Abstract
This article is intended to provide a review of clinically relevant neurovascular anatomy. A solid understanding of the vascular anatomy of the brain and spine are essential for the safe and effective performance of neurointerventional radiology. Key concepts to master include collateral pathways and anastomoses between the external and internal carotid circulation, the Circle of Willis as a route to otherwise inaccessible intracranial vascular distributions, and the origin of spinal arterial blood supply. These concepts will be highlighted using clinical angiographic examples with discussion of relevant embryology and pathology as needed.
Keywords: angiography, neurointerventional radiology, neuroanatomy, interventional radiology
Objectives: Upon completion of this article, the reader will be able to identify the relevance of vascular neuroanatomy as it relates to basic neurointerventions.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Endovascular therapies require a thorough understanding of the vascular anatomy in the target area of the intervention as well as the appropriate and safe route. The pathology and natural history of the disease and the structure of the arteries or veins supplying the target predicate the technique and intervention that must be employed during therapy.
The purpose of this article is to introduce the reader to basic neurovascular anatomy as it can be employed in everyday clinical practice. Common neurointerventions that the practicing general interventional radiologist may be called upon to perform include diagnostic cerebral angiography, ischemic stroke rescue, carotid stenting, external carotid artery (ECA) embolization for epistaxis or other bleeding, and preoperative tumor embolization in the spine and calvarium. The anatomical descriptions are tailored to these interventions. This article will add to the readers' foundation of knowledge and provide reference for further study.
Basic Neurovascular Histology
Arteries throughout the body are composed of three histologically distinct layers. The intima is the innermost layer composed of a single layer of flat epithelium. The media is the middle layer that consists primarily of smooth muscle and fibrous tissue. The adventitia is the outermost layer that includes fibrous tissue, loose connective tissue, vasa vasorum, and nerves. The arteries in the brain share this structure with arteries throughout the body, but there are also significant differences worth discussing.
Arteries of the central nervous system lack an external elastic lamina and are relatively deficient in terms of the thickness of the middle and outer histological layers in addition to the percentage of elastic fibers within these layers.1 The transition from the cervical to cerebral vessels occurs where the arteries pierce the dura mater. The angiographic landmark for this transition is the ophthalmic artery (OA) in the anterior cerebral circulation. The posterior inferior cerebellar artery (PICA), which arises from the distal vertebral artery (VA), is the first major intradural branch of the posterior cerebral circulation.
Diseases of the cerebral vasculature are different from those of other vascular distributions due in large part to the structural differences of the cerebral arteries. One interesting example is aneurysms.
Cerebrovascular aneurysms form at points of high vessel wall shear stress. The relative lack of constraining tissues in the vessel wall of cerebral arteries means that cerebral aneurysm formation owes something of its natural history to the structure of the vessel itself. Stress applied to a structurally vulnerable vessel predisposes to further weakening and aneurysm formation.1 Furthermore, the therapy for aneurysms is predicated by this histopathology, namely, protecting the focal weakening of the vessel wall from intravascular pressure. This fragility in turn dictates the design of the endovascular devices ensuring that they are atraumatic enough to avoid perforation as a common complication but robust enough to be passed over a wire or through a microcatheter into the brain.
Cervicocerebral Vascular Anatomy
The brain receives arterial blood from the bilateral internal carotid artery (ICA) and VA. The ICA carries blood into the anterior cerebral circulation including the middle and anterior cerebral arteries; the VA carries blood into the posterior circulation including the basilar artery (BA) and posterior cerebral arteries (PCAs). The anterior and posterior circulations communicate through the Circle of Willis via anterior and posterior communicating arteries (Fig. 1). This circle theoretically allows cerebral perfusion to continue via collaterals in the event of trauma or disease to one or more arteries supplying that territory. A complete Circle of Willis is, however, only seen in up to 24% of individuals.2
Figure 1.
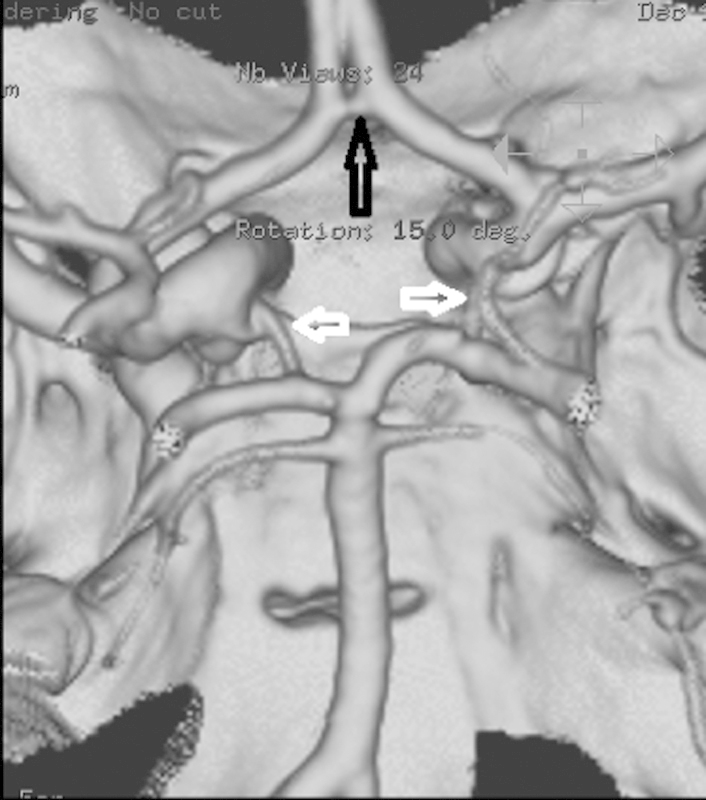
Circle of Willis anatomy: Surface volume rendered image from a CT angiography (CTA) showing the anterior communicating artery (open black arrow) and bilateral posterior communicating arteries (open white arrows). Orientation is as though the brain has been removed and we are looking down at the inside of the skull from above.
Structures perfused by the anterior circulation include evolutionarily younger parts of the brain including the neocortex of the cerebral hemispheres. These structures are highly sensitive to ischemia and reperfusion hemorrhage if the occluded vessel is opened later than 6 to 8 hours after symptom onset. Structures perfused by the posterior circulation include the more primitive brainstem and cerebellum, which may be more resistant to ischemia and the clinical sequelae of infarction. Infarction in the posterior circulation can lead to the “locked-in” syndrome, in which nearly all voluntary actions as well as the involuntary function of the diaphragm during respiration are impaired. However, the brain is still aware of the patient's surroundings and grim situation because of continued function of the visual and auditory systems as well as preservation of higher cortical integration. Because of these, attempts to reperfuse the posterior circulation during ischemic stroke are not as strictly limited by time from symptom onset.
Common and Internal Carotid Artery
The right common carotid artery (CCA) arises from the right brachiocephalic artery (BCA) in the majority of patients. The right BCA is the first of the great vessels to arise from the aortic arch in the majority of individuals. Variant origin of the right CCA directly from the aortic arch occurs in 3% of the population, seen in patients with aberrant origin of the right subclavian artery (SCA).3 The left CCA arises directly off of the aortic arch and is most frequently the second of the great vessels to do so; the right and left VAs arise from their respective SCAs in the majority of patients. The left VA can be seen to arise from the aortic arch between the left CCA and SCA in 6% of individuals.4
The CCA bifurcates into the internal and external divisions at the level of the second or third cervical vertebrae. ECA branches supply the face, scalp, and neck, while the ICA carries blood into the brain.
Several clinically important anastomoses exist between the branches of the ECA and the intracranial branches of the ICA.5 Once again, these connections allow cerebral perfusion to continue via collaterals in the event of trauma or disease to one or more arteries supplying that territory. These connections are always present but may not be evident on angiography unless there is pathology. Chief among these are the connections between the internal maxillary artery (IMAX) and the OA, the IMAX and the arteries of the foramens rotundum and ovale, the ascending pharyngeal artery and the inferolateral trunk, and the superficial temporal artery and the OA.
Bearing in mind that these anastomoses always exist, it is less important to memorize the names of the involved branches and more important to be alert to the possibility that injections in the ECA circulation may collateralize to intracranial vessels. In general, if one does not opacify intracranial vessels during external carotid angiography, it is unlikely that careful external carotid embolization will result in such nontarget embolization. Vigorous embolization, however, should be avoided at all times as this can result in reflux of particles, abrupt occlusion of the target external branch, and increased flow through angiographically occult collaterals. Increased flow through these collaterals is more common at the end of an embolization procedure when there can be a precipitous drop in antegrade flow in the embolized territories.
The ICA can be subdivided into segments on the basis of surrounding anatomy. There are multiple numbering systems for the ICA segments that can lead to confusion, and therefore, it is recommended to avoid these and instead use anatomical descriptors. In this way, we may talk about the cervical ICA, the petrous portion, the cavernous carotid, and the supraclinoid carotid (Fig. 2).
Figure 2.
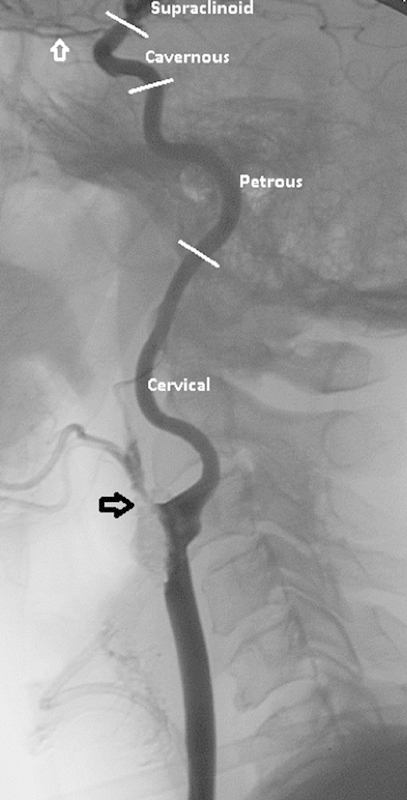
Cervical internal carotid artery anatomy: Lateral common carotid angiogram showing anatomic segments of the internal carotid artery. Note that there is near complete occlusion of the external carotid artery because of a heavily calcified lesion (open black arrow). The open white arrow denotes the ophthalmic artery.
It is commonly remarked that the ICA has no branches. This is not strictly true. There are several small but clinically significant branches of the ICA that are routinely identified on angiography including the vidian artery which arises from the horizontal petrous segment, and the inferolateral and meningohypophyseal trunks that arise from the cavernous carotid.6 These branches were mentioned previously as important anastomotic collateral pathways from the extracranial to the intracranial circulation.
The OA is the first intradural branch of the ICA and therefore is of unique clinical significance. Aneurysms at or above the OA can result in subarachnoid hemorrhage and therefore require neurointerventional or neurosurgical consideration. Aneurysms below the OA will not cause subarachnoid hemorrhage and therefore are generally not treated unless they cause other problems such as carotid cavernous fistulae or cranial nerve palsies.7
The terminal ICA bifurcates at the origin of the middle cerebral artery (MCA) and the anterior cerebral artery (ACA). The location of diseases of these vessels is roughly described with reference to the branch order of the vessel such that the first order, or parent vessel, is denoted by the number “1,” second order vessels are denoted “2,” and so on (Fig. 3).
Figure 3.
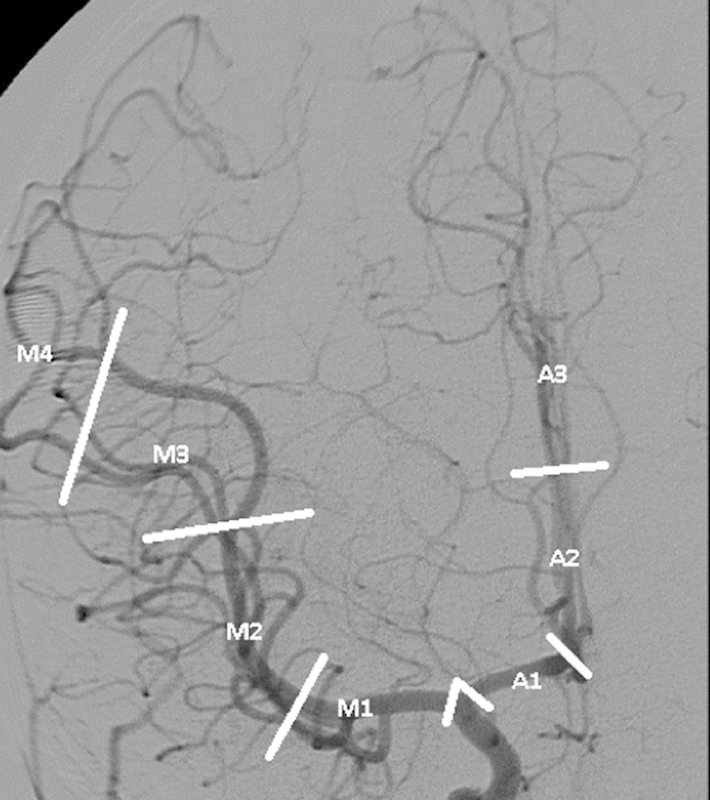
Anterior circulation anatomy: anteroposterior (AP) right internal carotid angiogram showing divisions of the middle (M) and anterior (A) cerebral arteries. There is reflux into the external branches as well.
The ACA courses medially, and turns cephalad and posterior between the cerebral hemispheres. The right and left halves of the anterior cerebral circulation are connected through the anterior communicating artery. This artery connects the two ACAs to one another and defines the transition from the first to the second portion of the ACA.
The MCA courses laterally into the Sylvian fissure. It bifurcates at its lateral extent before coursing posterior and cephalad within the fissure. The transition between the M1 and M2 segments occurs at the bifurcation. The MCA is the most frequently involved artery in ischemic stroke and the third most commonly affected artery in aneurysm disease, distinctions that make the MCA the most commonly catheterized artery in the brain. The M1 segment gives rise to lenticulostriate perforators, which are end arterioles supplying the basal ganglia and the internal capsule. Lack of collaterals to these structures renders them particularly vulnerable to reperfusion hemorrhage in the setting of ischemic stroke. The MCA also supplies the majority of the cerebral hemispheres.
Vertebral and Basilar Artery
The VAs supply the posterior circulation of the brainstem, cerebellar hemispheres, and posterior cerebral hemispheres. These arteries typically arise from the SCAs bilaterally. Anatomic segments of the VA from proximal to distal include the extraosseous segment (V1), the foraminal segment (V2), the extraspinal segment (V3), and the intradural segment (V4) (Fig. 4).
Figure 4.
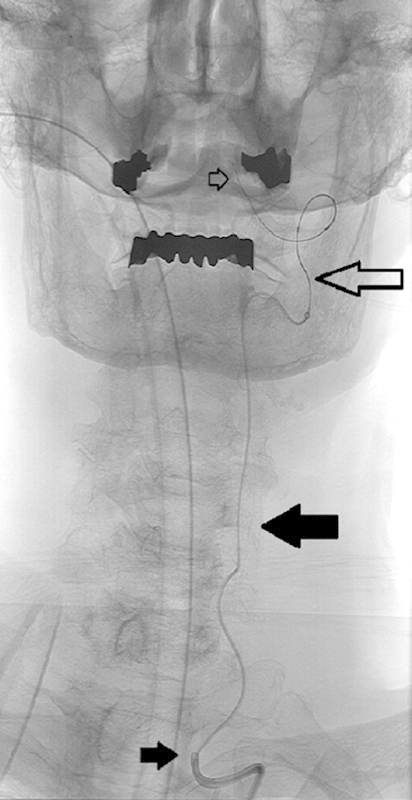
Vertebral artery anatomy: AP fluoroscopic image of a patient undergoing treatment of a symptomatic basilar artery stenosis. A microcatheter and guidewire are within the cervical vertebral artery. The small solid black arrow indicates the extraosseous (V1) segment, the large solid black arrow indicates the foraminal (V2) segment, the large open black arrow indicates the extraspinal (V3) segment, and the small open black arrow indicates the intradural (V4) segment.
As they ascend in the posterior neck, they enter into the vertebral foramen transversarium within the lateral mass of cervical vertebrae C6 through C2. The foraminal segment of the VA gives rise to numerous muscular branches but no large named branches. Above the C1 level the VA passes through the dura mater at the foramen magnum and gives rise to the PICAs, which are the first intradural branches of the VAs, before anastomosing at the midline in front of the brainstem as the BA. The BA supplies the anterior inferior cerebellar arteries, the brainstem perforators, the superior cerebellar arteries, and finally the PCAs (Fig. 5). The anterior spinal artery of the cervical enlargement arises from the distal VAs.
Figure 5.

Posterior circulation anatomy: AP angiogram of the posterior cerebral circulation demonstrating the segments of the posterior cerebral artery; open black arrows show P1 segments, solid black arrows show P2 segments, open white arrows show P3 segments, and solid white arrows show P4 segments. The bilateral superior cerebellar arteries are seen immediately below the posterior cerebrals. Note that the left vertebral artery is slightly larger than the right, consistent with a left dominant vertebrobasilar system (the most common variation).
The larger of the two VAs is sometimes denoted as “dominant,” making the most common relationship a “left dominant” vertebrobasilar system. Common variants of the distal vertebrobasilar system include VA termination as the PICA, conjoined origin of the ipsilateral posterior inferior and anterior inferior cerebellar arteries, and contralateral supply to the cerebellar arteries.
External Carotid Artery
The external carotid circulation includes several high resistance arteries supplying the face and scalp. The CCA bifurcates in the neck at or slightly below the angle of the mandible. Branches of the ECA include the lingual, facial, IMAX, ascending pharyngeal, posterior auricular, and superficial temporal arteries (Fig. 6). The IMAX is essentially the terminal portion of the ECA and is important for its role in epistaxis. This artery gives rise to the middle meningeal artery (MMA); the MMA has a characteristic 90° turn on lateral projection angiographically, and the goal of safe IMAX artery embolization includes avoiding nontarget intracranial reflux via this artery.
Figure 6.
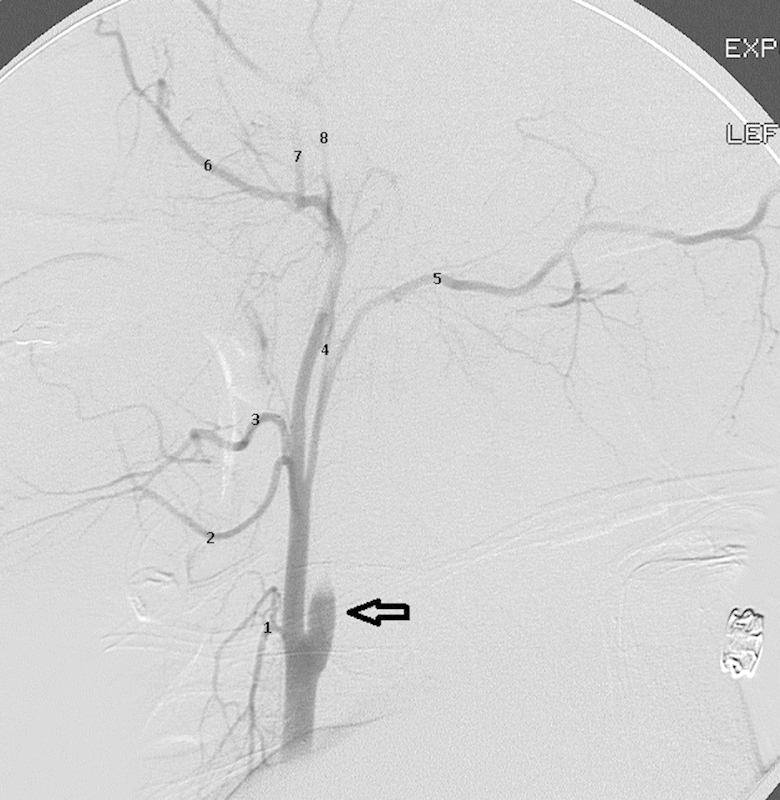
External carotid artery anatomy: Lateral angiogram in a patient with acute traumatic dissection of the internal carotid artery (open arrow) demonstrating the branches of the ECA. 1, superior thyroidal artery; 2, lingual artery; 3, facial artery; 4, ascending pharyngeal artery; 5, occipital artery; 6, internal maxillary artery; 7, middle meningeal artery; 8, superficial temporal artery.
Spinovertebral Vascular Anatomy
Arterial blood supply to the vertebral body and intervertebral discs comes from the radicular arteries.8 These arteries are branches from the segmental intercostal and lumbar arteries in the thorax and abdomen, and from the VAs in the cervical region. The radicular arteries enter the vertebral column through the intervertebral neural foramen. The intimate association between blood supply to the vertebral body and spinal cord is highlighted by concomitant infarction to both anatomic structures following disruption of their shared blood supply8 (Fig. 7). The presence of vertebral body ischemia, indicated by abnormal signal on the STIR (short tau inversion recovery) sequence, is a helpful secondary sign in cases with subtle or delayed findings of spinal cord ischemia. The proximity between the radicular arteries and veins within the confines of the neural foramen underlies the pathogenesis of spinal dural arteriovenous malformations. These malformations are most often the result of innocuous trauma related to degenerative changes in the spine. The radicular artery and vein become interconnected in the confines of the neural foramen, and venous hypertension slowly causes spinal cord ischemia.
Figure 7.
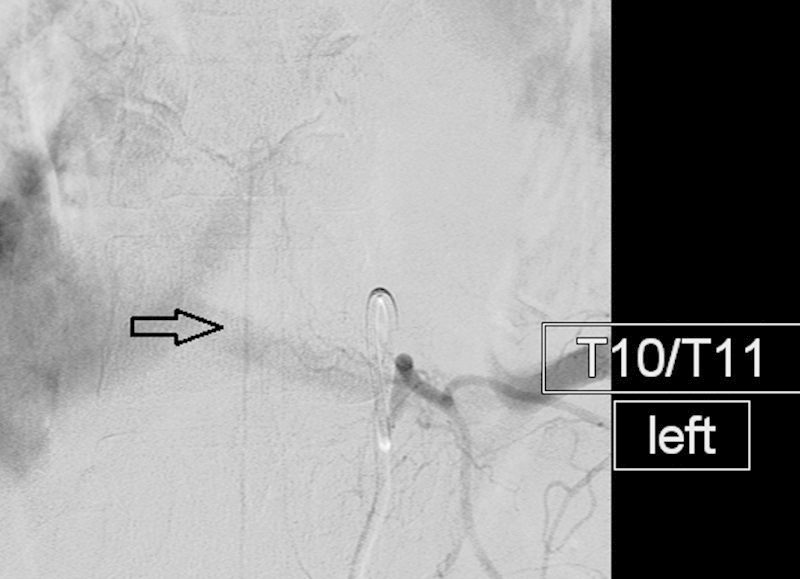
Anterior spinal artery: AP angiogram demonstrating the typical appearance of the artery of Adamkiewicz (open arrow). Note small somatic branches traveling lateral to medial and paralleling the vertebral body end plate.
Once inside the spinal canal, the radicular arteries travel in the epidural space from lateral to medial along the posterior margin of the vertebral body and branch to supply their anatomic targets. Somatic branches supply the disc and bone of the vertebral column, whereas the radiculomedullary branches supply the cord. In general, the primitive intervertebral disc is more vascular than the adjacent vertebral bodies, and therefore, a concentration of vessels exists in the discs and endplates of the bones. This disco-centric concentration of vessels leads to the phenomenon of dual blood supply to each vertebral body arising at the disc spaces above and below it.9 This anatomic relationship supports the technical consideration for embolization of the blood supply above and below the level of a hypervascular vertebral metastasis to minimize hemorrhage during subsequent surgical intervention.
The spinal cord receives both an anterior and a posterior blood supply from respectively named spinal arteries.10 Classical teaching observes that the anterior spinal arteries are fewer in number and receive fewer collateral feeders, and therefore, injury to these vessels poses a greater risk for neurological injury. Although recent investigations refute this simplistic explanation, the anatomic relationships remain and provide a basis for education.11,12,13
There is one continuous anterior spinal artery found at the midline on the ventral surface of the cord. Blood supply to this artery comes from bilateral branches of the distal VA as well as a single dominant anterior radiculomedullary artery or a dominant anterior radiculomedullary artery in conjunction with several subdominant anterior radiculomedullary arteries in the low thoracic or upper lumbar region. The vessels providing blood to the anterior spinal artery are found at the cervical and lumbar enlargements of the spinal cord. The more caudal supply often perfuses two-thirds of the cord and carries the eponym Adamkiewicz. The artery of Adamkiewicz is most commonly found at the L1 level (range, T9-L5) and arises from the left of midline in > 60% of the patients.9 Contrary to classical teaching that the Artery of Adamkiewicz is a terminal artery without collateralization, human clinical and cadaver studies as well as animal model studies support the concept of a rich collateral network to Adamkiewicz and the anterior spinal artery.12,13
The posterior spinal arteries are greater in number and richer in collateralization than the anterior spinal artery, and therefore, neurological injury resulting from vascular insult to the posterior spinal arteries is much less common.14 The posterior spinal arteries are paired and paramedian in location along the dorsal aspect of the cord. They receive arterial blood from the vertebral arteries, 10 to 20 posterior segmental radicular arteries, and collaterals from the anterior spinal artery at the conus medullaris. The arteries are seldom visualized on angiography and tend to have a cruciate, or “crossed,” configuration.
The artery of Adamkiewicz (arteria radicularis magna) has a classic “hairpin loop” configuration that is because of the differential growth of the vertebral column and the spinal cord. The artery perforates the meninges at the level of the primitive disc during early embryological development. However, as the embryo elongates, the vertebral column lengthens more rapidly and to a greater extent than the spinal cord such that the cord terminates at about T12 or L1 in the mature individual. This growth drags the spinal artery upward from the original level of its blood supply and forms the characteristic course of the artery with the top of the “hairpin” coinciding with the location where the artery perforates the meninges. Occasionally, the posterior spinal arteries will have this same appearance, but they will be located off of midline.
Conclusions
A thorough understanding of neurovascular anatomy is crucial for the safe performance of basic neurointerventions. Intracranial interventions include restoring blood flow during ischemic stroke and preoperative embolization of meningiomas (addressed elsewhere in this issue). The MCA is the most commonly affected vessel in ischemic stroke. Extracranial carotid embolization is frequently employed for management of acute hemorrhage in the head and neck, as is the case in epistaxis. The interventional radiologist should be aware of the existence of extracranial to intracranial collaterals in the carotid circulation and practice safe embolization technique to avoid complications. Spinal interventions are most frequently employed during the performance of preoperative embolization to prevent significant blood loss during resection of hypervascular metastases to the vertebral column. Interventional radiologists should be aware of the shared blood supply to the vertebral column and the spinal cord and should look for evidence of collateral flow to the cord, before attempting tumor embolization.
References
- 1.Idowu O E, Shokunbi M T, Malomo A O, Akang E EU. Anomalies, aneurysm and histology of the middle cerebral artery in Nigerian Africans. Int J Morphol. 2008;26(4):1023–1027. [Google Scholar]
- 2.Krishnaswamy A, Klein J P, Kapadia S R. Clinical cerebrovascular anatomy. Catheter Cardiovasc Interv. 2010;75(4):530–539. doi: 10.1002/ccd.22299. [DOI] [PubMed] [Google Scholar]
- 3.Yang C, Shu C, Li M, Li Q, Kopp R. Aberrant subclavian artery pathologies and Kommerell's diverticulum: a review and analysis of published endovascular/hybrid treatment options. J Endovasc Ther. 2012;19(3):373–382. doi: 10.1583/11-3673MR.1. [DOI] [PubMed] [Google Scholar]
- 4.Meila D, Tysiac M, Petersen M. et al. Origin and course of the extracranial vertebral artery: CTA findings and embryologic considerations. Clin Neuroradiol. 2012;22(4):327–333. doi: 10.1007/s00062-012-0171-0. [DOI] [PubMed] [Google Scholar]
- 5.Geibprasert S, Pongpech S, Armstrong D, Krings T. Dangerous extracranial-intracranial anastomoses and supply to the cranial nerves: vessels the neurointerventionalist needs to know. AJNR Am J Neuroradiol. 2009;30(8):1459–1468. doi: 10.3174/ajnr.A1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tubbs R S, Hansasuta A, Loukas M. et al. Branches of the petrous and cavernous segments of the internal carotid artery. Clin Anat. 2007;20(6):596–601. doi: 10.1002/ca.20434. [DOI] [PubMed] [Google Scholar]
- 7.Lee N, Jung J Y, Huh S K, Kim D J, Kim D I, Kim J. Distinction between intradural and extradural aneurysms involving the paraclinoid internal carotid artery with T2-weighted three-dimensional fast spin-echo magnetic resonance imaging. J Korean Neurosurg Soc. 2010;47(6):437–441. doi: 10.3340/jkns.2010.47.6.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng M Y, Lyu R K, Chang Y J. et al. Concomitant spinal cord and vertebral body infarction is highly associated with aortic pathology: a clinical and magnetic resonance imaging study. J Neurol. 2009;256(9):1418–1426. doi: 10.1007/s00415-009-5126-2. [DOI] [PubMed] [Google Scholar]
- 9.Biglioli P, Roberto M, Cannata A. et al. Upper and lower spinal cord blood supply: the continuity of the anterior spinal artery and the relevance of the lumbar arteries. J Thorac Cardiovasc Surg. 2004;127(4):1188–1192. doi: 10.1016/j.jtcvs.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 10.Etz C D, Kari F A, Mueller C S. et al. The collateral network concept: a reassessment of the anatomy of spinal cord perfusion. J Thorac Cardiovasc Surg. 2011;141(4):1020–1028. doi: 10.1016/j.jtcvs.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakami H, Kawahara N, Tomita K, Demura S, Kato S, Yoshioka K. Does interruption of the artery of Adamkiewicz during total en bloc spondylectomy affect neurologic function? Spine (Phila Pa 1976) 2010;35(22):E1187–E1192. doi: 10.1097/BRS.0b013e3181e215e5. [DOI] [PubMed] [Google Scholar]
- 12.Kato S, Kawahara N, Tomita K, Murakami H, Demura S, Fujimaki Y. Effects on spinal cord blood flow and neurologic function secondary to interruption of bilateral segmental arteries which supply the artery of Adamkiewicz: an experimental study using a dog model. Spine (Phila Pa 1976) 2008;33(14):1533–1541. doi: 10.1097/BRS.0b013e318178e5af. [DOI] [PubMed] [Google Scholar]
- 13.Ueda Y, Kawahara N, Tomita K, Kobayashi T, Murakami H, Nambu K. Influence on spinal cord blood flow and function by interruption of bilateral segmental arteries at up to three levels: experimental study in dogs. Spine (Phila Pa 1976) 2005;30(20):2239–2243. doi: 10.1097/01.brs.0000182308.47248.59. [DOI] [PubMed] [Google Scholar]
- 14.Struhal W, Seifert-Held T, Lahrmann H, Fazekas F, Grisold W. Clinical core symptoms of posterior spinal artery ischemia. Eur Neurol. 2011;65(4):183–186. doi: 10.1159/000324722. [DOI] [PubMed] [Google Scholar]


