Abstract
Percutaneous vertebroplasty has become widely accepted as a safe and effective minimally invasive procedure for the treatment of painful vertebral body compression fractures refractory to medical therapy. In this article, the authors review the indications and contraindications for vertebroplasty, principles of appropriate patient selection, useful techniques to achieve optimal outcomes, and the potential risks and complications of the procedure.
Keywords: vertebral compression fracture, osteoporosis, vertebroplasty, kyphoplasty, vertebral augmentation, interventional radiology
Objectives: Upon completion of this article, the reader will be able to identify indications and contraindications for vertebroplasty, techniques for thoracic and lumbar vertebroplasty, expected outcomes, and suggested thresholds for complications.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Vertebral body compression fractures (VFs) frequently result in severe and disabling back pain. Many patients may experience significant morbidity and decreased quality of life secondary to severe pain, prolonged immobilization, kyphosis, pulmonary deterioration, depression, and loss of independence.1,2,3 Patients with VF are also at higher risk for chronic back pain and demonstrate increased mortality rates.4,5,6
The most common etiology of VF is osteoporosis. Other causes include primary and metastatic malignancies, trauma, hemangioma, and osteonecrosis. More than 700,000 osteoporosis-related VF are diagnosed each year in the United States, resulting in approximately 115,000 hospital admissions.7 Worldwide, 1.4 million people are affected by VF annually8; the lifetime risk for VF is 16% in women and 5% in men.7,9
The standard treatment for painful VF consists of conservative medical therapy utilizing analgesics, bed rest, and external bracing. Symptoms typically improve in 4 to 6 weeks; approximately two-thirds of patients will respond to conservative measures alone. However, up to a third of patients managed with conservative medical therapy may not improve and will require alternative therapy.10
Vertebroplasty (VP), first described by Galibert and colleagues in 1987,11 has become a widely used alternative treatment for symptomatic VF refractory to medical therapy. VP is a minimally invasive image-guided procedure involving the injection of bone cement into a vertebral body fracture in an effort to improve pain and stability of the fracture.12 The most widely used cement product remains polymethyl methacrylate (PMMA). Kyphoplasty is a similar procedure that utilizes an inflatable balloon tamp, in an effort to reduce the fracture and create a space to theoretically allow safer injection of cement into the fractured vertebral body.13 Although this review will focus on VP, both procedures have been shown to be effective and safe.
Patient Selection
Indications
The most common indication for VP is treatment of painful acute and subacute VF in patients who have failed to respond to a 4- to 6-week course of appropriate medical therapy. Failure to respond to medical therapy is defined as minimal or no pain relief with prescribed analgesics, or inadequate pain relief in patients who are unable to tolerate narcotics secondary to unwanted side effects such as sedation, confusion, and constipation.14
By far, the most common underlying etiology of painful VF is osteoporosis.15,16,17,18,19,20 Other frequently encountered causes are metastatic disease, multiple myeloma, and painful aggressive hemangiomas.11,21,22,23,24,25,26,27,28,29,30 Less common indications for treatment include pain related to osteonecrosis (Kummel disease), Paget disease, Langerhans cell histiocytosis, osteogenesis imperfecta, spinal pseudarthrosis, and intravertebral vacuum phenomena.31 VP has also been performed in the treatment of painful Schmorl nodes32 and for reinforcement of pathologically weak vertebral bodies prior to surgical stabilization.
Contraindications
Absolute contraindications include treatment of asymptomatic VF and treatment of patients improving with conservative medical care. Prophylactic treatment in osteoporotic patients without a VF is not viewed as an acceptable indication. Additional absolute contraindications include uncorrectable coagulopathy, and active local or systemic infection. Allergy to PMMA or other bone cement products preclude VP. Relative contraindications include disruption of the posterior vertebral body wall or tumor extension into the spinal canal. The treatment of very severely compressed VF, defined as vertebral body collapse to less than one-third of the original height, is also considered a relative contraindication. Treatment of these fractures is more technically challenging and is often associated with increased rates of complications.33 Although studies assessing the response to VP in these patients are scarce, a recent clinical study demonstrated that patients with such severe compression fractures can be successfully treated and may benefit from VP.33
Assessment and Workup
A history and physical exam must be performed as part of any VP consultation. VF can occur with minimal or no trauma and are typically (but not always) associated with acute onset of severe back pain. Pain related to a VF is often midline, localized to the level of fracture, and typically not radicular in character. Pain may radiate bilaterally to the anterior abdomen in a “belt-like” fashion. Discomfort is usually exacerbated with sitting, standing, weight bearing, or movement. Patients may be unable to perform their usual activities of daily living. In severe cases, hospital admission for pain control with intravenous narcotics may be required. Bowel or bladder incontinence may be seen in VF with associated spinal cord compression.
On physical examination, pain often may be reproduced by firm palpation over the spinous process at the effected or adjacent level. However, the presence of tenderness is not necessarily associated with more favorable outcomes.34 Kyphotic posturing may be present in patients with severe or multiple compression deformities. Patients may rarely have numbness, tingling, or weakness suggesting a possible nerve-related injury.
Preprocedural blood work should be obtained including platelet and coagulation studies. In the authors' practice, a platelet count < 50,000/dL and an INR > 1.8 are used as strict cutoffs. The procedure may be performed in patients on nonsteroidal anti-inflammatory medications or aspirin; however, when possible, clopidogrel and anticoagulation medications should be held. An immediate baseline preprocedural neurological exam should be performed and documented. Any existing diagnostic imaging tests should be reviewed and other potential causes of back pain should be excluded such as degenerative disk disease, facet disease, spinal stenosis, or infection. Clinical evaluation should also include an assessment of the patient's underlying osteoporosis, assessment of bone density, and appropriate pharmacotherapy. In the authors' practice, the interventional radiologists work together with primary care physicians in a multidisciplinary approach for comprehensive osteoporosis management. In addition, one should be reminded that physical therapy often is an important component of recovery from symptomatic VF.
Imaging
Diagnostic imaging is crucial to VF evaluation. Conventional radiographs of the thoracic and/or lumbar spine are most often used to diagnose VF. However, as the acuity of the VF is unable to be determined without serial radiographs, alternative imaging studies are often required. Magnetic resonance imaging (MRI) is preferred for several reasons. MRI can accurately confirm the presence of acute or subacute VF, assess the morphology of the VF, and exclude existence of concomitant disease that may preclude VP. In addition, radiographically occult VF may be detected on MRI, helping to avoid incomplete therapy. Because of the presence of bone marrow edema, acute, subacute, or nonhealing VF appears hypointense on T1W sequences and hyperintense on T2W and STIR sequences (Fig. 1).
Figure 1.
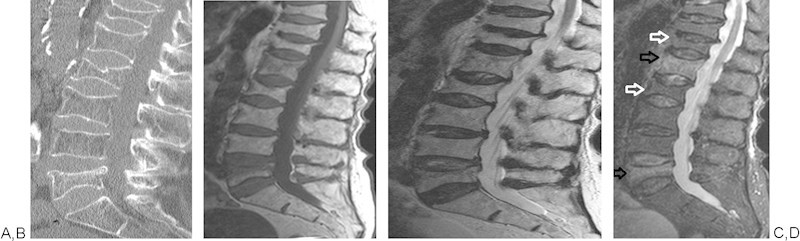
Sagittal CT reconstruction (A) showing multiple vertebral body fractures of unknown age. MRI T1, T2, and STIR images (B-D, respectively) confirm fractures, demonstrating marrow edema at L1 and L5 compatible with acute vertebral body fractures (black arrows). Lack of marrow edema at T12 and L2 suggests more chronic vertebral body fractures (white arrows).
For patients who are unable to have an MRI, nuclear medicine bone scans can be used to determine the acuity of a VF (Fig. 2). Computed tomography (CT) can also demonstrate the presence of VF. Moreover, CT allows evaluation of the integrity of the posterior wall of the vertebral body and exclusion of any retropulsion of fracture fragments. Although some practitioners routinely obtain follow-up imaging, most have found that it is not cost-effective. The current authors do not routinely perform postprocedural imaging.
Figure 2.
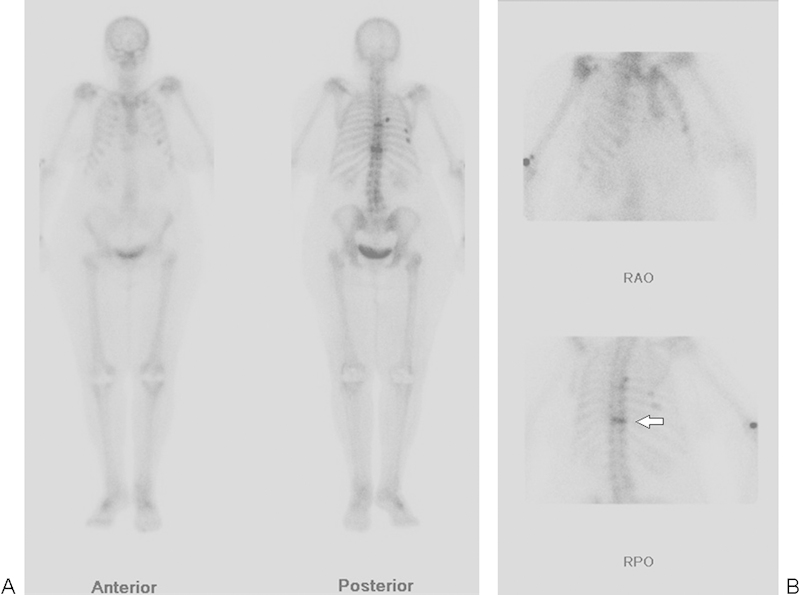
(A, B) Nuclear medicine bone scan, with anterior, posterior, right anterior oblique (RAO), and right posterior oblique (RPO) views, in a patient unable to have an MRI, showing increased radiotracer uptake at T10 consistent with an acute vertebral body fracture (arrow). Uptake is seen to a lesser degree at the mid-thoracic spine compatible with a second acute fracture. Multiple rib fractures are also present.
Technique
VP is best performed using high-quality fluoroscopy for needle placement, and cement injection should be performed under continuous fluoroscopy. Although not as ideal, a portable c-arm unit can be used. Biplane fluoroscopy, if available, can decrease procedure time. In some technically challenging cases, CT guidance combined with CT fluoroscopy or conventional fluoroscopy may be necessary.
The procedure is performed using maximum surgical barrier technique. Preprocedural prophylactic antibiotics should be used in all immunocompromised patients. Although there is no clear consensus on routine antibiotic use in non-immunocompromised patients, many practitioners administer prophylactic intravenous antibiotics to all patients immediately before the start of the procedure.12,35 The authors typically administer 1 g of cefazolin 1 hour before the start of the procedure; in patients with an allergy to penicillin or cefazolin, 500 mg of vancomycin can be substituted.
Thoracic and lumbar VP is performed with the patient in the prone position. Padding should be utilized to maximize patient comfort and reduce curvature of the spine. The skin, subcutaneous tissues, and periosteum are locally anesthetized with lidocaine, which may require the use of a spinal needle for length. The patient's heart rate, blood pressure, and oxygen saturation are continuously monitored throughout the duration of the procedure. In many practices, including the authors', VP is performed under conscious sedation using intravenous versed and fentanyl. Rarely, deeper sedation with an anesthesiologist may be required for patients with severe pulmonary compromise or those unable to tolerate prone positioning without general anesthesia.
In the thoracic and lumbar spine, VP is typically performed using a transpedicular or parapedicular approach.36 In the authors' practice, both unilateral and bilateral transpedicular approaches are utilized. Unilateral technique may reduce procedural times and minimize trauma to paraspinal soft tissues, but often requires greater degree of obliquity that may obscure visualization of the pedicle. The authors find that as the posterior spinous process is overlapped to the contralateral pedicle with an exaggerated ipsilateral oblique projection, the needle will almost always reach the opposite side of the vertebral body. Bilateral technique often requires longer procedural time but is typically technically less challenging. Many different types of VP needles are commercially available; the authors most often use 11-gauge needles for lumbar levels and 13-gauge needles for thoracic levels. Both sizes are available in 10 and 15 cm lengths with diamond-shaped multi-beveled and or single-beveled stylets.
Proper positioning of the fluoroscopic C arm is important to the success of the procedure. The C arm should be skewed cranially or caudally to view the vertebral body “straight on,” that is, anterior wall projected directly over the posterior wall. To access the vertebral body using the transpedicular technique, the VP needle is advanced “down the barrel” of the pedicle under intermittent fluoroscopic guidance (Fig. 3). Prior to needle placement, the C arm is positioned in a 20- to 30-degree ipsilateral oblique view. The pedicle, especially the medial and the inferior walls, must be clearly visualized. Single-shot radiographs may be required for higher image resolution. In the author's experience, needle advancement is best performed with a diamond-shaped multi-beveled stylet while using a mallet to gently tap and drive the needle into place. Initial use of a single-beveled stylet may be more difficult and lead to problems with the needle having a tendency to slide or skid off the pedicle. Taking the extra time to properly seat the needle on the pedicle is prudent, as the needle will tend to continuously select the original “hole,” making repositioning difficult if the original needle placement is suboptimal. While advancing the needle, it is imperative that the needle does not violate the medial and inferior walls of the pedicle to decrease the risks of nerve root or spinal cord injury. As such, the needle should be advanced toward the upper and outer half of the face of the pedicle. Intermittent lateral views should also be obtained to ensure appropriate cranial to caudal angulation, access into the fractured vertebral body, and to determine when the needle reaches the posterior (dorsal) wall of the vertebral body (Fig. 3). Until the needle enters the posterior vertebral body, AP (ipsilateral oblique) projection should be used to advance the needle. With experience, the posterior wall can often be detected with slight increased resistance in the needle, as well as a subtle change in the “tapping sound” encountered while advancing the needle with a mallet. Once the needle has been advanced just beyond the posterior wall of the vertebral body, a biopsy can be performed as necessary through the outer cannula with a bone biopsy needle. Once the posterior vertebral body has been reached, the single-beveled stylet may assist by steering the tip of the VP needle to the intended position, or toward midline. Typically, the needle will advance preferentially toward the pointed edge of the bevel.
Figure 3.
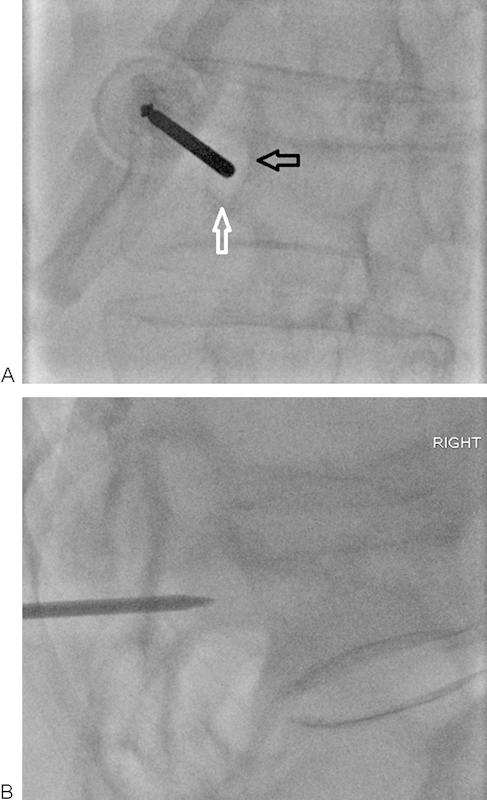
(A, B) Ipsilateral oblique and lateral fluoroscopic views of a T10 vertebral fracture. The needle should be advanced at the upper and outer quadrant of the pedicle. The needle must be advanced without violating the inferior (white arrow) and medial (black arrow) walls of the pedicle to avoid potential nerve or cord injury. Intermittent lateral views should be obtained to ensure appropriate angle of entry into the vertebral body.
From this point, the needle should be advanced in the lateral projection with continuous visualization. Ultimately, the tip of the needle should be positioned at the junction of the anterior (ventral) and middle third of the vertebral body, and as close to midline as possible (Fig. 4). Opposite direction traction on the outer portion of the needle to drive the tip in the desired direction can help ideal tip positioning. Advancing the needle further anteriorly than the junction of anterior and middle third of the vertebral body could result in violating the anterior cortex of the vertebral body. Final needle positioning should be confirmed in both the lateral and frontal projections prior to bone cement injection.
Figure 4.
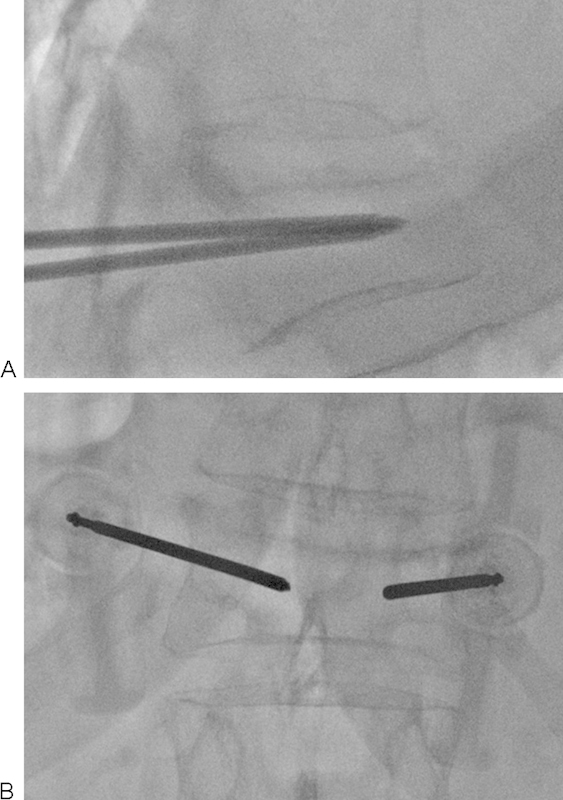
(A, B) Bilateral transpedicular access obtained for vertebroplasty. Needle positioning should be confirmed in both projections prior to the injection of cement. Ideally, the tips of the needles should be positioned as close to midline as possible at the junction of the anterior and middle third of the vertebral body.
Numerous types of bone cement are commercially available, and vary in terms of cost, radio-opacity, rate of polymerization, and biocompatibility. The advantages and disadvantages of various different cements have been previously described.37 Although newer composite and calcium phosphate cements exist, PMMA remains the most widely used in the treatment of osteoporotic and malignancy-related vertebral body fractures.
Intraosseous venography prior to the injection of PMMA has been utilized by some practitioners as a way of ensuring no direct communication with central or epidural venous structures, and potentially predicting the direction of cement flow within the vertebral body. However, contrast from intraosseous venography can ultimately obscure the ability to confidently visualize cement at the time of its injection. More recently, authors have shown no significant difference in clinical outcomes or significant added benefit with intraosseous venography, and the practice has subsequently decreased in prevalence.38,39,40 The authors do not routinely perform intraosseous venography.
Cement injection should be performed in the lateral projection using continuous fluoroscopy. The goal is delivery of cement evenly in the vertebral body or to the targeted area while avoiding extra-vertebral cement delivery, for example, epidural vein or beyond the posterior wall of the vertebral body. Care should be taken to deliver the cement at a controllable pace and to avoid over pressurization of the cement delivery system. Cement may “shoot out” when the pressure build up eventually overcomes the obstruction and lead to cement spreading to unintended places. Turning the outer cannula intermittently will release any pressure that may build. As cement is injected, the needle is slowly pulled backward to facilitate even distribution of cement throughout the vertebral body. To further minimize the risk of cement leakage, PMMA should ideally be injected when it reaches the consistency of a paste. Injection of cement is discontinued once cement reaches the posterior one-quarter of the vertebral body. If cement is noted to travel into a vein, injection should be temporarily stopped to “cast” the vein, preventing further cement accumulation in the vein and to avoid pulmonary embolism. Typically, once the cement has hardened within the vein, injection can be continued. Similarly if leakage outside the vertebral body is observed, the injection should be temporarily stopped. Pulling the needle back or twisting the needle to redirect the cement may be helpful in halting leakage when it is encountered.
The ideal cement volume for VP remains controversial and is not known.36,41 A large prospective study of 403 patients, in which average filling volumes were 3.5 mL for T3-T8, 5 mL for T9-T12, and 6 mL of L1-L5, showed no correlation between the injected volume of cement and clinical outcomes.42 Conversely, some authors have suggested that low cement volumes may be associated with worse clinical outcomes.43
As mentioned above, the goal is cement delivery across the midline (if unilateral) and even distribution in the superior-inferior and anterior-posterior vertebral body (Figs. 5 and 6). Bilateral access may be necessary when compression is severe or if cement does not spread to the midline when utilizing a unilateral approach.35 With experience, a contralateral needle can be quickly placed if a unilateral approach is inadequate. When doing so, it is recommended that the first needle be left in place within the pedicle to prevent leakage of cement backward along the initial needle tract. Studies have failed to show any difference in clinical outcomes when using a unilateral versus bilateral approach.44 When removing the needle, care must be taken to avoid leaving a “cement tail” (Fig. 7). This can be avoided by clearing the needle of cement using a stylet, or by gently spinning and rocking the needle once retracted to the edge of the pedicular margin.
Figure 5.
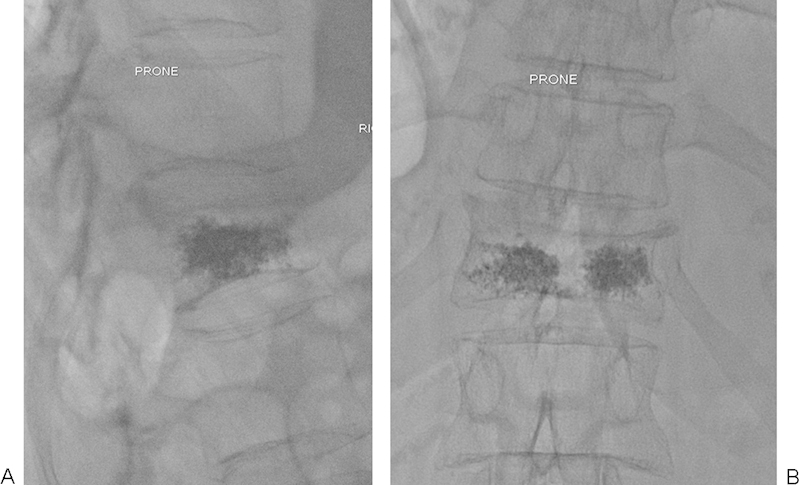
(A, B) Satisfactory vertebroplasty endpoint with even distribution of cement.
Figure 6.
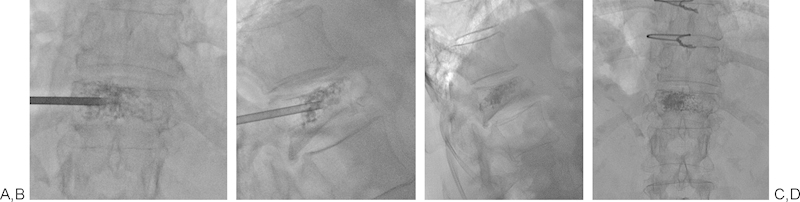
(A-D) Left unilateral transpedicular approach with ideal needle positioning and cement distribution.
Figure 7.
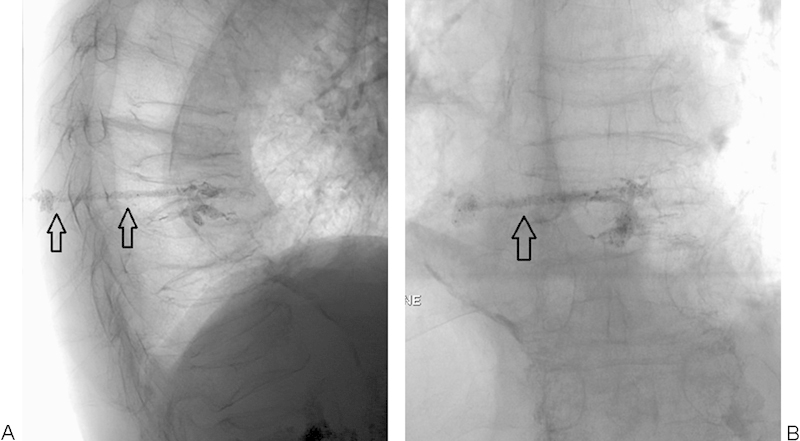
(A, B) Poorly filled mid-thoracic vertebral body after vertebroplasty. Notice the cement tail (black arrows) extending to the subcutaneous soft tissues. This can be avoided by clearing the needle of cement using the stylet, or by breaking the tail off by gently rocking and spinning the needle prior to removal. In this case, a small incision could be made to cut down on the tail and, using a hemostat, to physically remove as much as possible.
Rarely, in cases of severe compression fractures or at higher levels of the thoracic spine where pedicles are often more perpendicular in configuration to the vertebral body, a para-vertebral or extra-pedicular approach may be needed. The authors prefer transpedicular approach and use para-vertebral or extra-pedicular approach only if the transpedicular approach is not technically possible. Although beyond the scope of this article, treatment of cervical VFs may require an alternative anterolateral, transoral, or transfacial approach and more advanced image guidance. Care must be taken to avoid the carotid jugular complex. Treatment of these fractures should be reserved for the most experienced practitioners.
Postprocedural Care
After removing the VP needles, manual pressure should be applied for at least several minutes to decrease the risk of a hematoma, after which a sterile dressing is applied. Some operators prefer to allow the bone cement to further polymerize in the prone position before transferring the patient to supine position. Left over cement at the end of the procedure, and its degree of polymerization, can be used to help guide how long a patient should remain on the table after the procedure. In the case of PMMA, setting times are typically 8 to 10 minutes.37 However, the authors routinely transfer the patients to his or her stretcher once hemostasis is achieved. Patients are kept supine for approximately 2 hours after completion of the procedure. A postprocedural neurological exam should be documented and compared with the patient's preprocedural exam. Any change in the patient's baseline neurological exam should prompt further evaluation and often additional imaging. The patient's level of pain should be recorded (visual analogue scale) at the end of the procedure and at 2 hours postprocedure prior to discharge. Patients are discharged home after ambulation. Persistent pain after VP is not uncommon, and patients may require a prescription for pain medications at the time of discharge. Patients should be encouraged to remain at bed rest or keep activity minimal for 24 hours. Significant variation in the timing for scheduled outpatient follow-up after VP has been described. In the authors' practice, patients are seen in follow-up at 1 month and additionally as needed. In the authors' experience, majority of patients will gain significant pain improvement within 2 to 7 days. Some patients may also require muscle relaxants and physical therapy due to muscle spasms and/or physical deconditioning.
Complications and Clinical Outcomes
Most complications associated with VP are minor and rarely require intervention. However, major complications that may require a surgical intervention can occur and result in significant disability or death. In a review of 30 studies with more than 2,000 patients with osteoporotic VF treated with VP, major complications occurred in 0.9% of patients, and no deaths were reported.45 It is important to note that VP performed in the setting of painful malignant VF is associated with higher complication rates (upward of 10%).12,14 Major complication thresholds of 2% or less have been recommended for the treatment of osteoporotic fractures, and 10% or less for the treatment of malignancy-associated VF.46
Reported complications include infection, bleeding, transient radiculopathy, spinal stenosis, pulmonary embolization, and death. Infection and bleeding has been reported in fewer than 1% of cases. To decrease the risk of bleeding and trauma, published quality guidelines have advised against treating more than three to four levels at any one time.12 More than 3 to 6% of patients may experience transient radiculopathy, which often can be treated successfully with steroids or anti-inflammatory medications.47,48 Local trauma can occur in fewer than 1% of cases, with injury to the pleura, kidneys, nerve roots, and spinal cord, as well as fractures to lamina and pedicles. Rib fractures have been reported and often attributed to patient positioning. Rare allergic reactions to PMMA and transient hypotension believed to be related to the PMMA monomer have also been described. VP may also be associated with an increased risk of subsequent VF immediately adjacent to a treated level, although the data on this are debatable.
One of the most common findings, which is typically asymptomatic, is the leakage of cement from the vertebral body into adjacent structures. Extravasation of cement has been reported to occur in 30 to 80% of cases.48 A review of 69 clinical studies revealed cement leakage in 41% of cases, with 96% being asymptomatic. Of these, 32.5% of leakages were paravertebral, 32% involved the epidural space, 30.5% were into the disc, 3.3% were neuroforaminal, and 1.7% resulted in pulmonary emboli.49 Although the overwhelming majority of cement leakage is asymptomatic, few patients may experience problems related to the event. Cement extravasation into the disc and paravertebral tissues may result in pain from exothermic heat related to cement polymerization or from actual mass effect (Fig. 8). Cement leakage can also extend into epidural and vertebral veins and result in potentially life-threatening pulmonary emboli (Figs. 9 and 10). Leakage of PMMA into the epidural space has been reported and can result in spinal cord or nerve root compression and subsequent neurological symptoms and deficits. Patients with cement extravasation and new neurological deficits should undergo further evaluation with CT and consideration for consultation with neurosurgical service. This is a rare occurrence and often alleviated with short-term steroid therapy.
Figure 8.
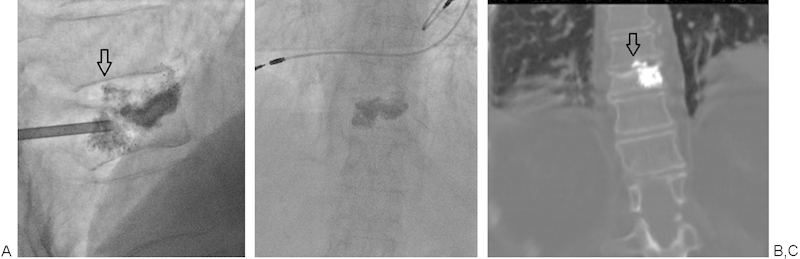
(A-C) Intradiscal extravasation of cement (arrows) in this case was asymptomatic, but uncommonly can be associated with pain. Cement injection should be paused when extravasation is encountered. Often waiting a minute or two will allow the cement more time to thicken and for the leak to seal.
Figure 9.
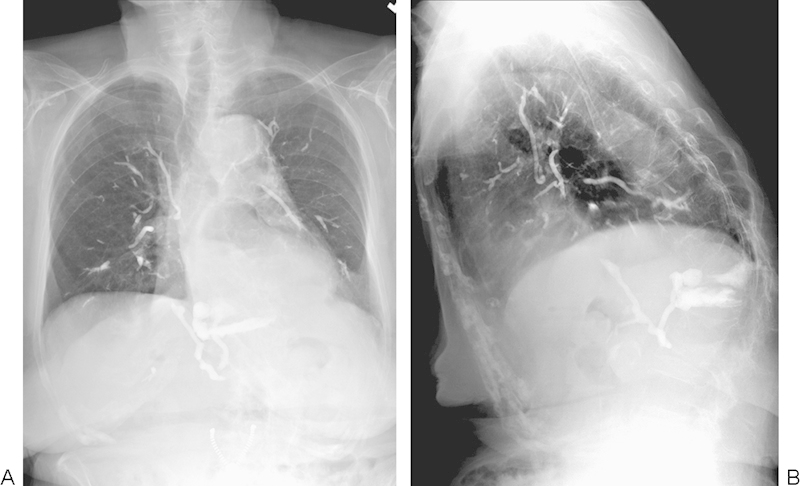
(A, B) A rare case of massive pulmonary emboli related to PMMA from a vertebroplasty performed in an outpatient setting. This patient survived, but eventually developed pulmonary hypertension. Images courtesy of Dr. Michael Jay.
Figure 10.
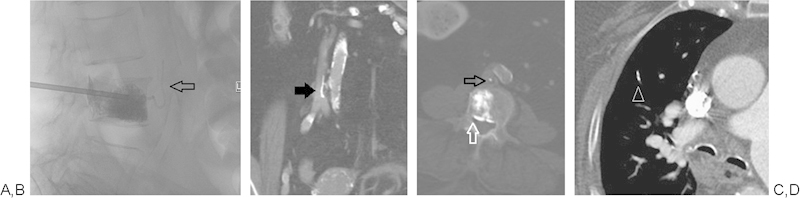
(A-D) Unipedicular vertebroplasty with extravasation of cement anteriorly via a vertebral vein and ultimately extending into the IVC (black arrows). Cement also extends beyond the posterior wall of the vertebral body and into epidural veins (white arrow). CT chest shows a small cement PE in the right middle lobe (white arrow head). The patient remained asymptomatic.
Many observational studies have shown VP to be effective and safe for the treatment of osteoporotic and malignancy-related VF that have failed to respond to medical therapy.49,50,51,52,53,54,55,56,57,58,59 Studies have shown significant pain relief following VP in more than 90% of patients with osteoporotic VF, 70% of patients with malignant VF, and 80% of patients with painful hemangioma.38 Improved quality of life, decreased dependence on pain medications, and increased physical mobility, as well as durable mid- and long-term pain relief have been demonstrated after VP.38
Recently, two randomized controlled trials were published in which patients were randomized to VP or a sham procedure.60,61 These studies, with pain reduction as the primary outcome, failed to show increased benefit of VP compared with sham procedures that consisted of an injection of local anesthetic into the periosteum. These two trials have questioned the efficacy of VP and have been challenged with much criticism given their conflicting results with prior studies, as well as with the clinical experiences of referring and treating physicians. Specifically, the large discrepancy in number of patients screened versus those who were ultimately enrolled and the fact that screening MRIs and bone scans were not required in one of the studies are the main criticisms. There was also a large crossover to the VP group in one of the studies, raising the possibility of some benefit of VP over the sham procedure.60 Adding support to the efficacy of VP and in contrast to the aforementioned two studies, a recent nonblinded randomized trial showed VP to be superior to optimal conservative management.62 In this study, the VP group demonstrated significant improvement in pain, quality of life, and disability compared with those patients managed with conservative therapy alone. At the time of this manuscript preparation, another randomized sham control trial is in progress.63
Conclusion
Percutaneous VP is widely accepted as a safe and effective therapeutic option for the treatment of painful osteoporotic and malignant VFs that fail to respond to optimal medical therapy. Appropriate patient selection, preprocedural evaluation, and meticulous attention to proper technique are paramount to achieve best outcomes and to minimize complications. As existing studies have shown conflicting results, additional studies will likely be required to conclusively establish the efficacy of VP. Until then, patients should be treated in the context of a collaborative and multidisciplinary approach, and in the setting of an appropriate informed consent.
References
- 1.Leech J A, Dulberg C, Kellie S, Pattee L, Gay J. Relationship of lung function to severity of osteoporosis in women. Am Rev Respir Dis. 1990;141(1):68–71. doi: 10.1164/ajrccm/141.1.68. [DOI] [PubMed] [Google Scholar]
- 2.Borgström F, Zethraeus N, Johnell O. et al. Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int. 2006;17(5):637–650. doi: 10.1007/s00198-005-0015-8. [DOI] [PubMed] [Google Scholar]
- 3.Silverman S L. The clinical consequences of vertebral compression fracture. Bone. 1992;13 02:S27–S31. doi: 10.1016/8756-3282(92)90193-z. [DOI] [PubMed] [Google Scholar]
- 4.Röllinghoff M, Zarghooni K, Schlüter-Brust K. et al. Indications and contraindications for vertebroplasty and kyphoplasty. Arch Orthop Trauma Surg. 2010;130(6):765–774. doi: 10.1007/s00402-010-1083-6. [DOI] [PubMed] [Google Scholar]
- 5.Kado D M Browner W S Palermo L Nevitt M C Genant H K Cummings S R; Study of Osteoporotic Fractures Research Group. Vertebral fractures and mortality in older women: a prospective study Arch Intern Med 1999159111215–1220. [DOI] [PubMed] [Google Scholar]
- 6.Nevitt M C, Thompson D E, Black D M. et al. Effect of alendronate on limited-activity days and bed-disability days caused by back pain in postmenopausal women with existing vertebral fractures. Arch Intern Med. 2000;160(1):77–85. doi: 10.1001/archinte.160.1.77. [DOI] [PubMed] [Google Scholar]
- 7.Riggs B L Melton L J III The worldwide problem of osteoporosis: insights afforded by epidemiology Bone 199517(5, Suppl):505S–511S. [DOI] [PubMed] [Google Scholar]
- 8.Johnell O, Kanis J A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 9.Lips P Epidemiology and predictors of fractures associated with osteoporosis Am J Med 19971032A3S–8S., discussion 8S-11S [DOI] [PubMed] [Google Scholar]
- 10.Klazen C A, Verhaar H J, Lohle P N. et al. Clinical course of pain in acute osteoporotic vertebral compression fractures. J Vasc Interv Radiol. 2010;21(9):1405–1409. doi: 10.1016/j.jvir.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty [in French] Neurochirurgie. 1987;33(2):166–168. [PubMed] [Google Scholar]
- 12.Gangi A Sabharwal T Irani F G Buy X Morales J P Adam A; Standards of Practice Committee of the Society of Interventional Radiology. Quality assurance guidelines for percutaneous vertebroplasty Cardiovasc Intervent Radiol 2006292173–178. [DOI] [PubMed] [Google Scholar]
- 13.Garfin S R, Yuan H A, Reiley M A. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001;26(14):1511–1515. doi: 10.1097/00007632-200107150-00002. [DOI] [PubMed] [Google Scholar]
- 14.McGraw J K, Cardella J, Barr J D. et al. Society of Interventional Radiology quality improvement guidelines for percutaneous vertebroplasty. J Vasc Interv Radiol. 2003;14(9, Pt 2):S311–S315. doi: 10.1097/01.rvi.0000082822.75926.4c. [DOI] [PubMed] [Google Scholar]
- 15.Cortet B, Cotten A, Boutry N. et al. Percutaneous vertebroplasty in the treatment of osteoporotic vertebral compression fractures: an open prospective study. J Rheumatol. 1999;26(10):2222–2228. [PubMed] [Google Scholar]
- 16.Cotten A Boutry N Cortet B et al. Percutaneous vertebroplasty: state of the art Radiographics 1998182311–320., discussion 320-323 [DOI] [PubMed] [Google Scholar]
- 17.Cyteval C, Sarrabère M P, Roux J O. et al. Acute osteoporotic vertebral collapse: open study on percutaneous injection of acrylic surgical cement in 20 patients. AJR Am J Roentgenol. 1999;173(6):1685–1690. doi: 10.2214/ajr.173.6.10584820. [DOI] [PubMed] [Google Scholar]
- 18.Deramond H, Depriester C, Galibert P, Le Gars D. Percutaneous vertebroplasty with polymethylmethacrylate. Technique, indications, and results. Radiol Clin North Am. 1998;36(3):533–546. doi: 10.1016/s0033-8389(05)70042-7. [DOI] [PubMed] [Google Scholar]
- 19.Jensen M E, Evans A J, Mathis J M, Kallmes D F, Cloft H J, Dion J E. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol. 1997;18(10):1897–1904. [PMC free article] [PubMed] [Google Scholar]
- 20.Mathis J M, Petri M, Naff N. Percutaneous vertebroplasty treatment of steroid-induced osteoporotic compression fractures. Arthritis Rheum. 1998;41(1):171–175. doi: 10.1002/1529-0131(199801)41:1<171::AID-ART21>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Cotten A, Dewatre F, Cortet B. et al. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology. 1996;200(2):525–530. doi: 10.1148/radiology.200.2.8685351. [DOI] [PubMed] [Google Scholar]
- 22.Weill A, Chiras J, Simon J M, Rose M, Sola-Martinez T, Enkaoua E. Spinal metastases: indications for and results of percutaneous injection of acrylic surgical cement. Radiology. 1996;199(1):241–247. doi: 10.1148/radiology.199.1.8633152. [DOI] [PubMed] [Google Scholar]
- 23.McDonald R J, Trout A T, Gray L A, Dispenzieri A, Thielen K R, Kallmes D F. Vertebroplasty in multiple myeloma: outcomes in a large patient series. AJNR Am J Neuroradiol. 2008;29(4):642–648. doi: 10.3174/ajnr.A0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pflugmacher R, Schleicher P, Schröder R J, Melcher I, Klostermann C K. Maintained pain reduction in five patients with multiple myeloma 12 months after treatment of the involved cervical vertebrae with vertebroplasty. Acta Radiol. 2006;47(8):823–829. doi: 10.1080/02841850600812728. [DOI] [PubMed] [Google Scholar]
- 25.Deramond H, Darrasson R, Galibert P. Percutaneous vertebroplasty with acrylic cement in the treatment of aggressive spinal angiomas. Rachis. 1989;1:143–153. [Google Scholar]
- 26.Acosta F L Jr, Sanai N, Chi J H. et al. Comprehensive management of symptomatic and aggressive vertebral hemangiomas. Neurosurg Clin N Am. 2008;19(1):17–29. doi: 10.1016/j.nec.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Cotten A, Deramond H, Cortet B. et al. Preoperative percutaneous injection of methyl methacrylate and N-butyl cyanoacrylate in vertebral hemangiomas. AJNR Am J Neuroradiol. 1996;17(1):137–142. [PMC free article] [PubMed] [Google Scholar]
- 28.Feydy A, Cognard C, Miaux Y. et al. Acrylic vertebroplasty in symptomatic cervical vertebral haemangiomas: report of 2 cases. Neuroradiology. 1996;38(4):389–391. doi: 10.1007/BF00596600. [DOI] [PubMed] [Google Scholar]
- 29.Ide C, Gangi A, Rimmelin A. et al. Vertebral haemangiomas with spinal cord compression: the place of preoperative percutaneous vertebroplasty with methyl methacrylate. Neuroradiology. 1996;38(6):585–589. doi: 10.1007/BF00626105. [DOI] [PubMed] [Google Scholar]
- 30.Zapałowicz K, Radek A, Błaszczyk B, Koziński T, Zelechowski J. Percutaneous vertebroplasty with methyl methacrylate bone cement in the treatment of spinal angiomas and neoplasms. Ortop Traumatol Rehabil. 2003;5(2):185–188. [PubMed] [Google Scholar]
- 31.Cardon T, Hachulla E, Flipo R M. et al. Percutaneous vertebroplasty with acrylic cement in the treatment of a Langerhans cell vertebral histiocytosis. Clin Rheumatol. 1994;13(3):518–521. doi: 10.1007/BF02242955. [DOI] [PubMed] [Google Scholar]
- 32.Masala S, Pipitone V, Tomassini M, Massari F, Romagnoli A, Simonetti G. Percutaneous vertebroplasty in painful Schmorl nodes. Cardiovasc Intervent Radiol. 2006;29(1):97–101. doi: 10.1007/s00270-005-0153-6. [DOI] [PubMed] [Google Scholar]
- 33.Nieuwenhuijse M J, van Erkel A R, Dijkstra P D. Percutaneous vertebroplasty in very severe osteoporotic vertebral compression fractures: feasible and beneficial. J Vasc Interv Radiol. 2011;22(7):1017–1023. doi: 10.1016/j.jvir.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 34.Rad A E, Kallmes D F. Pain relief following vertebroplasty in patients with and without localizing tenderness on palpation. AJNR Am J Neuroradiol. 2008;29(9):1622–1626. doi: 10.3174/ajnr.A1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beall D P, Datir A, D'Souza S L. et al. Percutaneous treatment of insufficiency fractures : principles, technique and review of literature. Skeletal Radiol. 2010;39(2):117–130. doi: 10.1007/s00256-009-0722-x. [DOI] [PubMed] [Google Scholar]
- 36.Beall D P, Braswell J J, Martin H D, Stapp A M, Puckett T A, Stechison M T. Technical strategies and anatomic considerations for parapedicular access to thoracic and lumbar vertebral bodies. Skeletal Radiol. 2007;36(1):47–52. doi: 10.1007/s00256-006-0192-3. [DOI] [PubMed] [Google Scholar]
- 37.Katsanos K, Sabharwal T, Adam A. Percutaneous cementoplasty. Semin Intervent Radiol. 2010;27(2):137–147. doi: 10.1055/s-0030-1253512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peh W C Munk P L Rashid F Gilula L A Percutaneous vertebral augmentation: vertebroplasty, kyphoplasty and skyphoplasty Radiol Clin North Am 2008463611–635., vii [DOI] [PubMed] [Google Scholar]
- 39.Gaughen J R, Jensen M E, Schweickert P A. et al. Relevance of antecedent venography in percutaneous vertebroplasty for the treatment of osteoporotic compression fractures. Amer J Neuroradil. 2002;23(4):594–600. [PMC free article] [PubMed] [Google Scholar]
- 40.Kallmes D F, Jensen M E. Percutaneous vertebroplasty. Radiology. 2003;229(1):27–36. doi: 10.1148/radiol.2291020222. [DOI] [PubMed] [Google Scholar]
- 41.Belkoff S M, Mathis J M, Jasper L E, Deramond H. The biomechanics of vertebroplasty. The effect of cement volume on mechanical behavior. Spine. 2001;26(14):1537–1541. doi: 10.1097/00007632-200107150-00007. [DOI] [PubMed] [Google Scholar]
- 42.Al-Ali F, Barrow T, Luke K. Vertebroplasty: what is important and what is not. AJNR Am J Neuroradiol. 2009;30(10):1835–1839. doi: 10.3174/ajnr.A1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boszczyk B. Volume matters: a review of procedural details of two randomised controlled vertebroplasty trials of 2009. Eur Spine J. 2010;19(11):1837–1840. doi: 10.1007/s00586-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim A K, Jensen M E, Dion J E, Schweickert P A, Kaufmann T J, Kallmes D F. Unilateral transpedicular percutaneous vertebroplasty: initial experience. Radiology. 2002;222(3):737–741. doi: 10.1148/radiol.2223010718. [DOI] [PubMed] [Google Scholar]
- 45.Hochmuth K, Proschek D, Schwarz W, Mack M, Kurth A A, Vogl T J. Percutaneous vertebroplasty in the therapy of osteoporotic vertebral compression fractures: a critical review. Eur Radiol. 2006;16(5):998–1004. doi: 10.1007/s00330-005-0067-3. [DOI] [PubMed] [Google Scholar]
- 46.McGraw J K, Cardella J, Barr J D. et al. Society of Interventional Radiology quality improvement guidelines for percutaneous vertebroplasty. J Vasc Interv Radiol. 2003;14(7):827–831. doi: 10.1016/s1051-0443(07)60242-5. [DOI] [PubMed] [Google Scholar]
- 47.Chiras J, Depriester C, Weill A, Sola-Martinez M T, Deramond H. Percutaneous vertebral surgery. Technics and indications [in French] J Neuroradiol. 1997;24(1):45–59. [PubMed] [Google Scholar]
- 48.Vallejo R, Benyamin R. Vertebral augmentation techniques for the treatment of vertebral compression fractures: a review. Reg Anesth Pain Manag. 2010;14(3):133–141. [Google Scholar]
- 49.Hulme P A, Krebs J, Ferguson S J, Berlemann U. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine. 2006;31(17):1983–2001. doi: 10.1097/01.brs.0000229254.89952.6b. [DOI] [PubMed] [Google Scholar]
- 50.Berenson J, Pflugmacher R, Jarzem P. et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol. 2011;12(3):225–235. doi: 10.1016/S1470-2045(11)70008-0. [DOI] [PubMed] [Google Scholar]
- 51.Grafe I A, Da Fonseca K, Hillmeier J. et al. Reduction of pain and fracture incidence after kyphoplasty: 1-year outcomes of a prospective controlled trial of patients with primary osteoporosis. Osteoporos Int. 2005;16(12):2005–2012. doi: 10.1007/s00198-005-1982-5. [DOI] [PubMed] [Google Scholar]
- 52.Hadjipavlou A G, Tzermiadianos M N, Katonis P G, Szpalski M. Percutaneous vertebroplasty and balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures and osteolytic tumours. J Bone Joint Surg Br. 2005;87(12):1595–1604. doi: 10.1302/0301-620X.87B12.16074. [DOI] [PubMed] [Google Scholar]
- 53.Kasperk C, Hillmeier J, Nöldge G. et al. Treatment of painful vertebral fractures by kyphoplasty in patients with primary osteoporosis: a prospective nonrandomized controlled study. J Bone Miner Res. 2005;20(4):604–612. doi: 10.1359/JBMR.041203. [DOI] [PubMed] [Google Scholar]
- 54.Komp M, Ruetten S, Godolias G. Minimally invasive therapy for functionally unstable osteoporotic vertebral fracture by means of kyphoplasty: prospective comparative study of 19 surgically and 17 conservatively treated patients. J Miner Stoffwechs. 2004;11 01:13–15. [Google Scholar]
- 55.Ploeg W T, Veldhuizen A G, The B, Sietsma M S. Percutaneous vertebroplasty as a treatment for osteoporotic vertebral compression fractures: a systematic review. Eur Spine J. 2006;15(12):1749–1758. doi: 10.1007/s00586-006-0159-z. [DOI] [PubMed] [Google Scholar]
- 56.Taylor R S, Fritzell P, Taylor R J. Balloon kyphoplasty in the management of vertebral compression fractures: an updated systematic review and meta-analysis. Eur Spine J. 2007;16(8):1085–1100. doi: 10.1007/s00586-007-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bouza C, López T, Magro A, Navalpotro L, Amate J M. Efficacy and safety of balloon kyphoplasty in the treatment of vertebral compression fractures: a systematic review. Eur Spine J. 2006;15(7):1050–1067. doi: 10.1007/s00586-005-0048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Voormolen M H, Mali W P, Lohle P N. et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. AJNR Am J Neuroradiol. 2007;28(3):555–560. [PMC free article] [PubMed] [Google Scholar]
- 59.Wardlaw D, Cummings S R, Van Meirhaeghe J. et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet. 2009;373(9668):1016–1024. doi: 10.1016/S0140-6736(09)60010-6. [DOI] [PubMed] [Google Scholar]
- 60.Kallmes D F, Comstock B A, Heagerty P J. et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361(6):569–579. doi: 10.1056/NEJMoa0900563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buchbinder R, Osborne R H, Ebeling P R. et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361(6):557–568. doi: 10.1056/NEJMoa0900429. [DOI] [PubMed] [Google Scholar]
- 62.Klazen C A, Lohle P N, de Vries J. et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet. 2010;376(9746):1085–1092. doi: 10.1016/S0140-6736(10)60954-3. [DOI] [PubMed] [Google Scholar]
- 63.Firanescu C, Lohle P N, de Vries J. et al. A randomised sham controlled trial of vertebroplasty for painful acute osteoporotic vertebral fractures (VERTOS IV) Trials. 2011;12:93. doi: 10.1186/1745-6215-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]


