Abstract
Background
Local delivery is required to achieve the high antimicrobial concentrations needed to treat biofilm-forming infections. The delivery site is commonly either in the intramedullary canal or at the periosteal surface. It is unknown whether locally delivered antimicrobials are transported transcortically between the endosteal and periosteal surfaces when the infection involves the opposite surface.
Questions/Purposes
(1) Are antimicrobials transported transcortically between the endosteal and periosteal surfaces over time? And (2) are transcortical antimicrobials transported uniformly over the cortical surface?
Methods
To study transcortical antimicrobial transport, 12 human cadaveric femoral segments obtained from two women aged 63 and 64 years and one man aged 64 years were filled with antimicrobials. Three diaphyseal segments were filled with 5 wt% vancomycin in an N-isopropylacrylamide-based hydrogel and eluted in phosphate-buffered saline under infinite-sink conditions for 5 days; vancomycin was assayed by high-performance liquid chromatography. Nine segments (three infraisthmal diaphysis, three metaphysis, three epiphysis) embedded in 0.1% agarose gel were filled with aqueous doxycycline (400 μg/mL) and imaged under ultraviolet light for fluorescence on the periosteal surface at 15-minute intervals for 3 days.
Results
Transcortical vancomycin elution occurred: 8.65 mg during Day 1 and 26.5 mg by Day 5. Fluorescence from transcortical doxycycline transport was only visualized at focal locations corresponding to vascular foramina, appearing first at 5 to 10 minutes, with none over the majority of the periosteal surface for up to 24 hours.
Conclusions
Transcortical transport of locally delivered antimicrobials occurs primarily through vascular foramina.
Clinical Relevance
Transcortical antimicrobial transported may not be adequate to achieve therapeutic levels for infection on the far side of an intact cortex.
Introduction
All organisms that cause orthopaedic implant infections form biofilms on the implant surfaces. As the infection permeates adjacent bone, biofilm is established on dead bone surfaces. In established biofilm, organisms have decreased susceptibility to antimicrobials, requiring higher antimicrobial levels by hundreds of times [2]. High-dose antimicrobial-loaded bone cement (ALBC) is typically the vehicle used to deliver the high local levels of antimicrobials needed to kill organisms in established biofilm. Local delivery is typically placed adjacent to débrided bone surfaces in surgical wounds after débridement. Antimicrobials are also locally delivered to the endosteal surfaces of long bones when implants are fixed with low-dose ALBC. Orthopaedic implant infections and long-bone osteomyelitis generally involve the cortex and to varying degrees the periosteal surface, endosteal surface, and intramedullary canal [2]. After removal of implants and complete débridement, ALBC is frequently placed against the débrided bone surface in the intramedullary canal or on the periosteal surface, but often not both. It is unknown whether antimicrobials permeate though cortical bone to reach the far surface from local delivery sites.
We therefore asked: (1) Are antimicrobials transported transcortically between the endosteal and periosteal surfaces over time? And (2) are transcortical antimicrobials transported uniformly over the cortical surface?
Materials and Methods
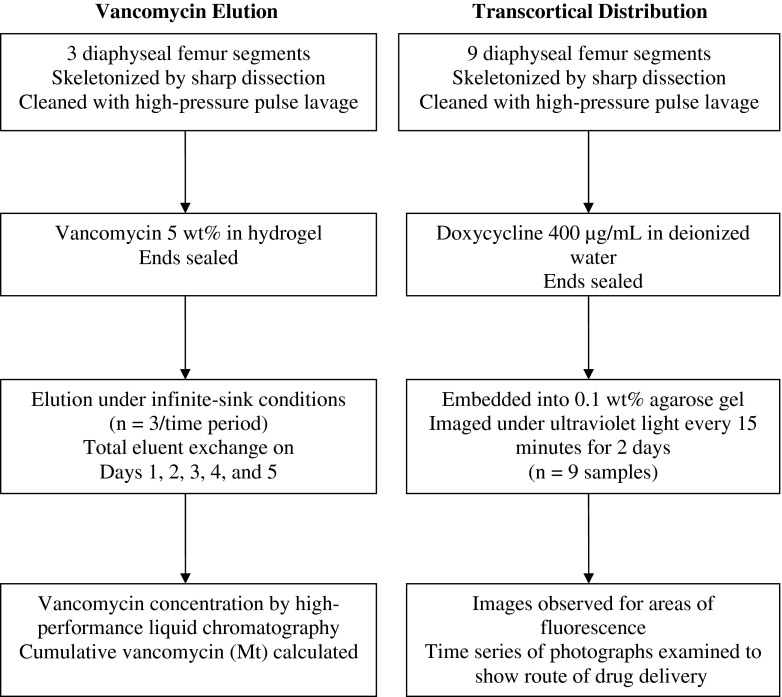
We studied the transcortical transport of antimicrobials from the intramedullary canal to the periosteal surface and into the extraosseous compartment. The study was performed in two parts: (1) transcortical elution, using an intramedullary depot of vancomycin in hydrogel; and (2) visualization of the periosteal distribution after transcortical transport, using intramedullary doxycycline solution (Fig. 1). Vancomycin was chosen because it is used to treat implant infections caused by resistant Staphylococcus [5], and it is a common choice for local delivery. It is detectable by high-performance liquid chromatography (HPLC). Doxycycline was chosen for its innate fluorescence, and it is known to release from ALBC [6, 11, 13]. This study is a benchtop pilot study intended to establish effect size, and as such, no a priori power calculation was performed.
Fig. 1.
A flow diagram illustrates the study protocol.
We used human cadaveric femurs obtained from two women aged 63 and 64 years and one man aged 64 years, all of average height and weight. Specimens were stored frozen before cleaning and experimentation. Human femurs were chosen for their true human ultrastructure, with separate segments utilized to isolate these anatomic regions of a long bone. All segments had grossly intact cortices of normal thickness. For transcortical elution, three 8-cm-long midshaft femoral diaphyseal segments from three femurs (two from the 63-year-old woman, one from the 64-year-old woman) were used. For visualization, nine femoral segments from three femurs (two from the 64-year-old man, one from the 64-year-old woman) were used: three infraisthmal diaphysis, three metaphysis, and three epiphysis. The femurs were skeletonized by sharp dissection. No reaming or other method that would be destructive to bone was performed on either the endosteal surface or the periosteal surface. High-pressure pulse lavage (InterPulse Irrigation System™; Stryker, Kalamazoo, MI, USA) with saline (for elution) or deionized water (for visualization), a high flow tip at 19 psi, and a flow rate of 1000 mL/minute was used to remove the intramedullary contents and clean the periosteal surface.
For transcortical elution, vancomycin was delivered to the intramedullary canal in N-isopropylacrylamide (NIPAAm)-based hydrogel [7, 8], which has been shown to release loaded antimicrobials [8]. The hydrogel consisted of NIPAAm and polyetheramine (Jeffamine®; Huntsman International LLC, Salt Lake City, UT, USA) copolymerized by free radical polymerization, which gelled on warming to temperatures near 37°C. After preparation as described above, each femoral diaphyseal specimen was filled with 30 wt% NIPAAm-based hydrogel containing 5 wt% vancomycin by sealing one end of the intramedullary canal with GE® waterproof silicone sealant (Momentive Performance Materials Inc, Huntersville, NC, USA), filling the intramedullary canal with vancomycin-loaded hydrogel (Fig. 2A–B), and sealing the second end of the canal (Fig. 2C). Each specimen was entirely submerged in 500 mL phosphate-buffered saline (PBS) at 37°C (Fig. 3). The PBS eluant was fully exchanged each day for 5 days. Vancomycin concentration was assayed once for each eluant specimen at each time point by isocratic HPLC on a Beckman System Gold® (Beckman Coulter, Brea, CA, USA) using a 5-μm, octadecylsilyl-coated, 100-Å pore diameter, 250- × 4.6-mm column (Prodigy™; Phenomenex, Torrance, CA, USA) at a flow rate of 2 mL/minute. The mobile phase was 8:92 vol/vol acetonitrile:0.2% aqueous triethylamine, titrated to pH 3.0 with phosphoric acid. Detection was performed at 220 nm [12]. The cumulative released mass of vancomycin was calculated.
Fig. 2A–C.
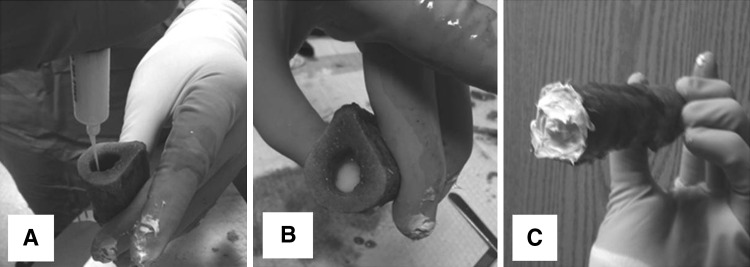
(A) A femoral diaphysis segment is loaded with vancomycin-loaded hydrogel after the distal end is sealed with silicone. (B) When the segment is filled, (C) the proximal end is sealed.
Fig. 3.
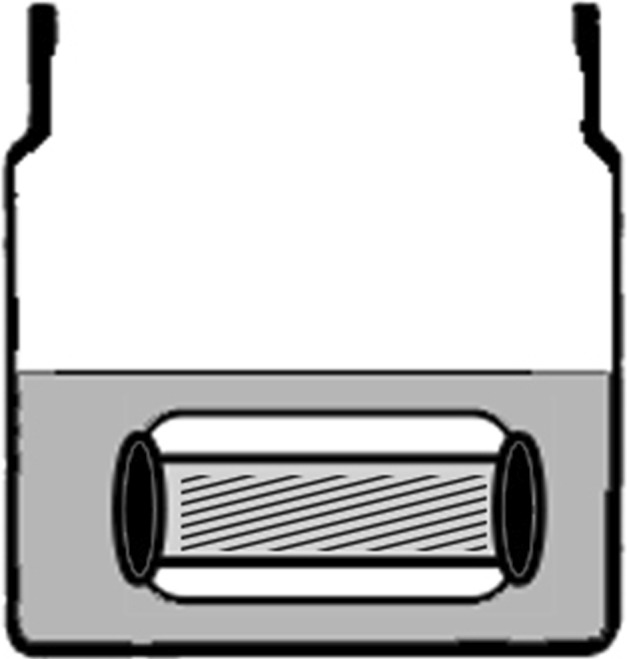
A diagram illustrates the transcortical transport experiment setup. The femoral diaphyseal segment (white) is filled with vancomycin-loaded hydrogel (diagonal strips), the ends are sealed with silicone (black), and it is submerged in PBS (gray). PBS was completely exchanged daily for 5 days, and the vancomycin concentration was measured by HPLC.
For the fluorescence visualization of the distribution of transcortical transport, the three segments (infraisthmal diaphysis, metaphysis, and epiphysis) from each of three femurs were prepared as described above. Then the distal end of each diaphyseal and metaphyseal specimen was sealed with silicone. The epiphyseal segments with intact subchondral bone did not need sealing distally. A dark box was constructed to eliminate ambient light and maximize visualization of fluorescence. Each specimen was studied individually. Images were captured using two Nikon® D40™ digital cameras with 18- to 55-mm lenses and X-1 green filters (Nikon, Melville, NY, USA), one axial and one transverse, at a focal distance of 20 and 30 cm, respectively. A Blak-Ray® ultraviolet lamp (UVP, LLC, Upland, CA, USA) was positioned 75 cm above the test specimen, offset by 20° as the sole light source. Each specimen was placed in the dark box and embedded in 0.1 wt% agarose gel (Sigma Aldrich, St Louis, MO, USA) in deionized water, leaving the proximal unsealed medullary canal of the specimen above the level of the agarose gel (Fig. 4). The intramedullary space of each specimen was filled with doxycycline solution (400 μg/mL) in deionized water (386 nm excitation, 530 nm emission [6]) and refilled daily as necessary to keep them full of doxycycline solution. Deionized water was used to prevent quenching of the fluorescence by chelation of the doxycycline [9]. The doxycycline concentration was chosen empirically to maximize visibility. Images were acquired under ultraviolet light at Time 0, before being placed in agarose gel, every 15 minutes for 3 days after filling the intramedullary canal with doxycycline, and after removal from the agarose gel. The pattern of doxycycline delivery to the periosteal surface of the bone was determined qualitatively on observation of the images.
Fig. 4.
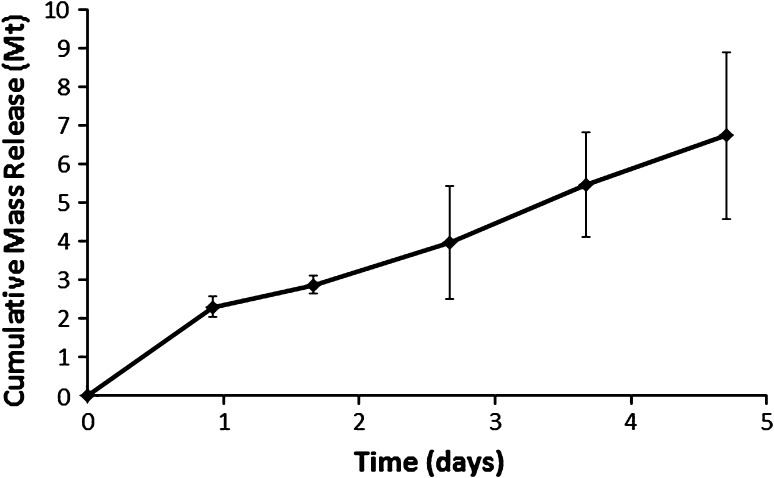
A graph shows recovered vancomycin versus time from transcortical transport into PBS. A mean of 8.65 mg (2.3% of the total vancomycin load) was recovered on Day 1 and a mean of 26.5 mg (6.7% of the total vancomycin load) was recovered by Day 5. Values are expressed as mean ± SD.
We determined whether vancomycin was transported transcortically over time using repeated-measures ANOVA. Standard normal plots of residuals were employed to confirm the normality of the data evaluated with ANOVA [1]. The route of doxycycline egress was observational data and not statistically analyzable. We performed all statistical analyses using Minitab® Statistical Software (Minitab Inc, State College, PA, USA).
Results
Vancomycin was transported from the intramedullary depot into the extraosseous eluant. The amount of released vancomycin increased over time (p < 0.001) from a mean of 8.65 mg (2.3% of the total vancomycin load) on Day 1 to a mean of 26.5 mg (6.7% of the total vancomycin load) by Day 5 (Fig. 4).
Fluorescence from transcortical transport of doxycycline was observed at the periosteal surface, at focal points along the linea aspera, the epicondylar ridges, the epicondyles, and capsular attachments (Fig. 5). There were no points of fluorescence seen on the smooth periosteal surfaces of the specimens between soft tissue attachment sites. Many of the locations where fluorescence appeared were located at visible vascular foramina (Fig. 6). Fluorescence was first seen at 5 to 10 minutes and then dispersed into the surrounding agarose gel, becoming confluent in the agarose gel by 24 hours and limiting further interpretation.
Fig. 5A–B .
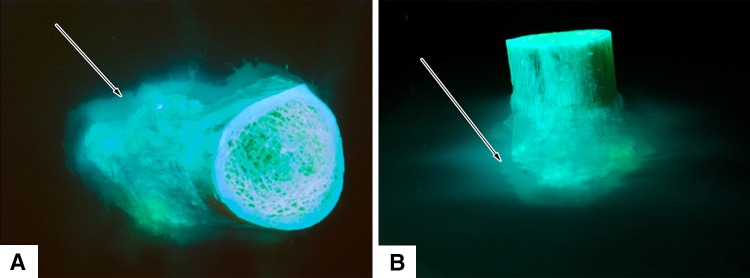
A distal femoral metaphysis loaded with doxycycline for visualization of fluorescence at the periosteal surface is shown in (A) axial and (B) transverse views. Doxycycline egress is seen as bright green fluorescence. Arrows indicate a cloud of fluorescence that has diffused into the agarose gel and become confluent from focal areas of doxycycline released from vascular channels.
Fig. 6A–B.
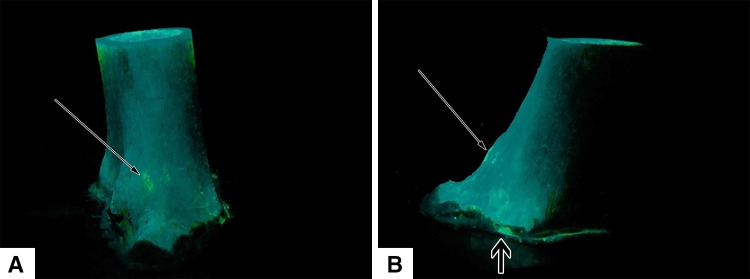
A femoral metaphyseal specimen loaded with doxycycline for visualization of fluorescence at the periosteal surface is shown in (A) lateral and (B) posterior views after removal from the agarose hydrogel. Bright points (long arrows) are fluorescence from doxycycline around vascular foramina at soft tissue attachment sites. Fluorescence from minimal leakage can be seen along the distal seal (short arrow).
Discussion
Antimicrobials are locally delivered in postdébridement surgical sites to achieve the high antimicrobial levels needed to treat organisms in biofilms [10]. Infection has the potential for transcortical extension to involve both surfaces of cortical bone. It is unknown whether locally delivered antimicrobials penetrate cortical bone sufficiently to be transported from one surface to the other. We therefore asked: (1) Are antimicrobials transported transcortically between the endosteal and periosteal surfaces? And (2) are transcortical antimicrobials transported uniformly over the cortical surface?
This study had several limitations. First, cadaveric bone is a static environment that does not have fluid flow caused by functional loading, intraosseous pressure gradients, or vascular pulse waves. Physiologic fluid flow and interstitial pressure changes would likely have some effect on the distribution of locally delivered antimicrobials, possibly augmenting transcortical transport; however, determining the effect of these factors would require in vivo investigation beyond the scope of this pilot study. Second, the duration was short: 5 days for transcortical elution and 2 days for fluorescence visualization. Five days includes the time during which it is important to achieve high local antimicrobial levels. Although total cumulative recovered vancomycin was continuing to increase over time, the 5-day transcortical elution data provided sufficient evidence to establish that locally delivered antimicrobials are transported from the intramedullary canal to the extraosseous eluant. Third, we did not directly examine the distributions of vancomycin or doxycycline in the substance of cortical bone. The pattern and extent of penetration that can occur under passive diffusion or from artificially driven convection has been reported by Elson et al. [4] and DiResta et al. [3]. Fourth, the cleaned cadaver bone had all soft tissues removed, which could have potentially increased the transport of antimicrobials through the vascular foramina. The space in vascular foramina is not entirely filled by the vessels. Loose connective tissue in the available space allows antimicrobial transport. The vessels were not removed in our specimens but likely occupied less space than when filled with blood, possibly allowing greater transport than occurs in vivo. We do not believe this would meaningfully affect our observations. Fifth, confluence of fluorescence in the agarose gel at 2 days prevented visualization of any continuing changes. At 24 hours, it was obvious that cortical bone was a substantial barrier to antimicrobial transport. Although unlikely, if doxycycline did reach the periosteal surface through the substance of cortical bone after 24 hours, we would not have been able to see it. Sixth, infinite-sink, well-mixed conditions, as present in our transcortical elution study, are not present in human tissues. It is unlikely that antimicrobials will distribute widely in tissues from the focal sites where transport occurs, as occurred in the PBS eluant or the agarose gel used in our studies.
Vancomycin from an intramedullary depot was measurable in extraosseous fluid (6.7% of the deliverable load in the hydrogel by Day 5) and increased over time. The delivery rate was much slower than would be expected from the same NIPAAm-based hydrogel when it is not surrounded by cortical bone, which was 316 mg at Day 5 (80% of the load) in a previous study [8]. Cortical bone is an important barrier limiting antimicrobial transport. Our data are consistent with Elson et al. [4] who reported transcortical delivery of gentamicin over 13 days using a similar model; however, thick lamellar bone, such as that found in the human femur, does not have sufficient porosity to expect the passive transport of antimicrobials by diffusion over several days found by Elson et al. [4]. Neither the transcortical elution in our study nor that in the study by Elson et al. [4] addresses how the antimicrobial gets into the extraosseous fluid. DiResta et al. [3] studied penetration into cortical bone but not transport through the full cortical thickness to the opposite surface or transport into the extraosseous compartment.
Our observations of where fluorescence appeared at the periosteal surface are consistent with the transport of the antimicrobial from the intramedullary delivery site to the extraosseous compartment through vascular foramina. Infraisthmal segments of the diaphysis were chosen to maximize visualization of fluorescence over areas of cortical bone free of vascular foramina. The metaphyseal and epiphyseal segments were chosen to maximize visualization of fluorescence at multiple vascular foramina. These three segmental types represent the major regions of human long bones. The lack of fluorescence visualization over smooth areas free of vascular foramina is consistent with DiResta et al. [3] who reported minimal penetration of cortical bone from the endosteal surfaced after 1 hour of passive diffusion or incomplete penetration after 20 minutes of pressurized infusion in an ovine tibial model. They pressurized an intramedullary infusion of toluene blue and Tc-99m-labeled pamidronate with a surfactant, up to +250 mm Hg, with simultaneous application of extraosseous vacuum at −50 mm Hg. These conditions increased the penetration of bisphosphonates by 6000-fold over passive diffusion (20 minutes versus 2000 hours for complete perfusion). Penetration was variable, reaching the periosteal surface in several locations around the circumference of the tibia by 20 minutes. Although these data are valuable information about penetration of fluid into lamellar bone, they do not represent potential clinical performance for transcortical transport of antimicrobials after local delivery. Unlike a human femur, the cortex in their model was only 2 mm thick and composed of fewer than 20 osteons. Pressurized intramedullary infusion, negative pressure at the periosteal surface, and surfactants are not clinical options for local antimicrobial delivery. Their histologic cross-sectional images did not reveal a transcortical route consistent with a flow of 1.2 mL/second, and their experimental model provided no possibility of detecting flow through vascular foramina. Elson et al. [4] reported gentamicin levels present broadly throughout lamellar bone, but no determination was made for a transport route into the extraosseous compartment.
Our study illustrated cortical bone is an important barrier to transport of locally delivered antimicrobials. After release, transcortical transport of antimicrobial is limited to tissues near vascular foramina for at least 24 hours, which is the critical time clinically when high local levels are needed. If infection is present at the far surface of cortical bone from a local antimicrobial depot, it is unlikely that timely transport of antimicrobial in adequate amounts will occur.
Acknowledgments
The authors thank Derek Overstreet, PhD, and Francis Calara, BSE, for their support with design, polymer synthesis, data gathering, and analysis.
Footnotes
One or more of the authors (AM) have received, during the study period, funding from the Herbert J. Lewis fund at the Orthopaedic Research and Education Foundation (Rosemont, IL, USA) and from the Southwest Orthopaedic Trauma Association (Albuquerque, NM, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Banner Good Samaritan Medical Center (Phoenix, AZ, USA) and Arizona State University (Tempe, AZ, USA).
References
- 1.Altman DG. Practical Statistics for Medical Research. 2. Boca Raton, FL: Chapman & Hall/CRC Press; 2006. [Google Scholar]
- 2.Diefenbeck M, Mückley T, Hofmann GO. Prophylaxis and treatment of implant-related infections by local application of antibiotics. Injury. 2006;37:S95–S104. doi: 10.1016/j.injury.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 3.DiResta GR, Manoso MW, Naqvi A, Zanzonico P, Smith-Jones P, Tyler W, Morris C, Healey JH. Bisphosphonate delivery to tubular bone allografts. Clin Orthop Relat Res. 2008;466:1871–1879. doi: 10.1007/s11999-008-0259-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elson RA, Jephcott AE, McGechie DB, Verettas D. Antibiotic-loaded acrylic cement. J Bone Joint Surg Br. 1977;59:200–205. doi: 10.1302/0301-620X.59B2.873980. [DOI] [PubMed] [Google Scholar]
- 5.Joseph TN, Chen AL, Di Cesare PE. Use of antibiotic-impregnated cement in total joint arthroplasty. J Am Acad Orthop Surg. 2003;11:38–47. doi: 10.5435/00124635-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Lu HT, Jiang Y, Li HB, Chen F, Wong MH. Simultaneous determination of oxytetracycline, doxycycline, tetracycline and chlortetracycline in tetracycline antibiotics by high-performance liquid chromatography with fluorescence detection. Chromatographia. 2004;60:259–264. [Google Scholar]
- 7.Overstreet D, McLemore R, Doan B, Farag A, Vernon B. Temperature-responsive graft copolymer hydrogels for controlled swelling and drug delivery. Soft Materials. 2012 [Google Scholar]
- 8.Overstreet DJ, Huynh R, Jarbo K, McLemore RY, Vernon BL. In situ forming, resorbable graft copolymer hydrogels providing controlled drug release. J Biomed Mater Res A. 2012 October 31 [Epub ahead of print]. [DOI] [PubMed]
- 9.Schneider S, Schmitt MO, Brehm G, Reiher M, Matousek P, Towrie M. Fluorescence kinetics of aqueous solutions of tetracycline and its complexes with Mg2+ and Ca2+ Photochem Photobiol Sci. 2003;2:1107–1117. doi: 10.1039/b304523b. [DOI] [PubMed] [Google Scholar]
- 10.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 11.Tamimi F, Torres J, Bettini R, Ruggera F, Rueda C, López-Ponce M, Lopez-Cabarcos E. Doxycycline sustained release from brushite cements for the treatment of periodontal diseases. J Biomed Mater Res A. 2008;85:707–714. doi: 10.1002/jbm.a.31610. [DOI] [PubMed] [Google Scholar]
- 12.Xu Q, Trissel L. Stability-Indicating HPLC Methods for Drug Analysis. London, UK: Pharmaceutical Press; 2003. [Google Scholar]
- 13.Zilberman M, Elsner J. Antibiotic-eluting medical devices for various applications. J Control Release. 2008;130:202–215. doi: 10.1016/j.jconrel.2008.05.020. [DOI] [PubMed] [Google Scholar]