Abstract
Purpose
Participation in therapeutic clinical trials rarely reflects the race and ethnic composition of the patient population. To meet National Institutes of Health-mandated goals, strategies to increase participation are required. We present a framework for institutional enhancement of minority clinical trial accrual.
Methods
We implemented structural changes on four levels to induce and sustain minority accrual to clinical trials: 1) leadership support, 2) center-wide policy change, 3) infrastructural process control, data analysis and reporting and 4) follow up with clinical investigators. A Protocol Review and Monitoring Committee reviews studies and monitors accrual, and the Program for the Elimination Cancer Disparities leads efforts for proportional accrual, supporting the system through data tracking, web tools and feedback to investigators.
Results
Following implementation in 2005, minority accrual to therapeutic trials increased from 12.0% in 2005 to 14.0% in 2010. The “rolling average” minority cancer incidence at the institution during this timeframe was 17.5%. In addition to therapeutic trial accrual rates, we note significant increase in the number of minorities participating in all trials (therapeutic and non-therapeutic) from 2005 to 2010 (346 to 552, 60% increase, p < 0.05) compared to a 52% increase for Caucasians.
Conclusions
Implementing a system to aid investigators in planning and establishing targets for accrual, while requiring this component as a part of annual protocol review and monitoring of accrual, offers a successful strategy that can be replicated in other cancer centers; an approach that may extend to other clinical and translational research centers.
Keywords: Clinical trial, Therapeutic trial, Participation, Enrollment, Minority, Disparities
INTRODUCTION
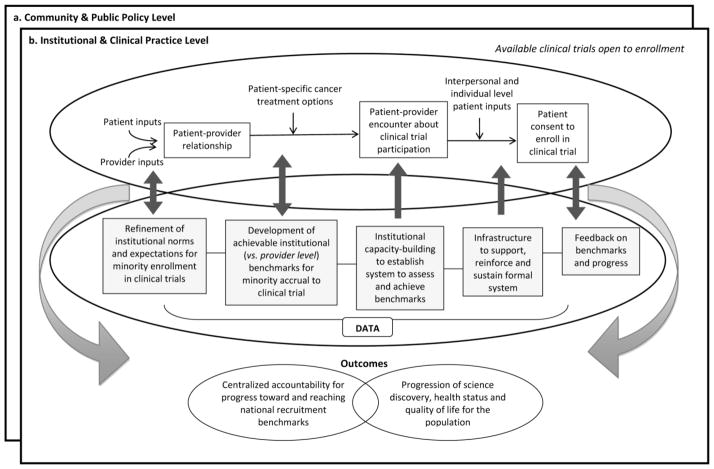
Clinical trials are crucial to advancing science across the cancer continuum. Comprehensive inclusion of diverse participants in clinical trials is essential to assuring generalizability of prevention, diagnostic and treatment recommendations and ultimately the identification of effective treatments for all sectors of society. Although federal mandates require investigators to demonstrate sufficient representation of minorities in study samples (1–4), there continues to be disparity in the representation of racial and ethnic minorities in clinical trials (5–10). Data available from 2000 show that accrual to National Cancer Institute-sponsored clinical trials for African Americans, Hispanics or Latinos, Asian/Pacific Islanders and American Indians was 8.2%, 4.5%, 1.8% and 0.3%, respectively (5). An update for the period 2003 to 2005 indicates that NCI and publically funded phase 1, 2, and 3 trials showed a national average of 8% African American participants among the total enrollment. Addition of Asian Pacific Islander (2.8%) and Native American Alaska Native (0.5%) and multiple (.1%) brought the total minority participation by race to 11.4% (11). For all aforementioned groups except American Indians, clinical trial accrual percentages are considerably lower than each group’s respective make-up of the United States population (12.3%, 12.5%, 3.7% and 0.9%, respectively) (12). Additionally, other groups have been under-represented in clinical trials, including uninsured and underinsured adults, adults with lower socioeconomic status and those living in underserved or rural areas (8, 13–15). Factors that contribute to this disproportional representation are well-documented and are influenced by multiple levels of interaction in the clinical trial recruitment process. Figure 1 illustrates four interrelated levels that interact to influence enrollment of minorities to clinical trials. They range from individual level influences on patient trial participation (e.g. mistrust of research, faith beliefs, or fear of side effects; Box a) to interpersonal level factors (e.g. physician-patient relationships/communication or communication about trials between patients and friends or other patients; Box b) to institutional and clinical practice level influences (e.g. organizational infrastructure to reinforce minority recruitment, lack of physician awareness of available clinical trials, systemic lack of time for recruiting, or small minority patient pools; Box c), and finally, community and public policy level influences (e.g. federal mandates or inhibitive inclusion criteria that restrict participation due to a range of factors including co-morbidities; Box d) (16–31), These discrete, but interrelated obstacles clearly indicate opportunities for intervention including careful consideration of required inclusion and exclusion criteria (19, 32–35); structured evaluation of cancer trial results on strata such as age (or ethnicity) to understand tumor biology, treatment tolerability and the effects of comorbid conditions to help refine mandatory eligibility criteria for future studies (36, 37); targeted patient communication via patient trusted communication vectors (38); use of peer coaches (39); and increased trust building between patient/provider and with communities (40, 41).
Fig. 1.
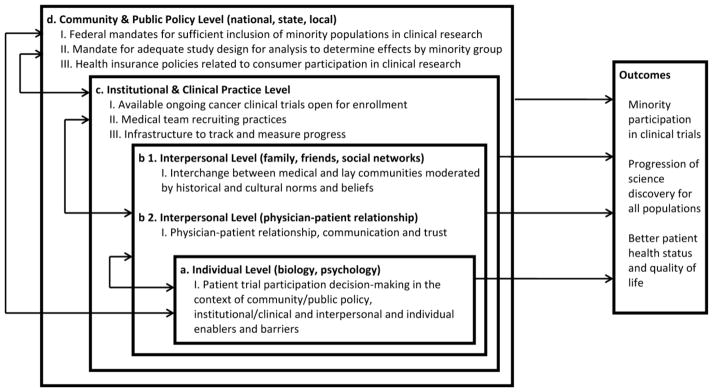
Levels that impact minority clinical trial enrollment
Such interventions are essential in the path to achieve federal mandates for minorities in clinical trials. Emphasis is usually placed on individual and interpersonal levels of influence respective to both patients and providers. While this approach has merit and is part of the solution (42), closer linkage across levels of influence from individual to policy will speed attainment of minority clinical trial recruitment benchmarks over time. Such has been argued by Sorensen et al. (43) in relation to cancer health behavior interventions across social contexts, and for achieving population-wide health advances in the United States (44). Without this linkage, interventions focused on individual and interpersonal levels of influence to increase minority participation are disconnected from parallel interventions at the community and public policy level of influence. Consequently, an implementation gap results, which diffuses both progress toward and accountability for reaching national recruitment benchmarks, and makes way for slower progression of science discovery reinforcing poorer health status and quality of life for the population. Fundamental to making the link across the continuum from individual to policy levels of influence is a focus on institutional level influences (i.e. Fig. 1 Box c), more specifically, Box c, Item III. Usually, there is an absence of any formal structure for ongoing monitoring of organizational/institutional progress in minority recruitment, beyond cancer center wide reporting at competitive peer review. To bridge this disconnect and underscore centralized accountability, tracking and monitoring across an organization, systematic adjustments to improve organizational infrastructure for minority clinical trial enrollment are required. Here, we describe a framework for centralized organizational accountability through systematic benchmark development, continuous progress monitoring and responsive adjustments at provider and organizational levels to enhance organizational infrastructure to induce and sustain increased minority participation in clinical trials. We also describe the application of this framework at a National Cancer Institute (NCI) Comprehensive Cancer Center and recruitment outcomes to date.
METHODS
A Framework for Centralized Implementation, Organizational Accountability and Monitoring for Minority Clinical Trial Recruitment
Figure 2 presents a framework with which to approach implementation strategies to achieve centralized minority recruitment to clinical trials, organizational accountability and monitoring. This framework draws upon principles of organizational change, implementation science research and behavioral and social science intervention (43–47). For institutionalizing an organizational-level change, three elements must interact: 1) refinement of a culture; 2) capacity-building efforts; and 3) a supportive, reinforcing, and sustaining infrastructure. We adopt and extend this approach for application with minority recruitment to clinical trials. Illustrated in the top oval of Fig. 2, clinical practice strategies to recruit individuals to clinical trials proceed along traditional patient-provider interaction points. Concurrently, illustrated in the lower oval of Fig. 2, phases of organizational advancement are transitioned to refine, build, reinforce and sustain a system that supports minority accrual to clinical trials toward national benchmarks. Data inputs along the phases of transition guide and help reform advancements made within the organization. Each organizational phase is important to ensuring centralized accountability for minority recruitment and perceived efficacy among providers of the infrastructure developed to reinforce that accountability. These organizational refinements intersecting with patient-provider and clinic practices create the opportunity for more centralized accountability toward institutional recruitment outcomes and progress to reaching national benchmarks for minority recruitment. This framework may be applied to many organizations and institutions. We describe its application in a Comprehensive Cancer Center.
Fig. 2.
Framework for centralized implementation, organizational accountability and monitoring for minority clinical trial recruitment
Application of Framework to a Comprehensive Cancer Center
The following describes methods to build and implement key structural changes (Fig. 2 lower oval) into the institutional/organizational infrastructure of a Comprehensive Cancer Center and details the interaction of these changes with clinical practice strategies for clinical trial recruitment (Fig. 2 top oval). We implemented structural changes on four levels to induce and sustain minority accrual to clinical trials: 1) leadership support, 2) center-wide policy change, 3) infrastructural process control, data analysis and reporting and 4) follow up with clinical investigators. Our goals were to increase minority recruitment through active interventions and monitoring, and to increase minority participation in clinical trials at the Cancer Center until participation rates among minorities mirrored the minority incidence rates observed for individual cancers. A long-term goal is to equalize Cancer Center patient demographics with those of the service region.
At the conceptualization of our system-level changes, like many others, we had no centralized accountability system for planning and monitoring minority accrual to clinical trials in our Center. However, there were existing organizational structures through which such a system could be created. Like other NCI-designated Comprehensive Cancer Centers, our Center has disease-oriented working groups and a Protocol Review and Monitoring Committee (PRMC), which is the Center’s NCI-required Protocol Review and Monitoring System (PRMS). In our Center, the PRMC operates separately from our institutional review board (IRB). Prior to PRMC submission all trial protocols are prioritized by the relevant disease-oriented working group(s) and approved by the appropriate working group leader(s). Our PRMC reviews and monitors all cancer-related trials conducted at the Cancer Center or partner institutions at initial submission and annual renewal to assure that clinical trials are scientifically sound and that studies maintain scientific and accrual progress. Decisions regarding study continuation are made by this committee. Oversight of data and safety monitoring is the responsibility of our PRMC and Quality Assurance and Safety Monitoring Committee (QASMC)1. After PRMC review, the IRB reviews all studies for ethical considerations, protection of human subjects in research and recruitment plans.
The first step in implementing structural changes to enhance minority accrual was to obtain explicit support and a mandate for change from senior-leadership at the Cancer Center. This was formally demonstrated in a letter to all investigators from Cancer Center leadership in support of the structural changes to enhance minority accrual. This directive not only laid the groundwork for refining norms and expectations for enhancement of minority clinical trial participation, but gave way to forthcoming initiatives to build institutional capacity in our system to support the new changes. Endorsement at the level of senior-leadership of the Cancer Center was championed by faculty within the Cancer Center with interest in minority clinical trial participation. With the mandate from the Cancer Center senior-leadership, we conducted a series of meetings with the PRMC leadership, to gain buy-in with the committee. Such meetings revealed an important issue to consider. Although the PRMC serves as a gatekeeper for cancer clinical research, the committee had both political and operational concerns in being charged with the new review responsibilities that would be needed. Cancer Center leaders discussed committee concerns at length, and subsequently developed operating procedures and data collection tools (described later) to streamline the review process. These procedures and tools included standards, benchmarks, evaluation, and making the process of review, monitoring, and accountability transparent.
With leadership support underway and active consideration of stakeholder concerns, we subsequently formalized a center-wide policy change that involved 1) defining disease-specific accrual targets by race (and gender) based on the race-specific (and gender-specific) incidence rates seen at the Center; and 2) a requirement for investigators to set specific accrual targets based on the cancer being studied. The policy was incorporated into the Cancer Center’s data safety and monitoring plan (48) and requires investigators, as standard procedure, to set minority accrual targets at the time of protocol development for initial submission to the PRMC. These requirements apply to 1) all therapeutic and imaging studies that are investigator initiated—classified as “institutional primary” and 2) all studies that are expected to accrue ≥ 25 participants (including investigator-initiated studies from other centers, national cooperative group and industry-initiated). To alleviate PRMC concern of additional review responsibility, our Cancer Center’s Program for the Elimination of Cancer Disparities (PECaD) reviews protocol accrual targets at the time of PRMC submission to make certain the targets correspond to the appropriate disease-specific institutional benchmarks. We divide cancers by primary disease site stratified by race, gender and stage. In general, the division of disease sites is based on the World Health Organization International Classification of Diseases for Oncology (ICD-O) (49). However, in some cases data is grouped with that from similar disease types for low incidence sites at the Cancer Center. If investigators set a lower accrual target than expected for specific protocols, they are required to justify that decision based on their particular study or to resubmit targets that match the institutional benchmarks for minority accrual. At the time of annual review of protocols as part of our PRMC, PECaD monitors actual minority accrual numbers over the course of trial implementation. In addition to changes made to trial submission and review processes, a key specification in the center-wide policy is that PECaD can recommend closure of any study to senior leadership if minority accrual is consistently poor and the study team takes no corrective action.
Before the center-wide policy change could be implemented fully with clinical investigators and faculty, we needed to build support for our proposed changes with the disease-oriented working groups. We held individual meetings with leaders of each working group at the Cancer Center to assess and raise their awareness of the importance of minority recruitment to strengthening the science and also to the overall success of the Center. This served to stimulate dialogue regarding obstacles to participation. As part of these meetings, we presented current minority clinical trial accrual rates for a disease area and compared that with data on the matching clinical population. This clearly demonstrated underrepresentation in most disease areas, and defined targets on which to establish institutional benchmarks. For our benchmark, we compare minority participation in clinical trials to site-specific cancer incidence at the Cancer Center to evaluate impact of the system-level changes. This approach is an appropriate and realistic benchmark for therapeutic clinical trials (50). Initial benchmarks were based on the preceding year’s clinical incidence data. Subsequently, we based the accrual benchmarks for treatment trials on five-year rolling average site-specific incidence rates at the Cancer Center, stratified by race, stage and gender. Using standard statistical methods and data from the Cancer Center tumor registry (known as Oncology Data Services), we calculate five-year rolling average incidence rates over the preceding five-year period. Each year, the earliest year drops off to add the most recent year to the calculation. We use rolling averages over five years to account for variability in the clinical population from year to year, and to give an adequate number of observations for rarer diagnoses. For prevention and screening studies, we use population data of the primary catchment area (Census data) to determine the benchmark on which investigators set accrual targets (51). To facilitate investigator access to accrual benchmarks, we post data on the Center web site.
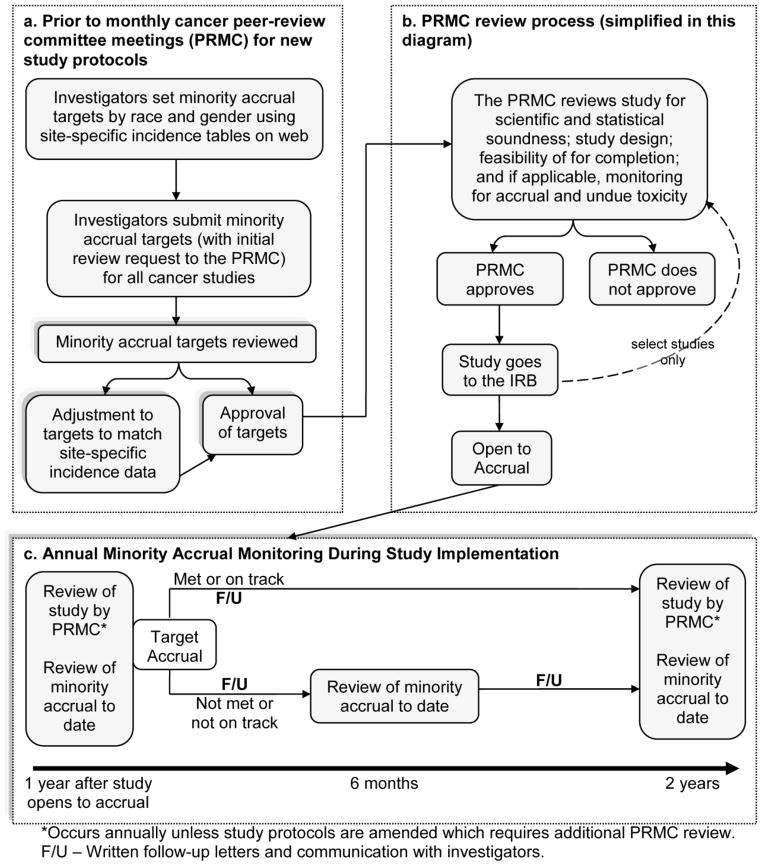
Finally, a formal process was established within PRMC procedures to provide ongoing monitoring and feedback to investigators on the status of minority accrual and retention in their individual trials. After minority accrual targets are submitted to the PRMC and approved by PECaD, investigators receive formal communication through written letters from the PECaD clinical trials team. This communication begins annually and is adjusted to bi-annually if minority accrual status is below the minority accrual targets. The letters summarize each investigator’s progress toward meeting minority accrual targets for the disease site(s) being studied. Letters include commendation for progress in meeting or exceeding target goals and suggestions for improvement and support services for underperforming studies (see Appendix). Investigators are advised of whether a shortened review period (less than annual) is warranted. In addition to written letters, our PECaD clinical trials outreach team leader communicates with investigators through in-person meetings or conference calls to better understand the nature of issues limiting minority clinical trial participation on a specific study and help find ways with that investigator to address the issues. The flow of studies through review and monitoring is outlined in Figure 3. The PECaD team meets at least monthly to review and process studies within our scope.
Fig. 3.
Flow of studies through custom review process for minority accrual at Siteman Cancer Center
Outcome Measures and Analysis
Our main outcome measure is change in minority accrual to therapeutic trials and all trials (i.e. therapeutic and non therapeutic) over time. We assessed trends in minority accrual to clinical trials over time in yearly increments and compared these rates to five-year rolling-average incidence rates at the Cancer Center. We defined minority as African American and Other including American Indian/Alaska Native, Asian and Native Hawaiian or Other Pacific Islander. Other races are combined because individually their proportions at the Cancer Center are too small for individual analysis. Participants with unknown race were removed from analysis; for all trials and therapeutic trials, about 3.1% and 2.5% participants with unknown race removed, respectively. Chi-square tests for trend were used to determine if there was a significant increase in minority participation in trials over the study time period. In analysis of working group distribution for trials, chi-square tests of homogeneity were used to assess variance in the number of trials in each working group per year. Linear regression analysis was used to assess the trend in the percent participation in each racial category (white, minority, and unknown race) over the study time period.
RESULTS
Table 1 shows minority participation rates in therapeutic trials and all trials per year compared to five-year rolling average incidence rates from 2005 to 2010 during implementation of the system-level changes. There is a significant increasing trend in the number of minority participants enrolling in therapeutic trials (p = 0.04), but not among all trials (p = 0.09). There is a very small, yet significant increasing trend in minority cancer incidence between the years 2005 and 2010 (p = 0.04). In addition to accrual rates, we note significant increase in the number of minorities participating in all trials in the years 2005 to 2010 (from 346 to 552, 60% increase) compared to a 52% increase for Caucasians during the same time period. A two-sample test of proportion results indicate that this difference in increase is significant, z = 2.0416, p = 0.0412. Examining the number of minority patients participating in clinical trials, relative to the total number of minority patients seen in the clinic population each year, provides another summary of participation. This percentage has remained stable over the years (with the exception of 2007). Presented this way, there has not been an increase in the actual proportion of the minority population participating in clinical trials, but rather the absolute number of minorities participating in clinical trials has increased over the five-year course (33% increase between 2005–2010-[(170−128)/128]). This has followed a similar increase in the number of minority cancer patients seen at the institution (30% (1137−869)/869). The proportion of minorities participating in clinical trials in relationship to the minorities receiving care at the facility (e.g. 128/869=14.7% in 2005 vs 170/1137=15% in 2010) has remained quite stable (except in 2007 when a greater proportion of minorities were recruited 16.2%).
Table 1.
Minority clinical trial participation by year, 2005–2010 at Siteman Cancer Center
| n (%) | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Avg. annual % change |
|---|---|---|---|---|---|---|---|
| Therapeutic trials | 128 (12.0) | 128 (12.0) | 154 (15.2) | 146 (14.4) | 159 (13.9) | 170 (14.0) | 0.43 |
| All trials | 346 (14.0) | 402 (14.7) | 489 (16.4) | 630 (17.5) | 599 (15.4) | 552 (14.6) | 0.18 |
|
| |||||||
| Minority cancer incidence | 869 (17.8) | 856 (17.1) | 949 (17.4) | 1021 (17.1) | 1094 (17.6) | 1137 (18.1) | 0.08 |
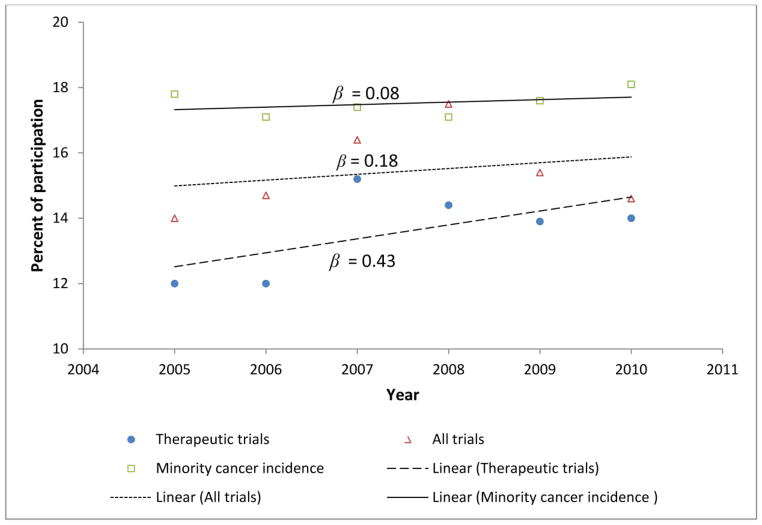
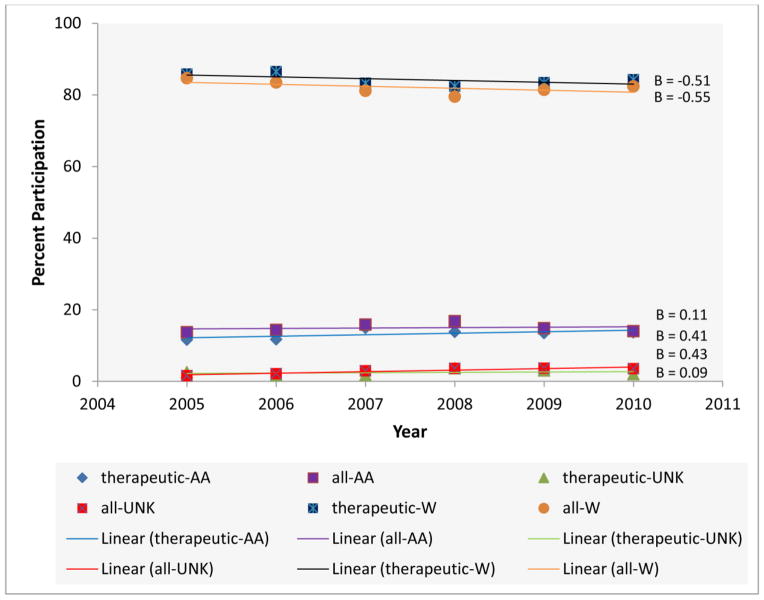
Figure 4 shows that across the study time period minority cancer incidence was fairly stable (β = 0.08), the percent of minority participation for all trials was stable with a very slight increasing trend (β = 0.18), and the regression line for percent of minority participation for therapeutic trials indicated a positive trend with a slope (β = 0.43) about five times (the magnitude) the slope for percent of minority cancer incidence and about 2.4 times the slope for percent of minority participation in all trials. Therefore, the Cancer Center is increasing the percent of minorities enrolled in therapeutic trials at a rate five times faster than the increase in the cancer incidence for this patient population. The beta in these analyses was not significant however. Further, shown in Figure 5, the percent of white participants in all trials and therapeutic trials is decreasing while the percentages of minority and unknown races are increasing over the study period. Notable, there is a large increase among the percent of minorities enrolled in therapeutic trials (β = 0.41).
Fig. 4.
Percent of trial participation and cancer incidence for minorities, 2005–2010 at Siteman Cancer Center
Fig. 5.
Percent participation in all trials and in therapeutic trials, 2005–2010 at Siteman Cancer Center
Since our program only reviews a subset of protocols at the Cancer Center (note: the Methods section describes criteria for protocols that fall under the center-wide review policy change), we compared the percent of potential enrollment slots available for protocols that do not fall under the policy review criteria to slots for protocols that do fall under the criteria to illustrate the organizational grasp of our clinical trial review and monitoring program. In the time period from 2005 to 2010, the ratio of potential enrollment slots for protocols not under our review compared to those that fall under our review was relatively equal (ratio of 1 to 0.90), which indicates that the policy review change adequately captures enrollment capacity of trials available in the Cancer Center. Additionally, we assessed the types of trials available for accrual at our center from 2005 to 2010. Over the study time period and of all phase designations at our Cancer Center (i.e. pilot; phase I, I/II, II, II/III, III, or IV; and phase inapplicable), except phase inapplicable which had the highest average per year (210.5, 62.1%), there were on average more Phase I, II, III, and pilot studies per year (18.0, 37.2, 31.8, and 18.0, respectively). These made up an average percent of 5.5%, 12.7%, 11.0%, and 6.0% of trials available per year, respectively. We also assessed the types of trials available by their study category. At our Cancer Center, study category is used to provide a general description of the nature of a trial. During our time period of analysis, there were 20 different study categories2. The average number of trials classified as therapeutic per year was 99.2 (33.3%), which accounted for 30.6% of trials in all years. Other study category classifications have evolved over time; less specific in the early years, they have become more refined over time as the types of studies offered by the cancer center have evolved. Finally, we assessed the trial types available by working group. As mentioned earlier, a working group at our center is disease-oriented and all trials must be initially approved and prioritized by the appropriate working group leader prior to submission to the PRMC and IRB. We have 14 primary working groups. These are listed in our data safety and monitoring plan (48). We have nine additional working groups3. Each working group classification is mutually exclusive. Also, each primary working group includes sub-classifications. For example, the breast oncology working group has sub-classifications for adjuvant chemo, imaging, metastatic chemo, and so on. Tables 2 and 3 describe the number of trials in each working group per year during the study period. Table 2 shows that the numbers of therapeutic trials in any single working group were stable and there is no significant difference among the numbers of trials in any working group over the study period. However, over the study period the numbers of all trials (therapeutic and non-therapeutic) in any single working group (Table 3) were not stable and there were statistically significant differences in ten working groups across the study period. Comparing Tables 2 and 3, we show that while the number of trials have increase over time, the number of therapeutic trials in each working group per year has been relatively stable. We also note that 190 new trials were reviewed from 2005 through 2010 along with 392 annual or 6-month reviews. The proportion of studies needing follow-up varied over time and a total of 11 Principal Investigator interviews were scheduled to review steps that could be taken by the PI to improve minority accrual.
Table 2.
Number of therapeutic trials in each working group by year, 2005–2010 at Siteman Cancer Center
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Chi-square | df | p | |
|---|---|---|---|---|---|---|---|---|---|
| Leukemia/lymphoma/transplant | 20 | 21 | 24 | 26 | 24 | 31 | 3.18 | 5 | 0.6726 |
| Breast oncology | 16 | 19 | 19 | 20 | 17 | 16 | 0.83 | 5 | 0.9750 |
| Gynecologic oncology | 8 | 8 | 7 | 9 | 10 | 11 | 1.23 | 5 | 0.9423 |
| Gastrointestinal - lower | 9 | 11 | 10 | 10 | 8 | 4 | 3.62 | 5 | 0.6060 |
| Gastrointestinal - upper | 9 | 8 | 6 | 4 | 7 | 11 | 3.93 | 5 | 0.5591 |
| Thoracic oncology | 3 | 5 | 9 | 9 | 9 | 8 | 4.58 | 5 | 0.4691 |
| Genitourinary oncology | 9 | 6 | 7 | 7 | 5 | 4 | 2.42 | 5 | 0.7883 |
| Developmental therapeutics | 5 | 4 | 2 | 5 | 7 | 7 | 3.60 | 5 | 0.6083 |
| Head & neck oncology | 3 | 2 | 5 | 7 | 4 | 4 | 3.56 | 5 | 0.6143 |
| Pediatric oncology | 5 | 3 | 3 | 3 | 3 | 0 | 4.53 | 5 | 0.4760 |
| Neuro-oncology | 0 | 0 | 1 | 3 | 5 | 3 | 10.00 | 5 | 0.0752 |
| N/A | 1 | 1 | 1 | 1 | 1 | 3 | 2.50 | 5 | 0.7765 |
| Musculoskeletal oncology | 0 | 0 | 0 | 1 | 3 | 3 | 9.29 | 5 | 0.0982 |
| Endocrine oncology | 2 | 1 | 1 | 1 | 1 | 0 | 2.00 | 5 | 0.8491 |
| Melanoma | 0 | 0 | 0 | 2 | 2 | 2 | 6.00 | 5 | 0.3062 |
working groups not listed here had no therapeutic trials
Table 3.
Number of all trials (therapeutic and non-therapeutic) in each working group by year, 2005–2010 at Siteman Cancer Center
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Chi-square | df | p | |
|---|---|---|---|---|---|---|---|---|---|
| Leukemia/lymphoma/transplant | 28 | 35 | 45 | 59 | 69 | 74 | 33.59 | 5 | <.001 |
| Breast oncology | 27 | 41 | 40 | 51 | 54 | 50 | 11.38 | 5 | <.05 |
| Gynecologic oncology | 10 | 16 | 26 | 32 | 39 | 36 | 24.89 | 5 | <.001 |
| Gastrointestinal - lower | 17 | 24 | 28 | 33 | 32 | 23 | 6.99 | 5 | |
| Gastrointestinal - upper | 14 | 15 | 16 | 19 | 30 | 29 | 12.56 | 5 | <.05 |
| Pediatric oncology | 12 | 17 | 19 | 20 | 21 | 12 | 4.68 | 5 | |
| Thoracic oncology | 9 | 23 | 38 | 42 | 48 | 43 | 32.60 | 5 | <.001 |
| Genitourinary oncology | 18 | 19 | 26 | 33 | 43 | 32 | 15.77 | 5 | <.01 |
| Psychosocial factors in cancer care | 8 | 8 | 7 | 8 | 7 | 6 | 0.45 | 5 | |
| Head & neck oncology | 7 | 10 | 20 | 27 | 27 | 23 | 19.47 | 5 | <.01 |
| Developmental therapeutics | 5 | 4 | 2 | 5 | 7 | 7 | 3.60 | 5 | |
| Early detection and screening | 4 | 5 | 3 | 2 | 2 | 2 | 2.67 | 5 | |
| Endocrine oncology | 3 | 4 | 6 | 7 | 9 | 10 | 5.77 | 5 | |
| Melanoma | 2 | 6 | 9 | 15 | 15 | 11 | 13.59 | 5 | <.05 |
| N/A | 2 | 4 | 9 | 14 | 13 | 16 | 16.69 | 5 | <.01 |
| Aids related | 1 | 1 | 1 | 1 | 1 | 1 | 0.00 | 5 | |
| Musculoskeletal oncology | 1 | 2 | 2 | 4 | 4 | 4 | 3.12 | 5 | |
| Nicotine dependence | 1 | 1 | 1 | 0 | 0 | 1 | 2.00 | 5 | |
| Community outreach/prevention | 1 | 2 | 2 | 1 | 3 | 3 | 2.00 | 5 | |
| Chemoprevention related | 0 | 0 | 0 | 1 | 1 | 1 | 3.00 | 5 | |
| Neuro-oncology | 0 | 4 | 7 | 9 | 13 | 13 | 17.13 | 5 | <.01 |
| Ecological aspects & cancer care | 0 | 0 | 0 | 1 | 1 | 0 | 4.00 | 5 | |
| Other | 0 | 0 | 1 | 2 | 3 | 1 | 5.86 | 5 |
DISCUSSION
Our structural changes to the clinical trial review process in the Cancer Center resulted in increased minority participation in therapeutic trials. Overall, the increasing trend in minority therapeutic trial participation shows incremental progress that with continued intervention and monitoring we expect to continue. We note some important issues and challenges that other institutions might face. Before reviewing such issues, we first discuss the critical challenges and decision points that were critical to implementing and sustaining our systems-level changes successfully.
Clinical trial accrual underpins the science of cancer and is especially valuable to the long-term goals of the NCI and its Cancer Centers program. Our clinical trial accrual monitoring program was framed as both an NCI requirement and a critical success factor for both scientific and institutional goals. This framing was key in obtaining center-wide support from leaders and investigators. The program has enhanced long-term sustainability within the Center because we hard-wired it within investigator requirements and PRMC policies and procedures. Prior to implementing our system-level changes, investigators were aware of national mandates and priorities for inclusion of racial and ethnic minorities in clinical trials (1–4), but no centralized accountability system for monitoring minority accrual existed in the Cancer Center. This fueled diffusion of accountability for minority accrual at the trial level and perpetuated a situation in which investigators were neither held accountable nor offered support in meeting goals. We found that the system-level changes increased investigator awareness of their role and responsibility for minority accrual to trials. It also created accountability by requiring investigators to set minority accrual targets which drove increases in minority accrual. Matching this accountability by providing support services for investigators made the entire process seem less punitive and more productive. However, the ultimate success of the initiative hinged on the policy provision that any study could be shut down by the Cancer Center’s senior leadership if minority accrual was consistently poor and the study team failed to develop a corrective action plan.
Obtaining early backing from Cancer Center leadership and formalizing that support in a letter from leadership to all investigators was also critical in prioritizing the issue of minority accrual and to building support or at least buy-in of disease-oriented working group leaders. This allowed for broader dialogue about ways to enhance minority accrual across the institution. Moreover, the accrual monitoring program has stimulated more productive physician-patient dialogue regarding clinical trial participation. PECaD sponsors at least one clinical trial or research participation focused continuing medical education talk per year with a local forum of African-American physicians from the cancer center and from primary care and referral clinics that serve predominately minority and underserved patient populations.
Connecting minority accrual to and broadening the existing PRMC review system at the Cancer Center established institutional capacity for long-term sustainability and linked the minority accrual monitoring process to other initiatives to enhance the quality of the science. In order to facilitate the PRMC’s involvement and reduce any antagonistic perceptions, PECaD led monitoring and enforcement responsibilities. This allowed the PRMC to be the vector of implementation while preserving its focus on scientific quality and review. The process of setting and reviewing study specific minority accrual targets also needed to be timely to avoid delay in the initial protocol review process by the PRMC. The duration of time for the action steps described in Box a of Fig. 3 for new studies occur before submission to PRMC for review. PECaD review of minority accrual targets occurs in the days and weeks prior to monthly PRMC review meetings once protocols are flagged as new in the Cancer Center’s clinical trial database. Over the time frame of this report, the review team followed up by letter with 10 to 20 percent of principal investigators submitting protocols for annual review, to remind investigators of strategies and resources available to increase accrual to meet approved minority accrual goals.
Enhancing strategies for improvement and support services for underperforming studies and those having difficulty with minority accrual is interconnected with the complexities inherent in the broader obstacles to minority accrual discussed earlier such as patient fear and distrust. As part of support services offered to members, we work on a case-by-case basis with investigators to identify study-specific barriers to minority accrual and ways to overcome those barriers. For example, in some cases we link investigators with referring physicians that have high minority populations. Other times we have discussed recruitment practices of individual clinics that investigators recruit from and helped to identify opportunities for enhanced minority recruitment. What we have learned is that every trial and recruitment situation is different. We approach strategies for improving minority trial participation in this vain, while accrual benchmarks underpin those strategies as well as expectations. Additionally, strategies for minority accrual improvement are combined with strategies to maintain minorities in the clinical population pool by increasing awareness and utilization of cancer screening and maintaining linkage with safety net referral clinics. We are implementing initiatives at the Cancer Center to make education and training resources more readily available to investigators as well as engage the community in learning about clinical trials and other research. Toward our long-term goal of equalizing Siteman patient demographics with those of the service region, we are making steady headway as noted by an 18.1% minority patient population in 2010 compared to the 22.4% make-up of minorities in our primary catchment area. We note that the population in the catchment area has been stable, and the level of health insurance and of poverty have not changed over time. Other PECaD initiatives use community-based participatory approaches to engage community members in outreach and education activities about clinical trials and general cancer information.
Despite having had success within our organization, achieving representative participation in clinical trials is only part of the solution to eliminating disparities in cancer incidence and mortality among racial and ethnic minorities and other groups. Assessing differences by race and ethnicity and reporting them in the scientific literature is critical to expanding knowledge about disparities, but is not standard in practice (37, 52, 53). Furthermore, merely having proportional representation within trials may not provide sufficient sample size and statistical power to evaluate race/ethnicity-specific therapy and outcomes. Many investigators at our Cancer Center have specific interest in cancer disparities. Thus there is increased development of studies that over-sample or target minorities which may be helping to increase minority accrual.
In conclusion, our approach to implementing structural changes at four levels—leadership support; center-wide policy change; infrastructural process control, data analysis and reporting; and follow up with clinical investigators—to induce and sustain minority accrual to clinical trials has been effective in increasing awareness and accountability for minority clinical trial accrual. To achieve improvement in recruitment, this approach should be combined with institutional support for investigators during the planning and recruitment stages of clinical trials. Our system level approach to improve minority accrual responds to the recommendation of leading medical and public health experts for better a system of coordination to integrate innovations and build synergy across multiple levels to advance science and public health (54, 55). It bridges activity and innovations directed to individuals with those at the community and public policy level through structural intervention in at the organizational/institutional level. Our approach can be a model for other NCI-designated Cancer Centers and institutions implementing clinical trials.
Acknowledgments
This work was supported by the National Cancer Institute at the National Institutes of Health (U01CA114594, U54CA153460, and P30CA91842).
Appendix
|
SAMPLE LETTER CONTENT
|
| RE: Protocol Number and Title |
| Dear Dr. (…): |
| As part of the annual PRMC renewal, the Program for the Elimination of Cancer Disparities (PECaD) reviewed the above-noted protocol on (date).
|
|
(Insert Applicable Statement)
|
| …Consistent with goal/target |
| We congratulate you on the reported total minority accrual of XX% which is consistent with the target accrual of XX%. |
| …Exceeding anticipated goal/target |
| We congratulate you on reaching total minority accrual of XX% thus far. This exceeds the target of XX% minority enrollment that we would expect based on our patient population. |
| …To soon to tell |
| With total recruitment at (<10%) of your target, it is difficult to assess this study’s demographics. We encourage you to continue recruitment with your target goal of XX% minority participation in mind. |
| …To soon to tell… but close |
| Though you are in the early stages of recruitment for this study, we see that the current total minority accrual is XX%, which is very close to the target accrual of XX%. |
| …Below anticipated goal/target |
| Currently minority accrual for this protocol is XX% which is XX% (approximately X patients) lower than your target accrual of XX%. Please keep this goal in mind as you continue to recruit and take advantage of the available services to assist you in achieving the targeted minority recruitment.
|
| We appreciate you and your staff’s efforts to date and wish you success in ongoing enrollment and maintenance of strong minority participation. If at any point you experience difficulty recruiting minorities to this trial, we are available to assist you with strategies to increase minority participation. |
| (or variation) |
| We appreciate you and your staff’s efforts to date and wish you success in ongoing enrollment and maintenance of strong minority participation. Are you experiencing any barriers to recruiting minorities to this trial? If so, we are available to assist you with strategies to increase minority participation.
|
| We will review your accrual again in one year (six months if significantly below targets). If you have any questions or concerns in the meantime, please contact….
|
|
Note
|
| Sometime special letter tailoring is used for unique characteristics such as small patient pool, eligibility limitations, etc… |
Footnotes
The Quality Assurance and Safety Monitoring Committee (QASMC) provides independent oversight and review of quality assurance audits, serious adverse event reporting, and interim data and safety monitoring reports.
ancillary, chart review, companion, correlative, detection (early), diagnostic (non-radiologic), diagnostic imaging, epidemiologic, exempt research, existing data/specimens, observational, prevention and control, registry, screening, standard treatment, supportive care, survey, therapeutic, tissue/specimen, tissue/survey
AIDS-related, chemoprevention-related, psychosocial factors in cancer care, nicotine-dependence, community outreach and prevention, early detection and screening, ecological aspects of early detection and cancer care, other, null
References
- 1.Freedman LS, Simon R, Foulkes MA, et al. Inclusion of women and minorities in clinical trials and the NIH Revitalization Act of 1993--the perspective of NIH clinical trials. Control Clin Trials. 1995;16:277–85. doi: 10.1016/0197-2456(95)00048-8. discussion 86–9, 93–309. [DOI] [PubMed] [Google Scholar]
- 2.Hohmann AA, Parron DL. How the new NIH Guidelines on Inclusion of Women and Minorities apply: efficacy trials, effectiveness trials, and validity. J Consult Clin Psychol. 1996;64:851–5. doi: 10.1037//0022-006x.64.5.851. [DOI] [PubMed] [Google Scholar]
- 3.(June 10, 1993) NIH Revitalization Act of 1993.
- 4.(October 9, 2001) Amendment: NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research - October 2001.
- 5.Christian MC, Trimble EL. Increasing participation of physicians and patients from underrepresented racial and ethnic groups in National Cancer Institute-sponsored clinical trials. Cancer Epidemiol Biomarkers Prev. 2003;12:S277–S83. [PubMed] [Google Scholar]
- 6.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–6. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 7.Stewart JH, Bertoni AG, Staten JL, Levine EA, Gross CP. Participation in surgical oncology clinical trials: gender-, race/ethnicity-, and age-based disparities. Ann Surg Oncol. 2007;14:3328–34. doi: 10.1245/s10434-007-9500-y. [DOI] [PubMed] [Google Scholar]
- 8.Baquet CR, Ellison GL, Mishra SI. Analysis of Maryland cancer patient participation in national cancer institute-supported cancer treatment clinical trials. J Clin Oncol. 2008;26:3380–6. doi: 10.1200/JCO.2007.14.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Cancer Institute. NCI-Supported clinical trials: facts and figures. Rockville: National Cancer Institute; 2000. pp. NCI clinical trials: an overview. [Google Scholar]
- 10.Movsas B, Moughan J, Owen J, et al. Who enrolls onto clinical oncology trials? A radiation Patterns Of Care Study analysis. Int J Radiat Oncol Biol Phys. 2007;68:1145–50. doi: 10.1016/j.ijrobp.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 11.Springfield S. Racial and Ethnic Minorites Accrual to NCI Clinical Trials. Bethesdda, MD: Center to Reduce Cancer Health Disparities, National Cancer Institute, NIH; 2010. [Google Scholar]
- 12.U.S. Bureau of the Census. Census 2000 Summary File 1 (SF 1) 100-Percent Data. U.S. Census Bureau; 2000. DP-1. Profile of General Demographic Characteristics: 2000. [Google Scholar]
- 13.Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20:2109–17. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 14.Elliott TE, Elliott BA, Renier CM, Haller IV. Rural-urban differences in cancer care: results from the Lake Superior Rural Cancer Care Project. Minn Med. 2004;87:44–50. [PubMed] [Google Scholar]
- 15.Klabunde CN, Springer BC, Butler B, White MS, Atkins J. Factors influencing enrollment in clinical trials for cancer treatment. South Med J. 1999;92:1189–93. doi: 10.1097/00007611-199912000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Ford JG, Howerton MW, Bolen S, et al. Knowledge and access to information on recruitment of underrepresented populations to cancer clinical trials. Evid Rep Technol Assess (Summ) 2005:1–11. doi: 10.1037/e439572005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tournoux C, Katsahian S, Chevret S, Levy V. Factors influencing inclusion of patients with malignancies in clinical trials. Cancer. 2006;106:258–70. doi: 10.1002/cncr.21613. [DOI] [PubMed] [Google Scholar]
- 18.Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112:228–42. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 19.Adams-Campbell LL, Ahaghotu C, Gaskins M, et al. Enrollment of African Americans onto clinical treatment trials: study design barriers. J Clin Oncol. 2004;22:730–4. doi: 10.1200/JCO.2004.03.160. [DOI] [PubMed] [Google Scholar]
- 20.Holcombe RF, Jacobson J, Li A, Moinpour CM. Inclusion of black Americans in oncology clinical trials: the Louisiana State University Medical Center experience. Am J Clin Oncol. 1999;22:18–21. doi: 10.1097/00000421-199902000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Baquet CR, Commiskey P, Daniel Mullins C, Mishra SI. Recruitment and participation in clinical trials: socio-demographic, rural/urban, and health care access predictors. Cancer Detect Prev. 2006;30:24–33. doi: 10.1016/j.cdp.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson SB, Ashley M, Haynes MA. Attitude of African-Americans regarding prostate cancer clinical trials. J Community Health. 1996;21:77–87. doi: 10.1007/BF01682300. [DOI] [PubMed] [Google Scholar]
- 23.Coyne CA, Demian-Popescu C, Brown P. Rural cancer patients’ perspectives on clinical trials: a qualitative study. J Cancer Educ. 2004;19:165–9. doi: 10.1207/s15430154jce1903_11. [DOI] [PubMed] [Google Scholar]
- 24.Brown DR, Fouad MN, Basen-Engquist K, Tortolero-Luna G. Recruitment and retention of minority women in cancer screening, prevention, and treatment trials. Annals of Epidemiology. 2000;10:S13–S21. doi: 10.1016/s1047-2797(00)00197-6. [DOI] [PubMed] [Google Scholar]
- 25.Pinto HA, McCaskill-Stevens W, Wolfe P, Marcus AC. Physician perspectives on increasing minorities in cancer clinical trials: an Eastern Cooperative Oncology Group (ECOG) Initiative. Ann Epidemiol. 2000;10:S78–S84. doi: 10.1016/s1047-2797(00)00191-5. [DOI] [PubMed] [Google Scholar]
- 26.Advani AS, Atkeson B, Brown CL, et al. Barriers to the participation of African-American patients with cancer in clinical trials: a pilot study. Cancer. 2003;97:1499–506. doi: 10.1002/cncr.11213. [DOI] [PubMed] [Google Scholar]
- 27.Outlaw FH, Bourjolly JN, Barg FK. A study on recruitment of black Americans into clinical trials through a cultural competence lens. Cancer Nurs. 2000;23:444–51. doi: 10.1097/00002820-200012000-00006. quiz 51–2. [DOI] [PubMed] [Google Scholar]
- 28.Hudson SV, Momperousse D, Leventhal H. Physician perspectives on cancer clinical trials and barriers to minority recruitment. Cancer Control. 2005;12:S93–S6. doi: 10.1177/1073274805012004S14. [DOI] [PubMed] [Google Scholar]
- 29.Giuliano AR, Mokuau N, Hughes C, et al. Participation of minorities in cancer research: The influence of structural, cultural, and linguistic factors. Annals of Epidemiology. 2000;10:S22–S34. doi: 10.1016/s1047-2797(00)00195-2. [DOI] [PubMed] [Google Scholar]
- 30.Linden HM, Reisch LM, Hart A, Jr, et al. Attitudes toward participation in breast cancer randomized clinical trials in the African American community: a focus group study. Cancer Nurs. 2007;30:261–9. doi: 10.1097/01.NCC.0000281732.02738.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Ann Epidemiol. 2002;12:248–56. doi: 10.1016/s1047-2797(01)00265-4. [DOI] [PubMed] [Google Scholar]
- 32.George SL. Reducing patient eligibility criteria in cancer clinical trials. J Clin Oncol. 1996;14:1364–70. doi: 10.1200/JCO.1996.14.4.1364. [DOI] [PubMed] [Google Scholar]
- 33.Fuks A, Weijer C, Freedman B, Shapiro S, Skrutkowska M, Riaz A. A Study in contrasts: Eligibility criteria in a twenty-year sample of NSABP and POG clinical trials. Journal of Clinical Epidemiology. 1998;51:69–79. doi: 10.1016/s0895-4356(97)00240-0. [DOI] [PubMed] [Google Scholar]
- 34.McCabe MS, Varricchio CG, Padberg RM. Efforts to recruit the economically disadvantaged to national clinical trials. Semin Oncol Nurs. 1994;10:123–9. doi: 10.1016/s0749-2081(05)80066-x. [DOI] [PubMed] [Google Scholar]
- 35.Elks ML. The right to participate in research studies. J Lab Clin Med. 1993;122:130–6. [PubMed] [Google Scholar]
- 36.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23:3112–24. doi: 10.1200/JCO.2005.00.141. [DOI] [PubMed] [Google Scholar]
- 37.Swanson GM, Bailar JC., 3rd Selection and description of cancer clinical trials participants--science or happenstance? Cancer. 2002;95:950–9. doi: 10.1002/cncr.10785. [DOI] [PubMed] [Google Scholar]
- 38.Wood CG, Wei SJ, Hampshire MK, Devine PA, Metz JM. The influence of race on the attitudes of radiation oncology patients towards clinical trial enrollment. Am J Clin Oncol. 2006;29:593–9. doi: 10.1097/01.coc.0000236213.61427.84. [DOI] [PubMed] [Google Scholar]
- 39.Fracasso PM, Walker MS, Mathews KJ, et al. Coaching intervention as a strategy for enhancing accrual to phase I/II clinical trials. Journal of Clinical Oncology. 2007 ASCO Annual Meeting Proceedings Part I. 2007;25:S6580. [Google Scholar]
- 40.Fouad MN, Partridge E, Green BL, et al. Minority recruitment in clinical trials: a conference at Tuskegee, researchers and the community. Ann Epidemiol. 2000;10:S35–S40. doi: 10.1016/s1047-2797(00)00199-x. [DOI] [PubMed] [Google Scholar]
- 41.Fouad MN, Partridge E, Wynn T, Green BL, Kohler C, Nagy S. Statewide Tuskegee Alliance for clinical trials. A community coalition to enhance minority participation in medical research. Cancer. 2001;91:237–41. doi: 10.1002/1097-0142(20010101)91:1+<237::aid-cncr11>3.3.co;2-l. [DOI] [PubMed] [Google Scholar]
- 42.Lai GY, Gary TL, Tilburt J, et al. Effectiveness of strategies to recruit underrepresented populations into cancer clinical trials. Clin Trials. 2006;3:133–41. doi: 10.1191/1740774506cn143oa. [DOI] [PubMed] [Google Scholar]
- 43.Sorensen G, Emmons K, Hunt MK, et al. Model for incorporating social context in health behavior interventions: applications for cancer prevention for working-class, multiethnic populations. Prev Med. 2003;37:188–97. doi: 10.1016/s0091-7435(03)00111-7. [DOI] [PubMed] [Google Scholar]
- 44.Emmons K. Behavioral and Social Science Contributions to the Health of Adults in the United States. In: Smedley BD, Syme SL, editors. Promoting Health: Intervention Strategies from Social and Behavioral Research. Washington DC: The National Academies Press; 2000. pp. 254–321. [PubMed] [Google Scholar]
- 45.Stetler CB, Mittman BS, Francis J. Overview of the VA Quality Enhancement Research Initiative (QUERI) and QUERI theme articles: QUERI Series. Implement Sci. 2008;3:8. doi: 10.1186/1748-5908-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stetler CB, McQueen L, Demakis J, Mittman BS. An organizational framework and strategic implementation for system-level change to enhance research-based practice: QUERI Series. Implement Sci. 2008;3:30. doi: 10.1186/1748-5908-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feussner JR, Kizer KW, Demakis JG. The Quality Enhancement Research Initiative (QUERI): from evidence to action. Med Care. 2000;38:I1–6. doi: 10.1097/00005650-200006001-00001. [DOI] [PubMed] [Google Scholar]
- 48.Siteman Cancer Center. Institutional data safety and monitoring plan. St. Louis: Siteman Cancer Center; 2011. pp. Institutional Data Safety and Monitoring Plan (PDF v 5.05 7/2011) [Google Scholar]
- 49.Percy C, Fritz A, Jack A, et al. International classification of diseases for oncology (ICD-O) 3. Geneva Switzerland: World Health Organization; 1976. (ICD-O-3) pp. Non-serial publication. [Google Scholar]
- 50.Morgenlander KH, Winters SB, Lin CJ, Robertson LB, Heron DE, Herberman RB. Novel method for benchmarking recruitment of African American cancer patients to clinical therapeutic trials. J Clin Oncol. 2008;26:5074–7. doi: 10.1200/JCO.2008.17.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siteman Cancer Center. Monitoring accrual by gender; race and ethnicity. St. Louis: Siteman Cancer Center; 2009. pp. Incidence cancer cases by disease site and demographic information. [Google Scholar]
- 52.Wright JR, Bouma S, Dayes I, et al. The importance of reporting patient recruitment details in phase III trials. J Clin Oncol. 2006;24:843–5. doi: 10.1200/JCO.2005.02.6005. [DOI] [PubMed] [Google Scholar]
- 53.Corbie-Smith G, St George DM, Moody-Ayers S, Ransohoff DF. Adequacy of reporting race/ethnicity in clinical trials in areas of health disparities. J Clin Epidemiol. 2003;56:416–20. doi: 10.1016/s0895-4356(03)00031-3. [DOI] [PubMed] [Google Scholar]
- 54.Committee on Assuring the Health of the Public in the 21st Century, Board on Health Promotion and Disease Prevention, Institute of Medicine of the National Academies. The Future of the Public’s Health in the 21st Century. Washington DC: The National Academies Press; 2002. [Google Scholar]
- 55.Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1:2–4. [PubMed] [Google Scholar]