Abstract
Background
Morbid obesity has been shown to be a risk factor for increased complications after THA and TKA; however, large studies that would determine the effect size are lacking.
Questions/purposes
The purposes of this study were to determine whether morbid obesity increased the risk of: (1) venous thromboembolism (VTE), (2) bleeding, (3) other adverse events, and (4) infections during the early postoperative period (up to 6 to 8 weeks) after THA or TKA?
Methods
Data from the REgulation of Coagulation in ORthopaedic surgery to prevent Deep vein thrombosis and pulmonary embolism (RECORD) clinical trial program of rivaroxaban for prevention of VTE after THA or TKA were analyzed retrospectively. Data for 12,355 patients were reviewed to identify complication rates in morbidly obese patients (BMI ≥ 40 kg/m2) compared with patients with a BMI less than 40 kg/m2. Explorative analyses compared the rates of asymptomatic deep vein thrombosis (DVT), symptomatic DVT, symptomatic pulmonary embolism, bleeding, and other adverse events by BMI group.
Results
There were no significant differences in asymptomatic DVT, symptomatic DVT, symptomatic pulmonary embolism, or bleeding, but there were increases in other adverse events (including receipt of blood transfusion, erythema, peripheral edema, diarrhea, gastrointestinal or abdominal pain) and infections (including respiratory tract or lung infections, wound inflammation or infection, and extrasurgical-site infections), in patients with a BMI of 40 kg/m2 or greater compared with patients with a BMI less than 40 kg/m2.
Conclusions
After THA or TKA, morbid obesity is not associated with an increased risk of VTE or bleeding but is associated with increased early postoperative complications, including erythema, peripheral edema, diarrhea and gastrointestinal or abdominal pain, wound inflammation or infection, extrasurgical-site infections, and respiratory tract or lung infections.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Obesity, with its associated increased mortality and morbidity [19], is rapidly becoming a worldwide epidemic. Obese patients are more likely to undergo joint arthroplasty [3], and as the incidence of obesity increases, the numbers of primary hip and knee arthroplasties performed each year also are increasing. The annual numbers of total arthroplasties performed in the United States are projected to increase to 572,000 primary THAs and 3.48 million TKAs by 2030 [11].
Surgeons need to have a clear understanding of the potential risk of perioperative complications so they can engage in a more informed discussion with their patients and appropriately manage expectations for surgical outcomes. The effects of obesity on outcomes such as venous thromboembolism (VTE), wound complications, infection, and bleeding in patients undergoing total joint arthroplasties have been documented in numerous studies [4, 6, 7, 13, 15, 18, 20, 21]. However, large studies to determine the effect size of morbid obesity on early postoperative complications after THA or TKA are lacking. Other studies have not found an association between BMI and the risk of complications in patients undergoing THA or TKA [1, 16, 17, 23].
The purpose of this observational analysis was to determine whether morbid obesity (BMI ≥ 40 kg/m2) increases the risk of (1) VTE, (2) bleeding, (3) other adverse events, and (4) infections during the early (up to 6 to 8 weeks) postoperative period after THA or TKA?
Patients and Methods
This was a post hoc analysis of rivaroxaban and enoxaparin data from the REgulation of Coagulation in ORthopaedic surgery to prevent Deep vein thrombosis and pulmonary embolism (RECORD) program, which comprised four Phase III, randomized, double-blind clinical trials. In the RECORD program, men and women at least 18 years old who were scheduled to undergo elective THA or TKA were eligible for inclusion. Eligible patients were randomized to receive 10 mg oral rivaroxaban once daily (6 to 8 hours after wound closure) or 40 mg subcutaneous enoxaparin once daily (RECORD1–3 trials; initiated 12 hours before surgery and restarted 6 to 8 hours after wound closure) or 30 mg enoxaparin twice daily (RECORD4 trial; initiated 12 to 24 hours after wound closure) [8, 10, 12, 24]. Study drugs were administered for 31 to 39 days in RECORD1, rivaroxaban was administered for 31 to 39 days and enoxaparin for 10 to 14 days plus placebo in RECORD2, and for 10 to 14 days in the RECORD3 and RECORD4 trials [8, 10, 12, 24]. Patients underwent mandatory bilateral venography the day after the last dose of the study drug. Patients were followed up for 30 to 35 days after the last dose of study medication. At the followup, adverse events, signs and diagnosis of VTE, and cardiovascular and bleeding events during the 30 days after cessation of treatment were recorded. Blood samples for clinical chemistry were taken, and physical examination assessment of vital signs (heart rate and blood pressure measurement of the patient in the supine position after 5 minutes’ rest) was performed. The primary efficacy end point was the composite of any deep vein thrombosis (DVT), nonfatal pulmonary embolism (PE), or death (total VTE). The primary safety outcome was the incidence of major bleeding beginning after the first dose of the study drug and up to 2 days after the last dose of the study drug. This cohort contained 12,355 patients, of whom 24.3% had a BMI less than 25 kg/m2, 39.8% had a BMI of 25 to 29 kg/m2, 32.3% had a BMI of 30 to 39 kg/m2, and 3.6% had a BMI of 40 kg/m2 or greater (Table 1).
Table 1.
Demographic and baseline data for the four RECORD studies
| Demographics | BMI < 25 kg/m2 | BMI 25–29 kg/m2 | BMI 30–39 kg/m2 | BMI ≥ 40 kg/m2 |
|---|---|---|---|---|
| Total number of patients (n = 12,355), number (%) | 3008 (24.3) | 4916 (39.8) | 3986 (32.3) | 445 (3.6) |
| RECORD1 + 2 (THA) (n = 6870), number (%) | 2183 (31.8) | 2800 (40.8) | 1764 (25.7) | 123 (1.8) |
| RECORD3 + 4 (TKA) (n = 5485), number (%) | 825 (15.0) | 2116 (38.6) | 2222 (40.5) | 322 (5.9) |
| Sex (male), % | 38.9 | 44.4 | 37.1 | 25.2 |
| Age, mean, years | 62.9 | 65.3 | 63.9 | 60.3 |
| Weight, mean, kg | 62.7 | 76.6 | 91.7 | 117.9 |
| RECORD1 + 2 (THA) | 62.6 | 76.9 | 92.3 | 115.5 |
| RECORD3 + 4 (TKA) | 62.8 | 76.3 | 91.2 | 118.8 |
| Hypertension, diabetes, and/or hyperlipidemia, % | 40.1 | 57.0 | 71.9 | 82.0 |
| Operative time, mean, minutes | ||||
| RECORD1 + 2 (THA) | 100 | 95 | 99 | 102 |
| RECORD3 + 4 (TKA) | 101 | 99 | 98 | 100 |
RECORD = REgulation of Coagulation in ORthopaedic surgery to prevent Deep vein thrombosis and pulmonary embolism.
In this analysis, we compared the early (up to 6 to 8 weeks) postoperative rates of asymptomatic DVT (confirmed by venography), symptomatic DVT, symptomatic PE, and bleeding events (all adjudicated by an independent central adjudication committee), and investigator-reported adverse events in morbidly obese patients (BMI ≥ 40 kg/m2) with those in normal weight (BMI < 25 kg/m2), overweight (BMI 25–29 kg/m2), and obese (BMI 30–39 kg/m2) patients. Bleeding events were defined as reported previously [8, 10, 12, 24]. The bleeding outcomes assessed were any bleeding events after the start of study medication and major or nonmajor clinically relevant bleeding events. Major bleeding included, among other types of blood loss, transfusion of 2 units of blood or more. Blood transfusion requirements also were recorded, but were classified as major bleeding only if 2 units or more of blood were transfused.
All adverse events occurring after the patient had signed the informed consent form were fully recorded in the patient’s case record form. Documentation was supported by an entry in the patient’s file. Each event was described in detail in addition to start and stop dates, severity, relationship to investigational product, action taken, and outcome. Adverse events of interest in this analysis were wound-related complications; erythema; peripheral edema; infections; respiratory, neurologic, gastrointestinal, and cardiac complications; falls; and other complications such as pain and discomfort and febrile disorders. All investigator-reported adverse events that either had or were expected to have an imbalance between morbidly obese patients and the other BMI groups, regardless of the overall frequency of these events, were classified according to the Medical Dictionary for Regulatory Activities (MedDRA) Version 10.0 (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland) and were grouped into medical categories of interest. Adverse events were analyzed regardless of treatment group.
Asymptomatic DVT was analyzed in the modified intention-to-treat population (patients who were valid for safety analysis, had undergone the planned surgery, and had an adequate assessment of thromboembolism); symptomatic DVT, symptomatic PE, and all other analyses were evaluated in the safety population, which comprised patients who had taken at least one dose of study medication.
To investigate the effect of BMI of 40 kg/m2 or greater on the rates of the various end points, unadjusted and adjusted odds ratios (ORs) for the effect of BMI of 40 kg/m2 or greater versus less than 40 kg/m2 were derived from logistic regression. The OR is a relative measure of risk that indicates how much less or more likely it is that a patient who is exposed to the factor being studied (in this study, morbid obesity) will have an outcome develop (ie, early postoperative complication) compared with a patient who is not exposed (ie, who is not morbidly obese). The risk is statistically significant if the CIs of the OR do not cross 1.0. The adjusted analysis was performed to remove the influence of demographic or baseline characteristics on the outcomes. The covariates adjusted for were type of surgery (TKA versus THA), age, sex, and region (United States and Canada versus other countries); the latter was done to address the multinational character of the trial program and geographic variation in event reporting.
Patients’ baseline demographics were as expected for the different weight groups; however, the proportions of patients with a BMI of 30 kg/m2 or greater were higher in patients who underwent TKA than THA (Table 1).
In the adjusted analyses, covariates were always included in the regression model regardless of statistical significance. The reported p value refers to Fisher’s exact test or to the likelihood ratio test from logistic regression (adjusted for covariates) for the effect of BMI (≥ 40 kg/m2 versus < 40 kg/m2). Probability values were not adjusted for the multiplicity of end points.
Results
There were no significant differences in the rates of asymptomatic DVT, symptomatic DVT, or symptomatic PE between the group with a BMI of 40 kg/m2 or greater and the other BMI groups combined in the adjusted analysis (Table 2).
Table 2.
Infections, adverse events, VTE, and bleeding complications by BMI
| Event | BMI < 25 kg/m2 n = 3008 | BMI 25–29 kg/m2 n = 4916 | BMI 30–39 kg/m2 n = 3986 | BMI ≥ 40 kg/m2 n = 445 | Unadjusted analysis | Adjusted analysis* | ||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |||||
| Venous thromboembolism | ||||||||
| Asymptomatic DVT per venography (mITT population) | 74/2109 (3.5) | 208/3502 (5.9) | 181/2626 (6.9) | 16/265 (6.0) | 1.1 (0.6 to 1.8) | 0.7745 | 1.0 (0.6 to 1.7) | 0.9475 |
| Symptomatic VTE (DVT or PE) (safety population), n/n (%) | 28/3008 (0.9) | 36/4916 (0.7) | 46/3986 (1.2) | 7/445 (1.6) | 1.7 (0.8 to 3.7) | 0.2019 | 1.3 (0.6 to 2.9) | 0.5333 |
| Symptomatic DVT (safety population) | 17/3008 (0.6) | 26/4916 (0.5) | 30/3986 (0.8) | 5/445 (1.1) | 1.8 (0.7 to 4.6) | 0.2271 | 1.5 (0.6 to 3.8) | 0.4260 |
| Symptomatic PE (safety population) | 12/3008 (0.4) | 12/4916 (0.2) | 16/3986 (0.4) | 2/445 (0.4) | 1.3 (0.3 to 5.6) | 0.6997 | 0.9 (0.2 to 4.0) | 0.9275 |
| Bleeding events | ||||||||
| Any bleeding after start of study drug, n (%) | 230 (7.6) | 329 (6.7) | 265 (6.6) | 39 (8.8) | 1.3 (0.9 to 1.8) | 0.1302 | 1.1 (0.8 to 1.5) | 0.6146 |
| Major or nonmajor clinically relevant bleeding after start of study drug, n (%) | 108 (3.6) | 134 (2.7) | 118 (3.0) | 15 (3.4) | 1.1 (0.7 to 1.9) | 0.6715 | 1.2 (0.7 to 2.0) | 0.5779 |
| Adverse events | ||||||||
| All serious adverse events after start of study drug, n (%) | 223 (7.4) | 336 (6.8) | 249 (6.2) | 42 (9.4) | 1.4 (1.0 to 2.0) | 0.0354 | 1.8 (1.3 to 2.5) | 0.0014 |
| Drainage from the wound, n (%) | 2228 (74.1) | 3751 (76.3) | 3066 (76.9) | 312 (70.1) | 0.7 (0.6 to 0.9) | 0.0057 | 1.1 (0.9 to 1.4) | 0.2542 |
| Mean postoperative volume in drain, mL (RECORD1 + 2) | 578.2 | 574.9 | 583.7 | 537.8 | – | – | – | – |
| Mean postoperative volume in drain, mL (RECORD3 + 4) | 623.9 | 653.9 | 645.0 | 589.7 | – | – | – | – |
| Other wound-related complication†, n (%) | 26 (0.9) | 76 (1.5) | 72 (1.8) | 11 (2.5) | 1.7 (0.9 to 3.2) | 0.1059 | 1.4 (0.7 to 2.7) | 0.3086 |
| Patients who received blood transfusion, n (%) | 1654 (55.0) | 2311 (47.0) | 1733 (43.5) | 167 (37.5) | 0.7 (0.5 to 0.8) | < 0.0001 | 0.8 (0.6 to 1.0) | 0.0142 |
| Mean volume of total transfusion, mL (RECORD1 + 2) | 798.3 | 686.6 | 640.8 | 606.3 | – | – | – | – |
| Mean volume of total transfusion, mL (RECORD3 + 4) | 663.2 | 637.6 | 607.1 | 548.1 | – | – | – | – |
| Respiratory, thoracic, and mediastinal disorders, n (%) | 116 (3.9) | 238 (4.8) | 260 (6.5) | 52 (11.7) | 2.4 (1.8 to 3.3) | < 0.0001 | 1.3 (1.0 to 1.8) | 0.0969 |
| Breathing abnormalities, bronchospasm and obstruction, abnormal gas exchange, or coughing, n (%) | 67 (2.2) | 147 (3.0) | 192 (4.8) | 37 (8.3) | 2.6 (1.8 to 3.6) | < 0.0001 | 1.4 (1.0 to 2.1) | 0.0721 |
| Cardiac disorders, n (%) | 135 (4.5) | 256 (5.2) | 245 (6.1) | 48 (10.8) | 2.1 (1.6 to 2.9) | < 0.0001 | 1.3 (0.9 to 1.8) | 0.1554 |
| Cardiac rate and rhythm disorders, n (%) | 69 (2.3) | 166 (3.4) | 148 (3.7) | 38 (8.5) | 2.8 (2.0 to 4.0) | < 0.0001 | 1.2 (0.9 to 1.8) | 0.2804 |
| Falls, n (%) | 15 (0.5) | 28 (0.6) | 23 (0.6) | 5 (1.1) | 2.0 (0.8 to 5.1) | 0.1123 | 1.3 (0.5 to 3.4) | 0.5803 |
| Pain and discomfort, n (%) | 46 (1.5) | 83 (1.7) | 70 (1.8) | 16 (3.6) | 2.2 (1.3 to 3.7) | 0.0052 | 1.5 (0.9 to 2.6) | 0.1539 |
| Febrile disorders, n (%) | 290 (9.6) | 559 (11.4) | 527 (13.2) | 72 (16.2) | 1.5 (1.1 to 1.9) | 0.0042 | 0.9 (0.7 to 1.1) | 0.2762 |
| Infections | ||||||||
| Wound inflammation or infection‡, n (%) | 44 (1.5) | 94 (1.9) | 75 (1.9) | 19 (4.3) | 2.4 (1.5 to 4.0) | 0.0009 | 2.4 (1.4 to 3.9) | 0.0021 |
| Infections excluding surgical site, n (%) | 167 (5.6) | 303 (6.2) | 323 (8.1) | 52 (11.7) | 1.9 (1.4 to 2.5) | 0.0002 | 1.7 (1.2 to 2.3) | 0.0015 |
| Respiratory tract infections or lung infections, n (%) | 50 (1.7) | 67 (1.4) | 65 (1.6) | 12 (2.7) | 1.8 (1.0 to 3.2) | 0.0752 | 2.0 (1.1 to 3.7) | 0.0391 |
| Bacterial infections, n (%) | 12 (0.4) | 35 (0.7) | 34 (0.9) | 8 (1.8) | 2.7 (1.3 to 5.6) | 0.0147 | 1.4 (0.7 to 3.1) | 0.3715 |
| Urinary tract infections, n (%) | 76 (2.5) | 116 (2.4) | 125 (3.1) | 18 (4.0) | 1.5 (1.0 to 2.5) | 0.0989 | 1.5 (0.9 to 2.5) | 0.1199 |
| Bone and joint infections¶, n (%) | 2 (0.1) | 11 (0.2) | 15 (0.4) | 4 (0.9) | 3.8 (1.3 to 11.0) | 0.0269 | 2.1 (0.7 to 6.5) | 0.2140 |
All comparisons are for BMI ≥ 40 kg/m2 versus BMI < 40 kg/m2; * adjusted for indication, age, gender, and region effects; †defined as wound necrosis, suture rupture, procedural complication, postprocedural complication, postprocedural swelling, wound complication, wound dehiscence, postoperative wound complication, or abnormal or impaired healing; ‡defined as inflammation of the wound, postoperative wound infection, wound infection, wound abscess, wound sepsis, bacterial wound infection, staphylococcal wound infection, wound decomposition, postprocedural cellulitis, postprocedural infection, incision-site cellulitis, incision-site infection, device-related infection, or stitch abscess; ¶osteomyelitis or infective arthritis; DVT = deep vein thrombosis; mITT = modified intention-to-treat; n = number; OR = odds ratio; PE = pulmonary embolism; VTE = venous thromboembolism.
There were no significant differences in the rates of any bleeding or major or nonmajor clinically relevant bleeding between the group with a BMI of 40 kg/m2 or greater and the other BMI groups combined in the adjusted analyses (Table 2).
Serious adverse events occurred more frequently in the group with a BMI of 40 kg/m2 or greater compared with the other BMI groups combined in the adjusted analysis (p = 0.0014; Table 2). The most frequent serious adverse events in the group with a BMI of 40 kg/m2 or greater were: device-related infection (0.67%), femur fracture (0.67%), hypoxia (0.45%), increased alanine aminotransferase (0.45%), infective arthritis (0.45%), nausea (0.45%), vomiting (0.45%), and wound dehiscence (0.45%). Some adverse events (not classified as serious) were significantly more frequent in the group with a BMI of 40 kg/m2 or greater compared with the other BMI groups combined in the adjusted analysis: the numbers of patients who received blood transfusion (p = 0.0142; Table 2), erythema (p = 0.0366; Fig. 1), peripheral edema (p = 0.0037; Fig. 1), diarrhea (p = 0.0080; Fig. 2), and gastrointestinal and abdominal pain (p = 0.0149; Fig. 2).
Fig. 1.
Rates of erythema and peripheral edema were significantly higher in morbidly obese patients than for patients in other weight groups combined in the adjusted analysis of the RECORD studies (safety population).
Fig. 2.
Rates of diarrhea and gastrointestinal and abdominal pain were significantly higher in morbidly obese patients than in patients in other weight groups combined in the adjusted analysis of the RECORD studies (safety population).
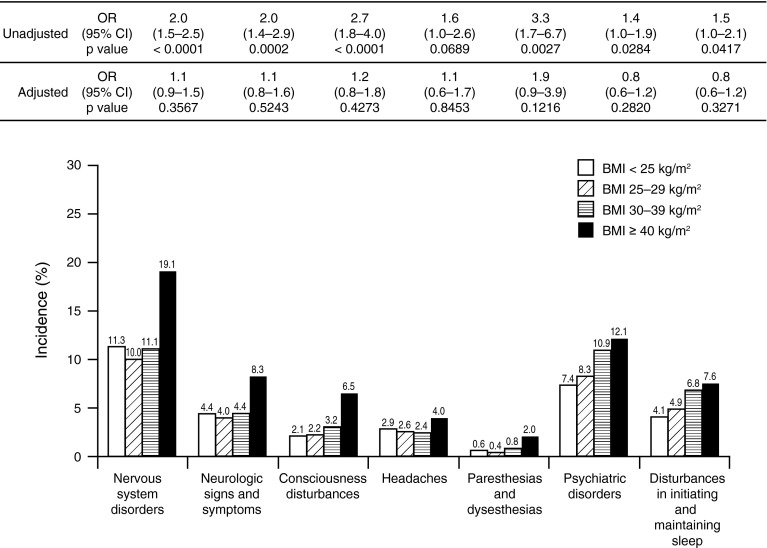
Rates of some infections (wound inflammation and infection, infections excluding the surgical site, and respiratory tract and lung infections) were significantly higher in the group with a BMI of 40 kg/m2 or greater relative to the other BMI groups combined in the adjusted analysis (p = 0.0021, p = 0.0015, and p = 0.0391, respectively) (Table 2); other types of infection (bacterial, urinary tract, and bone and joint) were no more common. Cardiac disorders, patients requiring wound drainage, and nervous system disorders also were not significantly different in the group with a BMI of 40 kg/m2 or greater than the other BMI groups combined (Table 2; Fig. 3).
Fig. 3.
Rates of neurologic complications did not differ significantly in morbidly obese patients compared with patients in other weight groups combined in the adjusted analysis of the RECORD studies (safety population).
Discussion
As the incidence of obesity increases globally, the demand for THAs and TKAs also will continue to increase. Therefore, although obese patients will gain worthwhile improvements in pain and function as a result of joint arthroplasty, it is increasingly important that orthopaedic surgeons be familiar with management issues pertinent to these patients [9]. In addition, preoperative education and discussion of potential complications can make patient expectations more realistic and can improve postoperative satisfaction [22]. We aimed to answer the following questions: does morbid obesity increase the risk of (1) VTE, (2) bleeding, (3) other adverse events, and (4) infections during the early postoperative period (up to 6 to 8 weeks) after THA or TKA?
This study has numerous limitations. It was a retrospective analysis based on a data source not designed to investigate surgical complications. Complications beyond the early postoperative period were not studied, and the analysis was not designed to investigate long-term complications or the clinical outcome of the arthroplasty. In addition, the cohort size of morbidly obese patients (n = 445) was small compared with the total number of patients, and complications were infrequent. There is the possibility that event rate differences were the result of differential reporting of adverse events in morbidly obese patients. Additionally, this study reports nominal p values (ie, no adjustment was made for multiple comparisons), which increases the probability that some of our findings that appear significant might not be so; furthermore, we made a large number of comparisons, further increasing the likelihood of this.
In a large population cohort of 12,355 patients we found no significant differences for morbidly obese patients compared with patients in the other BMI groups combined in early postoperative rates of asymptomatic DVT, symptomatic DVT, or symptomatic PE. This was consistent with other smaller studies that included morbidly obese patients, one of which prospectively analyzed 6-week complication rates after THA [16] and another that retrospectively assessed 30-day complications rates after THA and TKA [23]. However, some studies have shown that obesity is a risk factor for early postoperative DVT or PE, as was shown in a case-control study (with fewer patients than our analysis) including obese patients (BMI > 30 kg/m2) who experienced DVT or PE within 30 days of THA or TKA [14]. However, it is not clear if that study included morbidly obese patients. The disparity with our findings might be explained by the use of different anticoagulation (mainly warfarin) from that in our analysis (rivaroxaban or enoxaparin). Our findings might not apply to patients receiving less aggressive approaches to thromboprophylaxis.
We found no significant differences for morbidly obese patients compared with patients in other weight groups combined in any bleeding events and major or nonmajor clinically relevant bleeding events. In contrast, another small study that investigated blood loss after THA found that obese patients (BMI > 30 kg/m2) lost significantly more blood than nonobese patients (≤ 30 kg/m2) in the 48-hour postoperative period [4]. Unlike our analyses, that study did not include morbidly obese patients, and their comparison was obese versus nonobese patients (whereas ours compared morbidly obese with normal weight, overweight, and obese patients combined). Additionally, we included events over a longer period (6 to 8 weeks postoperatively). These differences between the studies might explain the disparity in findings.
Although serious adverse events were significantly more frequent in morbidly obese patients than in the other groups combined, these were rare and not life threatening. Some adverse events (eg, erythema, diarrhea) were significantly increased but were infrequent. In the literature, there is a lack of data regarding serious postoperative adverse events, particularly during the early postoperative period, in morbidly obese patients. A small prospectively matched study [5] of patients undergoing THA for osteoarthritis found that overall rates of complications (which included superficial wound infection, deep joint infection, dislocations, and other complications) were significantly higher in morbidly obese patients than in nonobese patients in the 5-year perioperative period.
Rates of surgical wound infection and inflammation were significantly higher in morbidly obese patients compared with patients in other weight groups combined. This is in contrast to a smaller study assessing 30-day complication rates after THA or TKA [23]. However, our study was larger and, therefore, more reliable. Our findings are in agreement with another smaller retrospective study that found significantly higher rates of acute deep wound infection within 1 year of THA in obese and morbidly obese patients compared with patients in other weight groups [7]. The correlation was independent of diabetes and cardiovascular disease, which we did not account for in our analyses. Another retrospective analysis with an average followup of 6 years and a smaller patient population than our study reported that morbid obesity and diabetes were significant risk factors for surgical wound infection after THA or TKA [13].
Our analyses support previous findings that morbidly obese patients are not at increased risk of DVT or PE, major bleeding, or life-threatening complications during the early postoperative period after THA or TKA. We studied a large number of morbidly obese patients (the largest group studied to date in this area), who are not routinely examined as a discrete population in clinical trials. Because few patients will lose a significant amount of weight before surgery, surgeons often operate on morbidly obese patients [2]. Consequently, surgeons and patients should know and understand the potential complications, which should be part of the informed consent process and discussion of these risks.
Acknowledgments
We thank Li Wan MSc who provided editorial support.
Footnotes
One author (RJF) certifies that he, or a member of his or her immediate family, has or may receive payments or benefits, during the study period, an amount of USD 10,000–USD 100,000, from DJ Orthopaedics (Austin, TX, USA) and an amount of USD 10,000–USD 100,000 from Tornier (Minneapolis, MN, USA) was received for a research grant. Three authors (SH, MH, SDB) are employees of Bayer HealthCare, which provided funding for editorial support for this study.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
RECORD was a multinational study.
References
- 1.Amin AK, Patton JT, Cook RE, Brenkel IJ. Does obesity influence the clinical outcome at five years following total knee replacement for osteoarthritis? J Bone Joint Surg Br. 2006;88:335–340. doi: 10.1302/0301-620X.88B3.16488. [DOI] [PubMed] [Google Scholar]
- 2.Bordini B, Stea S, Cremonini S, Viceconti M, De Palma R, Toni A. Relationship between obesity and early failure of total knee prostheses. BMC Musculoskelet Disord. 2009;10:29. doi: 10.1186/1471-2474-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourne R, Mukhi S, Zhu N, Keresteci M, Marin M. Role of obesity on the risk for total hip or knee arthroplasty. Clin Orthop Relat Res. 2007;465:185–188. doi: 10.1097/BLO.0b013e3181576035. [DOI] [PubMed] [Google Scholar]
- 4.Bowditch MG, Villar RN. Do obese patients bleed more? A prospective study of blood loss at total hip replacement. Ann R Coll Surg Engl. 1999;81:198–200. [PMC free article] [PubMed] [Google Scholar]
- 5.Chee YH, Teoh KH, Sabnis BM, Ballantyne JA, Brenkel IJ. Total hip replacement in morbidly obese patients with osteoarthritis: results of a prospectively matched study. J Bone Joint Surg Br. 2010;92:1066–1071. doi: 10.1302/0301-620X.92B8.22764. [DOI] [PubMed] [Google Scholar]
- 6.Dowsey MM, Choong PF. Early outcomes and complications following joint arthroplasty in obese patients: a review of the published reports. ANZ J Surg. 2008;78:439–444. doi: 10.1111/j.1445-2197.2008.04554.x. [DOI] [PubMed] [Google Scholar]
- 7.Dowsey MM, Choong PF. Obesity is a major risk factor for prosthetic infection after primary hip arthroplasty. Clin Orthop Relat Res. 2008;466:153–158. doi: 10.1007/s11999-007-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, Geerts W, RECORD1 Study Group Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–2775. doi: 10.1056/NEJMoa0800374. [DOI] [PubMed] [Google Scholar]
- 9.Guss D, Bhattacharyya T. Perioperative management of the obese orthopaedic patient. J Am Acad Orthop Surg. 2006;14:425–432. doi: 10.5435/00124635-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, Soglian AG, Pap AF, Misselwitz F, Haas S, RECORD2 Investigators Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet. 2008;372:31–39. doi: 10.1016/S0140-6736(08)60880-6. [DOI] [PubMed] [Google Scholar]
- 11.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 12.Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, Misselwitz F, Turpie AGG, RECORD3 Investigators Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358:2776–2786. doi: 10.1056/NEJMoa076016. [DOI] [PubMed] [Google Scholar]
- 13.Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 suppl):84–88. doi: 10.1016/j.arth.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Risk factors for clinically relevant pulmonary embolism and deep venous thrombosis in patients undergoing primary hip or knee arthroplasty. Anesthesiology. 2003;99:552–560. doi: 10.1097/00000542-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Memtsoudis SG, Besculides MC, Gaber L, Liu S, González Della Valle A. Risk factors for pulmonary embolism after hip and knee arthroplasty: a population-based study. Int Orthop. 2009;33:1739–1745. doi: 10.1007/s00264-008-0659-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michalka PK, Khan RJ, Scaddan MC, Haebich S, Chirodian N, Wimhurst JA. The influence of obesity on early outcomes in primary hip arthroplasty. J Arthroplasty. 2012;27:391–396. doi: 10.1016/j.arth.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Moran M, Walmsley P, Gray A, Brenkel IJ. Does body mass index affect the early outcome of primary total hip arthroplasty? J Arthroplasty. 2005;20:866–869. doi: 10.1016/j.arth.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89:33–38. doi: 10.2106/JBJS.F.00163. [DOI] [PubMed] [Google Scholar]
- 19.Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121:21–33. doi: 10.3810/pgm.2009.11.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarzkopf R, Thompson SL, Adwar SJ, Liublinska V, Slover JD. Postoperative complication rates in the ‘super-obese’ hip and knee arthroplasty population. J Arthroplasty. 2012;27:397–401. doi: 10.1016/j.arth.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Scott CE, Howie CR, Macdonald D, Biant LC. Predicting dissatisfaction following total knee replacement: a prospective study of 1217 patients. J Bone Joint Surg Br. 2010;92:1253–1258. doi: 10.1302/0301-620X.92B9.24394. [DOI] [PubMed] [Google Scholar]
- 23.Suleiman LI, Ortega G, Ong’uti SK, Gonzalez DO, Tran DD, Onyike A, Turner PL, Fullum TM. Does BMI affect perioperative complications following total knee and hip arthroplasty? J Surg Res. 2012;174:7–11. doi: 10.1016/j.jss.2011.05.057. [DOI] [PubMed] [Google Scholar]
- 24.Turpie AGG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, Cushner FD, Lotke PA, Berkowitz SD, Bandel TJ, Benson A, Misselwitz F, Fisher WD, RECORD4 Investigators Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009;373:1673–1680. doi: 10.1016/S0140-6736(09)60734-0. [DOI] [PubMed] [Google Scholar]