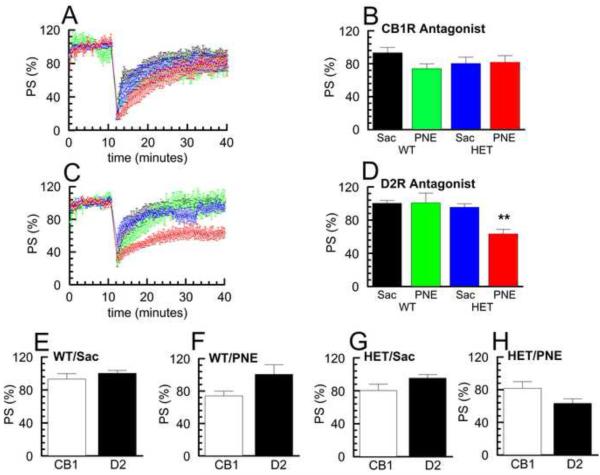
Both CB1 and D2 receptors, along with other receptors such as metabotropic glutamate receptors (mGluRs), have been shown to be critical in the process of induction of LTD in cortico-striatal synapses (Kreitzer and Malenka, 2005; Surmeier et al., 2007). Therefore, because of the differences that were found between the HET/PNE and other groups, both in terms of eliciting either LTD or STD and in the levels of recovery of LTD and STD, we tested whether the mechanisms responsible for induction of LTD were affected by either Snap25 genotype or PNE treatment. For these experiments, we used antagonists AM251 (2 μM) to block cannabinoid CB1Rs and sulpiride (10 μM) to block dopamine D2Rs in each of the four G × E groups. As shown in Fig. 4E, cluster analysis of data obtained for the WT/Sac control group in the presence of both CB1R and D2R antagonists yielded only a single population that was most similar in time course to our previously defined STD population, consistent with previous studies suggesting that the transition from STD to LTD requires both CB1Rs and D2Rs (Lovinger, 2010). Similarly, when we applied CB1R or D2R antagonists in the WT/PNE, HET/Sac, and HET/PNE groups, we again observed a single response that was similar to the STD population (Fig. 4 F, G, & H), This suggests that activation of CB1R and D2R pathways remain important for induction of LTD in WT/PNE, HET/Sac, and HET/PNE groups.
Fig. 4. Comparison of effects of D2R and CB1R antagonists on LTD induction in the four G × E groups.
A, C Time course of PS amplitude normalized to initial 10 minute baseline following HFS in the presence of CB1R antagonist, AM251, (2 μM) (A) and D2R antagonist, sulpiride, (10 μM) (C) for the four G × E experimental groups. Samples with D2R antagonist n = 6- 8 slices; samples with CB1 antagonist n = 6- 7 slices; values shown are mean ± SEM. (WT/Sac, black ○; WT/PNE, green □; HET/Sac, blue △; HET/PNE, red ◇). B, D Percent recovery of PS at 30 minutes following HFS in the presence of AM251 (B) and sulpiride (D) shown in A and C for the four G × E experimental groups. Post-hoc tests for D2R antagonists revealed HET/PNE group significantly less recovery than other groups (p < 0.01). E - H Effect of AM251 (CB1) or sulpiride (D2) on percent recovery of PS at 30 minutes following HFS in WT/Sac, WT/PNE, HET/Sac and HET/PNE groups.
Importantly, a two-way ANOVA comparing the response to HFS between these groups in the presence of the CB1R antagonist did not reveal any significant differences (Fig. 4 A, B), in keeping with the well-established central role of endocannabinoid feedback in the induction of LTD (Adermark et al., 2009; Lovinger, 2010). This finding suggests that the CB1R-dependent signaling responsible for modulating LTD mediated plasticity is not impaired in HET mice exposed to prenatal nicotine. In contrast, a two-way ANOVA comparing the response between these groups in the presence of the D2R antagonist revealed a significant effect of genotype (F(1,23)=10.07, p < 0.01), treatment (F(1,23)=5.71, p < 0.05), as well as a significant interaction of genotype × treatment (F(1,23)=5.97, p < 0.05). Post-hoc analysis showed that the response of the HET/PNE group in the presence of the D2R antagonist was significantly different from that of the other groups exhibiting decreased recovery (~0.6 − 0.7 fold) (Fig. 4 C, D; p < 0.01). This finding strongly suggests that the D2R-dependent signaling responsible for modulating LTD mediated plasticity is selectively impaired in HET mice exposed to prenatal nicotine.
2.4 D2R agonist affinity and/or receptor coupling is altered in Snap25 heterozygotes prenatally exposed to nicotine
To investigate further the role of D2R signaling in striatal synaptic depression, we first performed [35S]-GTPγS binding experiments to measure the agonist-stimulated response of G-protein coupled receptors (GPCRs) using the D2R/D3R-specific agonist quinelorane (Sovago et al., 2001) in coronal sections of P35-50 mice of the four G × E experimental groups. The intensity of the signal over the dorsal striatum was measured as previously described (Martinez et al., 2008) both to obtain the basal level of GTP binding and binding following half maximal and maximal agonist stimulation. As expected, preliminary experiments demonstrated a concentration-dependent increase in response to increasing quinelorane concentration over a range of 1 nM to 1000 μM that resulted in an approximate EC50 of 20 M and EC100 of 200 M from WT/Sac controls (data not shown). The specificity of the binding was tested by co-incubating slices with 100 M quinelorane and a saturating level of the D2R/D3R specific antagonist sulpiride (50 μM), which fully blocked the agonist stimulated GTP binding from the same WT/Sac controls (data not shown).
The results of these agonist-stimulated GTP binding experiments in the four G × E groups are summarized in Table 2. Under basal conditions without quinelorane, a two-way ANOVA revealed a significant effect of genotype on GTP binding (F(1,20)=5.89, p < 0.05), although a post-hoc test failed to show any significance difference between the groups. At half maximal stimulation with the agonist quinelorane (EC50; 20 μM), a two-way ANOVA revealed decreased [35S]-GTPγS binding compared to the WT/Sac control with significant effects of both genotype (F(1,20)=9.49, p < 0.01) and treatment (F(1,20)=7.78, p < 0.05), although no significant interaction of genotype × treatment was detected (F(1,20)=0.02, p =0.90). Post-hoc analysis of these data demonstrated that the stimulated receptor binding of GTP at the EC50 for quinelorane was significantly decreased in the HET/PNE group compared to WT/Sac controls (~0.6 fold; p < 0.01), indicating reduced activation of D2R signaling in the dorsal striatum of HET mice exposed to prenatal nicotine. In contrast to the effects obtained at the EC50 concentration, a two-way ANOVA of the data obtained at maximal stimulation (EC100; 200 μM ) revealed a significant effect of genotype (F(1,20)=7.20, p <0.05), but no significant effects for treatment (F(1,20)=0.30, p =0.59), or for a genotype × treatment interaction (F(1,20)=0.29, p =0.59). Moreover, post-hoc analysis of the EC100 data did not reveal any significant differences between these groups. These results suggest that the synaptic plasticity differences seen in the HET/PNE group may be due to changes in D2R signaling, which is revealed only under limited agonist concentrations and might result from either a decrease in receptor affinity and/or G-protein coupling.
Table 2. Dopamine agonist, quinelorane simulated [35S]-GTP-γ-S binding.
Comparative values of the four G × E experimental groups for basal [35S]-GTP-γ-S binding without quinelorane, net EC50 [35S]-GTP-γ-S binding with quinelorane at 20 μM, and net EC100 [35S]-GTP-γ-S with quinelorane at 200 μM respectively. All samples in quadruplicate are in femtomoles/105 μm2. (mean ± SEM, n = 6 animals from which quadruplicate sections were obtained). The net EC50 and EC100 binding represent net binding with basal binding subtracted prior to analysis. Post-hoc tests for EC50 revealed HET/PNE group had significantly less binding than other groups (p < 0.01).
| Group | Basal Binding | EC50 (20 μM quinelorane) | EC100 (200 μM quinelorane) |
|---|---|---|---|
| WT/Sac | 0.049 ± 0.005 | 0.104 ± 0.007 | 0.245 ± 0.011 |
| WT/PNE | 0.042 ± 0.006 | 0.085 ± 0.010 | 0.254 ± 0.018 |
| HET/Sac | 0.035 ± 0.005 | 0.083 ± 0.004 | 0.207 ± 0.011 |
| HET/PNE | 0.031 ± 0.004 | 0.066 ± 0.002** | 0.223 ± 0.016 |
2.5 Decreased [3H]-Quinpirole agonist saturation binding kinetics indicates decreases in D2R affinity in Snap25 heterozygotes prenatally exposed to nicotine
To distinguish between a reduced affinity or number of expressed D2Rs, we next performed agonist saturation binding experiments with the D2R selective radioligand [3H]-quinpirole in striatal tissue homogenates (Levant et al., 1992) to determine the Kd and Bmax for the four G × E groups. For each experiment, specific binding was determined at six agonist concentrations from 0.2 nM to 9 nM and the values of the Kd and Bmax were determined by least-squares fits of data from replicate experiments (see Experimental procedures). As summarized in Table 3, the Kd obtained for the HET/PNE group was markedly greater than the comparable values obtained for the other three groups. A two-way ANOVA of the Kd obtained from each of the four experimental groups revealed a significant effect of genotype (F(1,16)=20.01, p < 0.001), treatment (F(1,16)=5.36, p <0.05), as well as an interaction of genotype × treatment (F(1,16)=5.15, p <0.05). Importantly, post-hoc analysis demonstrated that the Kd for D2R receptor agonist binding of HET/PNE group was significantly higher (~1.6 −2.1 fold; versus WT/Sac and WT/PNE, p < 0.01; versus HET/Sac, p < 0.05) than the other groups (Table 3), indicating a reduced agonist affinity for D2Rs in HET mice exposed to prenatal nicotine. In contrast to the Kd results, no significant difference was found between the Bmax values determined for the four G × E groups indicating that the number of receptors remained unchanged. Taken together with the data obtained from the agonist stimulated GTP binding experiments, these results suggest that G × E interactions affecting synaptic plasticity in the striatum, which are reflected by impaired dopamine-dependent induction of LTD, are mediated through changes in agonist affinity and consequently G-protein signaling by these D2 receptors.
Table 3. [3H]-Quinpirole D2R agonist saturation binding kinetics.
Comparative values of Kd and Bmax for the four G × E experimental groups. All samples in triplicate and values (mean ± SEM, n = 5, each n represents dissected striatal homogenates from 2 male and 2 female brains). Post-hoc tests for the Kd revealed HET/PNE group significantly greater than WT/Sac and WT/PNE (p < 0.01) and HET/Sac (p < 0.05).
| Group | Kd (nM ± SEM) | Bmax (fmol/mg protein ± SEM) |
|---|---|---|
| WT/Sac | 3.322 ± 0.458 | 117.7 ± 15.5 |
| WT/PNE | 3.348 ± 0.599 | 128.0 ± 15.2 |
| HET/Sac | 4.562 ± 0.565 | 127.8 ± 15.7 |
| HET/PNE | 7.128 ± 0.606*,** | 119.5 ± 9.3 |
3.0 Discussion
3.1 Summary of findings
Collectively, our results argue strongly that the combination of the HET genotype and prenatal nicotine exposure (G × E interaction) leads to an impaired D2R GPCR signaling resulting from decreased agonist affinity of the D2R receptors. These receptor binding observations are consistent with our electrophysiological results showing that, in the presence of a D2R antagonist, cortico-striatal circuits in HET/PNE mice exhibit significantly less recovery following HFS than the other groups indicating that the induction of LTD is impaired. Importantly, our finding that CB1Rs are not similarly affected confirms that the deficit in synaptic plasticity precedes the endocannabinoid feedback control of cortical glutamatergic input to medium spiny neurons (MSNs) that is responsible for inducing LTD in the striatum (Lovinger, 2010). Additionally, we observed behaviorally that the HET/PNE group demonstrated hyperactivity and reduced social responsiveness. In contrast to previous observations of hyperactivity in coloboma mice (Hess et al., 1996), other factors in addition to the Snap25 haploinsufficiency, such as prenatal nicotine exposure are required to produce marked hyperkinetic behavior.
3.2 Role of D2Rs in LTD induction
The role of D2Rs in LTD induction in the striatum is well established, but their physiological function in this process is yet to be fully understood (Lovinger, 2010). The majority of D2Rs in the striatum are expressed post-synaptically in MSNs, which are output neurons of the striatum (Kreitzer and Malenka, 2007). D2Rs are also expressed pre-synaptically in both the glutamatergic and dopaminergic inputs to the MSNs where they modulate the release of glutamate and dopamine thereby contributing to the process of LTD induction. D2Rs are also expressed in other populations of striatal neurons, such as the cholinergic interneurons, and there is evidence for a role for these D2Rs in LTD induction (Wang et al., 2006). It is likely that our results reflect decreased affinity and signaling of D2Rs associated with MSNs, since MSNs are the predominant neuron in the striatum and would be expected to provide the majority of D2R binding found in our [3H]-quinpirole agonist binding and [35S]-GTP-γ-S GPCR signaling experiments. However, affinity and signaling of a smaller population of D2Rs found in cholinergic interneurons as well as in glutamatergic and dopaminergic terminals in the striatum could be decreased as well.
Because blockade of either D2Rs or metabotropic glutamate receptors (mGluRs) prevents LTD induction in the striatum with the HFS paradigm, the current understanding is that activation of both receptor types post-synaptically is necessary for this process to occur (Lovinger, 2010). During HFS induction, large amounts of glutamate are released in concert with dopamine and this leads to the transition of MSNs from the resting DOWN state with a membrane potential of ~−85 mV to an active UP state with a membrane potential of ~−55mV. The glutamate activation of mGluRs and dopamine activation of D2Rs independently contribute to the opening of Cav1.3 L-type Ca2+ channels and the subsequent production of endocannabinoids (Surmeier et al., 2007; Wang et al., 2006). Consequently, endocannabinoids produced post-synaptically in MSNs undergo retrograde diffusion to act on presynaptic CB1 receptors on glutamatergic terminals to depress long term glutamate release (Lovinger, 2010). This role of D2Rs in striatal LTD is underscored by our findings that the combination of HET genotype and prenatal nicotine exposure leads to a decreased affinity and signaling in one or more populations of D2Rs, and this appears to both impair the induction of striatal LTD and to reduce relative magnitudes of resultant LTD and STD.
3.3 HET genotype and PNE treatment affects on D2R affinity and receptor signaling
Our data suggest that developmental and/or use-dependent changes that can be attributed to underlying genetic and environmental factors may lead to long term changes in dopaminergic regulation and function. The SNAP-25 deficient coloboma mouse mutant that displays hyperkinetic behavior, as well as behavioral deficits reflecting inattention and impulsivity (Bruno et al., 2007; Hess et al., 1992; Hess et al., 1996) has been shown to fulfill the necessary criteria for a valid model of ADHD (Fan et al., 2012). Importantly, Hess and colleagues have shown that both the hyperactivity and amelioration of that hyperactivity by amphetamine exhibited by these mutant mice is D2R-dependent (Fan and Hess, 2007; Fan et al., 2010). Additionally, using microdialysis to assay dopamine efflux in freely moving mice, these investigators have demonstrated markedly increased basal levels of extracellular dopamine in striatum of this mouse model of ADHD that is further increased by amphetamine administration (Fan and Hess, 2007).
Our results are consistent with these findings and further associate deficits in D2R signaling, which is required for synaptic plasticity in the dorsal striatum, with the hyperactive and socially impaired heterozygous Snap25 null mutants that have been prenatally exposed to nicotine. Moreover, preliminary evidence from in vivo microdialysis assays has indicated that heterozygous Snap25 null mutants also appear to have increased evoked extrasynaptic dopamine release in the striatum compared to their wild type littermates (Fan, Wilson, Hess, unpublished observations). Taken together, the decreased expression of SNAP-25 may result in enhanced dopamine release and this might be a precondition for the decreased D2R agonist affinity and down-regulation of receptor signaling responsible for modulating striatal activity.
Deciphering how decreased SNAP-25 expression might lead to increased dopamine efflux and result in alterations in D2R affinity will require further investigation. However, it is interesting to note that nicotine-stimulated release of dopamine has been reported to be decreased in synaptosomes prepared from the striatum of adolescent rats prenatally exposed to nicotine (Gold et al., 2009). By down-regulating the cholinergic modulation of dopaminergic terminals, the persistent effect of early nicotine exposure could be expected to lead to an up-regulation of dopaminergic receptors. Dopamine release in response to nicotine and other drugs of abuse has been primarily associated with the nucleus accumbens of the ventral striatum (Gerdeman et al., 2003). In contrast, our measurements of D2R agonist affinity, signaling, and synaptic plasticity are focused on the dorsal striatum, which exerts control over motor functions and has been implicated in the cortico-striatal circuitry that underlies decision-making processes, particularly executive function (Balleine et al., 2007). Various subtypes of the nicotinic acetylcholine receptor (nAChR) are expressed in striatal glutamate and dopamine terminals, as well as on MSNs and cholinergic interneurons (Quik et al., 2007). Importantly, nAChR antagonists have been shown to block LTD induction (Partridge et al., 2002) suggesting that prenatal nicotine may act on these nAChRs to additionally impact the induction of LTD through interactions with dopamine release with subsequent effects on D2R affinity. Using an adenovirus carrying a cre-inducible channelrhodopsin gene targeted to cholinergic interneurons, Cragg and colleagues (Threlfell et al., 2012) were able to demonstrate that cholinergic interneurons can directly activate dopamine release through nAChRs expressed on dopaminergic terminals. Dani and colleagues have demonstrated similar effects with nicotine (Zhang et al., 2009). These findings further underscore the potential role of cholinergic interneurons in the striatum in regulating dopamine release, by which homeostatic changes could affect D2 affinity and signaling. Deficiencies of SNAP-25 and persistent effects of prenatal nicotine exposure, may, therefore, converge on dopaminergic transmission in the striatum, although they might be expected to result in an opposite effect on receptor activity. Thus, while the HET genotype and prenatal exposure to nicotine could have distinct effects on dopamine release through different mechanisms, the interaction between these two conditions could affect the homeostatic regulation of dopaminergic signaling to result in the reduced affinity of D2 receptors and the ability to regulate induction of LTD, which would then alter the balance between STD and LTD.
Our results with WT/Sac controls are consistent with those of Kreitzer and Malenka (Kreitzer and Malenka, 2005), which identified induction of striatal LTD requiring both endocannabinoid and dopaminergic signaling. Interestingly, our findings demonstrate that the G × E interaction (SNAP-25 deficiency and prenatal nicotine exposure) during brain development selectively affects the D2R-mediated, but not the CB1-mediated response to HFS. This suggests that endocannabinoid release has been decoupled from D2R involvement in LTD induction. Our field recording paradigm cannot distinguish whether this occurs in a subpopulation of MSNs or in all MSNs. It is interesting to note additional findings (Kreitzer and Malenka, 2005) that group I mGluRs and L-type calcium channels converge with D2Rs to facilitate endocannabinoid release. Future work will be needed to evaluate whether these components are also altered in this G × E interaction and if these changes may be factors in the decrease in D2R affinity and signaling observed.
Another important question regards the types of D2R receptors that are affected by the G × E interaction. Our [3H]-Quinpirole agonist saturation assay reflected changes in affinity of all D2-class receptors expressed in the striatum. Quinpirole is a selective D2-class receptor agonist with affinity for all three D2-class receptor types: D2, D3 and D4 receptors (Ki’s for D2- ~4.8 nM, D3- ~24 nM, D4- ~30 nM; Seeman and Van Tol, 1994). All D2-class receptors are expressed in MSNs in the dorsal striatum with D2 receptors normally expressed ~2 fold higher that D3 and D4 receptors combined (Surmeier et al., 1996). One interesting possibility is that our results reflect different changes in affinity or relative numbers of functional receptors for each of the D2-class receptor subtypes. Specific D2, D3, and D4 agonists could be used to determine if this has occurred. It is known that the D2 receptor can exist in either a state of low or high affinity for dopamine (D2Low or D2High, respectively) and methods exist to determine the relative levels of each affinity state (Seeman et al., 2006). A shift towards a higher proportion of D2Low states as a result of the G × E interaction could also account for our results. Finally, it has been also shown that the D2 receptor has two isoforms, generated by alternative splicing designated as the long D2L and short D2S isoforms, the former differentiated by an additional 29 amino acids in the third intercellular loop (Usiello et al., 2000). The D2S is predominantly expressed pre-synaptically on input terminals and D2L is expressed post-synaptically (Usiello et al., 2000). Additional experiments performed by cross breeding D2L null mice with our HET/PNE group, and then repeating the saturation binding assays would distinguish if either or both D2 isoforms undergo changes in affinity and thus whether these effects are pre-synaptic (on glutamatergic, cholinergic or dopaminergic inputs), postsynaptic (on MSNs) or both.
3.4 Implications for neuropsychiatric disorders
These studies were aimed at extending the SNAP-25 deficient coloboma model of ADHD by evaluating individual contributions of G, E, and combined G × E effects on behavior, as well as on synaptic plasticity and receptor function in adolescent mice. Because the hyperkinetic behavior and response to amphetamine treatment of coloboma mice could be genetically rescued by a Snap25 transgene (Hess et al., 1996), haploinsufficiency of Snap25 has been implicated in these behavioral abnormalities. Therefore, it was puzzling that mice heterozygous for selective ablation of the Snap25 gene (Snap25+/−) do not recapitulate the robust spontaneous hyperkinetic behavior of coloboma mutants (Washbourne et al., 2002). Our results corroborate this initial observation, showing no difference between the locomotor activity of Snap25 +/− mice (HET/Sac group) and Snap25+/+ littermates (WT/Sac group). Moreover, we found no significant effect on social interaction, or in the induction of STD in the striatum between heterozygote and homozygote mutants without prenatal exposure to nicotine. Notably, we did detect a small, but significantly less robust LTD for both HET/PNE and HET/Sac groups compared to wild type controls. This suggests that while heterozygous Snap25 null mutants did exhibit haploinsufficiency with subtle effects on synaptic plasticity, this alone was not sufficient to produce the pronounced behavioral impairments characteristic of the coloboma model of ADHD. Consequently, the contribution of additional factors, such as nicotine exposure during brain development, a risk factor implicated for ADHD (Linnet et al., 2003), appear necessary to produce this phenotype. Alternatively, genetic influences including those encoded by neighboring loci within the Cm deletion, a microdeletion including 10 or more genes (Hess et al., 1994), or the C3H/HeJ genetic background could produce the phenotype in coloboma mutant mice.
Interestingly, Matteoli and colleagues have reported that comparably aged heterozygote Snap25 null mutant mice (derived from the same line used here) fail to habituate and display increased activity in a novel testing environment (Corradini et al., 2012). While this behavior pattern differs from the immediate and very robust spontaneous hyperactivity exhibited by coloboma mutant mice (Hess et al., 1992; Hess et al., 1996), it does suggest that Snap25 heterozygotes have deficits in behavioral regulation. Moreover, these investigators found that the Snap25 heterozygote null mutants exhibit abnormal EEG profiles, including spontaneous spike discharges and more pronounced kainate-induced seizures, as well as deficiencies in associative learning when challenged with novel object and social recognition tasks. Treatment with antiepileptics that reduced the spike activity in these mice also ameliorated the impaired performance in object recognition, but not the deficit in social interactive behavior. While our behavioral and neurophysiological experiments did not capture these specific phenotypes, these findings, coupled with our results showing diminished induction of LTD in Snap25+/− mice, underscore the hypothesis that diminished levels of SNAP-25 expression can impair synaptic transmission and thereby contribute to neurophysiological and behavioral dysregulation that can resemble complex heritable neuropsychiatric disorders in humans.
Snap25 gene deficits that affect social interactions in mice (Corradini et al., 2012) may also be relevant to other neuropsychiatric disorders, such as schizophrenia. Social impairment, along with other cognitive and emotional dysfunctions, is characteristic of schizophrenia (American Psychiatric Association, DSM IV-TR, 2000). In addition to ADHD, SNAP25 has been identified as a candidate gene for schizophrenia (Lewis et al., 2003). Furthermore, transgenic mice bearing a variant of the human DISC1 gene encoding a truncated DISC1 protein associated with schizophrenia, also exhibit decreased expression of SNAP-25 and LIS proteins, which may contribute to deficits in social interactions, as well as to the spontaneous hyperactivity of these mutants (Pletnikov et al., 2008). Importantly, in another Snap25 mutant mouse Bdr, proposed to model elements of schizophrenia (Jeans et al., 2007), prenatal exposure to stress not only exacerbates impaired sensorimotor behaviors, but also produces deficits in social interaction (Oliver and Davies, 2009).
These observations suggest that genetically defined mouse models may contribute further to our understanding of the crucial role played by gene-environmental interactions in neuropsychiatric illnesses, such as depressive disorders, ADHD, schizophrenia, obesity, and substance abuse (Wermter et al., 2010). The growing evidence for the influence of long-term synaptic plasticity including LTD in the striatum on learning and behavior (Lovinger, 2010; Yin et al., 2009), further suggests that this could contribute to mechanisms that underlie these disorders. The significant genetic association of ADHD with single nucleotide polymorphic (SNPs) variants of SNAP25, among other genes involved in dopaminergic transmission (Faraone and Mick, 2010), implicates alterations in gene regulation and expression that may confer the genetic susceptibility for G × E interactions that can affect synaptic plasticity in the striatum required for the control of behavior in ADHD and other psychiatric disorders. Exploiting targeted mutations to direct genetic deficiencies to specific neural circuits in mice, which are responsive to genetic, environmental, and age-related factors, will be an important next step in dissecting out those mechanisms that lead to specific endophenotypes of neurocognitive disorders.
4. Experimental procedures
4.1 Mouse breeding
All experiments were approved by the University of New Mexico Health Sciences Center Laboratory Animal Care and Use Committees and the National Institutes of Health. Mice were maintained in a 12 hour reverse light/dark cycle; lights on at 8PM and lights off at 8AM. To approximate adolescent brain maturation, all experiments used mice between postnatal PN35-50 and WT mice were age matched to the other groups described below used in the experiments. The Snap25 heterozygote knockout mice were produced by the knockout of exon5 of the wildtype Snap25 gene in one of the alleles (Washbourne et al., 2002). Genotyping was performed by PCR as described previously (Washbourne et al., 2002). The prenatal nicotine exposed (PNE) dams were provided with 0.05 mg/ml nicotine and saccharine two weeks prior to pregnancy, during pregnancy, and gradually tapered down during postnatal days 1-7, while the saccharine control dams were provided with saccharine during the same period (Paz et al., 2007).
We used a 2 × 2 design to evaluate the interactions the genotype and environment on corticostriatal signaling. Snap25 heterozygote knockout male mice (HET) (Washbourne et al., 2002) were bred with wild type C57/B16 female mice (WT) under either saccharine bottle fed conditions or prenatal nicotine exposure (PNE) bottle fed conditions during pregnancy according to standard protocols (Paz et al., 2007) to produce four experimental groups of mice representing genotype (HET) × environmental (PNE) interactions designated as: WT/Sac, WT/PNE, HET/Sac and HET/PNE.
4.2 Behavioral tests
Locomotor activity was tested using an automated system (San Diego Instruments, San Diego, CA) following the method described previously (Paz et al., 2007). Male mice from the four experimental groups were placed in individual photocell activity cages (29.2 × 50.0 cm) coupled to 12 infrared beam detectors arranged in a 4 × 8 grid located 2 cm above the floor of the cage. Beam breaks were automatically recorded 18 times per second in 10 min intervals throughout the 180 min testing period. Mice were habituated in the sound-attenuated room for 1 hour prior to testing.
Social interaction was assessed based on a published paradigm with modifications (Allan et al., 2008). Briefly, male mice from each of the experimental groups were first placed in the clear Plexiglas test arena (40 cm × 40 cm) for 5 min, to allow time for habituation to the environment. On the following day, mice were tested for social interaction time during a 5 minute test. For the first 5 min session, a small colored plastic cube was placed inside a tent made of wire mesh so the test mouse could both see and smell through the wire mesh tent (the interaction area). For the second 5 minute session, the toy cube was replaced by an unfamiliar mouse of the same sex and age as the test mouse for 5 min. The number of visits and the time spent in proximity to the wire tent (social interacting area) by the test mouse and the length of time the animal spent in direct contact with the tent were recorded during each of the two 5-min periods. The mice were recorded by video camera and independently scored by two experimenters who were blind to the genotypes.
4.3 Slice preparation
Electrophysiology experiments were performed in 300 μm coronal striatal slices prepared from 30-50 day old male mice using standard techniques (Schiess et al., 2006). Briefly, animals were deeply anaesthetized by I.P. injection of 250 mg kg−1 ketamine, brains were rapidly removed, and slices cut at 300 to 400 μm with a vibroslicer (Pelco 101, St Louis, MO, USA) in an ice bath with a cutting solution containing (mM): 220 sucrose, 3 KC1, 1.2 NaH2PO4, 26 NaHCO3, 12 MgSO4, 0.2 CaCl2, 10 glucose and 0.01 mg ml−1 ketamine equilibrated with 95%O2–5%CO2. Slices were then transferred to artificial cerebrospinal fluid (ACSF) containing (mM): 126 NaCl, 3 KCl, 1.25 NaH2PO4, 1 MgSO4, 26 NaHCO3, 2 CaCl2 and 10 glucose equilibrated with 95%O2–5%CO2 at 30°C for 1 h and then maintained at room temperature until recording in a constant flow chamber (Warner Instruments, Hamden, CT, USA or Scientific Systems Design, Mercerville, NJ, USA) maintained at 32°C and continuously perfused at 2 ml min−1 with ACSF saturated with 95%O2–5%CO2.
4.4 Population Spike (PS) recordings
Standard electrophysiological techniques were used for population spike (PS) recordings (Schiess et al., 2006) in the in the dorsal striatum following stimulation of cortical layer V afferents from brains removed from 35-50 day old male mice. PSs were recorded with an Axoclamp 2B (Molecular Devices, Sunnyvale, CA), amplifier and a Digidata 1322A interface using pCLAMP 9.2 software (Molecular Devices) for experimental control and data analysis. Recordings were digitized at 500 kHz and filtered at 2 kHz. Population spike amplitudes were calculated by subtracting the average of both positive components in the fEPSP from the maximum intervening negative value. Presynaptic constant current pulses (150 μs duration) were applied at 20 second intervals with an Iso-Flex constant current stimulator (API Instruments, Jerusalem, Israel) through a concentric bipolar electrode (FHC, Bowdoinham, ME, USA) at a stimulus intensity (0.1 − 2.0 mA) adjusted to produce 50% of the maximum population spike amplitude, which was previously determined by an input-output series.
To assess long term synaptic plasticity in the cortico-striatal field, a 10 minute baseline was established at ½ maximum stimulus intensity, then a high frequency (HFS) paradigm consisting of 4 sets of 1 s 100 Hz pulses at the maximum intensity was applied, and finally 30 minutes of recordings were obtained again at ½ maximum stimulus intensity. The average amplitude of the final 10 population spikes during the 30 minute period was compared to the average amplitude of 32 population spikes during the 10 minute pre-HSF baseline in order to determine the percentage of change relative to the baseline.
For each of the four experimental groups described above, receptor blocking experiments were carried out in which,10 μM sulpiride was added to the ACSF bath to antagonize D2 receptors, or 2 μM AM251 to antagonize CB1 receptors in order to examine the contribution these receptors to the establishment or maintenance of long term synaptic plasticity. Slices were pretreated in the drug for 20 minutes prior to the start of each experiment and for the duration of each experiment. A minimum of 6 slices was recorded in the experiment for each condition both for drug and non-drug perfused experiments.
4.5 Dopamine agonist stimulated [35S]-GTP-γ-S binding assay
[35S]-GTP-γ-S binding assays were conducted by method described previously (Bailey et al., 2008; Martinez et al., 2008; Newman-Tancredi et al., 2001). Brains were removed from 35-50 day old male mice from each of the four experimental groups and immediately immersed in isopentane at −35°C, chilled in a dry ice/methanol bath and then stored in airtight containers at −80°C until sectioning. Coronal sections (10 m) that included the striatum (approximately from Bregma 0.98 to −0.94) were cut and the level of sectioning in each plane was verified by examination of Nissl-stained sections. Sections were thaw-mounted onto precleaned Superfrost- Plus® microscope slides (VWR Scientific, West Chester, PA) and stored at −80°C in airtight containers until assays were performed.
Slides were pre-incubated in assay buffer (50 mM Tris-HCl, 100 mM NaCl, 5 mM MgC12, pH 7.4 at 25 °C) containing 1 mM DL dithiothreitol, 0.2 mM EGTA and 2 mM GDP at 25°C for 10 min. Sections were then incubated with 100 pM [35S]-GTPγS (specific activity = 1250 Ci/mmole; Perkin Elmer Life Sciences, Boston, MA) for 90 minutes in the absence or presence of 10 μM unlabeled GTPγS and D2R agonist, quinelorane, as described below. After incubation, sections were rinsed twice for 15 seconds each in fresh incubation buffer at 4 °C, dipped for one second in 4 °C distilled water, dried under a stream of cool air, and then vacuum desiccated overnight. Autoradiograms (Biomax MR Film) were produced with a set of 14C standards following a 4 day exposure. Microdensitometry of ligand binding in the striatum was performed using Media Cybernetics Image Pro Plus® (Silver Spring, MD) on an Olympus BH-2 microscope at a total image magnification of 3.125×. In each assay, an optical density standard curve, expressed inpicoCuries/105 μm2 was established based on the autoradiograms of the standards.
The approximate EC50 determined for quinelorane-stimulated [35S]-GTPγS binding was 20 μM and the EC100 was 200 μM based upon initial binding studies performed with WT/Sac controls, and used in subsequent experiments with the four experimental groups. As a control, 50 μM sulpiride was used as an antagonist to a 100 μM quinelorane concentration and found to fully block the response in these WT/Sac controls. Total [35S]-GTPγS binding in the left and right sections from each brain region of interest was measured in quadruplicate sections incubated with varying levels of quinelorane. Non-specific [35S]-GTPγS binding was measured in duplicate sections incubated with the addition of 10 μM unlabeled GTPγS with varying levels of quinelorane. Total and non-specific binding were both determined by subtracting background binding from each autoradiogram. The specific binding was then determined by subtracting total binding from non-specific binding. Quinelorane-stimulated [35S]-GTPγS binding was measured in the same way to compare basal binding without quinelorane, and net binding for both the EC50 (20 μM) and EC100 (200 μM) quinelorane concentrations. The net binding permitted quantification of quinelorane binding at each concentration (EC50 and EC100) in order to determine the differential effects of the agonist-stimulated responses to GTP binding. Each experimental group included 4 coronal sections each for basal, EC50, and EC100 quinelorane levels as well as 2 sections for non-specific binding including 10 μM unlabeled GTPγS.
4.6 [3H]-Quinpirole dopamine agonist radioligand saturation binding assay
Saturation binding assays were conducted by methods described previously (Levant et al., 1992). Brains were removed from 35 – 50 day old mice and immediately immersed in isopentane at −35°C, chilled in a dry ice/methanol bath and then stored in airtight containers at −80°C until homogenation. Each brain homogenate consisted of 4 total brains of dissected striata (2 male and 2 female) in order to obtain sufficient tissue. Brain tissue was homogenized in 20 volumes of assay buffer (50 mM Tris-HCl, 5 mM KC1, 2 mM MgCl2, and 2 mM CaCl2, pH 7.4) at 23°C using fitted spherical glass homogenizers. The crude homogenate was centrifuged twice for 30 and 15 minutes respectively at 13,000 × g, to yield a final tissue concentration of 1.5 − 2.5 mg per ml buffer. Brain protein levels were quantified by Bradford assays and refrozen until binding assays were performed.
Saturation binding assays were performed in triplicate polystyrene tubes in a final volume of 0.3 ml with the assay buffer as described (Levant et al., 1992). The dopamine agonist, [3H]-quinpirole, was included at 6 concentrations ~0.2 nM to ~9 nM as determined from preliminary binding studies (Levant et al., 1992). Binding was initiated by the addition of membrane homogenate at room temperature (23°C) and maintained for 5 hours to allow saturation to occur. Non-specific binding was defined in the presence of 50μM spiperone. The reaction was terminated by the separation of the free from bound radioligand by rapid filtration over Whatman GF/B filters using a Brandel cell harvester. Filters were washed twice with 3 ml of ice-cold 50 mM tris-HCl, pH 8.0 and punched into mini-vials. After the addition of scintillation cocktail (Packard Ultima Gold), vials were shaken, allowed to equilibrate for at least 5 hours, and counted in a Packard model 2000 liquid scintillation counter. Individual Kd and Bmax measurements were determined for [3H]-quinpirole from least squares fits to Scatchard plots of triplicate samples using data from the specific and non-specific binding from samples (brain homogenates of 4 striata) per experiment. The mean and standard deviation of Kd and Bmax measurements were obtained from comparison of 5 individual experiments per group.
4.7 Drugs
The following drugs were stored frozen in aliquots and diluted to the appropriate concentration in ACSF for electrophysiology on the day of the experiment, or the appropriate concentrations as dictated by the pharmacological assays. S-sulpiride, AM251, quinelorane, dithiothreitol and spiperone were obtained from Tocris (Ellisville, MO, USA); GDP and GTPγS from Sigma-Aldrich; [35S]-GTPγS and [3H]-quinpirole from Perkin-Elmer. Aliquots of s-sulpiride were made in DMSO as required.
4.8 General Data Analysis Techniques
SPSS 16.0 (SPSS, Inc., Chicago, IL) was used tor statistical analysis ot data. Numerical values for data measurements are expressed as the mean ± standard error unless otherwise specified. Statistical p values were represented as follows: * p < 0.05; ** p < 0.01 and *** p <0.001 The experimental paradigm was set up as a 2 × 2 analysis to compare effects of genotype and environment and comparisons were made with a two-way ANOVA two genotype (HET v WT) × two prenatal treatment (PNE v Sac) followed by a Tukey's HSD post-hoc test.
A two-step cluster analysis with no pre-determined number of clusters (SPSS 16.0) was used to group the field potential experiments based on the level of recovery following high frequency stimulation. This analysis seeks to identify clusters within a data set that minimize within-group variation and maximize between-group variation. The result of the cluster analysis is the identification of either a single cluster or several (2 or greater) clusters within the data set as well as the mean and standard deviation for each identified cluster.
Highlights.
We used Snap25 null mutants with a prenatal nicotine exposure paradigm
Nicotine exposed Snap25 (HET/PNE) mutants show hyperactivity and social impairment
HET/PNE mutants show D2R- but not CBlR-dependent impairment of LTD induction
HET/PNE mutants show impairment of D2R affinity and GPCR signaling
These results reveal gene-environment interactions in behavior and neural signaling
Acknowledgements
We are grateful for the expert technical assistance of Amy Lucero in managing the mouse colony, genotyping, nicotine administration, and behavioral testing. We would like to thank Dan D. Savage II and Martina Rosenberg for help and guidance in the receptor signaling studies. We would also like to thank Conrad James for his support and helpful suggestions in all aspects of these studies. We are grateful to Fernando Valenzuela and Ellen Hess for their thoughtful comments on the manuscript. This work was supported by National Institutes of Health grant MH 091464 (M.C.W.) and a portion of the salary support for Michael Baca was from Sandia National Laboratories.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Adermark L, Talani G, Lovinger DM. Endocannabinoid-dependent plasticity at GABAergic and glutamatergic synapses in the striatum is regulated by synaptic activity. Eur J Neurosci. 2009;29:32–41. doi: 10.1111/j.1460-9568.2008.06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan AM, Liang X, Luo Y, Pak C, Li X, Szulwach KE, Chen D, Jin P, Zhao X. The loss of methyl-CpG binding protein 1 leads to autism-like behavioral deficits. Hum Mol Genet. 2008;17:2047–57. doi: 10.1093/hmg/ddn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 2000.
- Bailey A, Metaxas A, Yoo JH, McGee T, Kitchen I. Decrease of D2 receptor binding but increase in D2-stimulated G-protein activation, dopamine transporter binding and behavioural sensitization in brains of mice treated with a chronic escalating dose ‘binge’ cocaine administration paradigm. Eur J Neurosci. 2008;28:759–70. doi: 10.1111/j.1460-9568.2008.06369.x. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–5. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Fried R, Petty CR, Wozniak J, Doyle AE, Henin A, Corkum L, Claudat K, Faraone SV. Cognitive development in adults with attention-deficit/hyperactivity disorder: a controlled study in medication-naive adults across the adult life cycle. J Clin Psychiatry. 2011;72:11–6. doi: 10.4088/JCP.09m05420pur. [DOI] [PubMed] [Google Scholar]
- Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ Health Perspect. 2006;114:1904–9. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno KJ, Freet CS, Twining RC, Egami K, Grigson PS, Hess EJ. Abnormal latent inhibition and impulsivity in coloboma mice, a model of ADHD. Neurobiol Dis. 2007;25:206–16. doi: 10.1016/j.nbd.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradini I, Donzelli A, Antonucci F, Welzl H, Loos M, Martucci R, De Astis S, Pattini L, Inverardi F, Wolfer D, Caleo M, Bozzi Y, Verderio C, Frassoni C, Braida D, Clerici M, Lipp HP, Sala M, Matteoli M. Epileptiform Activity and Cognitive Deficits in SNAP-25+/− Mice are Normalized by Antiepileptic Drugs. Cereb Cortex. 2012. [DOI] [PubMed]
- Fan X, Hess EJ. D2-like dopamine receptors mediate the response to amphetamine in a mouse model of ADHD. Neurobiol Dis. 2007;26:201–11. doi: 10.1016/j.nbd.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Xu M, Hess EJ. D2 dopamine receptor subtype-mediated hyperactivity and amphetamine responses in a model of ADHD. Neurobiol Dis. 2010;37:228–36. doi: 10.1016/j.nbd.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Bruno KJ, Hess EJ. Rodent models of ADHD. Curr Top Behav Neurosci. 2012;9:273–300. doi: 10.1007/7854_2011_121. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003;2:104–13. [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–23. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Mick E. Molecular genetics of attention deficit hyperactivity disorder. Psychiatr Clin North Am. 2010;33:159–80. doi: 10.1016/j.psc.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–92. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Gold AB, Keller AB, Perry DC. Prenatal exposure of rats to nicotine causes persistent alterations of nicotinic cholinergic receptors. Brain Res. 2009;1250:88–100. doi: 10.1016/j.brainres.2008.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess EJ, Jinnah HA, Kozak CA, Wilson MC. Spontaneous locomotor hyperactivity in a mouse mutant with a deletion including the Snap gene on chromosome 2. J Neurosci. 1992;12:2865–74. doi: 10.1523/JNEUROSCI.12-07-02865.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess EJ, Collins KA, Copeland NG, Jenkins NA, Wilson MC. Deletion map of the coloboma (Cm) locus on mouse chromosome 2. Genomics. 1994;21:257–61. doi: 10.1006/geno.1994.1254. [DOI] [PubMed] [Google Scholar]
- Hess EJ, Collins KA, Wilson MC. Mouse model of hyperkinesis implicates SNAP-25 in behavioral regulation. J Neurosci. 1996;16:3104–11. doi: 10.1523/JNEUROSCI.16-09-03104.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–43. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Jeans AF, Oliver PL, Johnson R, Capogna M, Vikman J, Molnar Z, Babbs A, Partridge CJ, Salehi A, Bengtsson M, Eliasson L, Rorsman P, Davies KE. A dominant mutation in Snap25 causes impaired vesicle trafficking, sensorimotor gating, and ataxia in the blind-drunk mouse. Proc Natl Acad Sci U S A. 2007;104:2431–6. doi: 10.1073/pnas.0610222104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25:10537–45. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–7. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Levant B, Grigoriadis DE, DeSouza EB. Characterization of [3H]quinpirole binding to D2-like dopamine receptors in rat brain. J Pharmacol Exp Ther. 1992;262:929–35. [PubMed] [Google Scholar]
- Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, Williams NM, Schwab SG, Pulver AE, Faraone SV, Brzustowicz LM, Kaufmann CA, Garver DL, Gurling HM, Lindholm E, Coon H, Moises HW, Byerley W, Shaw SH, Mesen A, Sherrington R, O’Neill FA, Walsh D, Kendler KS, Ekelund J, Paunio T, Lonnqvist J, Peltonen L, O’Donovan MC, Owen MJ, Wildenauer DB, Maier W, Nestadt G, Blouin JL, Antonarakis SE, Mowry BJ, Silverman JM, Crowe RR, Cloninger CR, Tsuang MT, Malaspina D, Harkavy-Friedman JM, Svrakic DM, Bassett AS, Holcomb J, Kalsi G, McQuillin A, Brynjolfson J, Sigmundsson T, Petursson H, Jazin E, Zoega T, Helgason T. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet. 2003;73:34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, Kotimaa A, Moilanen I, Thomsen PH, Olsen J, Jarvelin MR. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 2003;160:1028–40. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Tyler EC, Merritt A. Short- and long-term synaptic depression in rat neostriatum. J Neurophysiol. 1993;70:1937–49. doi: 10.1152/jn.1993.70.5.1937. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58:951–61. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez EJ, Kolb BL, Bell A, Savage DD, Allan AM. Moderate perinatal arsenic exposure alters neuroendocrine markers associated with depression and increases depressive-like behaviors in adult mouse offspring. Neurotoxicology. 2008;29:647–55. doi: 10.1016/j.neuro.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Tancredi A, Cussac D, Brocco M, Rivet JM, Chaput C, Touzard M, Pasteau V, Millan MJ. Dopamine D2 receptor-mediated G-protein activation in rat striatum: functional autoradiography and influence of unilateral 6-hydroxydopamine lesions of the substantia nigra. Brain Res. 2001;920:41–54. doi: 10.1016/s0006-8993(01)02927-4. [DOI] [PubMed] [Google Scholar]
- Oliver PL, Davies KE. Interaction between environmental and genetic factors modulates schizophrenic endophenotypes in the Snap-25 mouse mutant blind-drunk. Hum Mol Genet. 2009;18:4576–89. doi: 10.1093/hmg/ddp425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver PL, Davies KE. New insights into behaviour using mouse ENU mutagenesis. Hum Mol Genet. 2012;21:R72–81. doi: 10.1093/hmg/dds318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JG, Apparsundaram S, Gerhardt GA, Ronesi J, Lovinger DM. Nicotinic acetylcholine receptors interact with dopamine in induction of striatal long-term depression. J Neurosci. 2002;22:2541–9. doi: 10.1523/JNEUROSCI.22-07-02541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Barsness B, Martenson T, Tanner D, Allan AM. Behavioral teratogenicity induced by nonforced maternal nicotine consumption. Neuropsychopharmacology. 2007;32:693–9. doi: 10.1038/sj.npp.1301066. [DOI] [PubMed] [Google Scholar]
- Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, Mori S, Moran TH, Ross CA. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2008;13:173–86, 115. doi: 10.1038/sj.mp.4002079. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–8. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Quik M, Bordia T, O’Leary K. Nicotinic receptors as CNS targets for Parkinson's disease. Biochem Pharmacol. 2007;74:1224–34. doi: 10.1016/j.bcp.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J, Sudhof TC. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices--guilty as charged? Annu Rev Cell Dev Biol. 2012;28:279–308. doi: 10.1146/annurev-cellbio-101011-155818. [DOI] [PubMed] [Google Scholar]
- Schiess AR, Scullin CS, Partridge LD. Neurosteroid-induced enhancement of short-term facilitation involves a component downstream from presynaptic calcium in hippocampal slices. J Physiol. 2006;576:833–47. doi: 10.1113/jphysiol.2006.118505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Van Tol HH. Dopamine receptor pharmacology. Trends Pharmacol Sci. 1994;15:264–70. doi: 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Seeman P, Schwarz J, Chen JF, Szechtman H, Perreault M, McKnight GS, Roder JC, Quirion R, Boksa P, Srivastava LK, Yanai K, Weinshenker D, Sumiyoshi T. Psychosis pathways converge via D2high dopamine receptors. Synapse. 2006;60:319–46. doi: 10.1002/syn.20303. [DOI] [PubMed] [Google Scholar]
- Sovago J, Dupuis DS, Gulyas B, Hall H. An overview on functional receptor autoradiography using [35S]GTPgammaS. Brain Res Brain Res Rev. 2001;38:149–64. doi: 10.1016/s0165-0173(01)00106-0. [DOI] [PubMed] [Google Scholar]
- Sung KW, Choi S, Lovinger DM. Activation of group I mGluRs is necessary for induction of long-term depression at striatal synapses. J Neurophysiol. 2001;86:2405–12. doi: 10.1152/jn.2001.86.5.2405. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–91. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–35. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, Taylor E, Casey BJ, Castellanos FX, Wadhwa PD. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev. 2007;17:39–59. doi: 10.1007/s11065-007-9019-9. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–52. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Bendito G, Molnar Z, Becher MW, Valenzuela CF, Partridge LD, Wilson MC. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci. 2002;5:19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- Wermter AK, Laucht M, Schimmelmann BG, Banaschweski T, Sonuga-Barke EJ, Rietschel M, Becker K. From nature versus nurture, via nature and nurture, to gene × environment interaction in mental disorders. Eur Child Adolesc Psychiatry. 2010;19:199–210. doi: 10.1007/s00787-009-0082-z. [DOI] [PubMed] [Google Scholar]
- Wilson MC. Coloboma mouse mutant as an animal model of hyperkinesis and attention deficit hyperactivity disorder. Neurosci Biobehav Rev. 2000;24:51–7. doi: 10.1016/s0149-7634(99)00064-0. [DOI] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12:333–41. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zhang L, Liang Y, Siapas AG, Zhou FM, Dani JA. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J Neurosci. 2009;29:4035–43. doi: 10.1523/JNEUROSCI.0261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]