Abstract
We report the use of liquid crystal (LC)-in-water emulsions for the synthesis of either spherical or non-spherical particles with chemically-distinct domains located at the poles of the particles. The approach involves the localization of solid colloids at topological defects that form predictably at surfaces of water-dispersed LC droplets. By polymerizing the LC droplets displaying the colloids at their surface defects, we demonstrate formation of both spherical and, upon extraction of the mesogen, anisotropic composite particles with colloids located at either one or both of the poles. Because the colloids protrude from the surfaces of the particles, they also define organized, chemical patches with functionality controlled by the colloid surface.
The synthesis of particles with either anisotropic shape or patterned surface chemistry is enabling new scientific and technological advances. For example, particle shape has been shown to influence (i) the efficiency of intracellular delivery of particles,1(ii) colloidal interactions between particles either adsorbed at liquid-liquid interfaces or dispersed in liquid crystalline (LC) solvents,2-6 (iii) the stability of particle-decorated (Pickering) emulsions,7 and (iv) Brownian motion.8 Additionally, particles with chemical surface patterns that mediate site-specific interactions are opening new routes to the synthesis of functional colloidal materials.9,10 While the above studies and others reveal the rich range of phenomena and materials that are enabled by non-spherical and/or surface-patterned particles,9-23 the synthesis of such particles remains challenging.
In this communication, we report a new approach to the synthesis of either spherical or non-spherical particles with organized surface-chemical patches that uses LC-based emulsions as templates. The method enables, in particular, the synthesis of either spherical or anisotropic (ellipsoid-like) composite particles with “poles” that are decorated by colloids possessing chemical compositions that are distinct from the remainder of the composite particle. Such composite particles are synthesized with dipolar (see top composite particle in Scheme 1B-D) or quadrupolar symmetry (see bottom composite particle in Scheme 1B-D), and the chemical functionality presented at the poles is shown to be easily tuned by varying the material that constitutes the colloids. The composite particles are, moreover, prepared in bulk solution and thus the method is potentially scalable.
Scheme 1.
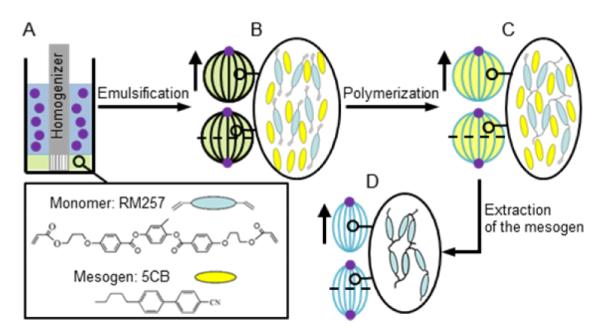
(A) Initial system before emulsification (blue color: aqueous phase; purple spots: colloids). (B) After emulsification, formation of bipolar nematic droplets with either 1 (dipolar symmetry) or 2 colloids (quadrupolar symmetry) located at the poles. (C) After polymerization of the monomer within the droplets, formation of spherical particles with dipolar or quadrupolar symmetry. (D) Upon extraction of the mesogen from the polymerized droplets, formation of anisotropic particles with dipolar or quadrupolar symmetry. The horizontal dashed line indicates a mirror plane in the quadrupolar composite particles.
We used the mesogen 4-cyano-4′-pentylbiphenyl (5CB, Merck®) which, below the nematic (N) – isotropic (I) transition temperature (TNI = 35°C, measured by differential scanning calorimetry (DSC), Figure S1 A - Supporting Information (SI)) forms a uniaxial nematic phase with a symmetry axis defined by the so-called nematic director n. This uniaxial symmetry underlies the formation of topological point and line defects in confined geometries (such as droplets and shells).24-29 Past studies have established that colloids will localize at defects in bulk LCs to minimize the free energy cost associated with both elastic strain of LCs near defects and diminished orientational ordering of mesogens in the cores of defects.30-33 In the experiments reported in this paper, we use well-defined surface (point) defects formed by LCs confined within micrometer-sized droplets as templates to direct the assembly of colloids on the surface of the droplets. Once assembled via defect-directed forces, we then use polymerization of monomers dissolved within the LC to preserve the internal organization of the composite particles.34
We prepared emulsions of 5CB in water by emulsifying 5CB in a mixture composed of water and glycerol (90% v/v) in the presence of 1-μm diameter fluorescent spherical polystyrene (PS) colloids (Scheme 1A; see SI for additional detail regarding the materials and experimental protocols). We used a low concentration of PS colloids (0.01% wt/v) to obtain 5CB droplets with, on average, no more than two PS colloids adsorbed at their surface. In addition, we note that glycerol was added to the aqueous phase so that it became sufficiently viscous (viscosity η24°C = 135 mPa.s) to permit imaging of the LC droplets with polarized light (PL), bright field (BF), and combined fluorescence (Fluo) and BF microscopy without substantial rotation of the LC droplets. For imaging, we used a 100x oil-immersion objective on an Olympus IX71 inverted epifluorescence microscope, equipped with crossed polarizers (see SI). Prior to emulsification, we confirmed that nematic 5CB was anchored homogeneously on the surfaces of the PS colloids (see Figure S2 (SI)).
After emulsification, we first sought to determine the size and internal ordering of the LC droplets with a single PS colloid at their surface (Scheme 1B). Figure 1A, E, I and Figure 1B, F, J show PL and BF micrographs, respectively, of three LC droplets with diameters of 9.9 μm, 6.2 μm or 4.8 μm. These micrographs are consistent with the presence of two point defects at the diametrically opposite poles of the droplets (so-called “boojums”), indicating the droplets to be in a bipolar configuration in which the director is anchored parallel to the LC/aqueous interface and links the poles (Figure 1D, H, L).25,26 Because the orientations of the three droplets with respect to the crossed polarizers are different, each droplet displays a distinct birefringence pattern in Figure 1. We note, in addition, that both droplets with and free of PS colloids were observed to assume a bipolar configuration.
Figure 1.
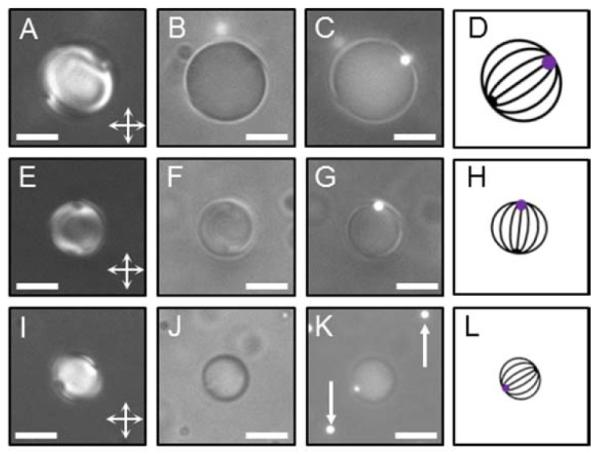
(A, E, I) PL, (B, F, J) BF, and (C, G, K) combined Fluo and BF micrographs of bipolar nematic 5CB droplets observed in a water/glycerol (90% v/v) mixture, with a diameter of (A, B, C) 9.9 μm, (E, F, G) 6.2 μm, and (I, J, K) 4.8 μm, and exhibiting a single 1-μm-diameter fluorescent PS colloid adsorbed at their surface (bright spot in C, G, K; the white arrows indicate free PS particles). Scale bars: 5 μm. (D, H, L) The corresponding illustrations of the director field configuration (dark lines) with the two “boojum” defects at the droplet poles; the purple spot represents the PS colloid (at a pole).
Next we determined the PS colloid location at the LC droplet surface using Fluo microscopy. From the combined Fluo and BF images (Figure 1C, G, and K) and the corresponding PL micrographs (Figure 1A, E, I), it is evident that the Fluo signal (the white spot in the combined Fluo and BF images) from a single PS colloid adsorbed at the droplet surface coincides with the location of one of the “boojums” of the bipolar nematic droplets. This correspondence was observed for all nematic droplets that possessed a single adsorbed colloid (Figure S3 A (SI)). We note also that measurements performed by depositing 5CB drops on a PS-coated silicon wafer immersed under water revealed a contact angle of ~ 10° between the water/5CB interface and the 5CB/PS-coated silicon wafer interface. This result is consistent with our observation of trapping of the PS colloids at the LC/aqueous interface of the droplets and leads us to conclude that each PS colloid presents a hemispherical patch to the aqueous phase at the pole of the LC droplet, the diameter of the surface patch being ~ 0.2 μm.
Using procedures similar to those reported above, we also characterized the sub-population of nematic 5CB droplets in the emulsions with two PS colloids adsorbed at their surfaces. Both PL (Figure 2A, E, I) and BF (Figure 2B, F, J) micrographs of these droplets (diameters of 7.8 μm (Figure 2A, B), 6.5 μm (Figure 2E, F), or 4.2 μm (Figure 2I, J)) were again consistent with the bipolar configuration. In addition, from the combined Fluo and BF images (Figure 2C, G, K) and the corresponding PL micrographs (Figure 2A, E, I), we conclude that the nematic 5CB droplets display a colloid at each “boojum” defect. A measurement of the angle subtended by pairs of colloids and the droplet center, performed over the range of diameters reported in Figure S3 B (SI), revealed an angular separation strongly peaked at 180° (Figure S4 (SI)), consistent with the separation between the “boojum” defects. We also observed two-colloid configurations in which both colloids were located at a single pole of a bipolar droplet (Figure S5 (SI)). Typically, half of the two-colloid droplets exhibited the latter configuration. We did not observe other configurations of the droplets with two PS colloids.
Figure 2.
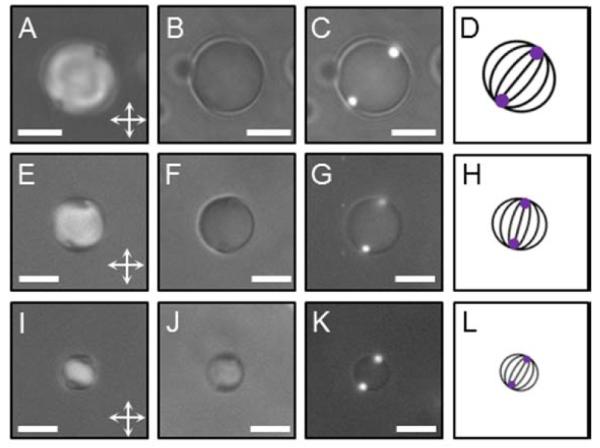
(A, E, I) PL, (B, F, J) BF, and (C, G, K) combined Fluo and BF micrographs of bipolar nematic 5CB droplets observed in a water/glycerol (90% v/v) mixture, with a diameter of (A, B, C) 7.8 μm, (E, F, G) 6.5 μm, and (I, J, K) 4.2 μm, and exhibiting two 1-μm-diameter fluorescent PS colloids adsorbed at their surfaces (bright spots in C, G, K). Scale bars: 5 μm. (D, H, L) The corresponding illustrations of the director field configuration (dark lines) with the two “boojum” defects at the droplet poles; the purple spots represent the PS colloids (at the poles).
The results described above demonstrate that PS colloids partition to the surface defects of LC droplets, thus localizing the colloids to the droplet poles. We note here, however, that the approach is generalizable to a range of colloidal materials. For example, we observed inorganic colloids such as 1-μm-diameter fluorescent silica colloids coated with N,N-dimethyl-N–octadecyl–3-aminopropyltrimethoxysilyl chloride (DMOAP; contact angle of 40°, as determined in a similar manner to PS, but with a DMOAP-coated glass slide; diameter of the hemispherical patch presented to water is ~ 0.6 μm) to assemble at the “boojum” defects of the LC droplets. In contrast to the PS colloids, the DMOAP-coated silica colloids caused perpendicular anchoring of nematic 5CB at their surface.35,36 This result indicates that localization of the colloids at the LC defects is not strongly dependent on the surface chemistry of the colloids and associated anchoring of the LC.33 Overall, our experiments show that facile control of chemical patterning of the droplet poles is possible by varying the material constituting the colloids.
We also demonstrated that the above-reported patterning of colloids on the surfaces of the LC droplets can be preserved by polymerization (Scheme 1C). Building from prior studies of polymerization of LCs,34 we mixed 5CB with 20% wt/wt of the mesogenic monomer 4-(3-acryloyloxypropyloxy) benzoic acid 2-methyl-1,4-phenylene ester (RM257, Merck) (see SI for more details). DSC experiments (Figure S1 B (SI)) indicated that the mixture is nematic at room temperature (TNI = 46.1°C) and PL micrographs were consistent with a bipolar configuration. Following polymerization, DSC revealed the TNI to decrease by 2°C with respect to that of pure 5CB (Figure S1 E (SI)) but PL observations showed that the overall bipolar configuration of the nematic droplets exhibiting two-diametrically opposite colloids was not perturbed (Figure 3I and J). Upon heating into the isotropic phase either pure (not polymerized) or polymerized 5CB droplets patterned with two-diametrically opposed PS colloids (Figure 3), we observed the colloids on the surfaces of the droplets of pure 5CB to diffuse towards one-another whereas the colloids on the surfaces of the polymerized droplets were immobile. These observations led us to conclude that the colloids are partially embedded in a polymer network that persists above TNI, and that the polymerized droplets provide a route to spherical LC particles that possess either one or two chemical patches at their poles.
Figure 3.
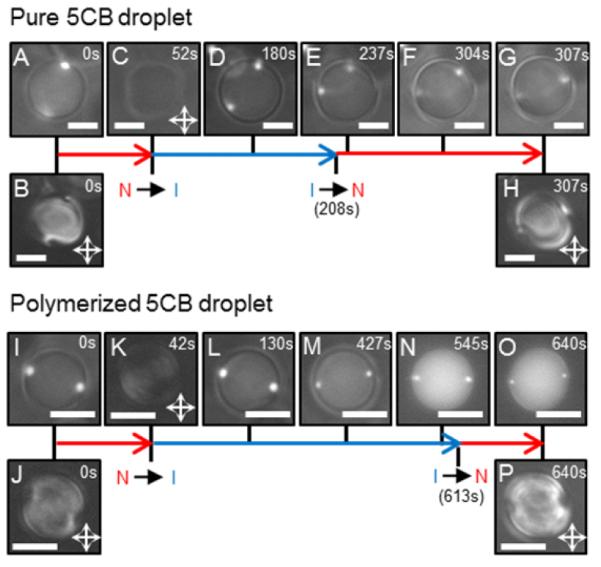
(A, D-G; I, L-O) Combined Fluo and BF images of LC droplets during their heating into the isotropic (I) phase and cooling into the nematic (N) phase. (B, C, H, J, K, and P) PL micrographs; B, H, J, and P are PL micrographs corresponding to A, G, I, and O, respectively. (A-H) Pure and (I-P) polymerized 5CB droplets, with a diameter of 9.4 μm and 6.7 μm, respectively, which initially exhibit two diametrically opposite 1-μm-diameter PS colloids located at their poles in the N phase. Scale bars: 5 μm. (M-O) The intensity of the fluorescent spots decreases over time; this decrease stems from diffusion and dissolution of the fluorescent dye contained in the PS colloids into 5CB.
To determine if it was possible to extend the methods reported above to the preparation of particles with non-spherical shapes, after polymerization of the nematic droplets in the water/glycerol mixture, 5CB was extracted from the particle core by washing twice with an excess of ethanol (EtOH; see SI) (Scheme 1D). DSC analysis of the particles after washing with EtOH (Figure S1 F (SI)) did not reveal evidence of a nematic-to-isotropic transition, unlike the polymerized droplets prior to washing (Figure S1 E (SI)). After redispersing the extracted particles in water, we observed the particles to exhibit an ellipsoid-like shape (ratio of major:minor axis of ~ 1.4), with either a single PS colloid located at one vertex (dipolar symmetry) or a single PS colloid at each of the two vertices (quadrupolar symmetry), respectively (Figure 4). These observations indicate that removal of 5CB was accompanied by deswelling of the polymerized particles in a direction perpendicular to the line joining the poles of the nematic droplets. In addition, PL micrographs in Figure 4C and H reveal that both the dipolar and the quadrupolar particles appear dark when their long axis is parallel to the axis of the polarizer (bright regions can also be observed near the tips of the quadrupolar particle), while they appear bright when tilted with respect to both polarizers (Figure 4D and I). From these observations, we conclude that the optical axis of the polymer chains is oriented preferentially along the long axis of the ellipsoid-like particles, and that the polymer network has been templated by the initial bipolar configuration of the nematic droplet.34 We obtained similar results using 1-μm-diameter DMOAP-coated silica colloids. We therefore anticipate that the above-described methods can be used to broadly tailor the physical and chemical properties of polymeric ellipsoid-like particles, simply by varying the material that constitutes the colloids located at the vertices.
Figure 4.
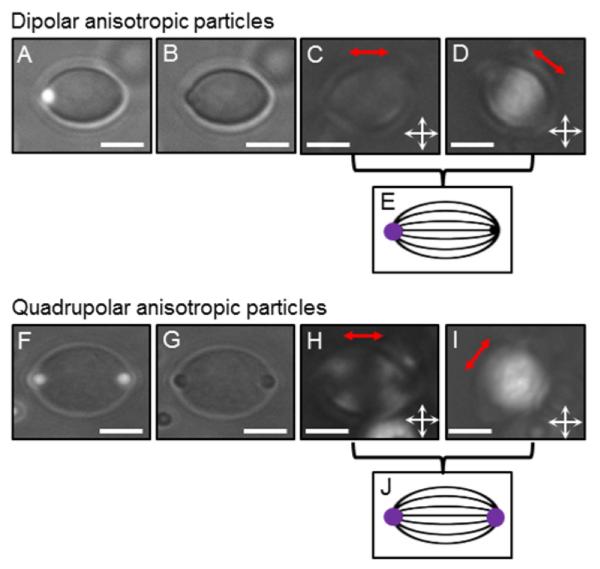
Typical dipolar (A-E) and quadrupolar (F-J) anisotropic particles synthesized using methods reported in this paper. The images were acquired in water, after extraction of 5CB. (A, F) are combined Fluo and BF micrographs, (B, G) are BF micrographs, and (C, D, H, I) are PL micrographs (the red double arrows indicate the particle long axis). Scale bars: 5 μm. (E) and (J) are schematics illustrating the average orientation of the optical axis of the polymer chains (black lines) within the anisotropic particles, as deduced from the PL pictures (C, D) and (H, I), respectively. The purple spots represent the PS colloids.
In conclusion, we have demonstrated that LC-based emulsions can be used as the basis of a general and facile method for the synthesis of spherical and non-spherical particles possessing organized chemically-distinct domains. Specifically, we show that LC droplets that possess two “boojums” can be used to direct the assembly of organic or inorganic colloids to the poles of the droplets (forming dipolar and quadrupolar droplets) independent of the type of anchoring of the LC at the colloid surface. The principle, however, is general and can be applied to other LC configurations observed within droplets.26 Furthermore, we anticipate that additional tailoring of the LC droplets will be possible by controlling key parameters involved in emulsification (shear rate, viscosity of the dispersing medium, and both volume fractions of LC and colloids (including high surface coverage of colloids)) or by using microfluidic systems that generate monodisperse LC droplets and control precisely the stoichiometry of the colloids and LC droplets. Finally, we also predict that control of the weight fraction of monomer in the LC/monomer mixture will provide a systematic method for tuning the anisotropy of the shapes of the composite particles. Overall, the multitude of possibilities offered by this approach will, we anticipate, enable synthesis of a large range of composite particles whose physical and chemical properties can be tailored as desired.
Supplementary Material
Footnotes
Supporting Information Available: Detailed experimental procedures, additional results. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
This work was primarily supported by NSF through DMR-1121288 (Materials Research Science and Engineering Center), the Army Research Office (W911NF-11-1-0251 and W911NF-10-1-0181), and the National Institutes of Health (CA108467, CA105730, AI092004.). Acknowledgment of support is also made to the Department of Energy, Basic Energy Sciences, Biomaterials Program (DESC0004025).
References
- (1).Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, Schuchman EH, Mitragotri S, Muzykantov VR. Mol. Ther. 2008;16:1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Loudet J-C, Alsayed AM, Zhang J, Yodh AG. Phys. Rev. Lett. 2005;94:018301. doi: 10.1103/PhysRevLett.94.018301. [DOI] [PubMed] [Google Scholar]
- (3).Lapointe CP, Mason TG, Smalyukh II. Science. 2009;326:1083–1086. doi: 10.1126/science.1176587. [DOI] [PubMed] [Google Scholar]
- (4).Mondiot F, Prathap Chandran S, Mondain-Monval O, Loudet J-C. Phys. Rev. Lett. 2009;103:238303. doi: 10.1103/PhysRevLett.103.238303. [DOI] [PubMed] [Google Scholar]
- (5).Botto L, Lewandowski EP, Cavallaro M, Stebe KJ. Soft Matter. 2012;8:9957–9971. [Google Scholar]
- (6).Yao L, Botto L, Cavallaro M, Bleier BJ, Garbin V, Stebe KJ. Soft Matter. 2013;9:779–786. [Google Scholar]
- (7).Madivala B, Vandebril S, Fransaer J, Vermant J. Soft Matter. 2009;5:1717–1727. [Google Scholar]
- (8).Han Y, Alsayed AM, Nobili M, Zhang J, Lubensky TC, Yodh AG. Science. 2006;314:626–630. doi: 10.1126/science.1130146. [DOI] [PubMed] [Google Scholar]
- (9).Glotzer SC, Solomon MJ. Nat. Mater. 2007;6:557–562. doi: 10.1038/nmat1949. [DOI] [PubMed] [Google Scholar]
- (10).Costi R, Saunders AE, Banin U. Angew. Chem., Int. Ed. 2010;49:4878–4897. doi: 10.1002/anie.200906010. [DOI] [PubMed] [Google Scholar]
- (11).Perro A, Reculusa S, Ravaine S, Bourgeat-Lami E, Duguet E. J. Mater. Chem. 2005;15:3745–3760. [Google Scholar]
- (12).Nie ZH, Li W, Seo M, Xu SQ, Kumacheva E. J. Am. Chem. Soc. 2006;128:9408–9412. doi: 10.1021/ja060882n. [DOI] [PubMed] [Google Scholar]
- (13).Suzuki D, Tsuji S, Kawaguchi H. J. Am. Chem. Soc. 2007;129:8088–8089. doi: 10.1021/ja072258w. [DOI] [PubMed] [Google Scholar]
- (14).Ling XY, Phang IY, Acikgoz C, Yilmaz MD, Hempenius MA, Vancso GJ, Huskens J. Angew. Chem., Int. Ed. 2009;48:7677–7682. doi: 10.1002/anie.200903579. [DOI] [PubMed] [Google Scholar]
- (15).Lee SY, Yang SM. Angew. Chem., Int. Ed. 2010;49:2535–2538. doi: 10.1002/anie.201000108. [DOI] [PubMed] [Google Scholar]
- (16).Jiang S, Chen Q, Tripathy M, Luijten E, Schweizer KS, Granick S. Adv. Mater. 2010;22:1060–1071. doi: 10.1002/adma.200904094. [DOI] [PubMed] [Google Scholar]
- (17).Hu SH, Gao XH. J. Am. Chem. Soc. 2010;132:7234–7237. doi: 10.1021/ja102489q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Nie ZH, Fava D, Kumacheva E, Zou S, Walker GC, Rubinstein M. Nat. Mater. 2007;6:609–614. doi: 10.1038/nmat1954. [DOI] [PubMed] [Google Scholar]
- (19).Nie ZH, Fava D, Rubinstein M, Kumacheva E. J. Am. Chem. Soc. 2008;130:3683–3689. doi: 10.1021/ja711150k. [DOI] [PubMed] [Google Scholar]
- (20).Liu K, Nie ZH, Zhao NN, Li W, Rubinstein M, Kumacheva E. Science. 2010;329:197–200. doi: 10.1126/science.1189457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Zhang Z, Pfleiderer P, Schofield AB, Clasen C, Vermant J. J. Am. Chem. Soc. 2011;133:392–395. doi: 10.1021/ja108099r. [DOI] [PubMed] [Google Scholar]
- (22).Duguet E, Désert A, Perro A, Ravaine S. Chem. Soc. Rev. 2011;40:941–960. doi: 10.1039/c0cs00048e. [DOI] [PubMed] [Google Scholar]
- (23).He J, Perez MT, Zhang P, Liu YJ, Babu T, Gong JL, Nie ZH. J. Am. Chem. Soc. 2012;134:3639–3642. doi: 10.1021/ja210844h. [DOI] [PubMed] [Google Scholar]
- (24).Fernández-Nieves A, Vitelli V, Utada AS, Link DR, Márquez M, Nelson DR, Weitz DA. Phys. Rev. Lett. 2007;99:157801. doi: 10.1103/PhysRevLett.99.157801. [DOI] [PubMed] [Google Scholar]
- (25).Gupta JK, Sivakumar S, Caruso F, Abbott NL. Angew. Chem. Int. Ed. 2009;48:1652–1655. doi: 10.1002/anie.200804500. [DOI] [PubMed] [Google Scholar]
- (26).Gupta JK, Zimmerman JS, de Pablo JJ, Caruso F, Abbott NL. Langmuir. 2009;25:9016–9024. doi: 10.1021/la900786b. [DOI] [PubMed] [Google Scholar]
- (27).Lopez-Leon T, Fernández-Nieves A, Nobili M, Blanc C. Phys. Rev. Lett. 2011;106:247802. doi: 10.1103/PhysRevLett.106.247802. [DOI] [PubMed] [Google Scholar]
- (28).Lopez-Leon T, Koning V, Devaiah KBS, Vitelli V, Fernández-Nieves A. Nature Phys. 2011;7:391–394. [Google Scholar]
- (29).Seč D, Lopez-Leon T, Nobili M, Blanc C, Fernández-Nieves A, Ravnik M, Zumer S. Phys. Rev. E. 2012;86:020705. doi: 10.1103/PhysRevE.86.020705. [DOI] [PubMed] [Google Scholar]
- (30).Pires D, Fleury J-B, Galerne Y. Phys. Rev. Lett. 2007;98:247801. doi: 10.1103/PhysRevLett.98.247801. [DOI] [PubMed] [Google Scholar]
- (31).Škarabot M, Ravnik M, Žumer S, Tkalec U, Poberaj I, Babič D, MuŠevič I. Phys. Rev. E. 2008;77:061706. doi: 10.1103/PhysRevE.77.061706. [DOI] [PubMed] [Google Scholar]
- (32).Fleury J-B, Pires D, Galerne Y. Phys. Rev. Lett. 2009;103:267801. doi: 10.1103/PhysRevLett.103.267801. [DOI] [PubMed] [Google Scholar]
- (33).Ravnik M, Alexander GP, Yeomans JM, Zumer S. Faraday Discuss. 2010;144:159–169. doi: 10.1039/b908676e. [DOI] [PubMed] [Google Scholar]
- (34).Crawford GP, Polak RD, Scharkowski A, Chien L-C, Doane JW, Zumer S. J. Appl. Phys. 1994;75:1968–1971. [Google Scholar]
- (35).Koenig GM, Lin I-H, Abbott NL. Proc. Natl. Acad. Sci. 2010;107:3998–4003. doi: 10.1073/pnas.0910931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Škarabot M, MuŠevič I. Soft Matter. 2010;6:5476–5481. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


