Abstract
Non-coding RNAs include small transcripts, such as microRNAs and piwi-interacting RNAs, and a wide range of long non-coding RNAs (lncRNAs). Although many lncRNAs have been identified, only a small number of lncRNAs have been characterized functionally. Here, we sought to identify lncRNAs differentially expressed during replicative senescence. We compared lncRNAs expressed in proliferating, early passage, ‘young’ human diploid WI-38 fibroblasts [population doubling (PDL) 20] with those expressed in senescent, late-passage, ‘old’ fibroblasts (PDL 52) by RNA sequencing (RNA-Seq). Numerous transcripts in all lncRNA groups (antisense lncRNAs, pseudogene-encoded lncRNAs, previously described lncRNAs and novel lncRNAs) were validated using reverse transcription (RT) and real-time, quantitative (q)PCR. Among the novel senescence-associated lncRNAs (SAL-RNAs) showing lower abundance in senescent cells, SAL-RNA1 (XLOC_023166) was found to delay senescence, since reducing SAL-RNA1 levels enhanced the appearance of phenotypic traits of senescence, including an enlarged morphology, positive β-galactosidase activity, and heightened p53 levels. Our results reveal that the expression of known and novel lncRNAs changes with senescence and suggest that SAL-RNAs play direct regulatory roles in this important cellular process.
Keywords: post-transcriptional gene regulation, transcriptome, non-coding, proliferation, senescence-associated gene expression patterns
INTRODUCTION
Untransformed cells divide in culture for a limited number of times, after which they cease proliferation and enter a state of long-term growth arrest named senescence (Hayflick, 1965). Cellular senescence can be triggered through two closely related processes: by telomere erosion (replicative senescence) and by exposure to damaging conditions (stress-induced or premature senescence) (Kuilman et al., 2010). Although senescent cells do not divide, they can remain viable and metabolically active for a long time and often display a flat, enlarged morphology, and elevated activity of lysosomal β-galactosidase activity (Kuilman et al., 2010). In addition, senescent cells exhibit nuclear senescence-associated heterochromatic foci (SAHF), cytoplasmic vacuoles, and enhanced autophagy (Campisi 2005; Gorospe & Abdelmohsen, 2011). Many senescent cells also display a characteristic senescence-associated secretory phenotype (SASP) (Rodier & Campisi, 2011; Tominaga et al., 2012); among the secreted factors are numerous cytokines and chemokines [e.g., granulocyte/macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-6, IL-8, and IL-1α] and several matrix metalloproteases (e.g., MMP-1, and MMP-3). In addition, senescent cells often different types of oxidative and genotoxic damage; accordingly, they frequently express DNA damage-response proteins such as γ-H2AX, NBS1, MDC1, and 53BP1. Finally, cellular senescence is characterized by the presence of factors that govern tumor suppression networks, including the p53/p21 and the pRB/p16 tumor suppressor pathways (Kuilman et al., 2010).
Although cellular senescence has been studied most extensively in cultured cells, there is broad appreciation that cellular senescence occurs in vivo. Since senescent cells accumulate in tissues as the organism ages, their metabolic behavior and their signature gene expression profile have been linked to a number of age-related physiologic and pathologic changes (Tyner et al., 2002; Baker et al., 2011). Among the age-related changes, the influence of senescence on carcinogenesis has been studied most extensively; it has revealed that senescence constitutes a strong tumor suppressive mechanism, although in some instances it can enhance different carcinogenic traits (e.g., tissue invasion) (Ohtani et al., 2012). The influence of senescence on other diseases has also been recognized, particularly in pathologies associated with the chronic inflammatory phenotype seen in the elderly, like diabetes, cardiovascular disease, neurodegeneration, and declined immune function (Kuilman et al., 2008; Freund et al., 2010; Luo et al., 2010; Gorospe & Abdelmohsen, 2011).
Given the impact of cellular senescence in age-associated processes, there is much interest in understanding how to modulate senescence for therapeutic purposes. A number of transcription factors have been implicated in driving senescence, including p53, and proteins in the (AP)-1, E2F, Id and Ets families. In addition, two prominent classes of post-transcriptional regulators of senescence have emerged in recent years. The first comprises RNA-binding proteins (RBP), such as human antigen R (HuR), AU-binding factor 1 (AUF1) and tristetraprolin (TTP), which associate with target mRNAs that encode senescence factors and influence cellular senescence (Abdelmohsen et al., 2008; Sanduja et al., 2009; Fabian et al., 2010; Bartel et al., 2009). The second comprises miRNAs, a large group of small (~22 nt), non-coding RNAs that are differentially expressed during senescence and control numerous senescence traits in cultured cells as well as in animal models (Lal et al., 2008; Maes et al., 2009; Marasa et al., 2010; Bonifacio & Jarstfer, 2010; Wang et al., 2010; reviewed in Gorospe & Abdelmohsen, 2011).
In addition to these two groups, long non-coding (lnc)RNA molecules (generally larger than 200 nucleotides), are increasingly recognized as a vast class of regulators of gene expression. LncRNAs comprise a highly heterogeneous group of transcripts with a wide range of sizes, structures, and subcellular locations. Accordingly, the emerging examples of lncRNA-controlled gene expression are presenting a complex spectrum of gene regulatory functions. LncRNAs can affect both transcriptional processes (including epigenetic activities) and post-transcriptional events (Lee et al., 2012; Yoon et al., 2012; reviewed by Schonrock et al., 2012). Among the transcriptional regulatory functions, lncRNAs can target chromatin remodeling factors, coactivate or co-repress transcription factors, modulate the structure/function of bound proteins, form triple helices of DNA:RNA, and have scaffold or decoy functions affecting gene transcription (as seen for lncRNAs NEAT1, ANRIL, GAS5, HOTAIR, MALAT1) (Lee et al., 2012). Among the post-transcriptional functions, lncRNAs that base-pair with mRNAs can modulate the translation and/or the stability of target mRNAs [e.g., 1/2-sbsRNAs, lincRNA-p21 (Gong and Maquat 2011; Yoon et al., 2012)] while lncRNAs that do not base-pair can affect precursor mRNA splicing and translation by acting as cofactors, competitors or decoys of RBPs or microRNAs [e.g., lincMD1 (Cesana et al., 2011)]. The lncRNA-modulated transcriptional and post-transcriptional mechanisms elicit differentiation, proliferation, and cell viability programs, highlighting the rising interest in the roles of lncRNAs in cell function, physiology and pathology. As reviewed comprehensively elsewhere (Wapinski & Chang, 2011; Harries et al., 2012; Yan & Wang, 2012), the physiologic functions of lncRNAs include embryogenesis, cell proliferation, chromosomal imprinting (X chromosome inactivation), while pathologies implicating lncRNA function include cancer, neurodegeneration, immune dysfunction, and cardiovascular and metabolic diseases.
Despite rapidly rising interest in the expression and function of lncRNAs, their possible implication in senescence remain virtually unexplored. Therefore, we sought to identify senescence-associated lncRNAs (SAL-RNAs) using the fetal lung-derived WI-38 human diploid fibroblasts (HDFs) as model system (Wang et al., 2001). Early-passage (proliferating, ‘P’) WI-38 cells can undergo a number of population doublings (PDLs) until they become terminally arrested (senescent, ‘S’). Comparison of the patterns of expressed transcripts by deep sequencing of RNA expressed in proliferating and senescent fibroblasts (RNA-Seq analysis) revealed numerous SAL-RNAs previously annotated, including antisense transcripts, pseudogene-encoded transcripts, and other known lncRNAs, as well as many novel lncRNAs that were differentially expressed in the senescent population. We provide a comprehensive account of previously known SAL-RNAs and novel SAL-RNAs differentially expressed in senescent fibroblasts. Among the novel SAL-RNAs, we identified several transcripts that modulate the onset of senescence and protect senescent cell viability.
RESULTS
Proliferating and senescent WI-38 fibroblasts express different levels of antisense lncRNAs
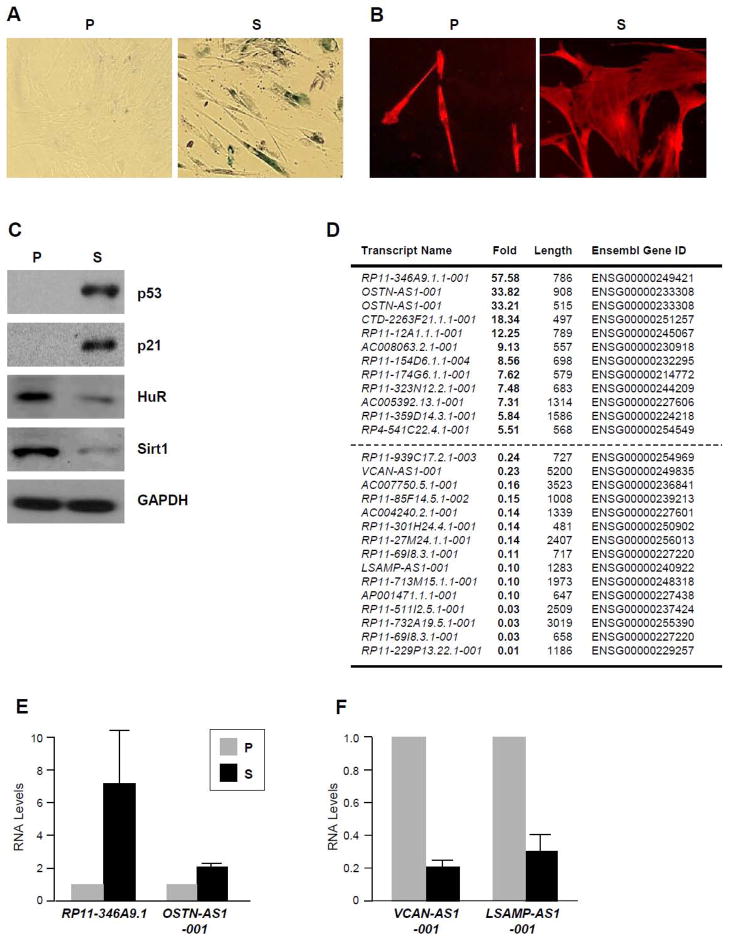
Compared with proliferating (P), early-passage [population doubling (PDL) 22], senescent (S, PDL 52) WI-38 HDFs displayed a flattened and enlarged morphology and increased senescence-associated β-galactosidase (SA-βgal) activity, a widely used senescence marker (Debacq-Chainiaux et al., 2009) (Fig. 1A). The flattened and enlarged morphology was also visualized by using rhodamine phalloidin, which stains filamentous (F-)actin (Fig. 1B). Western blot analysis revealed that S WI-38 cells expressed lower levels of Sirt1 and HuR, while they expressed higher levels of p21 and p53 (Fig. 1C), as previously reported (Marasa et al., 2010). RNA-Seq analysis was performed using these cell populations and the subsequent characterization was focused on differentially expressed, senescence-associated lncRNAs (SAL-RNAs).
Figure 1. Characterization of proliferating and senescent WI-38 human diploid fibroblasts.
(A) Micrographs to visualize senescence-associated β-galactosidase activity in early-passage, proliferating (P) [population doubling (PDL) 20], and late-passage, senescent (S) (PDL 52) WI-38 cells. (B) Detection of F-actin to visualize the cytoskeleton using rhodamine phalloidin in fixed and permeabilized WI-38 cells. (C) Western blot analysis of the levels of p21, p53, HuR, Sirt1, and loading control β-Actin in P and S cells. The even loading and transfer of the samples was confirmed by Coomassie blue and Ponceau red staining of the gel and membrane, respectively (not shown). (D) Naturally occurring antisense transcripts (NA) lncRNAs differentially expressed in P relative to S WI-38 fibroblasts, as identified by using RNA-Seq analysis; NA-SAL-RNAs elevated in S cells (top) and in P cells (bottom) are listed. (E,F) Individual validation of NA-SAL-RNAs displaying higher abundance in S cells (E) or in P cells (F).
Among the differentially expressed lncRNAs were a number of naturally occurring antisense (NA) lncRNAs. Listed in Fig. 1D (and supplemental Table S1) are WI-38 NA-SAL-RNAs showing >5-fold higher in S compared with P (top) and those showing >4-fold higher in P relative to S (bottom). These differences were validated for a handful of NA-SAL-RNAs by reverse transcription (RT) using random hexamers and real-time, quantitative (q)PCR amplification using transcript-specific primer pairs. Validated NA-SAL-RNAs showing higher expression levels in senescent cells (Fig. 1E) included RP11-346A9.1, targeting the metallopeptidase ADAMTS19, and OSTN-AS1-001, complementary to the mRNA encoding the secreted protein Osteocrin. Validated NA-SAL-RNAs exhibiting lower abundance in senescent cells (Fig. 1F) included VCAN-AS1-001, complementary to Versican mRNA (encoding an extracellular matrix proteoglycan) and LSAMP-AS1-001, antisense to the mRNA encoding a limbic system-associated membrane protein (LSAMP). In several instances, the levels of the NA-SAL-RNA and the complementary mRNA showed positive correlation (not shown); whether this finding reflects a positive influence of one transcript upon the other or simply joint transcriptional control awaits further investigation. In a different model of senescence, achieved by exposure of WI-38 fibroblasts to ionizing radiation (IR, 10 Gray) followed by culture for an additional 10 days (Fig. S1A), expression of NA-SAL-RNAs followed a similar trend, showing elevated levels of RP11-346A9.1 and OSTN-AS1-001 and reduced levels of VCAN-AS1-001 and LSAMP-AS1-001 in senescent (IR-treated) cells (Fig. S1B,C). These results indicate that the changes in lncRNA expression are not limited to the replicative exhaustion model (P and S fibroblasts).
Pseudogene-encoded and additional lncRNAs are differentially expressed with senescence
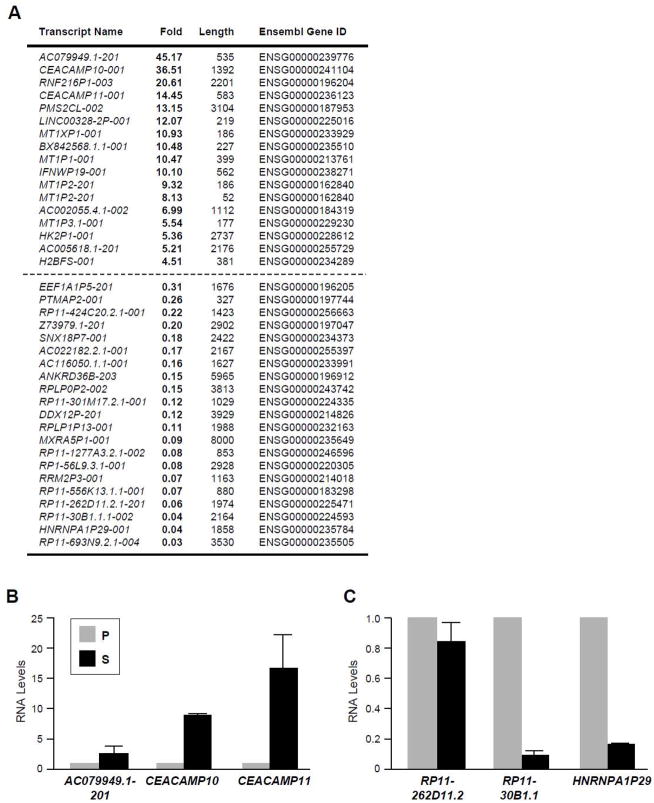
A number of lncRNAs encoded by pseudogenes were also found to be differentially expressed in senescent compared with proliferating WI-38 cells (Fig. 2A, supplemental Table S2). Validation of a subset of pseudogene-encoded (PE) transcripts using RT-qPCR analysis is shown in Fig. 2B (higher in senescent cells) and in Fig. 2C (higher in proliferating cells). Validated upregulated PE-SAL-RNAs include the carcinoembryonic antigen-related cell adhesion molecules CEACAMP10 and CEACAMP11, which are highly expressed in solid tumors, down in senescent cells (Fig. 2B). Among the downregulated PE-SAL-RNAs are transcripts expressed from the ribosomal protein L21 pseudogene and from the heterogeneous nuclear ribonucleoprotein A1 (HNRNPA1) pseudogene (high in cancer cells, low in senescent cells). In IR-induced fibroblast senescence, the relative expression levels of PE-SAL-RNAs in IR-treated relative to untreated fibroblasts was the same as that seen in S relative to P fibroblasts (Fig. S1D,E); in a different cell model of replicative senescence (early-passage IMR-90 and late-passage IMR-90), PE-SAL-RNAs showed a similar trend (Fig. S2A,B).
Figure 2. Pseudogene-encoded transcripts differentially expressed in proliferating and senescent WI-38 fibroblasts.
(A) Pseudogene-encoded (PE)-SAL-RNAs transcript lncRNAs differentially expressed in P relative to S WI-38 fibroblasts, as identified by using RNA-Seq analysis; PE-SAL-RNAs elevated in S cells (top) and in P cells (bottom) are listed. (B,C) Individual validation of PE-SAL-RNAs displaying higher abundance in S cells (B) or in P cells (C).
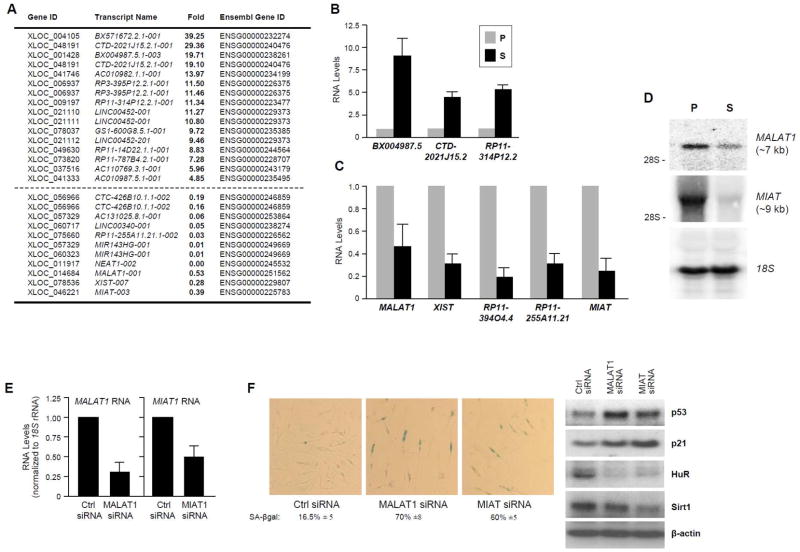
Other well-annotated lncRNAs are listed in Fig. 3A (and supplemental Table S3). Validation of the differential expression in S cells for a subset of them is shown. BX004987.5, CTD-2021J15.2, and RP11-314P12.2 were elevated with senescence (Fig. 3B) and MALAT1, XIST, RP11-394O4.4, RP11-255A11.21, and MIAT (also known as GOMAFU) were lower with senescence (Fig. 3C). Since the magnitude of changes in RNA levels differed between the RNA-Seq and RT-qPCR analyses, we assayed lncRNA changes by Northern blot analysis. As shown, MALAT1 and MIAT were indeed reduced in S relative to P cells (Fig. 3D). As above, in WI-38 fibroblasts rendered senescent by DNA damage (IR) and in S and P IMR-90 fibroblasts, the relative expression levels of several previously reported SAL-RNAs largely recapitulated the trend seen in P and S fibroblasts (Fig. S1F,G and Fig. S2C,D).
Figure 3. Known lncRNAs differentially expressed in proliferating and senescent WI-38 fibroblasts.
(A) LncRNAs differentially expressed in P relative to S WI-38 fibroblasts, identified by using RNA-Seq analysis; lncRNAs elevated in S cells (top) and in P cells (bottom) are listed. (B,C) Individual validation of lncRNAs displaying higher abundance in S cells (B) or in P cells (C). (D) Northern blot analysis of the levels of lncRNAs MALAT1, MIAT, and loading control 18S rRNA; the position of 28S rRNA is indicated for size reference. (E,F) Ten days after transfection with siRNAs to silence MALAT1 or MIAT, the levels of remaining lncRNA were monitored by RT-qPCR analysis (E), and the senescent phenotype was assessed by assessing SA-βgal-positive cells (quantified below) and protein senescence markers p21 and p53 (upregulated), as well as HuR and Sirt1 (downregulated) (F).
The impact of these two lncRNAs, MALAT1 and MIAT, was tested by lowering their abundance in early-passage (~PDL 22) HDFs; 5 days after transfection, cells were split and re-transfected to ensure the downregulation of the non-coding RNA. As shown in Fig. 3E, by 10 days of silencing MALAT1 or MIAT using small interfering (si)RNAs directed to the respective RNAs, which reduced their levels by 70% or 50%, respectively (Fig. 3E), cultures displayed an increase in the numbers of senescent, SA-βgal positive cells (Fig. 3F, left) and changes in protein markers of senescence [p53 and p21 were upregulated, HuR and Sirt1 were downregulated (Fig. 3F, right)]. These results suggest that the reduction in some of these SAL-RNAs could directly contribute to implementing the senescent phenotype.
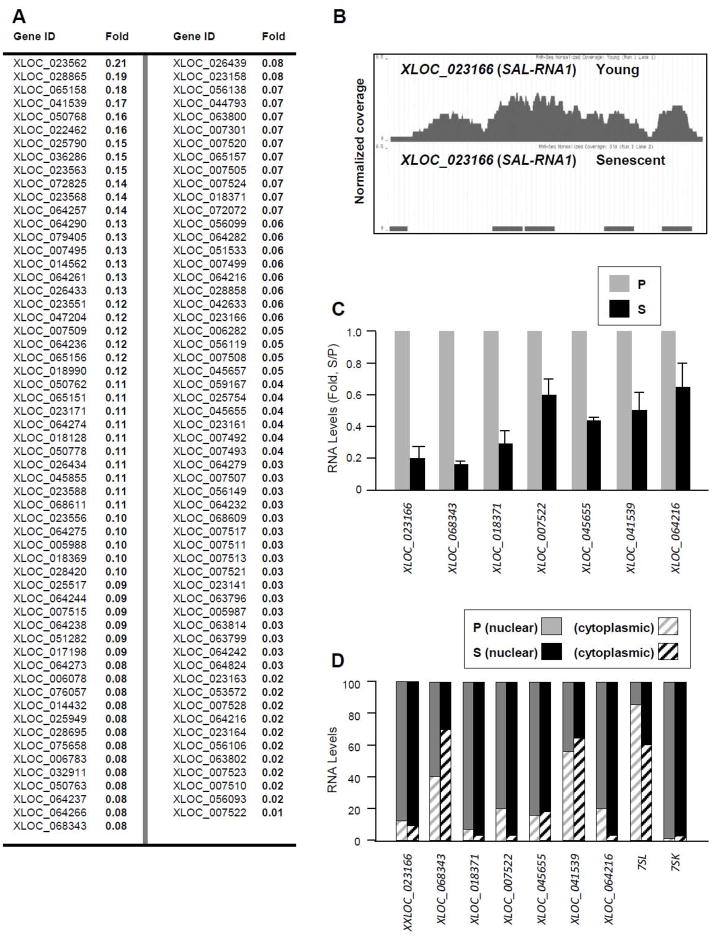
Identification of novel SAL-RNAs, both higher and lower in senescent cells
A number of novel SAL-RNAs were also identified in the RNA-Seq dataset as being upregulated in proliferating fibroblasts (Fig. 4A; supplemental Table S4); among them, an example of the RNA-Seq coverage plot (XLOC_023166; ‘SAL-RNA1’) and several validated transcripts of novel senescence-reduced lncRNAs are shown (Fig. 4B,C). Analysis of the subcellular distribution of these senescence-downregulated SAL-RNAs by RT-qPCR analysis indicates that several of the transcripts were predominantly nuclear (e.g., XXLOC_023166, XLOC_018371, XLOC_007522, XLOC_045655, and XLOC_064216), while others (XLOC_068343 and XLOC_041539) showed extensive presence in both the nucleus and the cytoplasm, with modest change in their subcellular distribution in senescence (Fig. 4D); the predominantly cytoplasmic lncRNA 7SL and nuclear lncRNA 7SK were included as controls in the fractionation experiment.
Figure 4. Novel SAL-RNAs preferentially expressed in proliferating WI-38 fibroblasts.
(A) SAL-RNAs more abundant in P than in S WI-38 fibroblasts, identified by using RNA-Seq analysis. (B) Example of a novel SAL-RNA discovered by RNA-Seq analysis of WI-38 fibroblasts; coverage plot illustrating the increased presence of XLOC_023166 (SAL-RNA1) in P compared with S cells. (C) Validation of seven novel SAL-RNAs preferentially expressed in P cells. (D) Relative abundance in nucleus and cytoplasm of the SAL-RNAs measured in (C). The mainly cytoplasmic lncRNA 7SL and the mainly nuclear lncRNA 7SK were included as controls for sample fractionation.
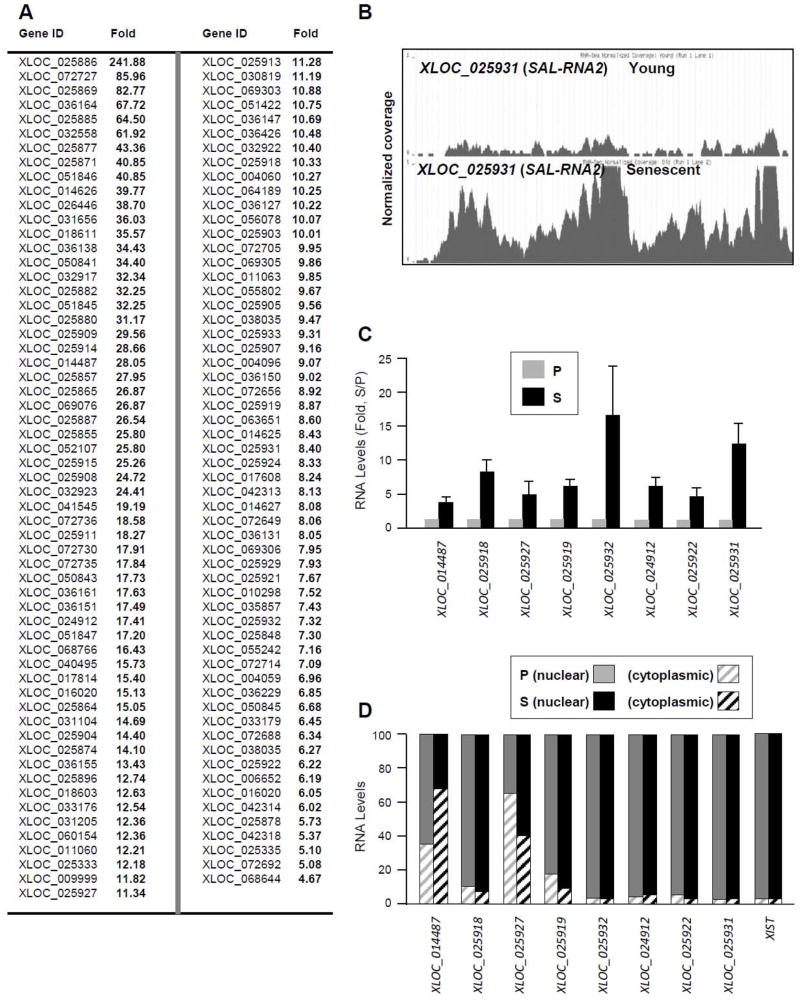
A comparably long list of SAL-RNAs was found to be upregulated in senescent fibroblasts (Fig. 5A, supplemental Table S5). A coverage plot for one such SAL-RNA showing higher levels in senescent cells (XLOC_025931; ‘SAL-RNA2’) and a number of validated SAL-RNAs are shown (Fig. 5B,C). Many validated lncRNAs in this group showed a primarily nuclear presence (e.g., XLOC_025932, XLOC_024912, XLOC_025922, XLOC_025931), but most of them are found in both the nucleus and the cytoplasm, with modest changes in the subcellular distribution during senescence. The nuclear lncRNA XIST was included as a control. Analysis in other models of senescence, the aforementioned IR-treated (senescent) versus untreated (proliferating) WI-38 fibroblasts and IMR-90 fibroblasts at early (proliferating, PDL 25) and late (senescent, PDL 49), indicated that the trends of expression of novel upregulated and downregulated SAL-RNAs (Fig. 4,5) were also conserved among senescence models (Fig. S3). Limited SAL-RNA analysis by Northern blotting is shown in Fig. S4. In sum, our results suggest that the RNA-Seq approach effectively identifies lncRNAs differentially expressed during senescence (Fig. 5D).
Figure 5. Novel SAL-RNAs preferentially expressed in senescent WI-38 fibroblasts.
(A) SAL-RNAs more abundant in S than in P WI-38 fibroblasts, identified by using RNA-Seq analysis. (B) Example of a novel lncRNA discovered by RNA-Seq analysis of WI-38 fibroblasts; coverage plot illustrating the increased presence of XLOC_025931 (SAL-RNA2) in S compared with P cells. (C) Validation of seven novel SAL-RNAs preferentially expressed in S cells. (D) Relative abundance in nucleus and cytoplasm of the SAL-RNAs measured in (C). The mainly nuclear lncRNA XIST was included as a control for sample fractionation.
Modulation of cell fate by SAL-RNAs
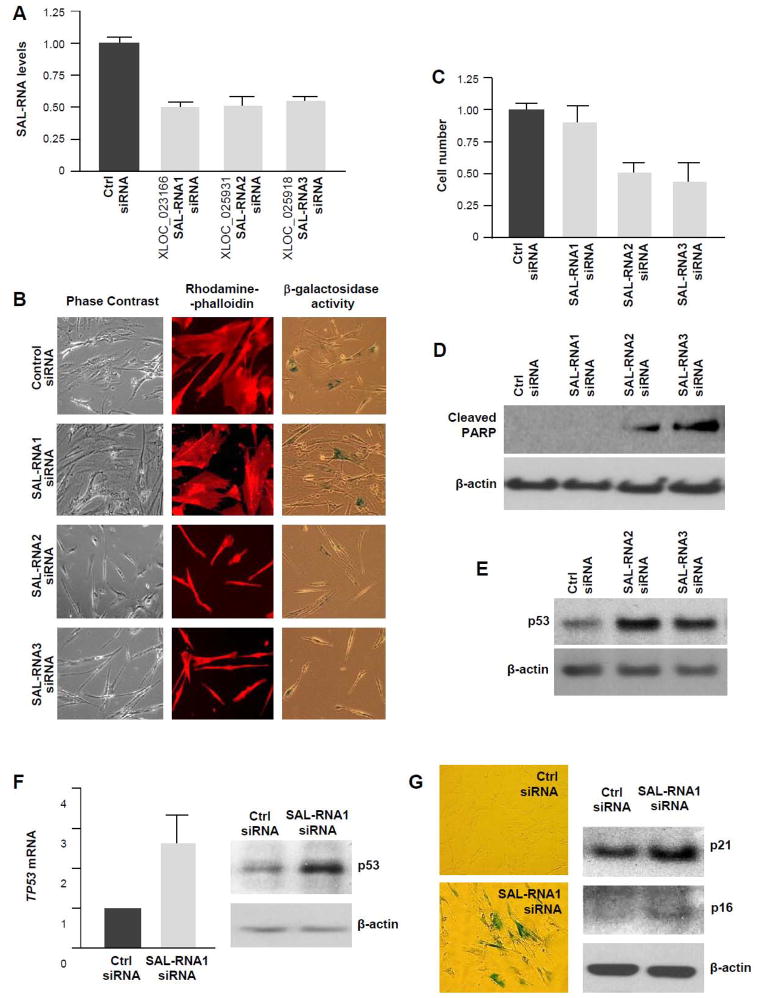
In order to begin to study if SAL-RNAs affect any aspect of cellular senescence (e.g., proliferation, survival, gene expression, SA-βgal activity), we designed siRNAs directed at SAL-RNA1, SAL-RNA2, or SAL-RNA3 (XLOC_ 025918, all three sequences in supplemental Fig. S5), which achieved reductions to ~50% of the original transcript concentration by 5 days after transfection (Fig. 6A). Other lncRNAs tested (XLOC_ 025932 and XLOC_ 018371) did not achieve sufficient silencing (not shown). As shown in Fig. 6B, by 5 days after transfecting senescent fibroblasts with the siRNAs, silencing SAL-RNA2 or SAL-RNA3 caused changes in cell morphology (Fig. 6B) and lowered cell numbers significantly (Fig. 6C). These changes were consistent with increased apoptosis, which was confirmed by the enhanced levels of cleaved PARP [Poly (ADP-ribose) polymerase], a marker of apoptosis (Fig. 6D) and by the increased expression of p53 (Fig. 6E). By contrast, 5 days after silencing SAL-RNA1, cells still displayed a senescent phenotype and cell numbers did not decline compared with the control group (Fig. 6B,C); no PARP cleavage was seen in these cells, underscoring the absence of apoptosis in this transfection group (Fig. 6D). However, the levels of p53 protein and mRNA also rose in early-passage fibroblasts 10 days after transfection with SAL-RNA1-directed siRNA (Fig. 6F); interestingly, in these fibroblasts, silencing SAL-RNA1 elicited a senescent phenotype, as revealed by the enhanced expression of senescence protein markers p21 and p16 and the accumulation of SA-βgal-positive cells (Fig. 6G). Together, these results indicate that SAL-RNA2 and SAL-RNA3 (XLOC_ 025931 and XLOC_ 025918) likely protect the survival of senescent fibroblasts, while SAL-RNA1 (XLOC_023166) helps to prevent the untimely onset of senescence.
Figure 6. Novel SAL-RNAs preferentially expressed in senescent WI-38 fibroblasts.
(A) Degree of silencing achieved 5 days after transfecting WI-38 cells with siRNAs directed at SAL-RNA1, SAL-RNA2, and SAL-RNA3, as assessed by RT-qPCR analysis. (B–D) In cells treated as explained in (A), F-actin was detected using rhodamine phalloidin in order to visualize the cytoskeleton (B), SA-βgal activity was assessed in order to monitor cellular senescence (B), proliferation was measured by monitoring changes in cell number (C) and apoptosis was examined by measuring the level of cleaved PARP using Western blot analysis (D). (E) Western blot analysis to detect the levels of p53 in WI-38 10 days after silencing SAL-RNAs preferentially expressed in senescent cells. (F,G) By 10 days after transfecting WI-38 fibroblasts (P), with SAL-RNA1-directed siRNA, the levels of TP53 mRNA and p53 were measured by RT-qPCR and Western blot analyses, respectively (F), and senescent cells were visualized by assessing SA-βgal-positive cells (G, left) and protein senescence markers p21 and p53 (upregulated), as well as HuR and Sirt1 (downregulated) (G, right).
DISCUSSION
We have used RNA-Seq to identify lncRNAs differentially expressed in senescent relative to proliferating human diploid fibroblasts. The collections of lncRNAs showing altered levels included several antisense transcripts (NA-SAL-RNAs), several pseudogene-encoded (PE-SAL-RNAs), and previously described lncRNAs (Figs. 1–3; Figs. S1–S3). Numerous novel lncRNAs were also found, both increased and decreased with replicative senescence (Figs. 4,5). Among this group, three randomly chosen SAL-RNAs were found to affect the fate of the cell. While lowering each of the three triggered an increase in p53 levels, only two of them (SAL-RNA2 and SAL-RNA3) were implicated in cell survival, while the third (SAL-RNA1) delayed senescence.
At present, it is unclear how many SAL-RNAs influence cellular senescence and what their relative impact is. Some SAL-RNAs likely do not affect directly the senescent phenotype. However, many other SAL-RNAs likely affect one or another senescence trait, including terminal growth arrest, cell survival, autophagy, DNA damage and repair, and senescence-associated protein expression patterns. Additional experiments are needed in order to establish which SAL-RNAs have stronger impact upon the process of cellular senescence and through which senescence trait. In this regard, SAL-RNAs are expected to function in coordination with other proteins (for example, RBPs and transcriptional regulators) and with other ncRNAs in order to control the aforementioned aspects of the senescence program. However, the most likely influence of SAL-RNAs is on senescence-associated gene expression programs. The specific gene expression pathways affected by the SAL-RNAs are unknown, but likely include those reported for other lncRNAs (Wapinski and Chang, 2011; Harries et al., 2012; Yoon et al., 2012), such as changes in chromatin structure, transcription factor activity, post-transcriptional gene regulation, and perhaps also post-translational gene regulatory processes. In a recent example of the latter function, the lncRNA MALAT1 was reportedly reduced in senescent fibroblasts, in keeping with the observation that lowering MALAT1 triggered cellular senescence in fibroblasts (Tripathi et al., 2013). This effect was attributed in part to the decline in the oncogenic transcription factor b-Myb/Mybl2 caused by aberrant B-MYB pre-mRNA splicing when MALAT1 levels were low. In turn, the reduced levels of b-Myb reduced the progression of cells through the G2/M cell cycle compartments and promoted cellular senescence (Tripathi et al., 2013). It was interesting to note that longer lncRNAs in all groups (including NE-SAL-RNAs, PE-SAL-RNAs and novel and previously reported SAL-RNAs) showed a tendency to be downregulated in S cells, while shorter lncRNAs showed a tendency to be upregulated in S cells. Additional work is needed to fully elucidate the mechanisms that explain this pattern, but it is tempting to speculate that lncRNAs follow the same general rules of stability as eukaryotic mRNAs (e.g., human) and prokaryotic mRNAs (e.g., E. coli), where a negative correlation was noted between long mRNAs and stability (Feng and Niu, 2007). Perhaps RNA endonucleases and/or mechanical damage could also lower the abundance of longer lncRNAs, as proposed by Feng and Niu for mRNAs (2007); in light of our results, this differential stability appears to be more pronounced in senescent cells (Fig. 1D, 2A, 3A, S4, S5).
The results of experiments testing three chosen examples of SAL-RNAs (SAL-RNA1, SAL-RNA2, and SAL-RNA3) indicate that these lncRNAs could have a direct impact on the senescent phenotype by promoting cell survival (SAL-RNA2, SAL-RNA3) and by preventing premature senescence (SAL-RNA1). However, the specific mediators through which they modulate these cellular processes are unknown. The identification of the proteins or RNAs that interact with these transcripts after they are labeled with MS2 RNA or with another RNA tag represents a systematic way forward in this analysis. Such an approach will reveal critical factors that help elicit SAL-RNA function, whether it be one of scaffold for assembly of macromolecular complexes (e.g., chromatin remodeling machinery), competitor of another gene expression factor (e.g., a microRNA), or other regulatory functions. High-throughput analyses to find the specific changes in gene expression programs (e.g., mRNA profiling or protein profiling) taking place after silencing or overexpression of these SAL-RNAs are also important. Down the road, searches for disease-associated mutations in these transcripts, as well as the generation of knockout mice in which these transcripts are deleted may also be potentially informative. Additionally, it will also be important to investigate the mechanisms that control the expression of SAL-RNA1-3, including the transcription factors and post-transcriptional regulators that affect their levels and their subcellular distribution.
Finally, it remains to be studied if the SAL-RNAs identified here in cultured models of cellular senescence are also differentially expressed and influence senescence in vivo in tissues and organs. With increasing appreciation that senescence underlies many of the changes that characterize the aging process (Kuilman et al., 2010; Baker et al., 2011) further studies of the possible implication of SAL-RNAs in age-associated disease are warranted.
EXPERIMENTAL PROCEDURES
Cell culture, transfection, small interfering RNAs and SA-β-galactosidase activity assay
WI-38 human diploid fibroblasts were obtained from Coriell Cell Repositories and in DMEM (Invitrogen) supplemented with 10% (v/v) FBS, antibiotics, and 0.1 mM non-essential amino acids (Invitrogen). All siRNAs were transfected at 20 nM final concentration using Lipofectamine-2000 (Invitrogen); UUCUCCGAACGUGUCACGUdTdT (Ctrl siRNA) was from Ambion. Duplexes (dsiRNA, each sense and antisense, respectively) obtained from Integrated DNA Technologies were used to silence MALAT1 [GGAGCAGAGAGGUAUGGGAAGCAGA and CCUCGUCUCUCCAUACCCUUCGUU], MIAT [CUGUUUAAACAUUUCCACUUGCCAG and GACAAAUUUGUAAAGGUGAACGGUC], XLOC_023166/SAL-RNA1 [CAUGCAUAUCAGCUCAGGUCUUAA and GUACGUAUAGUCAGUCCAGAAUU], XLOC_025931/SAL-RNA2 [UUCUUUCCAGAUUUGUGUCACCUG and AAGAAAGGUCUAAACACAGUGGAG], and XLOC_025918/SAL-RNA3 [UUUUGCUAAGUUCCCACGAUCAGC and AAAACGAUUCAAGGGUGCUAGUCG]. In each case, 5 days after transfection, cells were split and re-transfected to ensure the downregulation of SAL-RNAs. Senescence-associated β-galactosidase activity in WI-38 cells was assessed using a kit from Cell Signaling, following the manufacturer’s protocol.
Western blot analysis
Whole-cell lysates, prepared in RIPA buffer, were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred onto PVDF membranes (Invitrogen iBlot Stack). Incubations with primary antibodies recognizing p21 (Millipore), HuR, Sirt1, p53, p16, cleaved PARP, GAPDH (Santa Cruz Biotech) or β-actin (Abcam), were followed by incubations with the appropriate secondary antibodies conjugated with horseradish peroxidase (HRP; GE Healthcare) and by detection using enhanced luminescence (GE Healthcare).
RNA isolation, RT-qPCR and Sequencing
Trizol (Invitrogen) was used to extract total RNA from young proliferating and senescent cells according to the manufacturer’s protocol. Total RNA was used for gene expression analysis by reverse transcription (RT) followed by quantitative (q)PCR analysis or by RNA deep sequencing (RNA-Seq). RT was performed by using random hexamers and reverse transcriptase (Maxima Reverse Transcriptase, Fermentas) and qPCR was carried out using gene-specific primers and SYBR green master mix (Kapa Biosystems) in an Applied Biosystems 7300 instrument.
For RNA sequencing, total RNA quality and quantity was assessed using the Agilent 2100-Bioanalyzer; 100 ng of RNA was used for first-strand and second-strand cDNA synthesis followed by single-primer isothermal amplification (SPIA) using NuGEN Ovation RNA-Seq System V2 kits according to the manufacturer’s protocol. The kit amplified both polyA-tailed and non-polyA-tailed RNA and removed ribosomal RNA. The amplified cDNA was sheared using Bioruptor (Diagenode) to an average size of 250–450 bases. The sequencing library was prepared using Illumina ChIp-Seq kits according to the manufacturer’s protocol (Illumina, San Diego, CA). In short, the ends of the fragments were repaired using T4 DNA polymerase, E. coli DNA Pol I large fragment (Klenow polymerase), and T4 polynucleotide kinase (PNK) and adenines were added to the 3′ end. Adapters were ligated to the DNA fragments, which were size-selected (250–300 bases) after electrophoresis through a 4% agarose gel. Eighteen cycles of PCR amplification was performed, followed by cluster generation and sequencing with Illumina Genome Analyzer (GA-II). Sequencing was performed for 42 cycles and the images generated were analyzed with the Firecrest program followed by base calls using the Bustard program; Firecrest and Bustard are part of the Illumina Analysis Pipeline package.
For RNA-Seq analysis, the quality of the bases was checked using FASTQC program and called bases were aligned to the human HG19 genome using the Tophat program, the Bowtie1 algorithm, and Ensembl hg19 (v62) as gene model annotations followed by genomic mapping (Trapnell et al., 2010). The aligned reads were assembled into transcripts (both known and novel) using Cufflinks program with Ensembl hg19 (v62) transcripts as a guide (Roberts et al., 2011a). FPKM (fragments per kilobase of exon model per million mapped reads) values were calculated after fragment bias correction and normalization to total hits. Significant changes in transcript expression levels were calculated using Cuffdiff program with a cut-off of fdr < 0.1 and minimum number of 5 alignments. Data were visualized in the UCSC genome browser (Roberts et al., 2011b).
Northern blot analysis
Northern blot analysis was performed as previously described (Abdelmohsen et al., 2007). Briefly, whole-cell RNA was isolated by using Trizol (Invitrogen), denatured, size-fractionated using 1.2% agarose-formaldehyde gels, and transferred. Oligonucleotides TTGCCGACCTCACGGATT for MALAT1, CACCAACTCTCCCACTAGGCTATAA for MIAT, and CCAATGGATCCTCGTTAAAGGATTT for 18S rRNA were end-labeled with [α32P]dATP and terminal transferase and used to detect these RNAs.
lncRNA PCR sequencing
PCR sequencing primers TGCATGTGTGTGTGTGTGTG and CTCTGGGAATCTGGAACCAA (forward and reverse, respectively) were used to amplify SAL-RNA1/XLOC-166. The PCR product was resolved by electrophoresis through a 2% agarose gel and the DNA was extracted from the gel using QIAquick Gel Extraction Kit (Qiagen). The purified PCR product (500 ng) was combined with 6 μl of BigDye Terminator v1.1/3.1 Sequencing Buffer, and 3.2 pmol of forward or reverse primer as used for qPCR. The PCR sample was purified and analyzed using an ABI sequencer; sequences were analyzed by an ABI sequence scanner.
Primer pairs for lncRNA RT-qPCR
Primer pairs (all forward and reverse, respectively) were as follows:
CTD-2021J15.2: TGCTCATGACTCCTCTGTGG and GACCAGAAGTCGTGCCTAGC
BX004987.5: CCATCGGCTGTAAACTTGGT and CAGAATATGGGCCAGCCTTA
RP11-314P12.2: CACAGGGAGGATGTGTTGTG and GAGGCTGCTGCAAGTTCAAGGTC
RP11-394O4.2: TGCTTCCAGCACTGATGTTC and CCCCATCCTACCCTTCATTT
RP11-255A11.21: TATGTTGCCACCATCTTGGA and CATAGGCCCTGCAGAAACAT
XLOC_023166/SAL-RNA1: TGCATGTGTGTGTGTGTGTG and CTCTGGGAATCTGGAACCAA
OSTN-AS1-001: AGACGGGGTTTCACCATATT and GGTGCAGTGGTTCACAGTTG
LSAMP-AS1-001: ATGATGAGGCAGCAAGAAGG and ACAGTTCTGGGGTTGTTGGA
VCAN-AS1-001: TGCATTGATCGGTAACCTTATG and AGGCCTGAATGGAGGACTTT
RP11-346A9.1: CTCTGGACAGGCAGAGGACT and CCTAAGGAGCGTGAGGAGTG
AC079949.1: GCCTCGGATACCTCGGATAG and ATGGTTTAGCGCCAGGTTC
RP11-262D11.2: ATGCAAAGAGGGGAAAACCT and TTGGAAGGTGGGTCCTAGTG
RP11-30B1.1: GGCCACATACCTGCAAATCT and GTGCCCTGACCTTGTTTGTT
HNRNPA1P29: TGGTGGCATCAAAGAAAACA and GAATCACGGCCATCAAAAGT
CEACAMP10: TGGGAAAACCCTTGTACCTG and AGGCATGAGCAAGGACAGTT
CEACAMP11: CCCAGGCTAAAGATGTCCAA and GGGTCACTGTGGCTGGTACT
XLOC_068343: CACGTTCACCAAGTCGTCTG and GGAGAGGGGCAGGAATTAAG
XLOC_018371: AGGAGCAAAACCATCAGGAA and GGCAGTGATTGTCTCCGAAT
XLOC_007522: GCCAAGGCCTGTGTAAATGT and CTGGCCTGCTGATTTATGGT
XLOC_045655: CAAGGAAGGGCAGGAGATTA and CTTTTTGGGCAGCTAGCACT
XLOC_041539: GTCTCCAGCATGACGTCTCA and CCAGGCCTTAGGAGACAGG
XLOC_064216: GCTGGAAGCAAGGTTGAGAA and CATGAGGCTCAGTTCTGTCTG
XLOC_014487: CTTCCCCTCAAATCCTGACA and GGACAGAAGAACGGGTGAGA
XLOC_025927: TGATTTCTGGCCATTTCACA and ACACCAGTCCACCTCCTCAC
XLOC_025919: GCACAAATGGAGCAGTCAAG and GACAGGTAGAGGTGCACACG
XLOC_024912: ACAGAGAGCCTCGGTGGAT and AGGGTGCCTACATCCTTGGT
XLOC_025922: GTTGGAGTACAGTGGCATGA and TGGTGGTACACACTTATGGTCCT
TP53: AGGCCTTGGAACTCAAGGAT and TGAGTCAGGCCCTTCTGTCT
7SK: GACGACCATCCCCGATAGAG and GGGAGCGCAGCTACTCGTAT
7SL: CAAAACTCCCGTGCTGATCA and GGCTGGAGTGCAGTGGCTAT
Malat1: GCAGGCGTTGTGCGTAGAG and TTGCCGACCTCACGGATT
Xist: GTGTGTGAGTGTACCTACCGCTTT and CGACTAGCCCTAAGCCGAGTT
GAPDH: ATGGAAATCCCATCACCATCTT and CGCCCCACTTGATTTTGG
MIAT: TCCTGAAACTCCTCTTTGTTTAACTG and CACCAACTCTCCCACTAGGCTATAA
XLOC_025918/SAL-RNA3: ACTGCTGGGATAACGGTGAC and TCTGTGCTCAGCTCTGCAAT
XLOC_025932: TGAAGAAGCAAGAGCCATGA and TGGCACCACTGTGACTTGAT
XLOC_025931/SAL-RNA2: GGATGCTGTGAGCTTTGTGA and GAAACCCCCAGAGCTGAGAC
Supplementary Material
Acknowledgments
This work was supported in its entirety by the NIA-IRP, NIH.
Abbreviations
- CR
coding region
- RBP
RNA-binding protein
- RISC
RNA-induced silencing complex
- RNP
ribonucleoprotein complex
- UTR
untranslated region
- miRNA
microRNA
Footnotes
CONFLICT OF INTEREST
The authors do not have any conflicts of interest.
BIBLIOGRAPHY
- Abdelmohsen K, Kuwano Y, Kim HH, Gorospe M. Posttranscriptional gene regulation by RNA-binding proteins during oxidative stress: implications for cellular senescence. Biol Chem. 2008;389:243–255. doi: 10.1515/BC.2008.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacio LN, Jarstfer MB. MiRNA profile associated with replicative senescence, extended cell culture, and ectopic telomerase expression in human foreskin fibroblasts. PLoS ONE. 2010;5:e12519. doi: 10.1371/journal.pone.0012519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nature Protocols. 2009;4:1798–1806. doi: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]
- Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- Feng L, Niu DK. Relationship between mRNA stability and length: an old question with a new twist. Biochem Genet. 2007;45:131–137. doi: 10.1007/s10528-006-9059-5. [DOI] [PubMed] [Google Scholar]
- Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong C, Maquat LE. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3′ UTRs via Alu elements. Nature. 2011;470:284–288. doi: 10.1038/nature09701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorospe M, Abdelmohsen K. MicroRegulators come of age in senescence. Trends Genet. 2011;27:233–241. doi: 10.1016/j.tig.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries LW. Long non-coding RNAs and human disease. Biochem Soc Trans. 2012;40:902–906. doi: 10.1042/BST20120020. [DOI] [PubMed] [Google Scholar]
- Hayflick L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A, Kim HH, Abdelmohsen K, Kuwano Y, Pullmann R, Jr, Srikantan S, Subrahmanyam R, Martindale JL, Yang X, Ahmed F, Navarro F, Dykxhoorn D, Lieberman J, Gorospe M. p16(INK4a) translation suppressed by miR-24. PLoS ONE. 2008;3:e1864. doi: 10.1371/journal.pone.0001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JT. Epigenetic regulation by long noncoding RNAs. Science. 2012;338:1435–1439. doi: 10.1126/science.1231776. [DOI] [PubMed] [Google Scholar]
- Luo XG, Ding JQ, Chen SD. Microglia in the aging brain: relevance to neurodegeneration. Mol Neurodegener. 2010;5:12. doi: 10.1186/1750-1326-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes OC, Sarojini H, Wang E. Stepwise up-regulation of microRNA expression levels from replicating to reversible and irreversible growth arrest states in WI-38 human fibroblasts. J Cell Physiol. 2009;221:109–119. doi: 10.1002/jcp.21834. [DOI] [PubMed] [Google Scholar]
- Marasa BS, Srikantan S, Martindale JL, Kim MM, Lee EK, Gorospe M, Abdelmohsen K. MicroRNA profiling in human diploid fibroblasts uncovers miR-519 role in replicative senescence. Aging. 2010;2:333–343. doi: 10.18632/aging.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani N, Takahashi A, Mann DJ, Hara E. Cellular senescence: a double-edged sword in the fight against cancer. Exp Dermatol. 2012;21(Suppl 1):1–4. doi: 10.1111/j.1600-0625.2012.01493.x. [DOI] [PubMed] [Google Scholar]
- Roberts A, Pimentel H, Trapnell C, Pachter L. Identification of novel transcripts in annotated genomes using RNA-Seq. Bioinformatics. 2011a;27:2325–2329. doi: 10.1093/bioinformatics/btr355. [DOI] [PubMed] [Google Scholar]
- Roberts A, Trapnell C, Donaghey J, Rinn JL, Pachter L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011b;12:R22. doi: 10.1186/gb-2011-12-3-r22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanduja S, Kaza V, Dixon DA. The mRNA decay factor tristetraprolin (TTP) induces senescence in human papillomavirus-transformed cervical cancer cells by targeting E6-AP ubiquitin ligase. Aging. 2009;1:803–817. doi: 10.18632/aging.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonrock N, Harvey RP, Mattick JS. Long noncoding RNAs in cardiac development and pathophysiology. Circ Res. 2012;111:1349–1362. doi: 10.1161/CIRCRESAHA.112.268953. [DOI] [PubMed] [Google Scholar]
- Tominaga-Yamanaka K, Abdelmohsen K, Martindale JL, Yang X, Taub DD, Gorospe M. NF90 coordinately represses the senescence-associated secretory phenotype. Aging. 2012;4:695–708. doi: 10.18632/aging.100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotech. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi V, Shen Z, Chakraborty A, Giri S, Freier SM, Wu X, Zhang Y, Gorospe M, Prasanth SG, Lal A, Prasanth KV. Long Noncoding RNA MALAT1 Controls Cell Cycle Progression by Regulating the Expression of Oncogenic Transcription Factor B-MYB. PLoS Genetics. 2013 doi: 10.1371/journal.pgen.1003368. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Park SH, Thompson T, Karsenty G, Bradley A, Donehower LA. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- Wang Y, Scheiber MN, Neumann C, Calin GA, Zhou D. MicroRNA regulation of ionizing radiation induced premature senescence. Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2010.09.048. 0.1016/j.ijrobp.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Wang W, Yang X, Cristofalo VJ, Holbrook NJ, Gorospe M. Loss of HuR is linked to reduced expression of proliferative genes during replicative senescence. Mol Cell Biol. 2001;21:5889–5898. doi: 10.1128/MCB.21.17.5889-5898.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B, Wang Z. Long noncoding RNA: its physiological and pathological roles. DNA Cell Biol. 2012;31(Suppl 1):S34–41. doi: 10.1089/dna.2011.1544. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Abdelmohsen K, Gorospe M. Posttranscriptional Gene Regulation by Long Noncoding RNA. J Mol Biol. 2012 doi: 10.1016/j.jmb.2012.11.024. S0022-2836(12)00896-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.