Abstract
Background
Although testing and treatment for Staphylococcus aureus colonization before total joint arthroplasty (TJA) are well described and understood, the durability of decolonization has not been studied extensively.
Questions/purposes
The purpose of this pilot study is to determine the percentage of arthroplasty patients with S. aureus colonization despite previous decolonization at the time of TJA.
Methods
Over a 2-year period, all patients having TJA by one surgeon were screened and treated for nasal S. aureus. Of 634 patients, 139 had methicillin-sensitive S. aureus (15%) or methicillin-resistant S. aureus (6.6%) colonization before TJA. Fifty-eight of these 139 patients (42%) were retested at 3 to 30 months for persistent colonization by nasal culture. Data collection included age at time of TJA, type of TJA, and time from TJA to repeat testing. We performed no clonal analysis for strains.
Results
Thirty-nine of the 58 patients (67%) decolonized before surgery were negative on retesting and 19 (33%) were again positive for S. aureus colonization. Of the 19 patients who retested positive for colonization, 17 (89%) were colonized by bacteria with unchanged antibiotic sensitivity.
Conclusions
We demonstrate that 33% (19 of 58) of postoperative arthroplasty patients test positive for S. aureus colonization at 3 to 30 months after surgery despite preoperative decolonization. Arthroplasty surgeons must be aware that a decolonization treatment does not guarantee that a patient will remain decolonized in the future. Although unchanged antibiotic sensitivity in 89% of these patients suggests a substantial role for persistence as opposed to eradication and repeat colonization, we were unable to retrospectively perform clonal analysis to confirm this conclusion. This group of patients demonstrating continued colonization with S. aureus after arthroplasty deserves further study, because it remains unclear whether there is a higher risk of late infection in this population.
Level of Evidence
Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Despite attempts to improve care with the national implementation of the Surgical Care Improvement Project antibiotic guidelines [7], infection continues to be a common and devastating complication in patients who have undergone total joint arthroplasty (TJA). Staphylococcus aureus is the most common organism (35%–65%) isolated from periprosthetic joint infections [2, 8, 17, 18] with its methicillin-resistant forms demonstrating increased difficulty of eradication [19]. Growing concern surrounds the increasing prevalence of both community- and nosocomial-acquired methicillin-resistant S. aureus (MRSA) infection, because they carry both an increased burden on medical resources as well as morbidity and mortality [3].
The anterior nares are reportedly the most reliable location for isolation of S. aureus [24]. It is estimated that S. aureus is colonized within the anterior nares in approximately 31% (range, 6%–56%) of the general population at any given time [4, 14, 15]. Twenty percent are persistently colonized carriers, whereas approximately 60% may demonstrate intermittent colonization with changing of strains with varying frequency [1, 14, 24]. The remaining 20% of people are defined as noncarriers [14].
In 1999, the Centers for Disease Control and Prevention listed preoperative nasal carriage of S. aureus as a risk for surgical site infection (SSI) [12]. Two epidemiologic studies have demonstrated an increased risk (two to ninefold) of SSIs in patients who are carriers of S. aureus [3, 23]. In those with SSIs, up to 85% have S. aureus isolates that are identical by pulse electrophoresis techniques to those isolated from their nares [20].
Within the orthopaedic literature, recent focus has been on the prevention of SSIs by using various decolonization protocols in patients colonized preoperatively [12, 13, 21]. Multiple studies have demonstrated the ability to decolonize patients using 2% mupirocin ointment with a combination of chlorhexidine body wash and in some cases systemic antibiotics [9, 10, 13, 20, 21]. In an effort to decrease SSIs, decolonization protocols have been recently implemented for orthopaedic patients undergoing elective procedures [9, 13, 21]. Lee et al. have shown this method to be a cost-effective strategy for both the third-party payer and hospital systems. This was held true even with a low MRSA prevalence (1%), lower decolonization rate (25%), and a cost of USD 300 for decolonization per patient [16]. To date, most studies have focused on the end point of SSIs when discussing the role of decolonization protocols. These studies have all shown a major decline in SSIs with the use of decolonization protocols, which has generated much interest in their use [9, 13, 21]. However, to our knowledge, there is only one article within the TJA literature detailing the persistence of nasal methicillin-sensitive S. aureus (MSSA)/MRSA colonization after a decolonization protocol has been used: Immerman et al. [11] showed that at an average interval of 213 days postoperatively, 30% of their patients were not decolonized after a decolonization protocol of mupirocin and chlorhexidine.
The purpose of this pilot study is to determine the percentage of arthroplasty patients with S. aureus colonization despite previous decolonization at the time of TJA.
Patients and Methods
Over a 2-year period from 2008 to 2010, all 634 patients having hip or knee arthroplasty by one surgeon (CAD) were preoperatively screened by culture for nasal MSSA/MRSA and were decolonized as part of an infection reduction protocol at the author’s institution. Of 634 patients screened, 139 were identified to have MSSA (97 of 634 [15%]) or MRSA (42 of 634 [7%]) colonization before TJA, and 139 all had undergone the decolonization protocol. Fifty-eight of these positive patients were able to be contacted and were available and agreed to be retested for persistence of MSSA/MRSA colonization as part of this pilot study. Of the 97 patients colonized with MSSA, 40 (41%) were available and agreed to be retested. The average age of the patients within this cohort was 67 years (range, 57–85 years). The cohort consisted of 19 primary THAs, 18 primary TKAs, and three primary unicondylar knee arthroplasties (UKAs).
The decolonization protocol consisted of 2% mupirocin (Bactroban; GlaxoSmithKline, Middlesex, UK) nasal ointment and a 2% chlorhexidine (Hibiclens; Molnlycke Health Care, Norcross, GA, USA) body wash. The nasal ointment was used twice a day for 5 days leading up to, but not including, the day of surgery. To ensure patient compliance, a nurse called each patient 6 days before surgery to discuss and confirm that prescriptions for the decolonization protocol medications were obtained. On the day of surgery, confirmation of decolonization protocol completion was discussed with the patient in the preoperative holding area by the senior surgeon (CAD) before the patient entered the operating room. All patients testing positive for MRSA were also placed on isolation precautions on admission to the hospital for surgery. Additionally, patients who tested positive for MRSA colonization preoperatively were given vancomycin for infection prophylaxis at the time of surgery rather than the standard use of cefazolin.
Seven of the 40 patients were discharged to a rehabilitation facility with the remaining 33 being discharged to home after the index procedure. The average length of hospitalization during the index procedure was 2.1 days. Mean time from the index procedure to repeat nasal swab testing was 492 days (range, 103–885 days). Eighteen (42%) of the 42 MRSA-colonized patients were available and agreed to be retested. The average age of the patients in this cohort was 69 years (range, 56–80 years). This cohort consisted of six primary THAs, six primary TKAs, two primary UKAs, and four revision TKAs. Seven of these patients were discharged to rehabilitation, whereas 11 were discharged to home after the index procedure. The average length of stay after the index procedure was 2.83 days. The time from the index procedure to repeat nasal swab testing was 608 days (range, 299–907 days).
Two of the authors (DME, CAD) were responsible for the retesting of all patients and were trained to use the proper technique by the preadmission screening technicians. Cultures were obtained by applying a single culturette to bilateral nares. The culturettes were then taken directly to the microbiology laboratory. There they were plated on BBL CHROMagar MRSA II (BD, Franklin Lakes, NJ, USA) plates and incubated for 48 hours by a standard laboratory technique. Standard microbiologic culture methods were used to determine the presence of MSSA or MRSA.
Data retrospectively collected included age at the time of arthroplasty, type of arthroplasty conducted, time between procedure and retested cultures obtained (in days), average length of hospitalization, and disposition of the patient at discharge.
Results
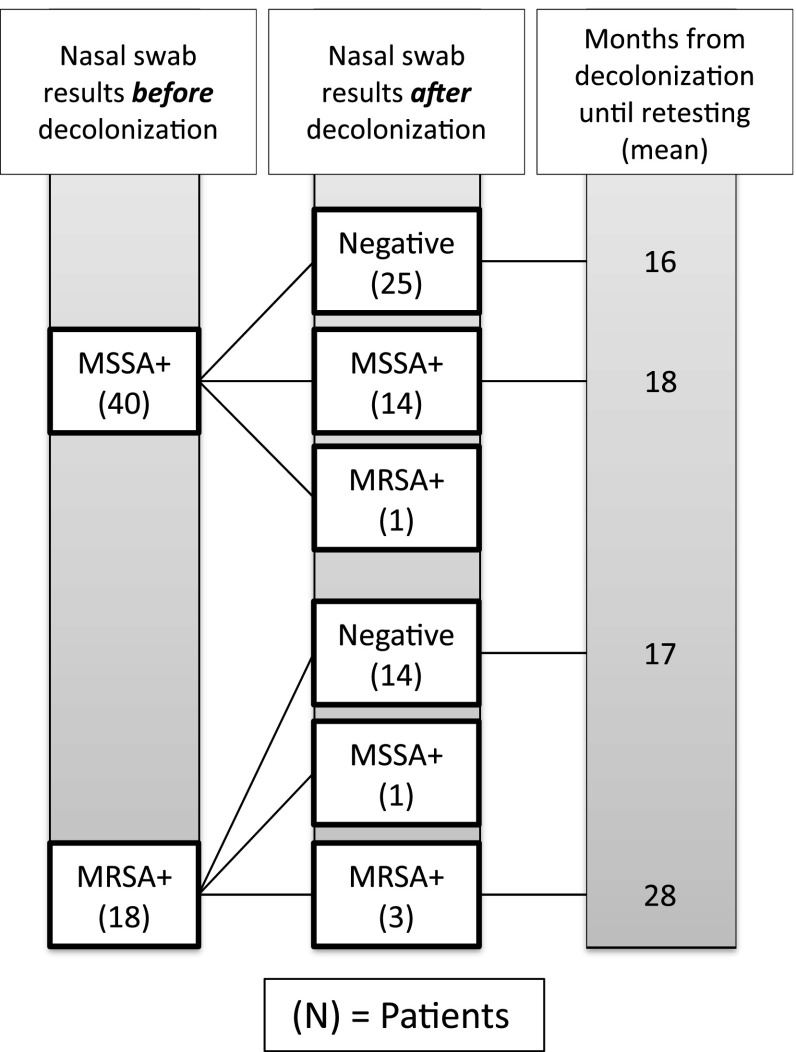
On retesting of the MSSA cohort, 15 (38%) patients were colonized with S. aureus (Fig. 1). The average age of those colonized in this subgroup was 67 years (range, 57–81 years). Of these 15, four had undergone primary THA, nine underwent primary TKA, and two underwent primary UKA. The average time from the index procedure until retesting was 549 days (range, 176–949 days). The average length of stay during after the index procedure was 2.4 days. After the index procedure, 12 patients were discharged home, whereas three were discharged to a rehabilitation facility. Of the 25 who tested negative for MSSA colonization on retesting, the average time from the index procedure to retesting was 473 days (range, 103–885 days).
Fig. 1.
Results are shown of nasal swab cultures in the study population before and after a decolonization protocol was used.
On retesting the MRSA cohort, four (22%) patients were colonized with S. aureus. The average age of these patients was 73 years (range, 68–81 years). This group consisted of one primary THA, one primary TKA, one primary UKA, and one revision TKA. The average time from the index procedure to retesting was 831 days (range, 689–905 days). The average length of stay after the index procedure was 3.5 days. Three of these patients were discharged to rehabilitation, whereas one was discharged home after their index procedure. Of the 14 patients who tested negative for MRSA colonization on retesting, the average time from the index procedure to retest was 521 days (range, 299–907 days).
Two patients (10%) who retested positive for S. aureus colonization demonstrated conversion in the antibiotic resistance pattern of the colonized S. aureus. One patient, who tested positive preoperatively for MSSA, converted to MRSA colonization at the time of retesting. This patient was 68 years old at the time of the index primary THA and was discharged to home from our institution on postoperative day 1 as part of a rapid recovery protocol. The time from the index procedure until retesting was 191 days. Another patient converted from MRSA to MSSA colonization at the time of retesting. This patient was 70 years old at the time of the index primary THA and was discharged to home on postoperative day 2. The time from the index procedure until retesting was 894 days.
Discussion
Nasal screening for S. aureus, with subsequent decolonization before hip and knee arthroplasty, has been demonstrated to decrease the perioperative infection rate. Investigations in the nonarthroplasty literature suggest that patients undergoing S. aureus decolonization may nevertheless demonstrate later evidence of persistent colonization and may be at an increased risk of late-onset infection. There is only one report in the arthroplasty literature describing the longer-term S. aureus colonization status among patients who were decolonized before arthroplasty [11]. The purpose of this pilot study was to determine the percentage of postoperative arthroplasty patients with S. aureus colonization despite previous decolonization at the time of TJA.
We recognize the limitations to this pilot study. First, we were only able to retest 42% of our decolonized patients as a result of a combination of factors including death and patient relocation, but mostly because of patient refusal to return on a testing date for followup testing. It is possible that this selection bias could have biased our data to demonstrate a lower rate of S. aureus colonization at followup considering that these patients have a higher likelihood of having comorbidities limiting their ability to followup. Second, the relatively low number of patients included in this pilot study limited our ability to perform multivariate analysis and identify risk factors for the presence of S. aureus colonization after arthroplasty. Third, given the retrospective nature of this study, we did not define the bacterial strain present at the time of preoperative nasal swab, thus making it impossible to definitively differentiate between intermittent colonization and persistent colonization. We do not believe these limitations compromise our conclusions. The demonstration that a large proportion of arthroplasty patients test positive postoperatively for S. aureus colonization, despite treatment to achieve decolonization, is important even without the definitive classification of persistent versus recurrent colonization. It is our intention that this pilot study will spur larger, more rigorous studies that very accurately define the population of arthroplasty patients with persistent and intermittent colonization and define the relevant infectious implications.
The conclusion of this study is that a large proportion (33%) of arthroplasty patients, preoperatively treated for decolonization of S. aureus, continues to test positive for colonization at 3 to 30 months after arthroplasty. This rate is consistent with the only other study in the arthroplasty literature, which demonstrated that 30% of patients decolonized before elective arthroplasty were colonized on subsequent nasal swabbing at a mean of 213 days [11]. This rate is also in general accordance with previous nonarthroplasty population studies revealing persistence after decolonization protocols of approximately 20%–30% [6, 22]. Based on these combined data, it is clear that surgeons should not place confidence in a patient’s prior colonization status but should retest for colonization at subsequent admissions for surgery as indicated.
In nonarthroplasty patients, newly acquired MRSA colonization is associated with a 29% risk of subsequent infection [10]. Furthermore, 23% of long-term carriers (those with known MRSA colonization > 1 year) will develop late-onset infections [5]. With the increasing numbers of implanted TJAs, understanding the importance and infectious consequences of the S. aureus carrier state is of critical importance necessitating clinical outcome studies.
Eighty-nine percent (17 of 19) of the S. aureus isolates cultured from those patients retesting positive for colonization matched the methicillin sensitivity profile of the S. aureus isolate that was presumed to be decolonized at the time of index arthroplasty. The only other study conducted on an arthroplasty population similarly found that 93% (37 of 40) of patients who retested positive for colonization demonstrated S. aureus with methicillin sensitivity consistent with the prior index cultures. This high concordance could have several possible causes. First, the strains of colonizing S. aureus may have never been eradicated, demonstrating persistent colonization. Second, the patients may be exposed to a consistent reservoir of bacteria at their household, resulting in recolonization with a bacterial strain of similar antibiotic sensitivities. Third, the patient may be recolonized by bacteria with similar antibiotic sensitivities by random chance. We cannot definitively make a conclusion without appropriate molecular characterization of the S. aureus strains.
It is hoped that a complete understanding of S. aureus colonization patterns in the arthroplasty population of patients can lead to more directed efforts at preventing both postoperative and late periprosthetic infections. From this pilot study, it appears that the patterns among arthroplasty patients are in concordance with those found in nonarthroplasty populations. Arthroplasty surgeons must be aware that decolonization treatment does not guarantee that a patient will remain decolonized in the future. Further research must demonstrate whether continued late S. aureus colonization has important infectious ramifications for our patients long after arthroplasty.
Footnotes
One of the authors certifies that he (CAD), or a member of his or her immediate family, has or may receive payments or benefits, during the study period, an amount of more than USD 1,000,001 from CD Diagnostics, Wynnewood, PA, USA.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Lankenau Medical Center, Wynnewood, PA, USA.
References
- 1.Armstrong-Esther CA, Smith JE. Carriage patterns of Staphylococcus aureus in a healthy non-hospital population of adults and children. Ann Hum Biol. 1976;3:221–227. doi: 10.1080/03014467600001381. [DOI] [PubMed] [Google Scholar]
- 2.Ayers DC, Dennis DA, Johanson NA, Pellegrini VD., Jr Common complications of total knee arthroplasty. J Bone Joint Surg Am. 1997;79:278–311. [Google Scholar]
- 3.Boucher H, Corey G. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46(Suppl):S344–S349. doi: 10.1086/533590. [DOI] [PubMed] [Google Scholar]
- 4.Boyce JM. Should we try to contain and control methicillin-resistant Staphylococcus aureus? Infect Control Hosp Epidemiol. 1991;12:46–54. doi: 10.1086/646237. [DOI] [PubMed] [Google Scholar]
- 5.Datta R, Huang S. Risk of infection and death due to methicillin-resistant Staphylococcus aureus in long-term carriers. Clin Infect Dis. 2008;47:176–181. doi: 10.1086/589241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dow G, Field D, Mancuso M, Allard J. Decolonization of methicillin-resistant Staphylococcus aureus during routine hospital care: efficacy and long-term follow-up. Can J Infect Dis Med Microbiol. 2010;21:38–44. doi: 10.1155/2010/590530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fry DE. Surgical infections and the Surgical Care Improvement Project (SCIP): evolution of the national quality measures. Surg Infect (Larchmnt). 2008;9:579–584. doi: 10.1089/sur.2008.9951. [DOI] [PubMed] [Google Scholar]
- 8.Fulkerson E. Della Valle CJ, Wise B, Walsh M, Preston C, DiCesare PE. Antibiotic susceptibility of bacteria infecting total joint arthroplasty sites. J Bone Joint Surg Am. 2006;88:1231–1237. doi: 10.2106/JBJS.E.00004. [DOI] [PubMed] [Google Scholar]
- 9.Hacek D, Robb W, Paule S, Kudrna J, Stamos VP, Peterson LR. Staphylococcus aureus nasal decolonization in joint replacement surgery reduces infection. Clin Orthop Relat Res. 2008;466:1349–1355. doi: 10.1007/s11999-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang S, Platt R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis. 2003;36:281–285. doi: 10.1086/345955. [DOI] [PubMed] [Google Scholar]
- 11.Immerman I, Ramos NL, Katz GM, Hutzler LH, Phillips MS, Bosco JA. The persistence of Staphylococcus aureus decolonization after mupirocin and topical chlorhexidine: implications for patients requiring multiple or delayed procedures. J Arthroplasty. 2012;27:870–876. doi: 10.1016/j.arth.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Kalmeijer M, Coertjens H, van Nieuwland-Bolland P, Bogaergs-Hofman D. deBaere GAJ, Stuurman A, van Belkum A, Kluytmans JAJW. Surgical site infection in orthopedic surgery: the effect of mupirocin nasal ointment in a double blind, randomized, placebo controlled study. Clin Infect Dis. 2002;35:353–358. doi: 10.1086/341025. [DOI] [PubMed] [Google Scholar]
- 13.Kim D, Spencer M, Davidson S, Li L, Shaw J, Gulczynski D, Hunter D, Martha J, Miley G, Parazin S, Dejoie P, Richmond J. Institutional prescreening for detection and eradication of methicillin-resistant Staphylococcus aureus in patients undergoing elective orthopaedic surgery. J Bone Joint Surg Am. 2010;92:1820–1826. doi: 10.2106/JBJS.I.01050. [DOI] [PubMed] [Google Scholar]
- 14.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms and associated risks. Clin Microbiol Rev. 1997;10:505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamikanra A, Paul BD, Akinwole OB, Paul MO. Nasal carriage of Staphylococcus aureus in a population of healthy Nigerian students. J Med Microbiol. 1985;19:211–216. doi: 10.1099/00222615-19-2-211. [DOI] [PubMed] [Google Scholar]
- 16.Lee B, Wiringa A, Bailey R, Goyal V, Tsui B, Lewis GJ, Muder R, Harrison LM. The economic effect of screening orthopedic patients preoperatively for methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2010;31:1130–1138. doi: 10.1086/656591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahomed NN, Barrett JA, Katz JN, Phillips CB, Losina E, Lew RA, Guadagnoli E, Harris WH, Poss R, Baron JA. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85:27–32. doi: 10.2106/00004623-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Meehan J, Jamali A, Nguyen H. Prophylactic antibiotics in hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91:2480–2490. doi: 10.2106/JBJS.H.01219. [DOI] [PubMed] [Google Scholar]
- 19.Odum S, Fehring T, Lombardi A, Zmistowski BM, Brown NM, Luna JT, Fehring KA, Hansen EN. Irrigation and débridement for periprosthetic infections—does the organism matter? J Arthroplasty. 2011;26(Suppl):114–118. doi: 10.1016/j.arth.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 20.Perl T, Cullen J, Wenzel R, Zimmerman MB, Pfaller MA, Sheppard D, Twombley J, French PP, Herwaldt LA. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. 2002;346:1871–1877. doi: 10.1056/NEJMoa003069. [DOI] [PubMed] [Google Scholar]
- 21.Rao N, Cannella B, Crossett L, Yates AS, McGough R. A preoperative decolonization protocol for Staphylococcus aureus prevents orthopaedic infections. Clin Orthop Relat Res. 2008;466:1343–1348. doi: 10.1007/s11999-008-0225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robicsek A, Beaumont J, Paule S, Hacek DM, Thomson RB, Kaul K, King P, Peterson LR. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med. 2008;148:409–418. doi: 10.7326/0003-4819-148-6-200803180-00003. [DOI] [PubMed] [Google Scholar]
- 23.Wenzel RP, Perl TM. The significance of nasal carriage of Staphylococcus aureus and the incidence of post operative wound infection. J Hosp Infect. 1995;31:13–24. doi: 10.1016/0195-6701(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 24.Williams R. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol Rev. 1963;27:56–71. doi: 10.1128/br.27.1.56-71.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]