Abstract
Medial temporal lobe (MTL) areas are crucial for memory tasks such as spatial working memory and temporal association memory, which require an active maintenance of memory for a short period of time (a few hundred milliseconds to tens of seconds). Recent work has shown that the projection from layer III neurons in the medial entorhinal cortex (MEC) to hippocampal region CA1, the temporoammonic (TA) pathway, might be specially important for these memory tasks. In addition, lesions to the entorhinal cortex disrupt persistent firing in CA1 which is believed to support active maintenance of memory. Injection of cholinergic antagonists and group I mGlu receptor antagonists to the MEC impairs spatial working memory and temporal association memory. Consistent with this, we have shown that group I mGlu receptor activation supports persistent firing in principal cells of the MEC layer III in vitro (Yoshida et al., 2008). However, it still remains unknown whether cholinergic receptor activation also supports persistent firing in MEC layer III neurons. In this paper, we tested this in MEC layer III cells using both ruptured and perforated whole-cell recordings in vitro. We report that the majority of cells we recorded from in MEC layer III show persistent firing during perfusion of the cholinergic agonist carbachol (2 – 10 μM). In addition, repeated stimulation gradually suppressed persistent firing. We further discuss the possible role of persistent firing in memory in general.
Keywords: acetylcholine, electrophysiology, patch-clamp, memory & learning, working memory
1. Introduction
Much evidence supports the involvement of medial temporal lobe (MTL) structures in tasks that require active maintenance of memory (duration: a few hundred milliseconds to tens of seconds) such as working memory, delayed match to sample and trace conditioning both in humans [1–4] and animals [5–10]. Active maintenance of memory may help association of temporally separated information which is thought to be necessary for the formation of long-term episodic and sequential memory [11–13]. In vivo recordings in animals [14–16] and fMRI in humans [14;15] have revealed persistent activity during the trace or delay period of memory tasks when information retention is necessary (reviewed in [16]). These indicate that persistent neural firing may support temporal association by providing a means to retain necessary information across the temporal gap. However, the specific MTL areas of importance and underlying mechanisms for persistent firing still remain unknown.
Persistent firing can be supported either by synaptic networks or the mechanisms within individual neurons (reviewed in [17]). Network based persistent firing was introduced by classical theoretical work which dates back to Donald Hebb's theory of cell assemblies [18] or to David Marr's theory of memory [19]. In these theories, synaptic excitation maintains neuron activity. Another line of studies supports the importance of mechanisms in individual cells to support persistent firing. This view is supported by the experimental observations of persistent firing in vitro in multiple areas in the MTL including the entorhinal cortex [20;21], post-subiculum [22] and perirhinal cortex [23]. In these areas, persistent firing is supported in individual cells by cholinergic and group I mGlu receptor activation which leads to the activation of the CAN current (reviewed in [24]).
Persistent firing in vivo has been shown in the EC [25;26], and hippocampal regions CA1 and CA3 [27–31] both in DMS tasks and trace conditioning. It has also been clear that cholinergic [10;32–34] and/or group I mGlu [35–37] receptor activation is necessary for performance in these memory tasks. These pharmacological studies are consistent with single cell mechanisms for supporting persistent firing.
Recently, using mutant mice, Suh et al. [38] showed the specific importance of the temporoammonic (TA) pathway, the direct projection from layer III of the medial entorhinal cortex (MEC) to the hippocampal CA1 field, in spatial working memory and temporal association memory. Although place cells and spatial reference memory were normal in these mutant mice, they exhibited a deficit in spatial working memory tasks such as the delayed matching-to-place (DMP) version of the water maze task (delay 30 s) and the delayed non–matching-to-place (DNMP) version of the T-maze task (delay 15 s). Mutants also had a deficit in a non-spatial temporal association task such as trace fear-conditioning with 20 s trace period. They further showed that infusion of a cholinergic receptor antagonist and a group I mGlu receptor antagonist in MEC layer III was effective in disrupting the task in control mice but no effect was seen in mutants. This study, therefore, indicated a particular importance of MEC layer III in active maintenance of memory possibly through persistent firing supported by individual cells.
Although we have reported that group I mGlu receptor activation supports persistent firing in principal cells in the MEC layer III, [39] it remains unknown whether cholinergic receptor activation can support persistent firing in these cells. Therefore, in this study, we tested the induction of persistent firing in rat MEC layer III cells using in vitro whole cell patch and perforated patch recordings in the cholinergic receptor agonist carbachol. We show that persistent firing can be greatly enhanced in carbachol in a dose dependent manner. We further show that multiple stimulations can gradually terminate persistent firing.
2. Materials and Methods
2.1 Ruptured whole-cell patch recording
All ruptured whole-cell patch recordings were conducted at Boston University. All experimental protocols were approved by the Institutional Animal Care and Use Committee at Boston University. Long-Evans rats (postnatal days 21 to 27; Charles River, Wilmington, MA) were deeply anesthetized with ketamine/xylazine (95 mg/Kg ketamine and 2.8 mg/Kg xylazine) through intraperitoneal injection. After the absence of both pedal and tail pinch reflex was confirmed, ice-cold modified artificial cerebrospinal fluid (ACSF) containing (in mM) 110 choline chloride, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 0.5 CaCl2, 7 MgCl2, 7 glucose, 3 pyruvic acid and 1 ascorbic acid (pH adjusted to 7.4 by saturation with 95% O2 - 5% CO2) was intracardially perfused. The brain was then removed from the cranium and placed in ice-cold modified ACSF. 350 μm-thick slices were cut horizontally using a Vibroslicer (World Precision Instruments, Sarasota, FL, USA). Slices were transferred to a holding chamber, where they were kept submerged at 30 degrees for 30 min and then at room temperature at least 30 more min before recording. The holding chamber was filled with ACSF containing (in mM) 124 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 1.6 CaCl2, 1.8 MgSO4, 10 glucose (pH adjusted to 7.4 by saturation with 95% O2 - 5% CO2).
Slices were transferred to a submerged recording chamber and superfused with ACSF, maintaining the temperature in between 34 to 36 °C for recordings. Patch pipettes were fabricated from borosilicate glass capillaries by means of a P-87 horizontal puller (Sutter Instrument, Novato, CA, USA). Patch pipettes were filled with intracellular solution containing (in mM) 120 K-gluconate, 10 HEPES, 0.2 EGTA, 20 KCl, 2 MgCl, 7 phosphocreatine-diTris, 4 Na2ATP, 0.3 TrisGTP and 0.1 % biocytin (pH adjusted to 7.3 with KOH). When filled with this solution, the patch pipettes had a resistance of 3–5 MΩ Slices were visualized with an upright microscope (Zeiss Axioskop 2), equipped with a ×40 water-immersion objective lens, and a near-infrared charge-coupled device (CCD) camera (JAI CV-M50IR, San Jose, CA, USA). Tight seals (>1 GΩ) were formed on cell bodies and the membrane was ruptured with negative pressure. Current-clamp recordings were made with a Multi Clamp 700B amplifier (Axon Instruments, Foster City, CA, USA). Signals were lowpass filtered at 5 kHz or 10 kHz and sampled at 10 kHz or 20 kHz, respectively, using Clampex 9.0 software (Axon Instruments, Foster City, CA, USA). A liquid junction potential of 10 mV was not corrected. All whole-cell recordings were performed in the presence of synaptic blockers to suppress ionotropic glutamatergic and GABAergic synaptic transmission by using kynurenic (2 mM) acid and picrotoxin (100 μM).
Stock solutions of carbachol (10 mM, in water) was prepared and diluted more than a thousand times in the ACSF. Kynurenic acid and picrotoxin were directly dissolved in the ACSF. Chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Tocris Bioscience (Ellisville, MO, USA).
2.2 Perforated whole-cell patch recording
All perforated patch recordings were performed at McGill University. All experimental procedures were approved by the McGill University Animal Care Committee and were in compliance with the guidelines of the Canadian Council on Animal Care. Long-Evans rats from young (P12) to adult (6 to 7 weeks old) ages were anesthetized with ketamine:xylazine cocktail (60:5 mg/kg), and intracardially perfused with ice-cold modified ACSF containing (in mM) 110 choline chloride, 1.25 NaH2PO4, 25 NaHCO3, 7 MgCl2, 0.5 CaCl2, 2.5 KCl, 7 glucose, 3 pyruvic acid, and 1.3 ascorbic acid. Horizontal slices (350 μm) were obtained using a VT1000 tissue slicer (Leica) with the same modified ACSF. Slices were transferred to a holding chamber, where they were kept submerged for ~1 h at room temperature (22 °C) before recording. The holding chamber was filled with ACSF consisting (in mM) 125 NaCl, 1.25 NaH2PO4, 25 NaHCO3, 2 MgCl2, 1.6 CaCl2, 2.5 KCl, 10 glucose, 3 pyruvic acid, and 1.3 ascorbic acid. ACSF was constantly bubbled with carbogen (95% O2-5% CO2).
Slices were transferred to a submerged recording chamber and superfused with ACSF. Slices were visualized with an upright microscope Axioskop (Zeiss, Oberkochen, Germany) equipped with a x63 water immersion objective and differential contrast optics. A near-infrared charged-coupled device (CCD) camera (Sony XC-75) was used to visualize the neurons.
Layer III medial entorhinal neurons selected for recording using layer II and lamina dissecans as references and were filled with biocytin for later identification. The cells were recorded using the current-clamp technique at 33 ± 1°C with an Axopatch 1D amplifier and Clampex 8.0 recording software (Axon Instruments, Foster City, CA). Whole cell recordings were performed using the perforated-patch technique. Perforated patch was obtained using amphotericin-B (175–200 μg/ml) [40]. Intracellular pipette solution was identical to that in the ruptured whole-cell patch method above. Drugs and chemicals were purchased from Sigma (St. Louis, MO).
Patch pipettes (5–7 MΩ) were pulled using a Sutter P-97 horizontal puller (Sutter Instrument, Novato, CA). Tight seals (>5 GΩ) were obtained by applying constant negative pressure. Electrical access to the cell was obtained by waiting ~30 min for amphotericin-B to attain a stable access resistance (perforated-patch configuration). Bridge correction was performed using the built-in circuit of the amplifier. Sampling rate was 20 kHz and the low-pass filter was set at 5 kHz. All recordings were performed in the presence of the same synaptic blockers as in the ruptured whole-cell patch recording (kynurenic (2 mM) acid and picrotoxin (100 μM)).
2.3 Data analysis
Clampfit 9.0 (Axon Instruments, Foster City, CA, USA) and Matlab (MathWorks, Natick, MA, USA) were used for data analysis. The latency of persistent firing was measured as the time from the offset of the stimulation and the first spike of persistent firing. The frequency of persistent firing was measured as an average firing frequency of the neuron during the period between 10 and 20 s after the termination of the current injection. The membrane potential difference after stimulation was measured as the difference between an average membrane potential during the same period (10 to 20 s) and the baseline membrane potential which was measured as an average of the membrane potential during the period between 1 and 5 s before current injection.
Significance levels were evaluated using paired and unpaired T-tests for comparisons between two groups. Comparisons of more than two groups were performed by either a one-way or repeated measures ANOVA followed by Tukey post-hoc tests. Significance level < 0.05 (ns: not significant, *: P < 0.05, **: P < 0.01, ***: P < 0.001) was used. Data are expressed as means ± SEM.
2.4 Identification of anatomical location
Locations of the cells were confirmed by biocytin staining or photos taken after recordings with the pipette still attached to the cell through the low magnification objective lens which was enough to approximate anatomical location of the cell. Figure 1A shows an example of a biocytin stained neuron in the MEC layer III. Figure 1B shows another example with a higher magnification in which pyramidal morphology can be seen.
Figure 1.
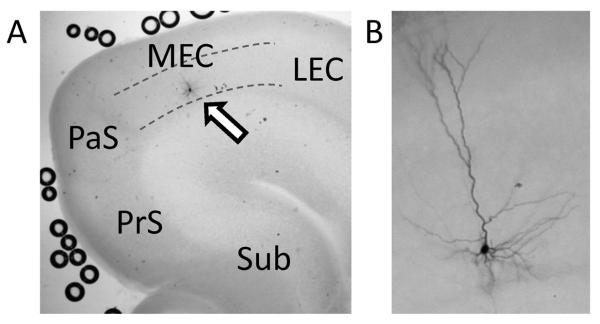
Location and morphology of MEC layer III neurons from which recordings were obtained. (A) A low magnification image showing a biocytin stained MEC layer III neuron (arrow). Dotted lines indicate the upper and the lower borders of the layer III. Sub: subiculum, PrS: presubiculum, PaS: parasubiculum, LEC: lateral entorhinal cortex. (B) A higher magnification image showing another example of biocytin stained MEC layer III neuron. Typical pyramidal cell morphology can be seen.
3. Results
3.1 Cholinergic receptor agonist supports persistent firing
We first tested induction of persistent firing using ruptured whole-cell patch clamp recordings. After obtaining stable recording, the membrane potential was adjusted to just below spike threshold in all conditions prior to testing the induction of persistent firing. This enabled us to compare persistent firing from equal conditions in different neurons and different experimental conditions. Induction of persistent firing was then tested by applying a current step (50 pA, 2 s) which mimics an afferent input signal.
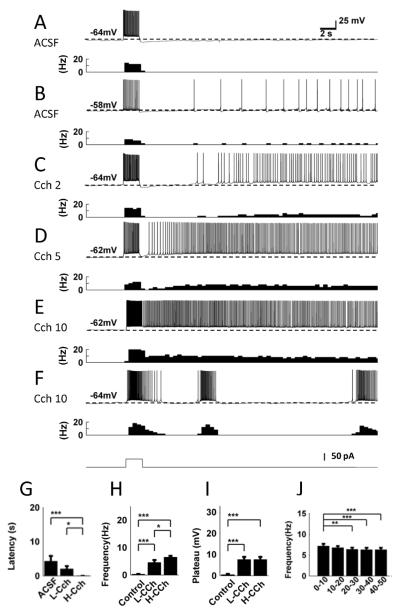
In the control condition in normal ACSF with synaptic blockers, an injection of current step (50 pA, 2 s) induced spiking in the theta domain (9 – 13.5 Hz). Following the termination of the stimulation, 8 out of 14 cells showed no spikes or fewer than 6 spikes in the 30 s period after the stimulation (Fig. 2A). We categorized these responses as no persistent firing response since the firing of these cells was not repetitive. In the rest of the cells (6 cells), the membrane potential gradually depolarized after the offset of the stimulation and persistent repetitive firing with a very low frequency was observed (more than 6 spikes in the 30 s period; Fig. 2B). The latency of the persistent firing was on average 4.28 ± 1.55 s (Fig. 2G), and the frequency of persistent firing in 6 cells that showed persistent firing was 0.43 ± 0.14 Hz (range: 0.4 – 1.4 Hz) and was 0.28 ± 0.12 Hz as an average of all 14 cells tested (Fig. 2H). The depolarization during persistent firing in the 6 cells that showed persistent firing was 2.05 ± 0.47 mV and 0.52 ± 0.33 mV as an average of all 14 cells (Fig. 2F). These agree with our previous observation of low-frequency persistent firing in the absence of cholinergic agonist [39].
Figure 2.
Cholinergic receptor agonist supports persistent firing. A, An example of no persistent firing response of MEC layer III neuron in normal ACSF. The upper trace shows membrane potential and the lower graph shows frequency of firing. A current injection (2 s, 50 pA; shown in F, bottom trace) drove spiking during stimulation but did not induce persistent firing. B, An example of a low-frequency long-lasting persistent firing in ACSF. C–E, Examples of long-lasting persistent firing in 2, 5, and 10 μM carbachol (Cch) respectively. F, An example of repetitive clustered firing in Cch (10 μM). G, Latency to the onset of persistent firing in different pharmacological conditions. One way ANOVA test, F(2, 36) = 12.45, p < 0.001. H, Frequency of persistent firing in different pharmacological conditions. One way ANOVA test, F(2, 45) = 36.82, p < 0.001. I, Plateau potential in different pharmacological conditions. One way ANOVA test, F(2, 45) = 36.3, p < 0.001. J, Average persistent firing frequency from different time intervals. Repeated measures ANOVA test, F(4, 72) = 7.647, p < 0.001.
Using 11 cells out of the 14 cells discussed above, we tested the effect of relatively low concentration of cholinergic receptor agonist carbachol (2 – 5 μM; L-Cch). In this condition, the same stimulation induced persistent firing in the majority of cells (91%; 10 out of 11 cells; 6 out of 7 cells in 2 μM, and 4 out of 4 cells in 5 μM carbachol; Fig. 2C and D). In these cells, persistent firing lasted more than 30 s. We call this type of response long-lasting persistent firing. The average frequency of long-lasting persistent firing was 5.00 ± 0.85 Hz and average depolarization during persistent firing was 8.34 ± 1.20 mV. In the remaining cell (1 cell) persistent firing was not observed. On average, although the latency of persistent firing was not significantly different from the control condition (3.53 ± 1.69 s; n = 10; Tukey post-hoc test, p > 0.05; Fig. 2G), the frequency of persistent firing (4.54 ± 0.90 Hz; n = 11; Tukey post-hoc test, p < 0.001, Fig. 2H) and depolarization during persistent firing (7.62 ± 1.31 mV; n = 11; Tukey post-hoc test, p < 0.001; Fig. 2I) were both significantly higher than the levels in the control condition.
We further tested a higher concentration of carbachol (10 μM; H-Cch) in an additional 23 cells. The same stimulation induced long-lasting persistent firing in 83 % (19 out of 23) of cells tested (Fig. 2E). The average frequency of long-lasting persistent firing was 5.60 ± 0.40 Hz and average depolarization during persistent firing was 9.82 ± 0.61 mV. Two cells (9 %) showed persistent firing which terminated by itself before reaching 30 s and repeated clustered firing as shown in Fig. 2F (self-terminating persistent firing). The rest of the cells (2 cells) showed no persistent firing. In this condition, the latency of persistent firing (0.03 ± 0.0034 s; n = 21) was significantly shorter than the control condition (Tukey post-hoc test, p < 0.001) and low carbachol conditions (Tukey post-hoc test, p < 0.05; Fig. 2G). The frequency of persistent firing (6.54 ± 0.48 Hz; n = 23) was also significantly higher than that in the control (Tukey post-hoc test, p < 0.001) and the low carbachol conditions (Tukey post-hoc test, p < 0.05; Fig. 2H). The depolariation during persistent firing (7.62 ± 1.31 mV; n = 23) was significantly higher than that in the control condition (Tukey post-hoc test, p < 0.001) but was not different from the low carbachol condition (Tukey post-hoc test, p > 0.05; Fig. 2I).
To address the stability of long-lasting persistent firing (n = 19), the recording was continued for a longer period of time in this condition (50 s). As shown in Fig. 2J, frequency of persistent firing was in general stable over a 50 s recording period, with some exceptions where the frequency went down during the first 10 – 20 s.
These observations indicate that cholinergic receptor activation via carbachol greatly increases the percentage of cells that show persistent firing and the frequency of persistent firing in MEC layer III cells. In addition, persistent firing is stable for more than 30 s in the majority of cells.
3.2 Persistent firing with perforated patch clamp method
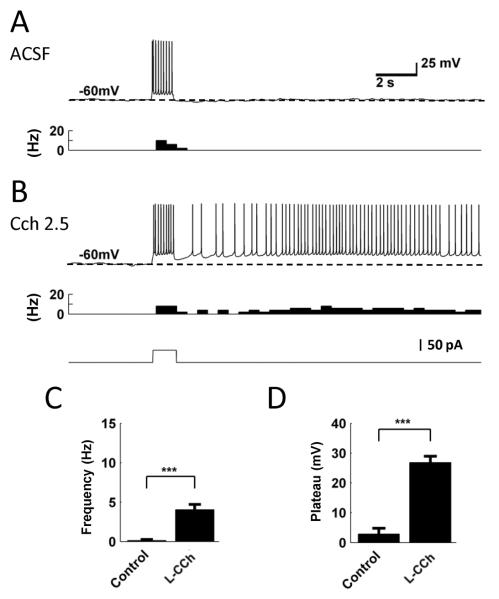
We next tested the induction of persistent firing using perforated whole-cell patch clamp recordings. Since this method keeps the chemical components of the cytoplasm intact, the recording condition is closer to the physiological condition compared to the ruptured whole-cell patch clamp method [41]. In the control condition in normal ACSF with synaptic blockers, 89 % (8 out of 9 cells) showed no persistent firing (Fig. 3A) and 11 % (1 out of 9 cells) showed persistent firing with a very low frequency (1 Hz).
Figure 3.
Persistent firing recorded with perforated patch clamp method. A, An example of no persistent firing response in normal ACSF. A current injection (1 s, 100 pA; shown in B, bottom trace) drove spiking during stimulation but did not induce persistent firing. B, An example of long-lasting persistent firing induced in Cch (2.5 μM). C, Frequency of persistent firing in different pharmacological conditions. D, Plateau potential in different pharmacological conditions.
Similar to the case of ruptured whole-cell recording, low concentrations of carbachol induced long-lasting persistent firing (4 out of 4 in 2.5 μM; 4 out of 4 in 5 μM; Fig. 3B) Compared to the control condition, both the frequency (unpaired T-test, p < 0.001; Control: n = 9, L-Cch: n = 8) and depolarization during persistent firing (unpaired T-test, p < 0.001; Control: n = 9, L-Cch: n = 8) increased significantly in carbachol (Fig 3C and D). These results indicate that persistent firing can be induced in MEC layer III cells using the perforated patch technique and carbachol significantly promotes persistent firing as in the whole-cell patch method. This suggests that persistent firing observed in ruptured whole-cell patch methods is not due to dilution of the cytoplasm.
3.3 Repeated stimulation suppresses persistent firing
In an in vivo condition, a neuron may receive similar stimulation multiple times. Repeated stimulation tested in vitro has been shown to facilitate persistent firing in MEC layer V neurons [21] and to terminate persistent firing in lateral entorhinal cortex layer III neurons [42]We tested repeated current injection (amplitude 10 to 100 pA; duration 1 to 4 s) using perforated patch recordings (n = 6). Interestingly, while the first stimulation consistently evoked persistent firing, subsequent repeated application of the same current steps consistently suppressed persistent firing (Fig. 4A). Figure 4B – D show latency, frequency and depolarization of persistent firing for 6 cells with repeated stimulation. The latency of persistent firing increased (Fig. 4A), the frequency of persistent firing decreased as the stimulation was repeated to almost 0 Hz after the 4th stimulation (Fig. 4C). The plateau potential also decreased with repeated stimulation (Fig. 4D).
Figure 4.
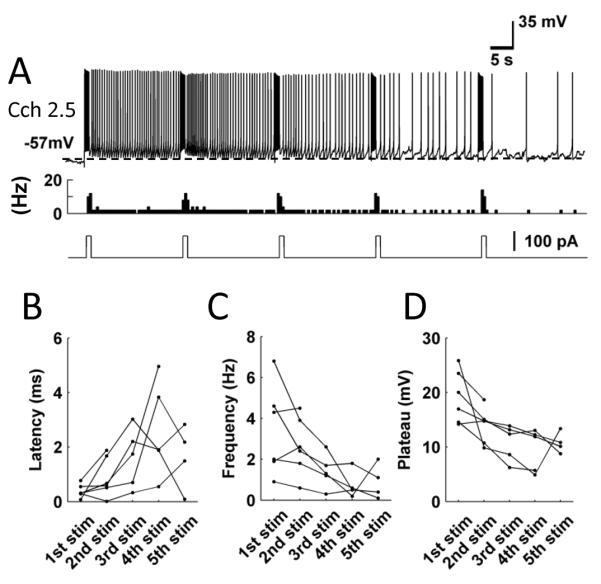
Repeated stimulation suppresses persistent firing. A, An example of persistent firing during repeated stimulations. From top to bottom, traces show the membrane potential, the firing frequency and the injected current. B – D, Latency, frequency and plateau potential of persistent firing of individual cells after repeated stimulation. These recordings were performed with perforated patch clamp method.
These results suggest that repeated input to these neurons may be able to gradually decrease the frequency of persistent firing.
4. Discussions
The entorhinal cortex has been shown to be crucial for memory tasks that involve association of temporally separated information. For example, lesions to the entorhinal cortex impair trace-conditioning in rabbits [10;43]. Motivated by the recent finding that the temporoammonic pathway from the MEC layer III to CA1 input is crucial for tasks that involve short-term information retention [38], we tested the induction of persistent firing in MEC layer III principal neurons. We found that persistent firing is observed in a majority of cells when cholinergic receptors are activated with carbachol. We demonstrated that persistent firing can be induced both in ruptured and perforated patch recordings with a carbachol concentration as low as 2 μM. We further showed that repeated stimulation may gradually inhibit persistent firing. These observations indicate that cholinergic receptor activation supports persistent firing in MEC layer III cells.
4.1 Comparison to persistent firing observed in other areas in vitro
In in vitro preparations of the EC, similar persistent firing has been reported in MEC layer II [20;44], layer V [21;45] and in lateral EC (LEC) layer III [42] neurons in the presence of the cholinergic receptor agonist carbachol. In addition, we have shown that activation of group I mGlu receptors by an agonist or by synaptic stimulation supports persistent firing in MEC layer III cells [39]. However, it is still unknown whether persistent firing can be supported through cholinergic receptor activation in MEC layer III cells. Our current study is therefore the first to report such persistent firing. Together with our previous study [39], our data suggest that persistent firing in individual MEC layer III neurons can be supported both through cholinergic and group I mGlu receptors.
Induction of persistent firing through both cholinergic and group I mGlu receptors is supported by intracellular mechanisms. In vitro studies using brain slices from the MTL areas have indicated that the following steps are involved in the induction of persistent firing under cholinergic receptor activation: (1) calcium influx to the cell, (2) activation of phospholipase Cβ (PLCβ), and (3) activation of the calcium activated non-specific cationic (CAN) current [21–23;42;46]. The CAN current is shown to be mediated by transient receptor potential cation (TRPC) channels [46;47], reviewed in [24] which can be activated both through muscarinic cholinergic receptor activation and through group I mGlu receptor activation [48–52]. This is consistent with the fact that tasks that require active maintenance of memory are impaired by the blockade of both cholinergic receptors and group I mGlu receptors [38].
4.2 Distinct properties of persistent firing in MEC layer III cells
In this study, MEC layer III cells showed persistent firing with low firing frequency even in normal ACSF in a subset of cells (Fig. 2). This is in agreement with our previous report where similar persistent firing with a low frequency was observed in the presence of the muscarinic receptor antagonist atropine without cholinergic or group I mGlu receptor agonists [39]. As discussed in our earlier paper, this could be because a small amount of the CAN current may be activated solely by the increase in intra-cellular calcium [48] due to spiking during current injection. Such persistent firing has not been reported from other areas of the EC without cholinergic agonist: MEC layer II [22;44] and V [21] cells and LEC layer III cells [42]. MEC layer III neurons have a high input resistance compared to other types of neurons in the EC (76.9 ± 20.1 MΩ; [53–55]) and very little or no voltage `sag' is observed at the soma of these cells indicating less membrane potential damping by the hyperpolarization-activated cation current compared to other entorhinal neurons [53]. Relatively high excitability due to these properties may enable the small amount of CAN current activated in the absence of the cholinergic agonist to depolarize the membrane potential of these cells sufficiently to induce persistent firing. MEC Layer III cells, therefore, could be more prone to induction of persistent firing compared to cells from other layers of the MEC. In addition, the percentage of cells with persistent firing in carbachol (83 – 100 %) was one of the highest among different MTL areas: 67 % in MEC layer II (unpublished observation by Yoshida, Jochems and Hasselmo), 84 – 98% in MEC layer V cells [21] and 79 – 84 % in the postsubiculum [22], Therefore, MEC layer III cells seem to have a low threshold for persistent firing and are prone to persistent firing.
Previous reports which tested repeated excitatory stimulation showed a gradual increase in firing frequency in MEC layer V [21] and complete termination of persistent firing by the second application of stimulation in LEC layer III neurons [42]. Our observation added a novel type of response where frequency of persistent firing gradually decreased with the repetition of identical excitatory stimulation. Although we did not test the mechanisms underlying the gradual decrease in the frequency of persistent firing, activation of calcium activated potassium current such as the large conductance calcium-activated (BK) channels may underlie the suppression [56]. This gradual suppression may serve several purposes. First, multistability of the firing rate of the persistent firing may allow neurons to code multiple memories using different firing rates, rather than using only `on' and `off' states. Such rate coding may increase the information which can be maintained by a limited number of cells. Second, gradual suppression might be used as a means to integrate repeated input. While the MEC layer V cells integrate repeated excitatory input as a positive increase in frequency of persistent firing [21], layer III cells may hold the same information as a decrease in firing. This, in theory, will enable layer III and layer V of the MEC to code novel and familiar information, respectively. Consider that the same stimulation activates a group of cells both in layer III and V. When this stimulation is given for the first time, layer III cells will be firing with a high frequency while layer V cells will be firing with a low frequency, resulting in a strong representation of the novel signal in the layer III. However, as this stimulation is repeated and becomes familiar, the layer V cells will code this with a higher frequency than the layer III cells do, resulting in a stronger representation of a familiar signal in the layer V. Therefore, a novel stimulus might be coded by the layer III initially and it could be gradually shifted to the layer V as this stimulus becomes more familiar. Third, the reduction of persistent firing frequency following subsequent excitatory stimulation could serve as a mechanism for implementing grid cell firing [57]. The theoretical framework for grid cell firing using graded persistent firing cells was described earlier by us [58–60] and this can be extended to graded persistent firing cells that reduce firing frequency upon subsequent stimulation. In the original framework, different graded persistent firing cells start out with the same baseline frequency of persistent firing and provide convergent input to a grid cell. The grid cell will fire when the inputs are in synchrony. Speed modulated head direction input to different graded persistent firing cells will alter the frequency of the graded persistent firing cells and thus also the phase of firing. A reduction in firing frequency of a graded persistent firing cell, following depolarizing input from head direction cells, can also support synchronization and desynchronization of multiple graded persistent firing cells. These graded persistent firing cells provide their input to a grid cell and thereby form grid cell firing.
4.3 Functional relevance of persistent firing in the MEC layer III
During trace-conditioning, the entorhinal cortex shows persistent firing similar to that observed in the hippocampus [61]. Persistent firing can also be found in the EC during the memory delay phase of delayed matching and nonmatching-to-sample tasks (DMS and DNMS tasks; [25;26]). The frequency of persistent firing observed in our study with the low concentration of carbachol (2 to 5 μM) is in agreement with that recorded in vivo (typically 0.5 – 4 Hz) in rat entorhinal neurons [25]. Moreover, lesions to the entorhinal cortex disrupt persistent firing in hippocampal region CA1 [43]. Cholinergic receptor blockade by scopolamine in the entorhinal cortex disrupts trace-conditioning [10] and cholinergic deafferentation of the entorhinal cortex impairs DNMS tasks with novel stimuli [32]. These findings are consistent with the idea that EC is important for active maintenance of memory and that persistent firing and cholinergic receptor activation may underlie this function.
Our observation is also in agreement with the idea that the MEC could serve as a temporal memory buffer [11]. Modeling studies have shown that temporally separated input signals could be maintained in the temporal buffer in the entorhinal cortex through persistent firing of groups of cells, and this could enable the hippocampus to associate these signals [60; 62]. Our observation provides the first evidence that the projection neurons from MEC layer III to the CA1 pyramidal cells support long-lasting persistent firing in the presence of the cholinergic agonist carbachol. Acetylcholine levels in the brain are higher during active waking and acetylcholine is believed to support memory encoding in the hippocampus [63]. Our findings thus suggest that during encoding, MEC layer III neurons can strongly support a temporal buffer function. An association of temporally separated signals may then be supported by hippocampal region CA1 which has been shown to be crucial for formation of associations across short delays between items [8;64;65].
Persistent firing may be supported by either network or intrinsic activity [17]. Since our recordings were performed in the presence of ionotropic synaptic blockers, our observations suggest that persistent firing observed in this study is supported by mechanisms intrinsic to cells as in other in vitro studies of persistent firing in the EC [20;21;42]. However, our data does not eliminate the possibility that ionotropic synaptic network can also support persistent firing. In fact, it has been shown that short-lasting plateau potentials (the UP state) during slow-wave-oscillation-like activity observed in the in vitro EC preparation depends on ionotropic synapses [64 – 66]. However, it should be noted that the duration of an UP state is 1 – 3 s [66]. This duration is much shorter than the persistent firing we observed (> 30 s) and the memory retention period in many memory tasks that require active maintenance of memory. In addition, while working memory and temporal association memory rely on intact cholinergic and group I mGlu receptor activation, it is not clear whether the ionotropic network origin of persistent firing could be supported by these receptors. In fact, cholinergic agonists in general suppress synaptic transmission [67–69] which will not support network based persistent firing.
Suh et al. [38] showed that the projection from MEC layer III to hippocampal region CA1 is necessary for encoding but not for recall after the animals has acquired a trace conditioning task. This is in accordance with the idea that the MEC serves as a temporal buffer for encoding as mentioned above. Interestingly, novel but not familiar stimuli increase acetylcholine levels in the hippocampus [70]. Persistent firing in trace conditioning studies is observed during the initial phase of learning but it disappears once the animal has acquired the task in hippocampal CA1 neurons [71]. These findings are consistent with the idea that persistent firing supported by cholinergic activation is the key for obtaining the association and once the association is obtained, the task does not rely on persistent firing. However, it may not be the case in other memory tasks where animals need to retain some information even after the acquisition of the tasks. For example, in a spatial working memory task, an intact hippocampus is required even after training [72].
Suh et al., [38] showed that the projection from region CA3 to region CA1 is not critically important for the trace conditioning task. This may indicate that the EC-CA1 pathway plays a more important role in short-term memory, as many other studies support the importance of region CA1 for encoding associations across short delays [8;64;65]. However, studies also support the role of CA3 in memory tasks such as spatial working memory [73] and sequential nonspatial memory [65]. In addition, persistent firing is observed in region CA3 in vivo as well [27;74]. We have recently shown that region CA3 pyramidal cells support persistent firing at a single cell level similar to current observation of persistent firing in EC layer III neurons in vitro [75]. It could be that region CA3 is involved when the task involves a spatial or sequential component in addition to an active maintenance of memory.
4.4 Concluding remarks
We have reported persistent firing supported by a cholinergic receptor activation in a single cell level in MEC layer III cells. This persistent firing may support active maintenance of memory in working memory and temporal association tasks that were recently shown to depend on the output of these cells [38]. The cholinergic dependency of these tasks supports single cell level persistent firing over the network based persistent firing as the mechanism for active maintenance of memory. Blockade of persistent activity may impair encoding into long-term memory [11]. This could contribute to the impairment of encoding of episodic memories caused by muscarinic cholinergic antagonists such as scopolamine [76;77]. In addition, this cholinergic modulation of the persistent firing property might support switching between encoding and consolidation functions of the MTL [16;63].
Research Highlights
We report persistent firing in MEC layer III cells in vitro.
Cholinergic stimulation increases the fraction of cells that show persistent firing.
Repeated stimulation gradually suppresses persistent firing.
Persistent firing in MEC layer III may help temporal association tasks.
Acknowledgements
This work was supported by NIMH R01 MH60013, R01 MH61492, Silvio O. Conte Center grant MH 094263 and ONR MURI M00014-10-1-0936, JSPS Postdoctoral Fellowship for Research Abroad, postdoctoral fellowship from the Spanish Ministry of Education, and the International Graduate School of Neuroscience at Ruhr-University Bochum.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ranganath C, Blumenfeld RS. Doubts about double dissociations between short- and long-term memory. Trends in Cognitive Sciences. 2005;9(8):374–80. doi: 10.1016/j.tics.2005.06.009. [DOI] [PubMed] [Google Scholar]
- [2].Hannula DE, Tranel D, Cohen NJ. The long and the short of it: relational memory impairments in amnesia, even at short lags. Journal of Neuroscience. 2006;26(32):8352–9. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Olson IR, Moore KS, Stark M, Chatterjee A. Visual working memory is impaired when the medial temporal lobe is damaged. Journal of Cognitive Neuroscience. 2006;18(7):1087–97. doi: 10.1162/jocn.2006.18.7.1087. [DOI] [PubMed] [Google Scholar]
- [4].Hartley T, Bird CM, Chan D, Cipolotti L, Husain M, Vargha-Khadem F, et al. The hippocampus is required for short-term topographical memory in humans. Hippocampus. 2007;17(1):34–48. doi: 10.1002/hipo.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behavioral Neuroscience. 2013;109(2):195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- [6].Weiss C, Bouwmeester H, Power JM, Disterhoft JF. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behavioural Brain Research. 1999;99(2):123–32. doi: 10.1016/s0166-4328(98)00096-5. [DOI] [PubMed] [Google Scholar]
- [7].McEchron MD, Tseng W, Disterhoft JF. Neurotoxic lesions of the dorsal hippocampus disrupt auditory-cued trace heart rate (fear) conditioning in rabbits. Hippocampus. 2000;10(6):739–51. doi: 10.1002/1098-1063(2000)10:6<739::AID-HIPO1011>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- [8].Tseng W, Guan R, Disterhoft JF, Weiss C. Trace eyeblink conditioning is hippocampally dependent in mice. Hippocampus. 2004;14(1):58–65. doi: 10.1002/hipo.10157. [DOI] [PubMed] [Google Scholar]
- [9].Kholodar-Smith DB, Boguszewski P, Brown TH. Auditory trace fear conditioning requires perirhinal cortex. Neurobiology of Learning and Memory. 2008;90(3):537–43. doi: 10.1016/j.nlm.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Esclassan F, Coutureau E, Di Scala G, Marchand AR. A cholinergic-dependent role for the entorhinal cortex in trace fear conditioning. Journal of Neuroscience. 2009;29(25):8087–93. doi: 10.1523/JNEUROSCI.0543-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hasselmo ME, Stern CE. Mechanisms underlying working memory for novel information. Trends in Cognitive Sciences. 2006;10(11):487–93. doi: 10.1016/j.tics.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wallenstein GV, Eichenbaum H, Hasselmo ME. The hippocampus as an associator of discontiguous events. Trends in Neurosciences. 1998;21(8):317–23. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- [13].Naya Y, Suzuki WA. Integrating what and when across the primate medial temporal lobe. Science. 2011;333(6043):773–6. doi: 10.1126/science.1206773. [DOI] [PubMed] [Google Scholar]
- [14].Schon K, Hasselmo ME, LoPresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: A functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. Journal of Neuroscience. 2004;24(49):11088–97. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Olsen RK, Nichols EA, Chen J, Hunt JF, Glover GH, Gabrieli JDE, et al. Performance-related sustained and anticipatory activity in human medial temporal lobe during delayed match-to-sample. Journal of Neuroscience. 2009;29(38):11880–90. doi: 10.1523/JNEUROSCI.2245-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yoshida M, Knauer B, Jochems A. Cholinergic modulation of the CAN current may adjust neural dynamics for active memory maintenance, spatial navigation and time-compressed replay. Frontiers in Neural Circuits. 2012;6(10) doi: 10.3389/fncir.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Major G, Tank D. Persistent neural activity: prevalence and mechanisms. Current Opinion in Neurobiology. 2004;14(6):675–84. doi: 10.1016/j.conb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- [18].Hebb DO, Martinez JL, Glickman SE. The organization of behavior - A neuropsychological theory - Hebb,do. Contemporary Psychology. 1994;39(11):1018–20. [Google Scholar]
- [19].Marr D. Simple memory: a theory for archicortex. Proc R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- [20].Klink R, Alonso A. Muscarinic modulation of the oscillatory and repetitive firing properties of entorhinal cortex layer II neurons. Journal of Neurophysiology. 1997;77(4):1813–28. doi: 10.1152/jn.1997.77.4.1813. [DOI] [PubMed] [Google Scholar]
- [21].Egorov AV, Hamam BN, Fransen E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420(6912):173–8. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- [22].Yoshida M, Hasselmo ME. Persistent firing supported by an intrinsic cellular mechanism in a component of the head direction system. Journal of Neuroscience. 2009;29(15):4945–52. doi: 10.1523/JNEUROSCI.5154-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Navaroli VL, Zhao YJ, Boguszewski P, Brown TH. Muscarinic receptor activation enables persistent firing in pyramidal neurons from superficial layers of dorsal perirhinal cortex. Hippocampus. 2012;22(6):1392–404. doi: 10.1002/hipo.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Reboreda A, Jimenez-Diaz L, Navarro-Lopez JD. TRP channels and neural persistent activity. Transient Receptor Potential Channels. 2011;704:595–613. doi: 10.1007/978-94-007-0265-3_32. [DOI] [PubMed] [Google Scholar]
- [25].Young BJ, Otto T, Fox GD, Eichenbaum H. Memory representation within the parahippocampal region. Journal of Neuroscience. 1997;17(13):5183–95. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. Journal of Neurophysiology. 1997;78(2):1062–81. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- [27].Colombo M, Gross CG. Responses of inferior temporal cortex and hippocampal-neurons during delayed matching-to-sample in monkeys (Macaca-Fascicularis) Behavioral Neuroscience. 1994;108(3):443–55. doi: 10.1037//0735-7044.108.3.443. [DOI] [PubMed] [Google Scholar]
- [28].Deadwyler SA, Hampson RE. Differential but complementary mnemonic functions of the hippocampus and subiculum. Neuron. 2004;42(3):465–76. doi: 10.1016/s0896-6273(04)00195-3. [DOI] [PubMed] [Google Scholar]
- [29].Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behavioral Neuroscience. 1986;100(5):729–44. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- [30].McEchron MD, Weible AP, Disterhoft JF. Aging and learning-specific changes in single-neuron activity in CA1 hippocampus during rabbit trace eyeblink conditioning. Journal of Neurophysiology. 2001;86(4):1839–57. doi: 10.1152/jn.2001.86.4.1839. [DOI] [PubMed] [Google Scholar]
- [31].McEchron MD, Tseng W, Disterhoft JF. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (fear) conditioning in rabbit. Journal of Neuroscience. 2003;23(4):1535–47. doi: 10.1523/JNEUROSCI.23-04-01535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McGaughy J, Koene RA, Eichenbaum H, Hasselmo ME. Cholinergic deafferentation of the entorhinal cortex in rats impairs encoding of novel but not familiar stimuli in a delayed nonmatch-to-sample task. Journal of Neuroscience. 2005;25(44):10237–81. doi: 10.1523/JNEUROSCI.2386-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bang SJ, Brown TH. Perirhinal cortex supports acquired fear of auditory objects. Neurobiology of Learning and Memory. 2009;92(1):53–62. doi: 10.1016/j.nlm.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pang MH, Kim NS, Kim IH, Kim H, Kim HT, Choi JS. Cholinergic transmission in the dorsal hippocampus modulates trace but not delay fear conditioning. Neurobiology of Learning and Memory. 2010;94(2):206–13. doi: 10.1016/j.nlm.2010.05.008. [DOI] [PubMed] [Google Scholar]
- [35].Naie K, Manahan-Vaughan D. Regulation by metabotropic glutamate receptor 5 of LTP in the dentate gyrus of freely moving rats: Relevance for learning and memory formation. Cerebral Cortex. 2004;14(2):189–98. doi: 10.1093/cercor/bhg118. [DOI] [PubMed] [Google Scholar]
- [36].Mikami A, Masuoka T, Yasuda M, Yamamoto Y, Kamei C. Participation of cholinergic system in memory deficits induced by blockade of hippocampal mGlu(1) receptors. European Journal of Pharmacology. 2007;575(1–3):82–6. doi: 10.1016/j.ejphar.2007.07.010. [DOI] [PubMed] [Google Scholar]
- [37].Hayashi K, Yoshihara T, Ichitani Y. Involvement of hippocampal metabotropic glutamate receptors in radial maze performance. Neuroreport. 2007;18(7):719–23. doi: 10.1097/WNR.0b013e3280d9e880. [DOI] [PubMed] [Google Scholar]
- [38].Suh J, Rivest AJ, Nakashiba T, Tominaga T, Tonegawa S. Entorhinal cortex layer III input to the hippocampus Is crucial for temporal association memory. Science. 2011;334(6061):1415–20. doi: 10.1126/science.1210125. [DOI] [PubMed] [Google Scholar]
- [39].Yoshida M, Fransen E, Hasselmo ME. mGluR-dependent persistent firing in entorhinal cortex layer III neurons. European Journal of Neuroscience. 2008;28(6):1116–26. doi: 10.1111/j.1460-9568.2008.06409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. Journal of Neuroscience Methods. 1991;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- [41].Spruston N, Johnston D. Perforated patch-clamp analysis of the passive membrane properties of three classes of hippocampal neurons. Journal of Neurophysiology. 1992;67(3):508–29. doi: 10.1152/jn.1992.67.3.508. [DOI] [PubMed] [Google Scholar]
- [42].Tahvildari B, Fransen E, Alonso AA, Hasselmo ME. Switching between “on” and “off” states of persistent activity in lateral entorhinal layer III neurons. Hippocampus. 2007;17(4):257–63. doi: 10.1002/hipo.20270. [DOI] [PubMed] [Google Scholar]
- [43].Ryou JW, Cho SY, Kim HT. Lesions of the entorhinal cortex impair acquisition of hippocampal-dependent trace conditioning. Neurobiology of Learning and Memory. 2001;75(2):121–7. doi: 10.1006/nlme.2000.3966. [DOI] [PubMed] [Google Scholar]
- [44].Magistretti J, Ma L, Shalinsky MH, Lin W, Klink R, Alonso A. Spike patterning by Ca2+-dependent regulation of a muscarinic cation current in entorhinal cortex layer II neurons. Journal of Neurophysiology. 2004;92(3):1644–57. doi: 10.1152/jn.00036.2004. [DOI] [PubMed] [Google Scholar]
- [45].Reboreda A, Raouf R, Alonso A, Seguela P. Development of cholinergic modulation and graded persistent activity in layer V of medial entorhinal cortex. Journal of Neurophysiology. 2007;97(6):3937–47. doi: 10.1152/jn.01233.2006. [DOI] [PubMed] [Google Scholar]
- [46].Zhang ZZ, Reboreda A, Alonso A, Barker PA, Seguela P. TRPC channels underlie cholinergic plateau potentials and persistent activity in entorhinal cortex. Hippocampus. 2011;21(4):386–97. doi: 10.1002/hipo.20755. [DOI] [PubMed] [Google Scholar]
- [47].Tai C, Hines DJ, Choi HB, MacVicar BA. Plasma membrane insertion of TRPC5 channels contributes to the cholinergic plateau potential in hippocampal CA1 pyramidal neurons. Hippocampus. 2011;21(9):958–67. doi: 10.1002/hipo.20807. [DOI] [PubMed] [Google Scholar]
- [48].Gee CE, Benquet P, Gerber U. Group I metabotropic glutamate receptors activate a calcium-sensitive transient receptor potential-like conductance in rat hippocampus. Journal of Physiology-London. 2003;546(3):655–64. doi: 10.1113/jphysiol.2002.032961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426(6964):285–91. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- [50].Tozzi A, Bengtson CP, Longone P, Carignani C, Fusco FR, Bernardi G, et al. Involvement of transient receptor potential-like channels in responses to mGluR-I activation in midbrain dopamine neurons. European Journal of Neuroscience. 2003;18(8):2133–45. doi: 10.1046/j.1460-9568.2003.02936.x. [DOI] [PubMed] [Google Scholar]
- [51].Bengtson CP, Tozzi A, Bernardi G, Mercuri NB. Transient receptor potential-like channels mediate metabotropic glutamate receptor EPSCs in rat dopamine neurones. Journal of Physiology-London. 2004;555(2):323–30. doi: 10.1113/jphysiol.2003.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Fowler MA, Sidiropoulou K, Ozkan ED, Phillips CW, Cooper DC. Corticolimbic expression of TRPC4 and TRPC5 channels in the rodent brain. Plos One. 2007;2(6) doi: 10.1371/journal.pone.0000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Dickson CT, Mena AR, Alonso A. Electroresponsiveness of medial entorhinal cortex layer III neurons in vitro. Neuroscience. 1997;81(4):937–50. doi: 10.1016/s0306-4522(97)00263-7. [DOI] [PubMed] [Google Scholar]
- [54].Hamam BN, Amaral DG, Alonso AA. Morphological and electrophysiological characteristics of layer V neurons of the rat lateral entorhinal cortex. Journal of Comparative Neurology. 2002;451(1):45–61. doi: 10.1002/cne.10335. [DOI] [PubMed] [Google Scholar]
- [55].Alonso A, Klink R. Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer-II. Journal of Neurophysiology. 1993;70(1):128–43. doi: 10.1152/jn.1993.70.1.128. [DOI] [PubMed] [Google Scholar]
- [56].Tahvildari B, Alonso AA, Bourque CW. Ionic basis of ON and OFF persistent activity in layer III lateral entorhinal cortical principal neurons. Journal of Neurophysiology. 2008;99(4):2006–11. doi: 10.1152/jn.00911.2007. [DOI] [PubMed] [Google Scholar]
- [57].Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, et al. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312(5774):758–62. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- [58].Hasselmo ME, Brandon MP. Linking cellular mechanisms to behaviour: Entorhinal persistent spiking and membrane potential oscillations may underlie path integration, grid cell firing and episodic memory. Neural Plasticity. 2008 doi: 10.1155/2008/658323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hasselmo ME. Grid Cell Mechanisms and Function: Contributions of entorhinal persistent spiking and phase resetting. Hippocampus. 2008;18(12):1213–29. doi: 10.1002/hipo.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jensen O, Lisman JE. Theta/gamma networks with slow NMDA channels learn sequences and encode episodic memory: Role of NMDA channels in recall. Learning & Memory. 1996;3(2–3):264–78. doi: 10.1101/lm.3.2-3.264. [DOI] [PubMed] [Google Scholar]
- [61].Berger TW, Clark GA, Thompson RF. Learning-dependent neuronal responses recorded from limbic system brain structures during classical-conditioning. Physiological Psychology. 1980;8(2):155–67. [Google Scholar]
- [62].Koene RA, Hasselmo ME. Reversed and forward buffering of behavioral spike sequences enables retrospective and prospective retrieval in hippocampal regions CA3 and CA1. Neural Networks. 2008;21(2–3):276–88. doi: 10.1016/j.neunet.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends in Cognitive Sciences. 1999;3(9):351–9. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- [64].Kesner RP, Hunsaker MR, Gilbert PE. The role of CA1 in the acquisition of an object-trace-odor paired associate task. Behavioral Neuroscience. 2005;119(3):781–6. doi: 10.1037/0735-7044.119.3.781. [DOI] [PubMed] [Google Scholar]
- [65].Farovik A, Dupont LM, Eichenbaum H. Distinct roles for dorsal CA3 and CA1 in memory for sequential nonspatial events. Learning & Memory. 2010;17(1):801–6. doi: 10.1101/lm.1616209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Shu YS, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423(6937):288–93. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- [67].Hasselmo ME, Schnell E. Laminar selectivity of the cholinergic suppression of synaptic transmission in rat hippocampal region Ca1 - computational modeling and brain slice physiology. Journal of Neuroscience. 1994;14(6):3898–914. doi: 10.1523/JNEUROSCI.14-06-03898.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Vogt KE, Regehr WG. Cholinergic modulation of excitatory synaptic transmission in the CA3 area of the hippocampus. Journal of Neuroscience. 2001;21(1):75–83. doi: 10.1523/JNEUROSCI.21-01-00075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kremin T, Hasselmo ME. Cholinergic suppression of glutamatergic synaptic transmission in hippocampal region ca3 exhibits laminar selectivity: Implication for hippocampal network dynamics. Neuroscience. 2007;149(4):760–7. doi: 10.1016/j.neuroscience.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Acquas E, Wilson C, Fibiger HC. Conditioned and unconditioned stimuli increase frontal cortical and hippocampal acetylcholine release: Effects of novelty, habituation, and fear. Journal of Neuroscience. 1996;16(9):3089–96. doi: 10.1523/JNEUROSCI.16-09-03089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].McEchron MD, Disterhoft JF. Sequence of single neuron changes in CA1 hippocampus of rabbits during acquisition of trace eyeblink conditioned responses. Journal of Neurophysiology. 1997;78(2):1030–44. doi: 10.1152/jn.1997.78.2.1030. [DOI] [PubMed] [Google Scholar]
- [72].Ainge JA, van der Meer MAA, Langston RF, Wood ER. Exploring the role of context-dependent hippocampal activity in spatial alternation behavior. Hippocampus. 2007;17(10):988–1002. doi: 10.1002/hipo.20301. [DOI] [PubMed] [Google Scholar]
- [73].Lee I, Kesner RP. Differential roles of dorsal hippocampal subregions in spatial working memory with short versus intermediate delay. Behavioral Neuroscience. 2003;117(5):1044–53. doi: 10.1037/0735-7044.117.5.1044. [DOI] [PubMed] [Google Scholar]
- [74].Watanabe T, Niki H. Hippocampal unit-activity and delayed-response in the monkey. Brain Research. 1985;325(1–2):241–54. doi: 10.1016/0006-8993(85)90320-8. [DOI] [PubMed] [Google Scholar]
- [75].Jochems A, Knauer B, Yoshida M. Intrinsic persistent firing in hippocampal CA3 pyramidal neurons in vitro. FENS. 2012 Abstr 827. [Google Scholar]
- [76].Ghoneim MM, Mewaldt SP. Effects of diazepam and scopolamine on storage, retrieval and organizational processes in memory. Psychopharmacologia. 1975;44(3):257–62. doi: 10.1007/BF00428903. [DOI] [PubMed] [Google Scholar]
- [77].Atri A, Sherman S, Norman KA, Kirchhoff BA, Nicolas MM, Greicius MD, et al. Blockade of central cholinergic receptors impairs new learning and increases proactive interference in a word paired-associate memory task. Behavioral Neuroscience. 2004;118(1):223–36. doi: 10.1037/0735-7044.118.1.223. [DOI] [PubMed] [Google Scholar]