Abstract
We investigated the role of ERK1/2 in the brain on the effects of centrally administered resistin on thermogenesis. Resistin (7 μg) into anaesthetized rats significantly decreased brown adipose tissue temperature by 1.0 ± 0.4 °C (P < 0.005). This response was significantly attenuated by over 60% when ERK1/2 was inhibited by U0126 (7 μg) (P < 0.05). Resistin reduced uncoupling protein-1 mRNA expression (0.11 ± 0.01 vs 1.24 ± 0.85 resistin vs control respectively) and the expression of peroxisome proliferator-activated receptor gamma co-activator 1-α, but the effects were not statistically significant. The results suggest that ERK1/2 in the brain contributes to resistin’s effects on thermogenesis.
Keywords: Resistin, brown adipose tissue, thermogenesis, ERK1/2
Introduction
Resistin is a newly discovered adipokine originally identified in adipose tissue [1], but now known to be expressed in a variety of tissues, including the hypothalamus in the brain [2,3]. Resistin belongs to a family of cysteine-rich proteins capable of inducing insulin resistance and affecting energy metabolism [4]. Plasma levels of resistin are high in conditions like obesity, a condition in which there is an imbalance between energy intake and expenditure. Resistin has been shown to reduce dietary intake, thereby reducing energy intake [5].
Energy expenditure is regulated by basal metabolic rate, physical activity and thermogenesis. Brown adipose tissue (BAT) is a key organ responsible for non-shivering thermogenesis, thereby influencing energy dissipation [6]. This function is linked to the unique presence of uncoupling protein-1 (UCP1) on the inner mitochondrial membrane of brown adipocytes. UCP1 ‘uncouples’ fuel oxidation from adenosine triphosphate synthesis resulting in dissipation of considerable energy as heat [6] and increased BAT and body core temperatures. The thermogenic function of BAT is tightly regulated by the sympathetic nervous system through activation of adrenoreceptors that elicit changes in UCP1 and other important thermogenic proteins, such as the transcriptional co-activator peroxisome proliferator-activated receptor gamma co-activator 1-α (PGC-1α) [7].
Resistin reduces sympathetic nerve activity innervating BAT, an effect mediated by Extracellular Regulated kinases 1/2 (ERK1/2) in the brain [10]. ERK1/2 are a family of cellular protein kinase enzymes involved in diverse cellular functions including cell growth, proliferation and neuronal activity. ERK1/2 has also been found to mediate the actions of resistin on cell proliferation, migration and hypertrophy [8,9]. Thus, in the present study, we investigated whether ERK1/2 in the brain mediates resistin’s effects on BAT temperature and body core temperature, and whether centrally administered resistin affected the mRNA expression of UCP1 and PGC-1α via brain ERK1/2.
Methods
Animals
All experiments were performed in accordance with the Prevention of Cruelty to Animals Act 1986 (Australia). They conformed with the Guiding Principles for Research Involving Animals and Human Beings (2) and guidelines set by the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 2007 (National Health and Medical Research Council of Australia). Experiments were approved by the Royal Melbourne Institute of Technology (RMIT) University Animal Ethics Committee.
Male Sprague Dawley rats were obtained from ARC animal resources centre (Western Australia). Rats were housed at 23 °C with a 12-h light/dark cycle and given free access to standard rat chow and water.
Procedures
General
Rats were fasted overnight before an experiment. On the day of the experiment, anaesthesia was induced using isoflurane gas (2.5–3%) in O2. A catheter was inserted into the femoral vein, to maintain anaesthesia with intravenous urethane (1–1.4 g/kg initially, followed by supplemental doses of 0.05 g/kg as required). The depth of anaesthesia was maintained to ensure the absence of corneal and pedal reflexes.
BAT temperature and body core temperature recording
The interscapular BAT was exposed and the tip of a thermistor probe was inserted into it to measure temperature (Fluke 73III, Fluke Australia, NSW, Australia). Body core temperature was measured by a thermometer placed in the rectum (Fluke 52II thermometer, Fluke Australia, NSW, Australia).
Microinjections into the lateral brain ventricle
Each animal was placed prone and its head was mounted in a Stoelting stereotaxic frame, such that bregma and lambda were on the same horizontal plane. A hole (4 mm diameter) centred 0.7 mm caudal and 1.8 mm lateral from bregma, was drilled into the skull. The hole was covered with cotton wool soaked in normal saline to cover the brain surface.
Intracerebroventricular (ICV) microinjections were made unilaterally using a fine glass micropipette (50–70 μm tip outer diameter) inserted into the lateral brain ventricle (stereotaxic coordinates: 0.7 mm caudal to bregma, 1.8 mm lateral to midline, and 3.7 mm ventral to the surface of the dura). After the ICV injections, the micropipette was left in place for 1 min. At the end of the experiment, a small amount of pontamine sky blue was microinjected using the same coordinates, to confirm injection into the lateral ventricle.
Experimental protocols
Resting levels of BAT and body core temperatures were recorded for at least 10 min before ICV injections. Temperature changes were monitored for 3–4 hr after ICV injection of resistin (7 μg in 5 μL) in rats pre-treated with the ERK1/2 inhibitor U0126 (7 μg in 2 μL, n=5) (administered 20–30 min earlier) or vehicle (DMSO, n=8). In separate groups, vehicle (n=7) or U0126 (n=5) were administered following pretreatment with artificial cerebrospinal fluid. Artificial cerebrospinal fluid contained NaCl 124 mM, KCl 3.0 mM, NaH2PO4.2H2O 1.3 mM, MgCl2.6H2O 2.0 mM, NaHCO3 26 mM, glucose 10 mM, CaCl2 2.0 mM in Milli-Q water, buffered with carbogen.Recombinant rat resistin (lot#-L16251/F) and U0126 (lot#3-Z5175W) were purchased from Sapphire Biosciences (Australia). DMSO (lot#109K2350) was obtained from Sigma (Australia).
BAT sampling and processing
Animals were euthanized 3–4 hr after ICV injections. BAT was rapidly excised, frozen in liquid nitrogen and stored at –80 °C for subsequent RNA extraction.
RNA extraction and quantification
BAT RNA extraction was performed using a TRIzol-based kit, following the manufacturer’s directions (Invitrogen, Melbourne, Australia, Cat No 12183-018A). Briefly, around 30 mg of BAT was put in 500 μL of TRIzol and homogenised with a handheld, motorised Teflon pestle. After elution through a spin cartridge, extracted RNA was quantified using a QUANT-iT analyzer kit (Invitrogen, Melbourne, Australia, Cat No Q32852), following the manufacturer’s directions. RNA samples were diluted as appropriate to equalise concentrations, and stored at minus 80 °C for subsequent reverse transcription.
Reverse transcription and real-time quantitative polymerase chain reaction (RT-PCR)
First-strand complementary DNA (cDNA) synthesis was done using commercially available TaqMan Reverse Transcription Reagents (Invitrogen, Melbourne, Australia) in a final reaction volume of 20 μL. All RNA and negative control samples were reverse transcribed to cDNA in a single run from the same reverse transcription master mix. A serially diluted pooled RNA sample from the control group was produced and included to make sure reverse transcription was efficient and calculate a standard curve for RT-PCR. Quantification of mRNA (in duplicate) was performed on a 72-well Rotor-Gene 3000 Centrifugal Real-Time Cycler (Corbett Research, Mortlake, Australia). Taqman-FAM-labelled primer/probes for UCP1 (Cat No. Rn 00562126_m1) and PGC-1α (Cat No. Rn00580241_m1) were used in a final reaction volume of 20 μL. PCR conditions were 2 min at 50 °C, 10 min at 95 °C, then 40 cycles at 95 °C for 15 s and 60 °C for 60 s.
Statistical analysis
BAT and body core temperatures
Resting levels of BAT and body core temperatures before the injections were compared between groups using one-way ANOVA. When a significant difference was observed, comparisons between groups were made using Tukey’s post-hoc test. Changes from resting levels of BAT and body core temperatures were compared between groups in each experimental series using two-way ANOVA with repeated measures. All results are expressed as means ± SE. P < 0.05 was considered to be statistically significant.
mRNA expression
18 S ribosomal RNA (18 S rRNA) (Cat No. Hs99999901_s1) was used as a housekeeping gene to normalize threshold cycle (CT) values. The relative amounts of mRNAs were calculated using the relative quantification (ΔΔCT) method [11]. Values were expressed relative to 18 S ribosomal RNA and presented in arbitrary units. Comparisons between the groups were performed using a one-way ANOVA. When a significant difference was observed, comparisons between groups were made using Tukey’s post-hoc test. Due to technical problems, mRNA levels could not be measured in BAT from all animals. All results are expressed as means ± SE. P < 0.05 was considered to be statistically significant.
Results
BAT and body core temperatures and the effects of ERK1/2 inhibition
Resting levels of BAT and body core temperatures before ICV injection of the drugs are shown in Figure 1. Compared with the control group, there was a small but statistically significant difference in resting BAT temperature in groups that received resistin. Resting body core temperatures before ICV injection of resistin were not different between the groups (Figure 1).
Figure 1.
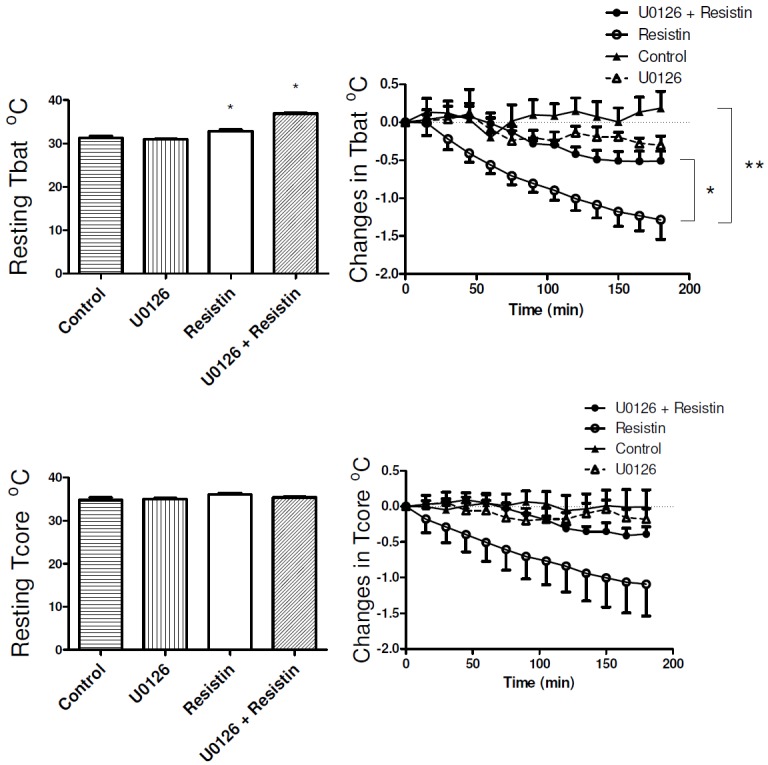
Bar graph shows brown adipose tissue temperature (Tbat) and body core temperature (Tcore) before intracerebroventricular administration of resistin alone (7 μg, n=7 for Tbat, n=8 for Tcore), resistin in the presence of U0126 (7 μg in 2 μL, n=5), U0126 alone (n=6) or vehicle (Control, n=8). Changes in Tbat and Tcore from resting levels over time in response to treatment are shown in the right panels. *p < 0.05, resistin alone vs resistin in the presence of U0126. **p < 0.005, resistin alone vs control.
ICV injection of resistin markedly reduced both BAT temperature (P < 0.005) and body core temperatures (Figure 1). In rats pre-treated with the ERK1/2 inhibitor, U0126, resistin’s effect on BAT temperature was significantly attenuated by over 60% (P < 0.05) (Figure 1). U0126 alone did not significantly change BAT temperature (Figure 1). Resistin decreased body core temperature by 1.09 ± 0.44 °C from resting levels, and although U0126 attenuated this effect by over 60%, the reduction did not reach statistical significance (Figure 1).
mRNA expression: UCP, PGC-1α
UCP1 mRNA expression was lower in the resistin-treated group (0.11 ± 0.01) than in the control group (1.24 ± 0.85), but this difference was not statistically significant. Inhibiting ERK1/2 in the brain blunted resistin’s effect on UCP1 expression, however, this effect was not statistically significant (Figure 2A). Figure 2B shows the levels of mRNA expression for PGC-1α in BAT. There were no statistically significant differences in PGC-1α expression between groups.
Figure 2.
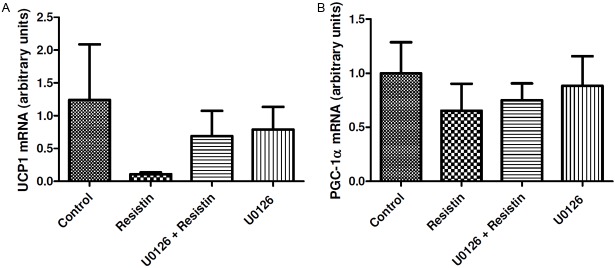
A: UCP1 and (B) PGC-1α mRNA abundance in BAT from control (n=4 for UCP1, n=6 for PGC-1α), resistin (n=5), U0126 (ERK1/2 inhibitor) + resistin (n=4) and U0126 (n=3) groups. Values are expressed relative to 18 S ribosomal RNA and presented in arbitrary units (mean ± SEM).
Discussion
This study reports that acute injections of resistin into the lateral ventricle of the brain markedly decreased BAT temperature and the response was significantly attenuated when central ERK1/2 was inhibited by U0126. We note that the resting BAT temperatures in the resistin group and in the U0126+resistin group were significantly greater than control. Although, one could argue that the fall in BAT temperature may be due to the higher basal level readjusting down to a lower level, it is more difficult to reconcile the fact that the reduction induced by resistin was significantly greater than that in the U0126+resistin group, as the latter group had a higher basal level. It is also noteworthy that we have shown previously that resistin significantly reduced BAT sympathetic nerve activity, a direct measure of thermogenesis.
The fall in BAT temperature was most likely responsible for the resistin-induced decrease in body core temperature. When central ERK1/2 was inhibited, the decrease in body core temperature induced by resistin was blunted (in excess of 60%) but the effect was not statistically significant, probably due to the variance seen in the control group. Nonetheless, the findings are in agreement with previous reports showing that central resistin reduces BAT sympathetic nerve activity [12], and that effect was mediated by central ERK1/2. It is not likely that a reduction in body core temperature was responsible for the fall in BAT temperature since (i) a reduction in body core temperature should reflexly increase BAT temperature, and (ii) resistin significantly lowered BAT sympathetic nerve activity when body core temperature was carefully maintained constant [12].
In contrast to resistin, leptin has been shown to increase BAT thermogenesis by increasing the BAT sympathetic nerve activity [13]. Interestingly, central ERK1/2 mediated the action of central leptin on BAT sympathetic nerve activity [14]. Together, the results suggest that central ERK1/2 signalling pathways are involved in the resistin-induced decrease in BAT thermogenesis and the leptin-induced increase in BAT thermogenesis. Given the two adipokines have opposing actions on thermogenesis, it seems likely that different cell groups mediate those actions.
In the present study, the average UCP1 mRNA expression was lower in the resistin-treated group than in the control group and this response was blunted when central ERK1/2 signalling pathways were inhibited. Although these changes were not statistically significant, the data are consistent with the findings that resistin reduces BAT temperature. There is considerable evidence indicating the presence of metabolically active BAT in adult humans [15,16], and it has been recently demonstrated that ablation of UCP1 induced obesity in mice fed a control diet under thermoneutral conditions [17]. The effects of resistin observed in the present study is consistent with reduced thermogenic capacity and energy expenditure and suggest furtile ground for further study on novel mechanisms to tackle obesity.
PGC-1α belongs to a family of nuclear transcriptional cofactors that regulate mitochondrial biogenesis and is involved in the regulation of brown adipocyte-specific genes [18]. PGC-1α is believed to control UCP1 expression, although this is controversial since studies have shown that PGC-1α and UCP1 gene expression in brown adipocytes are not necessarily correlated [19,20]. Our current study showed the average amount of PGC-1α mRNA was lower in the resistin-treated group than in the control group, but this was not statistically significant. Nonetheless, it is noteworthy that the expression pattern of PGC-1α in the different treatment groups followed the same expression pattern as UCP1.
In conclusion, the main finding of this study highlights the importance of ERK1/2 signalling pathways in the brain in mediating the reduction in BAT thermogenesis induced by resistin.
Acknowledgements
The authors wish to thank the School of medical sciences, RMIT University for support. S.K. was a recipient of an Australian Postgraduate Research Scholarship Award at the time of this work. D.M.C. is supported by a National Health and Medical Research Council Post-Graduate scholarship.
Disclosure of conflict of interest
The authors have nothing to disclose.
References
- 1.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 2.Morash BA, Willkinson D, Ur E, Wilkinson M. Resistin expression and regulation in mouse pituitary. FEBS Lett. 2002;526:26–30. doi: 10.1016/s0014-5793(02)03108-3. [DOI] [PubMed] [Google Scholar]
- 3.Yang RZ, Huang Q, Xu A, McLenithan JC, Eisen JA, Shuldiner AR, Alkan S, Gong DW. Comparative studies of resistin expression and phylogenomics in human and mouse. Biochem Biophys Res Commun. 2003;310:927–935. doi: 10.1016/j.bbrc.2003.09.093. [DOI] [PubMed] [Google Scholar]
- 4.Ahima RS, Lazar MA. Adipokines and the peripheral and neural control of energy balance. Mol Endocrinol. 2008;22:1023–1031. doi: 10.1210/me.2007-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tovar S, Nogueiras R, Tung LY, Castaneda TR, Vazquez MJ, Morris A, Williams LM, Dickson SL, Dieguez C. Central administration of resistin promotes short-term satiety in rats. Eur J Endocrinol. 2005;153:R1–5. doi: 10.1530/eje.1.01999. [DOI] [PubMed] [Google Scholar]
- 6.Cannon B, Nedergaard J. Brown Adipose Tissue: Function and Physiological Significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 7.Seale P. Transcriptional control of brown adipocyte development and thermogenesis. Int J Obes(Lond) 2010;34:S17–22. doi: 10.1038/ijo.2010.178. [DOI] [PubMed] [Google Scholar]
- 8.Di Simone N, Di Nicuolo F, Sanguinetti M, Castellani R, D’Asta M, Caforio L, Caruso A. Resistin regulates human choriocarcinoma cell invasive behaviour and endothelial cell angiogenic processes. J Endocrinol. 2006;189:691–699. doi: 10.1677/joe.1.06610. [DOI] [PubMed] [Google Scholar]
- 9.Kim M, Oh JK, Sakata S, Liang I, Park W, Hajjar RJ, Lebeche D. Role of resistin in cardiac contractility and hypertrophy. J Mol Cell Cardiol. 2008;45:270–280. doi: 10.1016/j.yjmcc.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kosari S, Rathner JA, Badoer E. Central Resistin Enhances Renal Sympathetic Nerve Activity via Pi3k but Reduces the Activity to Brown Adipose Tissue via Erk1/2. J Neuroendocrinol. 2012;24:1432–9. doi: 10.1111/j.1365-2826.2012.02352.x. [DOI] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Kosari S, Rathner JA, Chen F, Badoer E. Centrally Administered Resistin Enhances Sympathetic Nerve Activity to the Hindlimb but Attenuates the Activity to Brown Adipose Tissue. Endocrinology. 2011;152:2626–2633. doi: 10.1210/en.2010-1492. [DOI] [PubMed] [Google Scholar]
- 13.Hausberg M, Morgan DA, Mitchell JL, Sivitz WI, Mark AL, Haynes WG. Leptin potentiates thermogenic sympathetic responses to hypothermia: a receptor-mediated effect. Diabetes. 2002;51:2434–2440. doi: 10.2337/diabetes.51.8.2434. [DOI] [PubMed] [Google Scholar]
- 14.Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes. 2009;58:536–542. doi: 10.2337/db08-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 16.Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 Ablation Induces Obesity and Abolishes Diet-Induced Thermogenesis in Mice Exempt from Thermal Stress by Living at Thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic Coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 19.Lehr L, Canola K, Asensio C, Jimenez M, Kuehne F, Giacobino JP, Muzzin P. The control of UCP1 is dissociated from that of PGC-1α or of mitochondriogenesis as revealed by a study using β-less mouse brown adipocytes in culture. FEBS Lett. 2006;580:4661–4666. doi: 10.1016/j.febslet.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 20.Puigserver P, Spiegelman BM. Peroxisome Proliferator-Activated Receptor-γ Coactivator 1α (PGC-1α): Transcriptional Coactivator and Metabolic Regulator. Endocrine Reviews. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]


