Abstract
Background Bisphosphonates are powerful drugs used for the management of osteoporosis and metastatic bone disease to avoid skeletal-related complications. Side effects are rare but potentially serious such as the bisphosphonate-related osteonecrosis of the jaws (BRONJ). BRONJ impairs the quality of life and can even lead to pathologic fractures of the mandible. Management of BRONJ is difficult per se. If complicated with pathologic mandibular fractures in advanced stages, the treatment options are controversially discussed. This review delineates the epidemiology and pathogenesis of BRONJ to put the various modalities for the treatment of pathologic mandible fractures into perspective.
Methods Various case reports and case series in the literature were reviewed. Cases were reviewed of patients suffering from pathologic fracture due to bisphosphonate-related osteonecrosis of the jaw treated in the Department of Oral and Maxillofacial Surgery (Ludwig-Maximilians-University of Munich) from 2003 to 2010. Of 140 patients suffering from BRONJ, four were identified with pathologic fracture of the mandible.
Results Management of pathologic mandibular fractures in patients suffering from BRONJ is an unsolved issue. At present there is a paucity of information to establish reliable therapy guidelines. The published strategies range from conservative treatment to major bone resections with or without internal or external fixation and with or without autogenous reconstruction. There is no evidence for the superiority of a single therapeutic mode, however.
Conclusion Further understanding of BRONJ is mandatory to establish a sound rationale for the treatment of associated mandibular fractures.
Keywords: pathologic fractures, osteonecrosis of the jaw, bisphosphonates BRONJ
Bisphosphonates are the most widely used class of antiresorptive drugs. Binding selectively to hydroxyapatite and accumulating in bone, they inhibit osteoclast activity and thus bone remodeling. In higher doses they can also inhibit osteoblast activity and have antiangiogenic properties.1 Bisphosphonates play a key role in the management of metastatic bone disease as well as osteoporosis.2,3,4 In patients suffering from those conditions, bisphosphonates provide significant protection against skeletal complications, in particular against fractures,2,5,6 and therefore help to improve the quality of life.4 Bisphosphonates are generally well tolerated and severe side effects are rare.7
Bisphosphonate-Related Osteonecrosis of Jaw
Occurrence and Incidence of the Disease
Since the first description in 2003,8,9,10 bisphosphonate-related osteonecrosis of jaw (BRONJ) has become a well-acknowledged side effect of rising clinical importance. The incidence of BRONJ among patients receiving bisphosphonates differs depending on the route of application and the underlying disease with the highest incidence in patients receiving nitrogen-containing bisphosphonates intravenously due to metastatic bone disease. Even among these patients, the calculated incidence in retrospective studies ranges from less than 1 up to 18% and more,11,12 a fact that, among other reasons, can be attributed to different study designs (retrospective analysis with no intraoral investigation by a specialist). Recent prospective trials including oral and dental examinations have reported an incidence exceeding 10% (up to 28% in one study).11,13,14 The risk for patients under oral bisphosphonate treatment is considerably lower. In a large retrospective study, an incidence of ∼0.1% was reported.15
Diagnosis
According to the task force on bisphosphonate-related osteonecrosis of the jaw of the American Association of Oral and Maxillofacial Surgeons patients are considered to suffer from BRONJ if the following criteria are fulfilled16: (1) the history of current or previous bisphosphonate application; (2) the presence of exposed necrotic bone over a period of 8 weeks; (3) no history of irradiation to the jaws.
Recently, a new classification into the four stages has been introduced: stage 0 takes the fact into account that exposed bone is not mandatory for the diagnosis of BRONJ,17 but further nonspecific symptoms or clinical and radiologic findings may be present such as (1) odontalgia without odontogenic cause, (2) dull or aching pain that may radiate to the temporomandibular joint, (3) sinus pain and altered neurosensory function, (4) loosening of teeth not explained by parodontological reasons, and/or (5) changes to trabecular bone to dense woven bone and persistence of un-remodeled extraction sockets.
BRONJ stage 1 is characterized by exposed necrotic bone with no pain and no signs of infection, and stage 2 is defined by the presence of exposed necrotic bone with pain and clinical evidence of infection. Stage 3 is characterized by one or more of the further complications: (1) necrotic bone extending the alveolar bone to the inferior border or ramus in the mandible or to the maxillary sinus or zygoma, (2) pathologic fracture, (3) oral antral or oral nasal communication, or (4) extraoral fistula formation.
To date, neither the BRONJ localization nor the extent of exposed bone nor the histopathology are considered for the classification/staging, a circumstance that is considered as a drawback by the authors of this article.
Clinical Features
By far the most common clinical finding of BRONJ is exposed necrotic bone in the oral cavity. But BRONJ can have a variety of other clinical features such as pain, soft tissue swelling or ulceration, suppuration, intra- or extraoral fistulae, abscess formation.9,16,18,19,20 Even impairment of the inferior alveolar nerve function (Vincent's sign) can occur in the course of the disease.21
The most severe problems are caused by extended osteonecrosis with pathologic fractures of the mandible (stage 3), which can occur in an advanced stage of the disease because of structural changes and loss of bone and/or following extensive debridement of necrotic bone areas due to the necrosis that also impair the mechanical stability of the jaw.22
Risk Factors
Most of the published BRONJ cases occurred in patients who received aminobisphosphonates intravenously for the prevention or therapy of metastatic bone disease.12,23,24 Less frequently BRONJ has been reported in patients who receive oral aminobisphosphonates for the treatment of osteoporosis.3,15,25
Besides the mode of application (intravenous > oral), the dose, duration, and frequency of the treatment with bisphosphonates (cumulative dose) influence the risk for BRONJ.12,18,26,27 Other general risk factors are supposed to be chemotherapy, history of irradiation in the head and neck region, and comedication such as steroids, smoking habits, and poor oral hygiene.16,18 Indeed, most of the published BRONJ cases occurred after so-called trigger events such as tooth extractions and other dentoalveolar surgical procedures like insertion of dental implants but also nonsurgical procedures like endodontic or periodontal treatments. Even ill-fitting dentures can act as triggers for the manifestation of BRONJ.12,28,29
Pathogenesis
Numerous studies and case series have provided valuable insights into BRONJ but the exact pathogenesis remains elusive.30 The prevailing theory refers to an oversuppression of bone turnover once bisphosphonates have accumulated into toxic levels in the jawbone, resulting in necrosis.31,32 Another theory considers the antiangiogenic effects of bisphosphonates, which possibly lead to ischemia and necrosis of the bone.33,34 Reid et al hypothesized that accumulation of bisphosphonates in the jawbone could affect the covering mucosa and mucosal integrity as a consequence of the known soft tissue toxicity.35
Although all these theoretical mechanisms may contribute to the pathogenesis of BRONJ, none of them explain conclusively why almost all cases occur in the jawbones and why nitrogen-containing bisphosphonates seem to be associated with a higher risk than non-nitrogen-containing bisphosphonate derivates.
As a consequence our group recently suggested that localized pH reductions in the bone caused by local infections could be the missing piece in the pathogenesis puzzle.36 Bisphosphonates bind to bone at neutral pH values and are released in an acidic milieus,37 a mechanism that is well known and proved with regard to the therapeutic effects of bisphosphonates,38 but has not yet been linked to the pathogenesis of BRONJ. Depending on the concentration, soluble bisphosphonates are cytotoxic,39 and bone-bound bisphosphonates are inert.40 Furthermore, nitrogen-containing bisphosphonates are activated (by protonation) in acidic milieus and thus become more toxic.37,39,41
Acidic milieus are common in infections, and the jawbones are subjected to infections more frequently than other parts of the human skeleton; the vast majority of seniors suffer from moderate to severe inflammatory periodontal disease (periodontitis).42 Taken together this theory can explain why predominantly the jaws are affected and why nitrogen-containing bisphosphonates are associated with a higher risk than non-nitrogen-containing ones.36
BRONJ Therapy
Up to now there are no evidence-based treatment guidelines for the different stages of the BRONJ. Some authors prefer conservative treatment protocols consisting of long-term administration of antibiotics and local rinses.43,44 This approach can result in a temporary improvement but leads to healing in less than two-thirds of the cases.44,45,46 In contrast, surgical protocols have outcome results with success rates of 80% and more.47,48,49,50 An early and confined surgical removal of the necrotic bony portions is recommended to limit the defect and to avoid complications such as abscess or fistulae formation or pathologic fractures caused by a structural weakening or extensive resections.49,51 As a consequence, in localized BRONJ less invasive surgical approaches should be favored; however, controlled clinical trials are lacking. Recently, the tetracycline fluorescence-guided bone resection was introduced in the BRONJ therapy. It is a promising novel technique representing an improvement of surgical therapy, because viable bone can be distinguished from the osteonecrosis by bone fluorescence intraoperatively and therefore an adequate debridement becomes possible.48,52,53,54
Occurrence and Treatment of Pathologic Fractures Due to BRONJ
Pathologic fractures represent the severest degree of BRONJ in the mandible. As the number of reported pathologic fractures due to BRONJ is small, there are only very limited data concerning its incidence and management.22,55 Abu-Id and coworkers reported three pathologic fractures of the mandible among 78 patients suffering from BRONJ (3.8%).20 In our department among 140 patients with BRONJ treated from 2003 till 2010, four pathologic fractures of the mandible have occurred (2.9%). However, as BRONJ affects the mandible in the majority of cases and as the lesions tend to progress, an increasing frequency of pathologic fractures due to BRONJ can be anticipated. Because this complication has a tremendous impact on the quality of life of those patients, the treatment of this condition should be a target of further research.
The management of cases of extended BRONJ and pathologic fractures due to BRONJ is particularly difficult because there are several local and systemic problems. Pathologic fractures due to BRONJ are often accompanied by bone defects due to the osteonecrosis itself or following removal of necrotic bone portions. Another critical point for fracture healing is the suppression of bone remodeling caused by bisphosphonates. Additionally, there might also be an impairment of angiogenesis and soft tissue healing. Systemic factors influencing the treatment are that most of the patients suffer from advanced stages of malignancies with or at risk of metastatic bone disease. Due to the primary diseases, most of those patients have undergone or receive chemotherapies or irradiation, and immunosuppressive or antiangiogenic drugs are often administered.
Although there are no therapy guidelines and hardly any data, most of the cases are managed by an open surgical treatment because there is no convincing nonsurgical treatment option. But there are still several surgical treatment options under consideration, and various therapy concepts have been reported in the literature, ranging from local removal of necrotic bone with or without using plate osteosynthesis up to radical resections and complex microvascular reconstructions.22,52,56,57 As a principle, the treatment of BRONJ needs to take the general condition of the patient into account especially in cases due to malignancy with metastatic bone disease and limited life expectancy.
Some authors recommend performing local or radical resections without using plate osteosynthesis as a first step to control local infections and to avoid disturbances of wound healing such as plate exposure.56 This approach might be useful as first step followed by a rigid internal fixation or reconstruction. But functional and aesthetic results are unfavorable if the resection of the affected area is the only treatment, as it leads to deviation and severe limitation of the motility of the mandible as well as scar formation of soft tissues.
With regard to the bony defects with lack of buttressing and the compromised bone quality with remodeling suppression due to bisphosphonate effects in case of pathologic fractures, load-bearing osteosynthesis systems should be the treatment of choice. Furthermore, the osteosynthesis screws should not be placed in or too close to the necrosis. Due to the fact that the osseointegration of the screws can be reduced, screws with bigger diameter should be utilized. The presence of infections in or close to the fracture site can further complicate the local wound-healing conditions.
Details regarding the treatment and outcome of patients with pathologic fractures of the mandible due to BRONJ within our department are given in Tables 1 and 2 and the Figs. 1 to 234. We prefer to explore the fracture site, to remove necrotic bone parts, and to perform a load-bearing internal fixation using osteosynthesis plates (Figs. 1, 3 and 4) even though disturbances of wound healing and plate exposure can occur. But even in cases of impaired wound healing, the functional and aesthetic results are probably better than in cases that are only resected.
Table 1. General data of patients with bisphosphonate-related osteonecrosis of the jaws and pathologic fracture.
| Patient | Age | Sex | Underlying disease | Bisphosphonate | Localization of fracture |
|---|---|---|---|---|---|
| 1 | 65 | F | Breast cancer | Zoledronate (i.v.) | Right mandibular body/angle |
| 2 | 72 | M | Prostate cancer | Zoledronate (i.v.) | Left mandibular body |
| 3 | 91 | M | Prostate cancer | Zoledronate (i.v.) | Left mandibular angle |
| 4 | 73 | F | Multiple myeloma | Zoledronate (i.v.) | Left mandibular body |
Abbreviation: i.v., intravenously.
Table 2. Treatment and outcome of patients with bisphosphonate-related osteonecrosis of the jaws and pathologic fracture.
| Patient | Treatment | Outcome |
|---|---|---|
| 1 | Sequestrotomy and removal of necrotic bone parts including segmental resection, open reduction and rigid internal fixation (AO Titanium 2.4 unilock) (DePuy Synthes, Germany) | Delayed mucosal healing (initial wound dehiscence with complete mucosal healing after local disinfectant measurements) |
| 2 | Sequestrotomy and removal of necrotic bone parts and application of external fixation | Stable pseudarthrosis and mucosal healing |
| 3 | Sequestrotomy and removal of necrotic bone parts, open reduction and rigid internal fixation (AO titanium 2.4 unilock) | Delayed but complete mucosal healing |
| 4 | Sequestrotomy and removal of necrotic bone parts, open reduction and rigid internal fixation (Matrix mandible preformed plate) (DePuy Synthes, Germany) | Delayed mucosal healing with a small area of intraoral bone exposure (no plate exposure, no extraoral fistula) |
Figure 1.
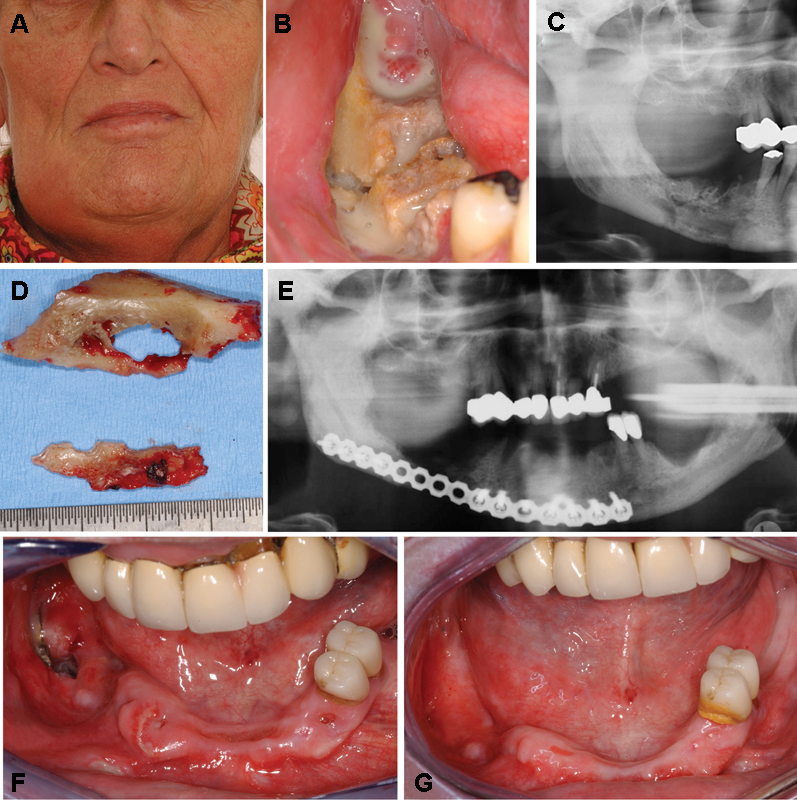
A 65-year-old woman suffering from metastases after breast cancer and receiving intravenous administrations of bisphosphonates (zoledronate) with an extended manifestation of bisphosphonate-related osteonecrosis of the right side of the mandible. (A) Extraoral view with a swelling of the right submandibular area, which was painful on palpation. (B) Intraoral view with a large area of exposed necrotic bone and sign of massive superinfection (swelling, pus) and a visible fracture of the mandible with mobile segments. (C) Panoramic radiograph of the patient with a mixed radiolucent and radiopaque appearance and a visible fracture line the right mandibular body. (D) Large bone sequesters that could be removed in the course of the treatment including segmental resection of the mandible, rigid internal fixation using a Synthes 2.4 unilock plate (DePuy Synthes, Germany), and complete closure of the wound. (E) Postoperative panoramic radiograph showing the resected area of the right mandible and the rigid internal fixation (Synthes 2.4 unilock plate). (F) Intraoral view 3 weeks postoperatively with a late dehiscence and plate exposure in region 47/48. (G) Intraoral view 4 months postoperatively with complete mucosal healing after local disinfectant measurements using disinfectant mouth rinses and activated photodynamic therapy.
Figure 2.
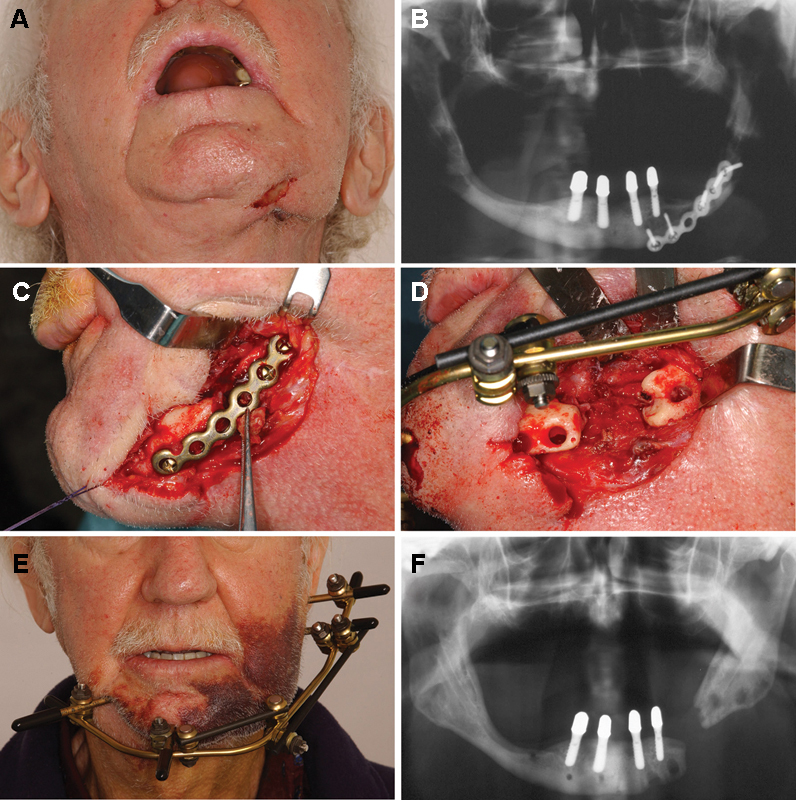
A 72-year-old man suffering from metastatic prostate cancer under intravenous treatment with bisphosphonates with a pathologic fracture due to bisphosphonate-related osteonecrosis of the left mandibular body, which was initially treated elsewhere using plate osteosynthesis. The patient was suffering from pain and swelling. (A) Extraoral view showing swelling and fistula formation and plate exposure in the left submandibular area. (B) Panoramic radiograph illustrating mixed radiopacities and radiolucencies in the affected area of the left mandibular body and the plate osteosynthesis, which was put in place elsewhere. (C) Intraoperative situation after extraoral approach and exploration of the left mandibular body with loosening of the screws and the osteosynthesis plate as well as bone necrosis and visible sequesters in the left mandibular body. (D) Intraoperative situation after sequestrectomy, removal of necrotic bone parts, and application of external fixation, which was intended to keep the segments in place until reconstruction. (E) Extraoral view 3 days postoperatively showing the external fixation in place. (F) Panoramic radiograph after removal of the external fixation illustrating that the distance between the mandibular segments was almost constant. The patient did not want any kind of reconstruction of the left mandibular body because he was free of symptoms and able to wear a denture.
Figure 3.
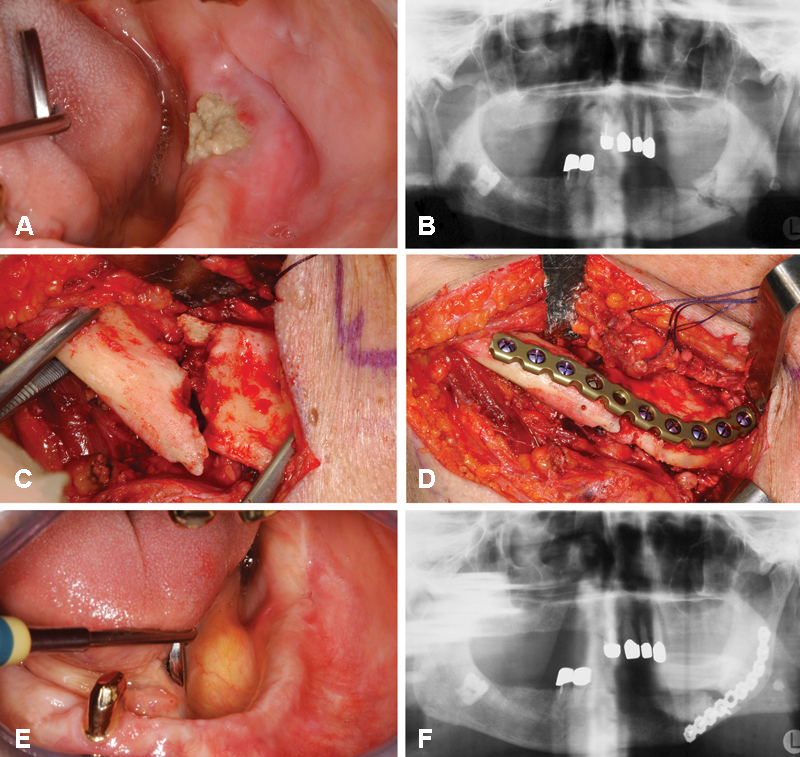
A 91-year-old man suffering from prostate cancer receiving intravenous administrations of zoledronate (4 mg every 4 weeks) who developed a pathologic fracture in the left mandibular angle due to bisphosphonate-related osteonecrosis of the jaws. (A) Intraoral view with exposed necrotic bone in the left mandibular angle. (B) Preoperative panoramic radiograph illustrating a fracture line in the left mandibular angle surrounded by mixed radiopaque and radiolucent lesions. (C) Intraoperative view after extraoral/submandibular approach before removal of necrotic bone parts and reduction. (D) Intraoperative view after removal of necrotic bone parts and fracture reduction. (E) Intraoral view after delayed mucosal healing. (F) Postoperative panoramic radiograph with AO titanium 2.4 unilock plate (DePuy Synthes, Germany) in place.
Figure 4.
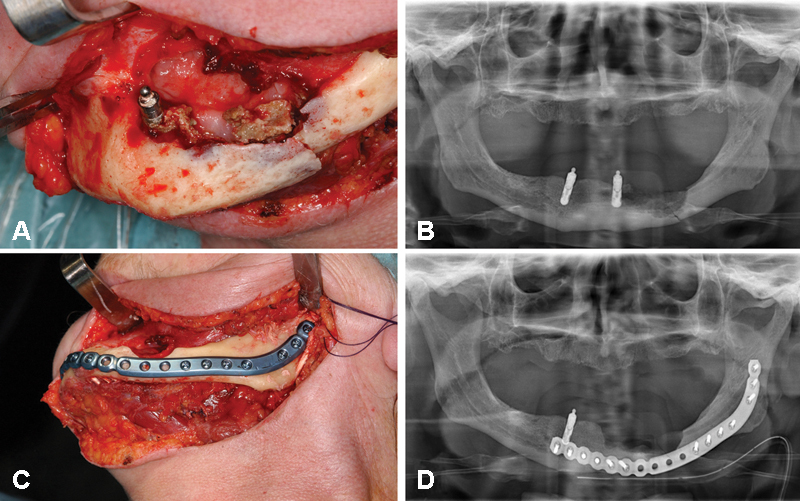
A 73-year-old woman suffering from multiple myeloma who received intravenous administrations of zoledronate. The patient developed bisphosphonate-related osteonecrosis of the jaws closely related to a dental implant in the left mandibular body and subsequently mandibular fracture in the area of jaw bone necrosis. (A) Intraoperative view after extraoral approach illustrating necrotic bone parts closely related to a dental implant in the left mandible (region 33) and corresponding preoperative panoramic radiograph (B). (C) Intraoperative view after removal of the implant and the necrotic bone parts, and fracture reduction and rigid internal fixation using a matrix mandible preformed plate with corresponding postoperative panoramic radiograph (D).
A promising alternative could be the use of external fixation (Fig. 2) after removal of the necrotic bone close to the fracture site to prevent extensive bone exposure.58 External fixation is also useful in cases with high risk of placing the osteosynthesis plates at infected necrotic bone areas. Indeed, the risk of wound dehiscence with consecutive exposure of osteosynthesis material or bone and the risk of scar formation influencing jaw function is diminished.
For extensive defects free or microvascular bone transfer might be a feasible alternative.56,57,59 However, the general limitations have to be considered especially the general condition of the patient suffering from bone metastasis as well as the prognosis and the stage of the underlying disease.
The most important local problems that should be taken into account are caused by disturbances of wound healing and especially remodeling suppression due to bisphosphonate effects.
Conclusion
We conclude that the treatment of mandibular fractures due to bisphosphonate-related osteonecrosis has to take special aspects of this disease into account. As removal of necrotic bone parts is necessary, the pathologic fractures become defect fractures. Besides that, bone remodeling is suppressed and fracture healing is delayed. Consequently principles of defect fracture treatment and load-bearing osteosynthesis should be applied.
However, further investigations with special emphasis on the pathogenesis and treatment of BRONJ and pathologic fractures due to BRONJ in particular are needed.
References
- 1.Green J R Antitumor effects of bisphosphonates Cancer 200397(3, Suppl):840–847. [DOI] [PubMed] [Google Scholar]
- 2.Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–2018. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 3.Yarom N, Yahalom R, Shoshani Y, Hamed W, Regev E, Elad S. Osteonecrosis of the jaw induced by orally administered bisphosphonates: incidence, clinical features, predisposing factors and treatment outcome. Osteoporos Int. 2007;18:1363–1370. doi: 10.1007/s00198-007-0384-2. [DOI] [PubMed] [Google Scholar]
- 4.Berenson J R, Lichtenstein A, Porter L. et al. Myeloma Aredia Study Group . Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. N Engl J Med. 1996;334:488–493. doi: 10.1056/NEJM199602223340802. [DOI] [PubMed] [Google Scholar]
- 5.Cranney A, Tugwell P, Adachi J. et al. Osteoporosis Methodology Group and The Osteoporosis Research Advisory Group . Meta-analyses of therapies for postmenopausal osteoporosis. III. Meta-analysis of risedronate for the treatment of postmenopausal osteoporosis. Endocr Rev. 2002;23:517–523. doi: 10.1210/er.2001-3002. [DOI] [PubMed] [Google Scholar]
- 6.Black D M, Cummings S R, Karpf D B. et al. Fracture Intervention Trial Research Group . Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 7.Mehrotra B. Safety profile of intravenous bisphosphonates. Semin Oncol. 2007;34(6) 04:S24–S27. doi: 10.1053/j.seminoncol.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Migliorati C A. Bisphosphanates and oral cavity avascular bone necrosis. J Clin Oncol. 2003;21:4253–4254. doi: 10.1200/JCO.2003.99.132. [DOI] [PubMed] [Google Scholar]
- 9.Marx R E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Goodger N M, Pogrel M A. Osteonecrosis of the jaws associated with cancer chemotherapy. J Oral Maxillofac Surg. 2003;61:1104–1107. doi: 10.1016/s0278-2391(03)00328-8. [DOI] [PubMed] [Google Scholar]
- 11.Walter C, Al-Nawas B, Grötz K A. et al. Prevalence and risk factors of bisphosphonate-associated osteonecrosis of the jaw in prostate cancer patients with advanced disease treated with zoledronate. Eur Urol. 2008;54:1066–1072. doi: 10.1016/j.eururo.2008.06.070. [DOI] [PubMed] [Google Scholar]
- 12.Bamias A, Kastritis E, Bamia C. et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23:8580–8587. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- 13.Vahtsevanos K, Kyrgidis A, Verrou E. et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J Clin Oncol. 2009;27:5356–5362. doi: 10.1200/JCO.2009.21.9584. [DOI] [PubMed] [Google Scholar]
- 14.Boonyapakorn T, Schirmer I, Reichart P A, Sturm I, Massenkeil G. Bisphosphonate-induced osteonecrosis of the jaws: prospective study of 80 patients with multiple myeloma and other malignancies. Oral Oncol. 2008;44:857–869. doi: 10.1016/j.oraloncology.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Lo J C, O'Ryan F S, Gordon N P. et al. Predicting Risk of Osteonecrosis of the Jaw with Oral Bisphosphonate Exposure (PROBE) Investigators . Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J Oral Maxillofac Surg. 2010;68:243–253. doi: 10.1016/j.joms.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons . American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65:369–376. doi: 10.1016/j.joms.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Ruggiero S L Dodson T B Assael L A Landesberg R Marx R E Mehrotra B American Association of Oral and Maxillofacial Surgeons American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update J Oral Maxillofac Surg 200967(5, Suppl):2–12. [DOI] [PubMed] [Google Scholar]
- 18.Khosla S, Burr D, Cauley J. et al. American Society for Bone and Mineral Research . Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 19.Abu-Id M H, Açil Y, Gottschalk J, Kreusch T. [Bisphosphonate-associated osteonecrosis of the jaw] Mund Kiefer Gesichtschir. 2006;10:73–81. doi: 10.1007/s10006-005-0670-0. [DOI] [PubMed] [Google Scholar]
- 20.Abu-Id M H, Warnke P H, Gottschalk J. et al. “Bis-phossy jaws”—high and low risk factors for bisphosphonate-induced osteonecrosis of the jaw. J Craniomaxillofac Surg. 2008;36:95–103. doi: 10.1016/j.jcms.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Otto S, Hafner S, Grötz K A. The role of inferior alveolar nerve involvement in bisphosphonate-related osteonecrosis of the jaw. J Oral Maxillofac Surg. 2009;67:589–592. doi: 10.1016/j.joms.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Wongchuensoontorn C, Liebehenschel N, Wagner K. et al. Pathological fractures in patients caused by bisphosphonate-related osteonecrosis of the jaws: report of 3 cases. J Oral Maxillofac Surg. 2009;67:1311–1316. doi: 10.1016/j.joms.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 23.Wang E P, Kaban L B, Strewler G J, Raje N, Troulis M J. Incidence of osteonecrosis of the jaw in patients with multiple myeloma and breast or prostate cancer on intravenous bisphosphonate therapy. J Oral Maxillofac Surg. 2007;65:1328–1331. doi: 10.1016/j.joms.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Dimopoulos M A, Kastritis E, Anagnostopoulos A. et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: evidence of increased risk after treatment with zoledronic acid. Haematologica. 2006;91:968–971. [PubMed] [Google Scholar]
- 25.Marx R E, Cillo J E Jr, Ulloa J J. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg. 2007;65:2397–2410. doi: 10.1016/j.joms.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Badros A, Weikel D, Salama A. et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol. 2006;24:945–952. doi: 10.1200/JCO.2005.04.2465. [DOI] [PubMed] [Google Scholar]
- 27.Diel I J, Fogelman I, Al-Nawas B. et al. Pathophysiology, risk factors and management of bisphosphonate-associated osteonecrosis of the jaw: Is there a diverse relationship of amino- and non-aminobisphosphonates? Crit Rev Oncol Hematol. 2007;64:198–207. doi: 10.1016/j.critrevonc.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Hoff A O, Toth B B, Altundag K. et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826–836. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hess L M, Jeter J M, Benham-Hutchins M, Alberts D S. Factors associated with osteonecrosis of the jaw among bisphosphonate users. Am J Med. 2008;(121):475–483000. doi: 10.1016/j.amjmed.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen M R Burr D B The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data J Oral Maxillofac Surg 200967(5, Suppl):61–70. [DOI] [PubMed] [Google Scholar]
- 31.Allen M R. Bisphosphonates and osteonecrosis of the jaw: moving from the bedside to the bench. Cells Tissues Organs. 2009;(189):289–294. doi: 10.1159/000151371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodan G A, Fleisch H A. Bisphosphonates: mechanisms of action. J Clin Invest. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scavelli C, Di Pietro G, Cirulli T. et al. Zoledronic acid affects over-angiogenic phenotype of endothelial cells in patients with multiple myeloma. Mol Cancer Ther. 2007;6(12 Pt 1):3256–3262. doi: 10.1158/1535-7163.MCT-07-0311. [DOI] [PubMed] [Google Scholar]
- 34.Hansen T, Kunkel M, Weber A, James Kirkpatrick C. Osteonecrosis of the jaws in patients treated with bisphosphonates—histomorphologic analysis in comparison with infected osteoradionecrosis. J Oral Pathol Med. 2006;35:155–160. doi: 10.1111/j.1600-0714.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 35.Reid I R, Bolland M J, Grey A B. Is bisphosphonate-associated osteonecrosis of the jaw caused by soft tissue toxicity? Bone. 2007;41:318–320. doi: 10.1016/j.bone.2007.04.196. [DOI] [PubMed] [Google Scholar]
- 36.Otto S, Hafner S, Mast G. et al. Bisphosphonate-related osteonecrosis of the jaw: is pH the missing part in the pathogenesis puzzle? J Oral Maxillofac Surg. 2010;(68):1158–1161. doi: 10.1016/j.joms.2009.07.079. [DOI] [PubMed] [Google Scholar]
- 37.Russell R G, Watts N B, Ebetino F H, Rogers M J. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 38.Sato M, Grasser W, Endo N. et al. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest. 1991;88:2095–2105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otto S, Pautke C, Opelz C. et al. Osteonecrosis of the jaw: effect of bisphosphonate type, local concentration, and acidic milieu on the pathomechanism. J Oral Maxillofac Surg. 2010;68:2837–2845. doi: 10.1016/j.joms.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 40.Agis H, Blei J, Watzek G, Gruber R. Is zoledronate toxic to human periodontal fibroblasts? J Dent Res. 2010;89:40–45. doi: 10.1177/0022034509354298. [DOI] [PubMed] [Google Scholar]
- 41.Nancollas G H, Tang R, Phipps R J. et al. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 2006;38:617–627. doi: 10.1016/j.bone.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Hoffmann T, John M, Kerschbaum T, MIcheels W, Potthoff P, Reich E, Reis U, Reiter F, Schiffner U, Schroeder E. Cologne, Germany: Deutscher Zahnärzte Verlag DÄV; 2006. Oral epidemiology. [Google Scholar]
- 43.Ruggiero S L, Fantasia J, Carlson E. Bisphosphonate-related osteonecrosis of the jaw: background and guidelines for diagnosis, staging and management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:433–441. doi: 10.1016/j.tripleo.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Montebugnoli L, Felicetti L, Gissi D B, Pizzigallo A, Pelliccioni G A, Marchetti C. Biphosphonate-associated osteonecrosis can be controlled by nonsurgical management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:473–477. doi: 10.1016/j.tripleo.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Badros A, Terpos E, Katodritou E. et al. Natural history of osteonecrosis of the jaw in patients with multiple myeloma. J Clin Oncol. 2008;26:5904–5909. doi: 10.1200/JCO.2008.16.9300. [DOI] [PubMed] [Google Scholar]
- 46.Van den Wyngaert T, Claeys T, Huizing M T, Vermorken J B, Fossion E. Initial experience with conservative treatment in cancer patients with osteonecrosis of the jaw (ONJ) and predictors of outcome. Ann Oncol. 2009;20:331–336. doi: 10.1093/annonc/mdn630. [DOI] [PubMed] [Google Scholar]
- 47.Stanton D C, Balasanian E. Outcome of surgical management of bisphosphonate-related osteonecrosis of the jaws: review of 33 surgical cases. J Oral Maxillofac Surg. 2009;67:943–950. doi: 10.1016/j.joms.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 48.Pautke C, Bauer F, Otto S. et al. Fluorescence-guided bone resection in bisphosphonate-related osteonecrosis of the jaws: first clinical results of a prospective pilot study. J Oral Maxillofac Surg. 2011;69:84–91. doi: 10.1016/j.joms.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 49.Markose G, Mackenzie F R, Currie W J, Hislop W S. Bisphosphonate osteonecrosis: a protocol for surgical management. Br J Oral Maxillofac Surg. 2009;47:294–297. doi: 10.1016/j.bjoms.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Carlson E R Basile J D The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws J Oral Maxillofac Surg 200967(5, Suppl):85–95. [DOI] [PubMed] [Google Scholar]
- 51.Vescovi P, Manfredi M, Merigo E, Meleti M. Early surgical approach preferable to medical therapy for bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2008;66:831–832. doi: 10.1016/j.joms.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 52.Pautke C, Bauer F, Bissinger O. et al. Tetracycline bone fluorescence: a valuable marker for the osteonecrosis characterization and therapy. J Oral Maxillofac Surg. 2010;68:125–129. doi: 10.1016/j.joms.2009.05.442. [DOI] [PubMed] [Google Scholar]
- 53.Pautke C, Bauer F, Tischer T. et al. Fluorescence-guided bone resection in bisphosphonate-associated osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67:471–476. doi: 10.1016/j.joms.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 54.Fleisher K E, Doty S, Kottal S, Phelan J, Norman R G, Glickman R S. Tetracycline-guided debridement and cone beam computed tomography for the treatment of bisphosphonate-related osteonecrosis of the jaw: a technical note. J Oral Maxillofac Surg. 2008;66:2646–2653. doi: 10.1016/j.joms.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 55.Abu-Id M H, Warnke P H, Gottschalk J. et al. “Bis-phossy jaws”—high and low risk factors for bisphosphonate-induced osteonecrosis of the jaw. J Craniomaxillofac Surg. 2008;36:95–103. doi: 10.1016/j.jcms.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Marx R E Reconstruction of defects caused by bisphosphonate-induced osteonecrosis of the jaws J Oral Maxillofac Surg 200967(5, Suppl):107–119. [DOI] [PubMed] [Google Scholar]
- 57.Engroff S L, Kim D D. Treating bisphosphonate osteonecrosis of the jaws: is there a role for resection and vascularized reconstruction? J Oral Maxillofac Surg. 2007;65:2374–2385. doi: 10.1016/j.joms.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 58.Cornelius C P, Augustin J B, Sailer L K. External pin fixation for stabilization of the mandible—comeback of a method: historical review and first experiences with the 'mandible external fixator.'. Oral Maxillofac Surg. 2009;13:1–14. doi: 10.1007/s10006-008-0142-4. [DOI] [PubMed] [Google Scholar]
- 59.Mücke T, Haarmann S, Wolff K D, Hölzle F. Bisphosphonate related osteonecrosis of the jaws treated by surgical resection and immediate osseous microvascular reconstruction. J Craniomaxillofac Surg. 2009;37:291–297. doi: 10.1016/j.jcms.2008.12.004. [DOI] [PubMed] [Google Scholar]


