Abstract
Introduction/Purpose
Previous work from our group demonstrated improved memory function in bariatric surgery patients at 12 weeks post-operatively relative to controls. However, no study has examined longer term changes in cognitive functioning following bariatric surgery.
Materials and Methods
A total of 137 individuals (95 bariatric surgery patients, 42 obese controls) were followed prospectively to determine whether post-surgery cognitive improvements persist. Potential mechanisms of change were also examined. Bariatric surgery participants completed self-report measurements and a computerized cognitive test battery prior to surgery and at 12-week and 12-month follow-up; obese controls completed measures at equivalent time points.
Results
Bariatric surgery patients exhibited cognitive deficits relative to well established standardized normative data prior to surgery, and obese controls demonstrated similar deficits. Analyses of longitudinal change indicated an interactive effect on memory indices, with bariatric surgery patients demonstrating better performance post-operatively than obese controls.
Conclusion
While memory performance was improved 12 months post-bariatric surgery, the mechanisms underlying these improvements were unclear and did not appear attributable to obvious post-surgical changes, such as reductions in BMI or co-morbid medical conditions. Future studies employing neuroimaging, metabolic biomarkers, and more precise physiological measurements are needed to determine the mechanisms underlying memory improvements following bariatric surgery.
Keywords: obesity, cognitive function, bariatric surgery, longitudinal assessment
Introduction
Obesity represents a significant public health problem [1] and is associated with comorbid medical conditions including hypertension, diabetes, and sleep apnea [2] and also represents a significant risk factor for neurocognitive sequelae. While these comorbid conditions are associated with adverse neurocognitive outcomes [3-5], midlife obesity is now established as an independent risk factor for Alzheimer’s disease, vascular dementia, and stroke [6,7]. While the pattern of cognitive deficits varies across studies, the most common deficits have been noted in the domains of memory and executive function amongst overweight and obese individuals of various ages, and regardless of gender [8-10]. Moreover, clinically meaningful levels of cognitive impairment are present in up to a quarter of obese participants [11].
Recent work suggests that weight loss is associated with improved cognitive function in some individuals. For example, a recent meta-analysis of intentional, behavioral weight loss reported modest improvements on memory (d = .13) and attention/executive function (d = .14) in obese persons. The studies included ranged from 28 days to 12.8 months in study duration and as a result the amount of weight lost was heterogeneous across studies [12]. In extending this work, our lab found that bariatric surgery patients exhibited improved memory test performance at 12 weeks post-operatively after an average change in BMI from 46.45 to 38.61. Specifically, bariatric surgery patients demonstrated improved learning, short delay recall, long delay recall, and recognition at 12 weeks post-operatively, while control participants exhibited no change or even a decline on these tasks. However, the mechanisms of change remained unclear as this improvement was found to be largely unrelated to the patients’ reported medical conditions or change in weight [11].
The current study sought to determine if cognitive improvements following surgery are transient or if long term cognitive benefits are attained. In addition, we aimed to identify potential mechanisms of change. Bariatric surgery patients were assessed at baseline, 12-weeks and 12-months post-operative time points to examine the impact of bariatric surgery on cognition function and performance compared to obese controls tested at similar intervals. BMI and the patient reported presence of diabetes, hypertension, and sleep apnea were also measured at each time point to evaluate their contribution to changes in cognitive performance following surgery.
Methods and Procedures
Participants
A total of 137 participants were included in the current study and were recruited from a multi-site longitudinal examination of the cognitive effects of bariatric surgery (95 bariatric surgery patients, 42 obese controls). All bariatric surgery patients were part of the Longitudinal Assessment of Bariatric Surgery (LABS) project and all participants were recruited from some of the existing LABS sites, including Columbia, Cornell, and Neuropsychiatric Research Institute [13]. Obese controls were recruited from outpatient clinics at the same locations through study advertisement (e.g., flyers). Individuals with a history of neurological disorder or injury or a history of severe psychiatric illness were excluded from the study. Control participants had not undergone bariatric surgery and reported no interest in pursuing surgery in the next two years. Additional study inclusion/exclusion criteria are described in detail elsewhere [11]. Bariatric surgery patients underwent either Roux-en-Y bypass procedure or adjustable gastric banding surgery. Within this sample, only two bariatric surgery patients underwent adjustable gastric banding. Thus, no comparisons for surgery type were made.
Interventions and Clinical Follow-Up
All procedures were approved by the appropriate Institutional Review Boards and all participants provided written informed consent prior to beginning study procedures. Bariatric surgery patients completed self-report measures of demographic and medical history information and as well a computerized cognitive test battery within 30 days prior to and 12-weeks and 12-months after surgery. Obese controls completed the same measurements at equivalent intervals of time. Regarding medical history, participants were asked to indicate if they currently have, previously had, or have no history of medical conditions including hypertension, diabetes, and sleep apnea based on diagnoses they have received from their healthcare providers. This information was then dichotomized to current presence/absence of the condition to facilitate analyses. Medical records were reviewed by research staff to corroborate patient report of medical conditions and to supplement participant self-report.
Cognitive test battery
The primary outcomes measures were change in cognitive test performance at 12 week and 12 month follow-up. Cognitive test performance was assessed using alternate forms of the Integneuro test battery, which has good psychometric properties and has been employed in past studies examining obesity and cognitive function [9,14,15].
Verbal List Learning
Participants are read a list of 12 words 4 times and asked to recall as many words as possible following each trial. Following presentation and recall of a distraction list, participants are asked to recall words from the original list. After a 20-minute filled delay, participants are asked to recall target words. Finally, a recognition trial comprised of target words and foils is completed.
Digit Span Forward
Participants are presented with a series of digits on the touch-screen, separated by a one-second interval. The subject is then immediately asked to enter the digits on a numeric keypad on the touch-screen. The number of digits in each sequence is gradually increased from 3 to 9, with two sequences at each level.
Switching of Attention
This test is a computerized adaptation of the Trail Making Test [16] and consists of two parts. First, participants are presented with a pattern of 25 numbers in circles and asked to touch them in ascending order. Then, an array of 13 numbers (1-13) and 12 letters (A-L) is presented. Participants are asked to touch numbers and letters alternately in ascending order.
Verbal Interference
This task taps the ability to inhibit automatic and irrelevant responses and has similarities to the Stroop Color Word Test [17]. Participants are presented with colored words one at a time. Below each colored word is a response pad with the four possible words displayed in black and in fixed format. First, the subject is required to identify the name of each word as quickly as possible after it is presented on the screen, thus providing a measure of attention. Then, the subject is required to name the color of each word as quickly as possible, assessing executive functioning. Each part lasts for 1 minute.
Maze Task
This task is a computerized adaptation of the Austin Maze [18] and assesses executive function. Participants are presented with a grid (8×8 matrix) of circles and asked to identify the hidden path through the grid. Distinct auditory and visual cues are presented for correct and incorrect responses. The trial ends when the subject completed the maze twice without error or after 10 minutes.
Letter Fluency
This test asks individuals to generate words beginning with a given letter of the alphabet for 60 sec. A different letter is used for each of the three trials.
Animal Fluency
Participants are asked to generate as many animals as possible in 60 sec.
Statistical Analyses
Raw test scores were converted into T-scores using well established normative data based on age, and when possible, education and gender. Missing cognitive data was excluded listwise. It is noted that there is missing data for clinical characteristics (e.g., medical variables, BMI) at various time points. This data is missing because it was not recorded at the site during the patient’s visit. Composite scores made up of individual tests were created for each cognitive domain at baseline to examine group differences. No group differences were found and baseline levels of cognitive performance were therefore not controlled for. Descriptive statistics were used to demographically and medically characterize the sample, as well as to determine the prevalence of cognitive impairment at each time point. Consistent with practice in many clinical settings, cognitive impairment was defined as performance falling > 1.5 standard deviations below the normative mean (i.e., performing meaningfully below expectations). It is noted that the term “cognitive impairment” is not meant to imply a dementia diagnosis. T-tests and chi-square analyses were used to examine potential demographic and medical differences between groups. Chi-square analyses were used to examine differences in prevalence rates of cognitive impairment within the sample over time; performance was dichotomized into impaired performance (i.e., performance below 1.5 standard deviations) and intact performance. Repeated measures MANOVA were used to compare neuropsychological test performance of the bariatric surgery patients and obese controls in each cognitive domain. Significant omnibus tests were further examined with Bonferroni-corrected post-hoc tests. Performance on cognitive tasks were examined for main effects of time (i.e., does performance change over time collapsed across groups) and group (i.e., is there a group difference in performance collapsed over time), as well as an interactive effect of group × time (i.e., does performance change over time as a function of group). Mixed modeling analyses were used to examine potential underlying mechanisms of change for significant cognitive measures. Specifically, the main effects for time and medical variables, as well as the time by medical variable interaction were examined. Each medical variable and memory task were examined individually. Specifically, for dichotomized medical variables we examined the linear and curvilinear main effect of time, the main effect of the medical variable, and the interactive effect. The same effects were examined for BMI with the appropriate changes made to accommodate a continuous variable. Non-significant time relationships were dropped from further analyses; if both linear and curvilinear time relationships were non-significant, the linear relationship was retained in further analyses.
Results
Demographic/Medical Characteristics
Bariatric surgery patients and obese controls were demographically similar (Table 1). However, at baseline bariatric surgery patients had greater BMI and were more likely to have hypertension, type 2 diabetes, and sleep apnea. By 12-month follow-up bariatric surgery patients had significantly decreased BMI from baseline [t(65)=28.64, p<.001] and significantly differed from the BMI of obese controls [t(87)=8.18, p<.001]. In addition, fewer bariatric surgery patients had type 2 diabetes and sleep apnea by 12-months and were significantly less likely to have type 2 diabetes than obese controls, who had demonstrated an increase in type 2 diabetes prevalence. Moreover, by 12-month follow-up, surgery patients were no more likely to have hypertension or sleep apnea than obese controls. At 12-week follow up the mean percentage of excess weight loss for bariatric surgery patients was 34.70% and 68.13% by 12-months. At 12-week follow up, bariatric surgery patients had lost a mean of 17.06% (n = 75) of their initial weight and a mean of 34.23% at 12-months (n = 66).
Table 1.
Demographic and Clinical Characteristics.
| Characteristic | Bariatric Surgery Patients (N=95) | Obese Controls (N=42) | Test statistic | p |
|---|---|---|---|---|
| Baseline | ||||
| Age (years; M ± SD; range) | 43.23 ±10.84; 21-41 | 39.93 ± 11.22; 19-61 | 1.63 | .11 |
| Women (%) | 89.48 | 90.48 | 0.03 | .86 |
| BMI (kg/m2) | 46.19 ± 5.90 | 40.77 ± 6.04 | 4.92 | < .001 |
| Hypertension (%) | 46.81(n=94) | 17.50 (n=40) | 10.22 | .001 |
| Type 2 Diabetes (%) | 27.66 (n=94) | 9.76 (n=41) | 4.31 | .04 |
| Sleep Apnea (%) | 38.95 | 9.76 (n=41) | 11.59 | .001 |
| 12-week follow-up | ||||
| BMI (kg/m2; M ± SD) | 37.44 ± 4.63 (n=75) | 41.37 ± 5.24 (n=22) | 3.40 | .001 |
| Excess Weight Loss (%) | 34.70 (n=75) | -0.65 (n=22) | 14.93 | < .001 |
| % of Initial Weight Lost | 17.06 (n = 75) | -0.11 (n=22) | 16.57 | < .001 |
| Hypertension (%) | 39.56 (n=91) | 25.00 (n=40) | 2.59 | .11 |
| Type 2 Diabetes (%) | 20.88 (n=91) | 12.82 (n=39) | 1.18 | .28 |
| Sleep Apnea (%) | 27.47 (n=91) | 5.00 (n=40) | 8.58 | .003 |
| 12-month follow-up | ||||
| BMI (kg/m2; M ± SD)* | 30.23 ±5.23 (n=66) | 40.79 ± 5.64 (n=23) | 8.18 | <.001 |
| Excess Weight Loss (%) | 68.13 (n=66) | 1.76 (n=23) | 15.84 | < .001 |
| % of Initial Weight Lost | 34.22 (n=66) | 0.89 (n=23) | 16.92 | < .001 |
| Hypertension (%)‡ | 38.37 (n=86) | 37.50 (n=32) | 0.01 | .93 |
| Type 2 Diabetes (%)*† | 15.12 (n=86) | 31.25 (n=32) | 3.87 | .05 |
| Sleep Apnea (%)*‡ | 18.82 (n=85) | 25.00 (n=32) | 0.54 | .46 |
Note.
denotes Baseline > 12 month for bariatric surgery patients;
denotes Baseline < 12 month for obese controls;
denotes trend for Baseline < 12 month for obese controls.
Prevalence of Impairment among Bariatric Surgery Patients and Obese Controls
Cognitive impairment was common in both bariatric surgery patients and matched controls at baseline (Table 2), though no between-group differences emerged in prevalence of impairment at baseline. However, by 12-months, the prevalence of impairment was significantly reduced on three of the four memory indices and on a measure of executive function within bariatric surgery patients. In contrast, there were no significant changes in the prevalence of impaired performance among the obese controls by 12-months. However, at 12-month follow-up, a between-group difference in the prevalence of impairment on the recognition memory task was observed, with obese controls demonstrating a significantly higher rate of impairment on this task when compared to bariatric surgery patients.
Table 2.
Percent of Participants with Cognitive Impairment (T < 35).
| Test | Bariatric Surgery Patients (n = 95)
|
Obese Controls (n = 42)
|
||||
|---|---|---|---|---|---|---|
| Baseline | 12-weeks | 12-month | Baseline | 12-weeks | 12-month | |
| Memory | ||||||
| Learning | 24.2 | 21.1 | 9.5† | 23.8 | 40.5 | 19.00 |
| Short Delay | 12.6 | 18.9 | 5.3 | 16.7 | 26.2 | 9.5 |
| Long Delay | 15.8 | 18.9 | 5.3† | 11.9 | 26.2 | 14.3 |
| Recognition* | 22.1 | 8.4 | 5.3† | 16.7 | 19.0 | 21.4 |
| Attention | ||||||
| Digit Span | 3.2 | 5.3 | 1.1 | 2.4 | 7.1 | 2.4 |
| SOA - Number | 10.5 | 4.2 | 5.3 | 9.5 | 2.4 | 7.1 |
| VI-Word* | 4.2 | 6.3 | 5.3 | 11.9 | 9.5 | 16.7 |
| Executive Function | ||||||
| SOA - Letter/Number | 9.5 | 6.3 | 8.4 | 9.5 | 4.8 | 7.1 |
| VI-Color-Word | 6.3 | 5.3 | 1.1† | 11.9 | 0.0 | 2.4 |
| Maze Errors | 12.6 | 6.3 | 7.4 | 21.4 | 11.9 | 16.7 |
| Language | ||||||
| Letter Fluency | 16.8 | 11.6 | 13.7 | 14.3 | 11.9 | 4.8 |
| Animal Fluency | 5.3 | 5.3 | 7.4 | 9.5 | 7.1 | 9.5 |
Note.SOA=switching of attention; VI=verbal interference;
Bariatric Surgery < Control at 12 months;
Baseline > 12 months within group.
Cognitive Differences between Bariatric Surgery Patients and Obese Controls
Memory
There was a significant group × time interaction [λ=.87, F(8,128) = 2.34, p= 02, ηp2=.13] with significant univariate differences between groups over time for learning [F(2,270)=5.63, p<.01], long delay [F(2,270)=6.36, p<.01], and recognition [F(2,270)=5.00, p=.01]. The effect for short delay was non-significant [F(2,270)=2.73, p=.07]. More specifically, Bonferroni-corrected comparisons indicated bariatric surgery patients had significantly improved on recognition at 12-weeks (p<.001) and on all 4 indices of memory by 12-months (p<.001 for all indices; see Table 3). Performance of obese controls on recognition improved from baseline to 12 weeks (p=.02) but declined from 12 weeks to 12 months (p=.03). Performance on all indices at 12 months did not differ from baseline performance for obese controls. When compared to each other, bariatric surgery participants performed significantly better than obese controls at 12-months on the long delay (p = .02) and recognition (p = .02) tasks; with the Bonferroni-correction, there was a trend for bariatric surgery patients to perform better than obese controls on the learning task (p = .07) at 12-months. In addition, a main effect for time [λ=.57, F(8,128)=11.30, p<.001, ηp2=.41] was found, but there was no effect for group [λ=.99, F(4,132)=0.33, p=.86, ηp2=.01]. See Table 3.
Table 3.
Comparison of Cognitive Test Performance T-scores of Bariatric Surgery Patients (n = 95) and Obese Controls (n = 42).
| Baseline | 12-week follow-up | 12-month follow-up | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cognitive Test | Bariatric surgery | Obese controls | Bariatric surgery | Obese controls | Bariatric surgery | Obese controls |
| Memory*† | ||||||
| Learning | 42.85 ± 12.85 | 45.10 ± 1.90 | 45.68 ± 14.20 | 40.87 ± 2.19 | 50.55 ± 13.22 | 46.50 ± 2.17 |
| Short Delay | 46.07 ± 10.48 | 47.26 ± 1.62 | 47.75 ± 12.58 | 44.07 ± 2.01 | 52.23 ± 10.43 | 49.38 ± 1.71 |
| Long Delay | 45.93 ± 10.89 | 48.76 ± 1.11 | 48.55 ± 13.14 | 44.44 ± 2.09 | 53.04 ± 9.80** | 48.53 ± 1.65 |
| Recognition | 41.33 ± 10.39 | 44.02 ± 1.63 | 51.37 ± 10.52 | 49.41 ± 1.73 | 49.09 ± 9.40** | 44.75 ± 1.57 |
| Attention* | ||||||
| Digit Span | 50.07 ± 9.06 | 49.69 ± 1.37 | 51.85 ± 10.80 | 50.97 ± 1.63 | 53.44 ± 10.94 | 51.56 ± 1.62 |
| SOA - Number | 54.67 ± 14.49 | 51.84 ± 2.17 | 58.49 ± 13.69 | 54.97 ± 2.06 | 61.39 ± 14.97 | 57.05 ± 2.35 |
| VI-Word | 53.20 ± 12.55 | 48.56 ± 2.13 | 55.01 ± 12.68 | 50.27 ± 2.09 | 56.40 ± 14.88 | 51.57 ± 2.30 |
| Executive Function* | ||||||
| SOA - Letter/Number | 52.29 ± 15.16 | 51.02 ± 2.21 | 57.83 ± 12.68 | 54.41 ± 1.92 | 58.04 ± 13.38 | 54.81 ± 2.08 |
| VI-Color-Word | 55.23 ± 12.40 | 53.58 ± 1.97 | 63.05 ± 13.03 | 60.33 ± 1.91 | 62.40 ± 12.18 | 60.67 ± 1.98 |
| Maze Errors | 49.81 ± 12.89 | 42.51 ± 2.15 | 55.69 ± 11.82 | 50.72 ± 1.87 | 53.77 ± 11.26 | 48.31 ± 1.99 |
| Language | ||||||
| Letter Fluency | 47.00 ± 11.51 | 48.69 ± 1.80 | 46.93 ± 10.14 | 49.60 ± 1.73 | 47.54 ± 11.25 | 48.02 ± 1.7 |
| Animal Fluency | 50.66 ± 10.64 | 47.95 ± 1.62 | 50.20 ± 10.14 | 48.91 ± 1.60 | 50.38 ± 11.07 | 48.22 ± 1.73 |
Note.
Main effect for time point;
Significant group × time interaction;
significant group difference at time point (p < .05);
SOA = switching of attention; VI = verbal interference
Attention
No group × time interaction (p= .99) or effect for group (p=.13) were demonstrated. However, a significant effect of time was observed [λ=.80, F(6,130)=5.31, p<.001, ηp2=.20]. Specifically, performance on digit span [F(2, 270)=4.52, p=.01] and switching of attention number task [F(2,270)=12.96, p<.001] significantly changed over time (see Table 3).
Executive Function
There was no group × time interaction (p=.74, ηp2=.03] or effect for group (p=.06). However, there was a significant effect of time [λ = .55, F (6,130) = 18.06, p < .001, ηp2 = .46]. Specifically, performance on the switching of attention letter/number task [F (2,270) = 15.12, p < .001], verbal interference color/word task [F(2,270)=26.26, p<.001], and maze errors [F(2,270)=25.47, p<.001] significantly changed over time (see Table 3).
Language
No significant group × time interaction (p=.97) or effects for group (p=.11) or time (p=.97) were found (see Table 3).
Predictors of Improved Memory Function in Bariatric Surgery Patients
Mixed modeling analyses were used to identify predictors of improved memory among bariatric surgery patients. Specifically, change in presence/absence of medical conditions, as well as change in BMI were examined individually for each memory index . Individuals missing the medical variable of interest at any time point were excluded from the analyses. See table 4 for significant parameter estimates.
Table 4.
Mixed Modeling Parameter Estimates of Significant Predictors of Memory Performance in Bariatric Surgery Patients.
| Medical Variable | Linear | |||
|---|---|---|---|---|
| Main Effect | Interaction | |||
| Cognitive & Medical Variables | Estimate | t | Estimate | t |
| Long Delay Recall & T2DMa | 4.22 | 2.73** | 0.04 | 0.63 |
| Learning & SAa | -0.01 | -0.01 | -0.13 | -2.05* |
| Short Delay Recall & BMIb | -0.02 | -0.21 | -0.01 | -2.19* |
Note. T2DM = type 2 diabetes; SA = sleep apnea.
indicates n = 84;
indicates n = 62;
indicates p ≤ .05;
indicates p ≤ .01.
Diabetes
Diabetes was related to performance on the long delay memory task with non-diabetic individuals performing better (T=50.10 ± 1.05) than diabetics (T=45.8 ± 1.56).
Sleep Apnea
Sleep apnea status was related to change in performance on the learning memory task. Baseline performance was similar among those with and without sleep apnea and was within low average range. Following surgery, both groups remained relatively stable though increased in performance by 12 months, with both groups performing within the average range. However, those with sleep apnea demonstrated a somewhat sharper increase in performance (see Figure 1).
Figure 1.
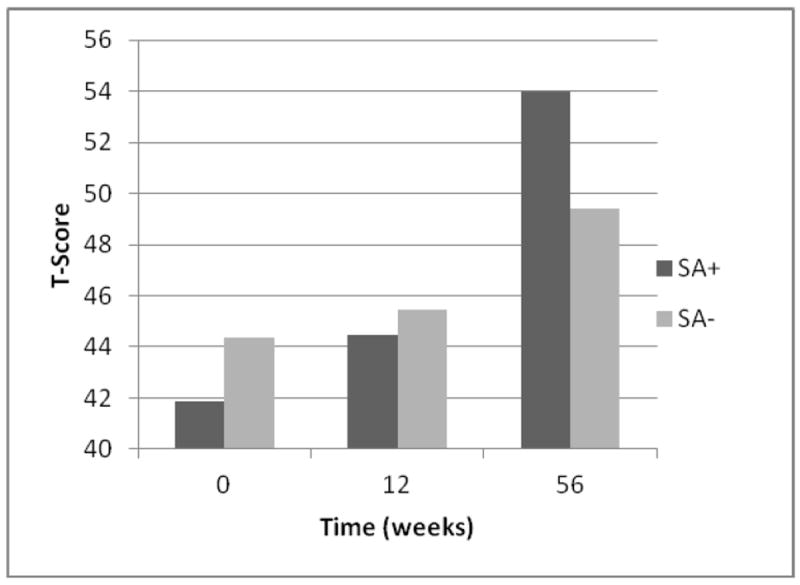
Sleep Apnea Status and Learning Performance (n = 84).
Note. SA+ indicates presence of sleep apnea; SA- indicated absence of sleep apnea.
Hypertension
Hypertension did not significantly influence memory performance.
BMI
BMI was related to the rate of change in performance on the short delay recall task. All patients demonstrated similar performance by 12-week follow-up. However, by 12-month follow-up, individuals who were 1SD below the mean BMI demonstrated a sharp increase in performance while those 1SD above the mean BMI declined in performance. Patients with the mean BMI showed a generally linear increase in performance (see Figure 2).
Figure 2.
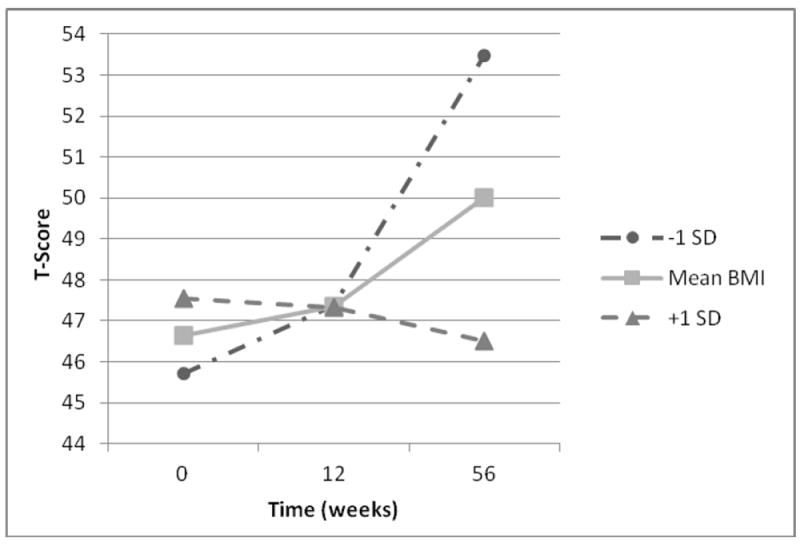
Interactive Effect of BMI and Time on Short Delay Recall (n = 62).
Note. BMI = Body Mass Index; -1SD indicates one standard deviation below the sample mean BMI; +1 SD indicates one standard deviation above the sample mean BMI.
Discussion
Obesity is a known risk factor for adverse neurocognitive outcomes [6,7] and is associated with cognitive deficits, particularly in aspects of memory and executive function [8-10]. Consistent with these findings, 24.2% of bariatric surgery candidates exhibited impaired performance (i.e., T < 35) on a measure of memory and 12.6% on an executive function measure. However, by 12-month follow-up bariatric surgery patients demonstrated average to high average performance and the rate of impaired performance significantly decreased on several indices of memory and on an executive function task. These findings extend beyond our previous work, which found improved performance on three indices of memory and one test of attention at 12 week follow-up [11]. Such findings suggest that bariatric surgery patients with the most severe cognitive impairment may be showing the most benefit on neuropsychological test performance; however, further work is needed to clarify this possibility.
Bariatric surgery patients demonstrated striking improvements on tasks of memory from baseline to 12-month follow-up. Prior to surgery, deficits were most notable for tasks of memory, with 24.2% of patients exhibiting decreased learning and 22.1% showing decreased recognition (i.e., T< 35). However, by 12 month-follow up, these rates of impairment significantly dropped to 9.5% and 5.3%, respectively, and had reached the average range for the group. These findings are interesting given the substantial evidence linking obesity to dementia, and in particular Alzheimer’s disease. As previously reviewed, elevated BMI in midlife is strongly related to an increase risk of dementia. However, in late-life, elevated BMI appears to serve as a buffer against cognitive decline [19], and these paradoxical findings may be explained by the weight loss that typically precedes dementia diagnosis [20,21]. The surgical candidates in this sample were in mid-life (mean age 43 years) suggesting that, based on weight, they may be at elevated risk for dementia. Moreover, when this is considered within the context of their reduced memory performance at baseline, they may have had a particularly increased vulnerability for developing Alzheimer’s disease, as decreased memory performance, including learning and recognition, is a hallmark of the disease.
Consistent with this possibility, obese individuals show cerebral atrophy, particularly in frontal and temporal brain regions, as well as white matter hyperintensities [22-28]. In addition, neuropathological changes similar to those seen in Alzheimer’s disease, have been found post-mortem in morbidly obese elderly individuals who had no history of dementia or neurological impairment. Specifically, increased levels of amyloid-β, amyloid- β precursor protein, and tau have been found in the hippocampus of morbidly obese individuals over the age of 65 [29]. Moreover, the levels found in some individuals approached, or was equal to, the levels seen in Alzheimer’s disease [29]. Taken together, the memory deficits noted at baseline suggest these individuals may have been at risk for a disease trajectory consistent with that of Alzheimer’s disease. However, following surgery the majority of these individuals demonstrated improved memory function, raising the possibility of an altered disease trajectory. To clarify this possibility, future studies should examine the cognitive effects of bariatric surgery and obese controls over extended follow-up intervals (e.g., 15-20 years) to determine whether surgery-related weight loss ultimately reduces risk of Alzheimer’s disease and other degenerative disorders.
In contrast to performance on tests of memory, no significant changes were demonstrated within the other cognitive domains. While both attention and executive function changed over time, no significant group differences emerged. It is possible that the observed effect of time was related to practice effects and further work is needed to clarify the effect of bariatric surgery on cognitive performance within these domains.
While substantial gains in memory were observed, these changes were largely unrelated to medical variables, as reported by the patients and corroborated through medical chart reviews, or change in BMI for bariatric surgery patients. Although some medical conditions and BMI were related to cognitive performance or influenced the rate of change over time, no clear pattern emerged to identify distinct contributory factors, and our limited findings may be, in part, due to Type I error. Hypertension, diabetes, sleep apnea are all known conditions that can negatively impact cognition [3-5], but some may have partly reversible effects. For example, improved cognitive function has been found in hypertensive overweight and obese individuals engaging in an exercise and dietary program targeted at reducing hypertension [30]. The reason as to why mechanisms of change were not identified remains unclear. One potential explanation for the lack of associations may be the nature by which patient medical history was obtained. More precise physiological analyses (e.g., continuous ambulatory blood pressure measurements, repeated mixed meal testing) is required to determine the exact extent of disease severity. It is also possible that the examination of individual medical variables is not sufficient for identifying potential underlying mechanisms of change and examination of a possible interactive effect is warranted. In addition, other potential risk factors known to impact cognition need to be assessed including measures of cerebral blood flow and endothelial function as well as circulating biomarkers (e.g., brain-derived neurotrophic factor). These factors are known contributors to cognitive function and are implicated in obese persons.
The current findings are limited in several ways. Due to the observational nature of the parent LABS project, individuals were not randomized to surgery or control groups. Although baseline cognitive performance was similar among controls and bariatric surgery candidates, the groups differed on several medical variables that are known to impact cognition. However, the presence of such conditions is often a requirement for bariatric surgery candidates in order to obtain payment coverage from third-party payors and a randomized trial may be impractical. In addition, while the Integneuro cognitive test battery is psychometrically sound, a more detailed assessment of specific aspects of memory (e.g., verbal versus visual memory) and more complex measures of executive function (e.g., inhibitory control) would likely aid in further clarifying the precise nature of the cognitive profiles of obese individuals pre- and postoperatively. Finally, future studies that longitudinally follow bariatric surgery patients for longer durations to fully understand the long-term effects of surgery on cognition are needed.
In sum, the current study found improved memory function in obese individuals following bariatric surgery. However, the underlying mechanisms of change remain unclear as change in memory was largely unrelated to medical variables or BMI. Further work, including neuroimaging and evaluation of biomarkers, is needed to elucidate contributing factors. A greater understanding of specific cognitive strengths and weaknesses that emerge following bariatric surgery will likely help guide treatment recommendations and provide insight into possible long-term neurocognitive outcomes in this population.
Acknowledgments
Data collection supported by DK075119. Manuscript supported in part by HL089311
Footnotes
Disclosures
No author has a conflict of interest for this work.
Contributor Information
Lindsay A. Miller, Email: lmille92@kent.edu.
Ross D. Crosby, Email: rcrosby@nrifargo.com.
Rachel Galioto, Email: rmgalioto@gmail.com.
Gladys Strain, Email: gls2010@med.cornell.edu.
Michael J. Devlin, Email: mjd5@columbia.edu.
Rena Wing, Email: Rena_Wing_PhD@brown.edu.
Ronald A. Cohen, Email: Ronald_Cohen@Brown.EDU.
Robert H. Paul, Email: paulro@umsl.edu.
James E. Mitchell, Email: jmitchell@nrifargo.com.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Athyros VG, Tziomalos K, Karagiannis A, Mikhaikidis DP. Cardiovascular benefits of bariatric surgery in morbidly obese patients. Obes Rev. 2011;12:515–524. doi: 10.1111/j.1467-789X.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 3.Paglieri C, Bisbocci D, Caserta M, Rabbia F, Bertello C, Canadè A. Hypertension and cognitive function. Clin Exp Hypertens. 2008;30:701–10. doi: 10.1080/10641960802563584. [DOI] [PubMed] [Google Scholar]
- 4.Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29:494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aloia MS, Arndett JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hyponea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772–85. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 6.Hassing LB, Dahl AK, Thorvaldsson V, et al. Overweight in midlife and risk of dementia: a 40-year follow-up study. Int J Obes. 2009;33:893–98. doi: 10.1038/ijo.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strazzullo P, D’Elia L, Cairella G, Garbagnati F, Cappuccio F, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke. 2010;41:e418–26. doi: 10.1161/STROKEAHA.109.576967. [DOI] [PubMed] [Google Scholar]
- 8.Gunstad J, Lhotsky A, Wendell CR, Ferrucci L, Zonderman AB. Longitudinal examination of obesity and cognitive function: results from the Baltimore Longitudinal Study of Aging. Neuroepidemiology. 2010;34:222–29. doi: 10.1159/000297742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007;48:57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Waldstein SR, Katzel LL. Interactive relations of central versus total obesity and blood pressure to cognitive function. Int J Obes. 2006;30:201–7. doi: 10.1038/sj.ijo.0803114. [DOI] [PubMed] [Google Scholar]
- 11.Gunstad J, Strain G, Devlin MJ, et al. Improved memory function 12 weeks after bariatric surgery. SOARD. 2011;7:465–72. doi: 10.1016/j.soard.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siervo M, Arnold R, Wells JCK, et al. Intentional weight loss in overweight and obese individuals and cognitive function: a systematic review and meta-analysis. Obes Rev. 2011;12:968–83. doi: 10.1111/j.1467-789X.2011.00903.x. [DOI] [PubMed] [Google Scholar]
- 13.Belle SH, Berk PD, Courcoulas AP, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis. 2007;3:116–26. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul RH, Lawrence J, Williams LM, Richard CC, Cooper N, Gordon E. Preliminary validity of “Integneuro”: a new computerized battery of neurocognitive tests. Int J Neurosci. 2005;115:1549–67. doi: 10.1080/00207450590957890. [DOI] [PubMed] [Google Scholar]
- 15.Williams LM, Simms E, Clark CR, Paul RH, Rowe D, Gordon E. The test-retest reliability of a standardized neurocognitive and neurophysiological test battery: “neuromarker”. Int J Neurosci. 2005;115:1605–30. doi: 10.1080/00207450590958475. [DOI] [PubMed] [Google Scholar]
- 16.Reitan R. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–76. [Google Scholar]
- 17.Golden C. Stroop color and word task: a manual for clinical and experimental uses. Chicago: Stoeling; 1978. [Google Scholar]
- 18.Walsh K. Understanding brain damage—a primer of neuropsychological evaluation. Melbourne: Churchill Livingstone; 1985. [Google Scholar]
- 19.Naderali E, Ratcliffe SH, Dale MC. Obesity and Alzheimer’s disease: A link between body weight and cognitive function in old age. Am J Alzheimers Dis Other Demen. 2009;24:445–49. doi: 10.1177/1533317509348208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch Neurol. 2006;63:1312–17. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- 21.Stewart R, Masaki K, Xue QL, et al. A 32-yearprospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 22.Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63:1990–1. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- 23.Gustafson DR, Steen B, Skoog I. Body mass index and white matter lesions in elderly women: An 18-year longitudinal study. Int Psychoger. 2004;16:327–36. doi: 10.1017/s1041610204000353. [DOI] [PubMed] [Google Scholar]
- 24.Gunstad J, Paul R, Cohen R, et al. Relationship between body mass index and brain volume in healthy adults. Int J Neurosci. 2008;118:1582–93. doi: 10.1080/00207450701392282. [DOI] [PubMed] [Google Scholar]
- 25.Pannacciulli N, Del Parigi A, Chen K, Le DNT, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. NeuroImage. 2006;31:1419–25. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 26.Taki Y, Kinomura S, Sato K, et al. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity. 2008;16:119–24. doi: 10.1038/oby.2007.4. [DOI] [PubMed] [Google Scholar]
- 27.Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC Neurol. 2005;5:23. doi: 10.1186/1471-2377-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jagust W, Harvey D, Mungas D, Haan M. Central obesity and the aging brain. Arch Neurol. 2005;62:1545–48. doi: 10.1001/archneur.62.10.1545. [DOI] [PubMed] [Google Scholar]
- 29.Mrak RE. Alzheimer-type neuropathological changes in morbidly obese elderly individuals. ClinNeuropathol. 2009;28:40–45. doi: 10.5414/npp28040. [DOI] [PubMed] [Google Scholar]
- 30.Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stopping hypertension diet, exercise, and caloric restriction in neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55:1331–38. doi: 10.1161/HYPERTENSIONAHA.109.146795. [DOI] [PMC free article] [PubMed] [Google Scholar]


