Abstract
Background
In presumed aseptic hip and knee revisions, it is common practice to send intraoperative cultures to screen for occult infection. Currently no guidelines exist for the routine use of acid-fast bacillus (AFB) and fungal cultures in this setting.
Questions/purposes
We established (1) the rate of positive fungal and AFB cultures in aseptic hip and knee revision arthroplasties, (2) factors associated with positive fungal and AFB cultures, (3) the likelihood that positive cultures represent true-positive results, and (4) the hospital charges of sending fungal and AFB cultures routinely.
Methods
We retrospectively evaluated all 1717 presumed aseptic hip and knee revisions performed from January 2006 to November 2011: 1139 patients had at least one intraoperative fungal culture and 1133 patients had at least one intraoperative AFB culture, with 923 and 920, respectively, achieving 1-year followup. The Musculoskeletal Infection Society criteria were used to classify subsequent infections. We attempted to identify risk factors for positive cultures.
Results
We observed six (0.5%) patients with positive AFB cultures and 19 (1.7%) with positive fungal cultures. Patients undergoing reimplantation procedures were more likely to have a positive fungal culture. The true-positive rate was 0% and 0.1% for AFB and fungal cultures, respectively. The total hospital charges for these cultures over the time frame of our study were USD 1,315,533.
Conclusions
Given the extremely low rate of true-positive AFB and fungal cultures in presumed aseptic revision joint arthroplasty and the charges associated with maintaining these cultures, we believe their routine use is unwarranted.
Level of Evidence
Level III, prognostic study. See the Instructions for Authors for a complete description of evidence.
Introduction
Before every hip and knee arthroplasty revision, Parvizi et al. [15] recommended patients be evaluated for the presence of infection. However, a negative preoperative workup does not completely eliminate the possibility of occult infection [6, 13, 14, 19, 20]. Intraoperative specimens are often sent for culture during presumed aseptic revision joint arthroplasty procedures to screen for such occult periprosthetic joint infection (PJI). Type I PJIs are those diagnosed when intraoperative cultures unexpectedly return positive results in this setting [9]. Several studies [11, 17, 21] on the epidemiology and outcomes of bacterial Type I PJI suggest the major causative organism is coagulase-negative Staphylococcus, accounting for 50% to 100% of all such infections. Bacterial Type I PJI reportedly occurs in 6.1% [3] of presumed aseptic knee revisions and these account for 3% to 11% of all PJI cases [11, 17, 21]. When diagnosed, Type I PJIs are typically treated with an extended course of antibiotics, yielding an infection control rate of 90% to 100% [17, 21].
To identify cases of occult fungal and mycobacterial infection, surgeons may send specimens for fungal and acid-fast bacillus (AFB) cultures during presumed aseptic revision joint arthroplasty procedures. Guidelines for this practice do not currently exist for screening for Type I infections. Atypical infections would be expected to represent a small fraction of Type I PJI given its low prevalence in the total PJI population. Shuman et al. [18] estimate atypical organisms account for approximately 1% of PJI cases, while Azzman et al. [2] found 46 reported cases of fungal PJI in a review of all available English language literature from 1979 to 2008. Given the charges associated with processing individual specimens for culture, the practice of screening for atypical infections in all presumed aseptic revision cases might substantially add to global healthcare costs [22].
We therefore determined (1) the rate of positive fungal and AFB cultures in the setting of presumed aseptic hip and knee revision arthroplasty, (2) factors associated with positive fungal and AFB cultures in this setting, (3) the likelihood that such cases represent true-positive results, and (4) the charges associated with this practice.
Patients and Methods
We crossreferenced ICD-9 procedure codes with our institutional joint arthroplasty database to identify 1717 presumed aseptic hip and knee arthroplasty revision cases between January 2006 and November 2011. The indications for revision varied (Table 1). For each patient, the operative report and microbiology results were reviewed. Patients were excluded from the study if they (1) did not have at least one fungal or AFB culture result, (2) did not have at least one component revised, or (3) received treatment for a known or suspected infection. For our analysis of fungal cultures, 127 patients were excluded due to treatment for active or suspected infection, 68 because no components were revised, and 383 because no specimens were sent for fungal culture. For our analysis of AFB cultures, six additional patients were excluded because no samples were sent for AFB culture. In total, we identified 1139 patients with at least one intraoperative fungal culture and 1133 patients with at least one intraoperative AFB culture (Table 2). Of the 1139 patients with at least one intraoperative fungal culture sent, 923 had a minimum followup of 12 months (mean, 36 months; range, 12–84 months). The remaining 216 patients died or did not return for routine followup beyond 12 months and could not be contacted. Of the 1133 patients with at least one intraoperative AFB culture sent, 920 had a minimum followup of 12 months (mean, 36 months; range, 12–84 months). The remaining 213 patients died or did not return for routine followup beyond 12 months and could not be contacted. The minimum followup for all patients was 1 year (mean, 3 years; range, 1–7 years). No patients were recalled specifically for this study; all data were obtained from medical records and radiographs.
Table 1.
Surgical indications
| Indication | Patients with fungal cultures (% of total 1139 patients with a culture) | Patients with AFB cultures (% of total 1133 patients with a culture) |
|---|---|---|
| Loosening | 672 (59%) | 668 (59%) |
| Periprosthetic infection (Stage 2) | 231 (20.3%) | 230 (20.3%) |
| Instability | 85 (7.5%) | 85 (7.5%) |
| Wear/Osteolysis | 77 (6.8%) | 76 (6.7%) |
| Periprosthetic fracture | 37 (3.2%) | 37 (2.8%) |
| Stiffness | 15 (1.3%) | 15 (3.3%) |
| Broken prosthesis | 7 (0.6%) | 7 (0.6%) |
| Metal corrosion | 4 (0.4%) | 4 (0.4%) |
| Synovitis | 4 (0.4%) | 4 (0.4%) |
| Patella resurfacing | 3 (0.3%) | 3 (0.3%) |
| Failed extensor mechanism | 1 (0.1%) | 1 (0.1%) |
| Leg length discrepancy | 1 (0.1%) | 1 (0.1%) |
| Ceramic fracture | 1 (0.1%) | 1 (0.1%) |
| Failed hemiarthroplasty | 1 (0.1%) | 1 (0.1%) |
| Total | 1139 | 1133 |
AFB = acid-fast bacillus.
Table 2.
Number of cultures sent
| Variable | Fungal | AFB |
|---|---|---|
| Total number of patients | 1139 | 1133 |
| Number with only one type of atypical culture sent | 16 | 10 |
| Number with fungal and AFB cultures sent | 1123 | 1123 |
| Total number of cultures sent | 3111 | 3106 |
| Average number of cultures sent | 2.7 (range, 1–9) | 2.74 (range, 1–9) |
| Number with ≥ 3 cultures sent | 763 (67.0%) | 766 (67.6%) |
AFB = acid-fast bacillus.
All procedures were performed at the same hospital by one of five fellowship-trained adult joint reconstruction surgeons. Most patients were limited to receiving 24 hours of routine perioperative antibiotics, although the 231 patients who were revised from an antibiotic spacer as part of a two-stage exchange received standing antibiotics until the third postoperative day when bacterial culture results were finalized. In all cases, clean instruments were used to obtain one or more intraoperative tissue specimens that were immediately sent to the microbiology laboratory for processing. Tissue samples sent for fungal cultures were divided and incubated at 30° C for an average of 29 days (range, 28–32 days) on trypticase soy agar with 5% sheep blood plate (Becton Dickinson & Co, Franklin Lakes, NJ, USA), Sabouraud dextrose agar (Remel Inc, Lenexa, KS, USA), brain heart infusion agar (Remel Inc), and Candida chrome agar (Becton Dickinson & Co). Tissue samples sent for AFB cultures were divided and incubated at 37° C for an average of 45 days (range, 12–50 days) on Lowenstein-Jensen Gruft (Remel Inc), 7H11 agar (Remel Inc), and Mycobacteria Growth Indicator Tubes (Becton Dickinson & Co). The surgical team followed fungal and AFB culture results until their finalization through the hospital electronic medical record system. When positive cultures were recognized after patient hospital discharge, the infectious disease team was consulted. Antibiotic treatment was rendered based on the organism, number of positive cultures, and recommendations of the infectious disease consultants.
Patients were evaluated for routine radiographic, clinical, and serologic evaluation (in cases of suspected infection) at 6 weeks, 6 months, 1 year, and every 3 years thereafter postoperatively. For all patients, we used hospital and office electronic medical records to collect data, including patient demographics, Charlson Comorbidity Index (CCI) [4], surgical indication, date of surgery, operative findings, number of cultures sent, number and results of positive cultures, and postoperative antibiotic treatment course. The Musculoskeletal Infection Society (MSIS) criteria for PJI were used to define patients who acquired a subsequent infection after their revision [12]. If subsequent surgeries for PJI were required, details of those surgeries were collected, including culture results.
For cases involving positive cultures, we considered the result false positive if only a single culture showed positive growth, the patient did not receive antimicrobial treatment, and the patient had no subsequent signs of infection or surgical intervention for PJI. We considered the result true positive if multiple cultures showed positive growth with the same organism and/or the patient developed a subsequent infection with cultures showing identical organism growth. The positive culture result was considered indeterminate if the patient received antimicrobial treatment. With these data, we determined the rates of any positive cultures, false-positive results, true-positive results, and indeterminate-positive results for fungal and AFB cultures in the setting of presumed aseptic hip and knee revision. All patients included in the analysis of true-, false-, and indeterminate-positive rates achieved a minimum of 1 year of followup, while all patients were considered when reporting the rate of positive intraoperative cultures.
To determine the charges associated with the practice of routinely sending fungal and AFB cultures in the setting of presumed aseptic hip and knee revision, we used the hospital billing database to determine the hospital charge for each individual fungal and AFB culture, which were USD 230.27 and USD 925.93, respectively. This figure was multiplied by the number of specimens sent per case and by the total number of cases in the time frame of our study.
We used descriptive statistics to determine the rates of positive culture. Multivariate logistic regression was used to determine whether age, sex, hip versus knee, CCI, and surgical indication were predictive of a positive AFB or fungal culture using R 2.15.1 (The R Project for Statistical Computing, Vienna, Austria).
Results
Of patients with AFB cultures (Table 3), six (0.5%) showed positive results, all of which showed growth in one of three specimens. Concomitant positive fungal or bacterial cultures were never present. Cultures were positive at an average of 19 days (range, 14–27 days) postoperatively. Three cases involving positive AFB cultures were processed from tissue samples taken by one surgeon on the same day and resulted in identical organism growth. Of patients with fungal cultures (Table 4), 19 (1.7%) showed a positive result. In all cases, one intraoperative tissue culture showed positive growth with a variable number of specimens sent. In total, 16 showed isolated positive fungal cultures and three showed concomitant bacterial growth. The fungal cultures were positive at an average of 5 days (range, 2–9 days) postoperatively.
Table 3.
Description of positive AFB culture cases and subsequent treatment*
| Case | Date of surgery | Hip/knee | Indication | Number of cultures sent | Number of positive cultures | Organism | Antibiotic treatment | Failure | Followup (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1/21/08 | Hip | Instability | 3 | 1 | MAC | No | No | 29 |
| 2 | 1/21/08 | Hip | Wear | 3 | 1 | MAC | No | No | 6 |
| 3 | 1/21/08 | Knee | Loosening | 3 | 1 | MAC | No | No | 8 |
| 4 | 10/3/08 | Knee | PJI Stage 2 | 3 | 1 | MM | No | No | 51 |
| 5 | 1/5/11 | Hip | PJI Stage 2 | 3 | 1 | MAC | No | No | 25 |
| 6 | 1/7/11 | Hip | Loosening | 3 | 1 | MM | No | No | 24 |
* Only patients with a minimum followup of 12 months were included in the analysis of true-/false positive rates; AFB = acid-fast bacillus; PJI = periprosthetic joint infection; MAC = Mycobacterium avium complex; MM = Mycobacterium mucogenicum.
Table 4.
Description of positive fungal culture cases and subsequent treatment*
| Case | Hip/knee | Indication | Number of cultures sent | Number of positive cultures | Organism | Treatment | Subsequent infection cultures | Followup (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | Knee | PJI Stage 2 | 4 | 1 | Candida albicans/Enterobacter cloacae | No | NA | 6 |
| 2 | Knee | Loosening | 4 | 1 | Alternaria/coagulase-negative Staphylococcus | No | NA | 62 |
| 3 | Hip | PJI Stage 2 | 4 | 1 | Penicillium | Voriconazole | NA | 32 |
| 4 | Knee | PJI Stage 2 | 3 | 1 | Aspergillus | No | NA | 79 |
| 5 | Hip | Loosening | 3 | 1 | Cladosporium | No | NA | 25 |
| 6 | Hip | Loosening | 3 | 1 | Penicillium | No | NA | 39 |
| 7 | Knee | Loosening | 3 | 1 | Aureobasidium | No | NA | 75 |
| 8 | Knee | PJI Stage 2 | 3 | 1 | Candida albicans | No | NA | 1 |
| 9 | Hip | PJI Stage 2 | 3 | 1 | Aspergillus fumigatus | No | NA | 3 |
| 10 | Hip | PJI Stage 2 | 3 | 1 | Dematiaceous | No | NA | 27 |
| 11 | Hip | Loosening | 3 | 1 | Penicillium | No | NA | 42 |
| 12 | Hip | PJI Stage 2 | 2 | 1 | Paecilomyces | No | NA | 82 |
| 13 | Hip | PPFx | 2 | 1 | Penicillium | No | NA | 2 |
| 14 | Hip | Instability | 2 | 1 | Rhodotorula minuta | Caspofungin | NA | 79 |
| 15 | Hip | PJI Stage 2 | 2 | 1 | Candida albicans | No | NA | 67 |
| 16 | Hip | Loosening | 1 | 1 | Rhodotorula | No | NA | 38 |
| 17 | Knee | PJI Stage 2 | 1 | 1 | Penicillium | No | NA | 15 |
| 18 | Hip | PJI Stage 2 | 2 | 1 | Candida albicans/VRE | Fluconazole | VRE | 39 |
| 19 | Knee | Loosening | 1 | 1 | Candida parapsilosis | No | Candida parapsilosis, MSSA | 47 |
* Only patients with a minimum followup of 12 months were included in the analysis of true-/false-positive rates; PJI = periprosthetic joint infection; PPFx = periprosthetic fracture; VRE = vancomycin-resistant Enterococcus; MSSA = methicillin-sensitive Staphylococcus aureus; NA = not applicable.
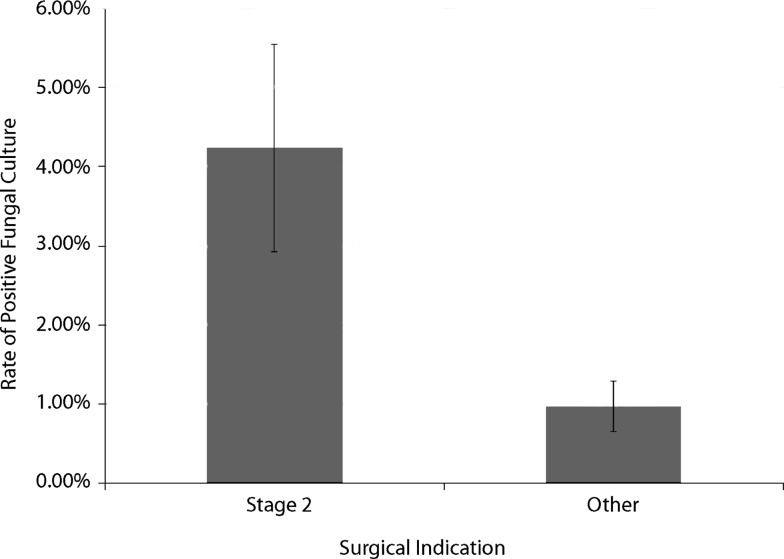
We found no association between the variables examined and positive AFB cultures. There was however increased odds of positive fungal cultures (odds ratio = 5.3; 95% CI = 2–13.7; p < 0.001) for patients receiving a reimplantation procedure (Fig. 1). None of these patients showed positive fungal growth at the time of their resection. There was no difference between groups in terms of culture incubation time. There was a difference (95% CI = −0.578 to 0.323; p < 0.001) in the number of cultures sent between reimplantation procedures (average, 3.1) and all other indications (average, 2.6). There were no associations between age, sex, CCI, or affected joint and positive fungal cultures.
Fig. 1.
Patients undergoing reimplantation procedures showed a 5.25-fold increase in the rate of positive fungal cultures compared with all other surgical indications for our cohort. Data are shown as mean ± SD.
In total, four of the six patients with positive AFB cultures achieved 12 months of followup (mean, 32 months; range, 24–51 months). We found none of the four positive AFB cultures represented true-positive results. Of cases involving positive AFB cultures, antibiotics were never administered. None of the patients showed subsequent clinical signs of infection or required infection surgery. Thus, by our criteria, all four cases represented false-positive cultures. For AFB cultures sent during presumed aseptic revisions, we found a false-positive rate of 0.4% (four of 920), an indeterminate-positive rate of 0%, and a true-positive rate of 0%. Of cases involving positive fungal cultures, 15 of the 19 total patients achieved 12 months of followup (mean, 50 months; range, 15–82 months). Three of these patients were treated with an extended course of an antifungal agent. Subsequent surgery for PJI was required in two patients, with one showing identical positive fungal cultures concomitant with methicillin-sensitive Staphylococcus aureus (Table 4). In 12 patients, there were no subsequent clinical signs of infection or infection surgery required. Thus, by our criteria, 11 cases represented false-positive cultures, three cases represented indeterminate results, and one case represented a true-positive result. For fungal cultures sent during presumed aseptic revisions, we found a false-positive rate of 1.2% (11 of 923), an indeterminate-positive rate of 0.3% (three of 923), and a true-positive rate of 0.1% (one of 923).
The total hospital charges associated with the routine use of AFB and fungal cultures in presumed aseptic revision procedures were USD 260,904 and USD 1,054,629, respectively. Thus, during the time frame of our study, the total charges for sending AFB and fungal cultures to screen for atypical Type I PJI was USD 1,315,533.
Discussion
At the time of revision hip and knee arthroplasty, it is common to screen for infections that eluded the preoperative workup. Infections identified in the postoperative period by these intraoperative cultures are termed Type I PJIs. The current literature suggests Type I bacterial PJIs occur at a rate that warrants the practice of screening for them in every presumed aseptic revision [11, 17, 21]. Yet, few studies have investigated Type I PJI involving atypical organisms, making the practice of screening for such infections unguided. Our study defined the rate of positive fungal and AFB cultures in the setting of presumed aseptic revision, determined the true- versus false-positive rate of these occurrences, established factors associated with positive cultures, and investigated the charges associated with the practice of screening for such atypical infections.
We recognize several limitations of our study. First, because of the retrospective nature of the study, we were unable to standardize several factors in our methods. For example, approximately 25% of cases were excluded because specimens were not sent for atypical cultures during the procedure. This could have resulted in selection bias of our data. Second, when cultures were sent, no standard practice existed to guide the number of specimens sent. In addition, when cultures returned positive, there are no standardized guidelines for the decision of antibiotic treatment. Due to our firm criteria for true-positive results and because serology was not obtained for patients with no clinical suspicion of infection, it is possible some subclinical infections were missed. It is also possible patients lost to followup could have sought treatment elsewhere for infection, resulting in a lower false-positive rate than actually was present. Third, we chose to perform a multivariate analysis despite the low number of positive culture results. While it has been suggested by others [1] that our data do not have an ideal ratio of adverse events to variables, we nonetheless believed determining risk factors for positive cultures was an important aspect of the study, and the multivariate logistic regression model that we constructed produced results that warrant consideration and future confirmation.
We found a rate of positive AFB (0.5%) and fungal (1.7%) cultures in the setting of presumed aseptic hip and knee revision lower than the rate of Type I bacterial PJI previously reported [17, 21]. Our numbers support the rarity of atypical infections in the general joint arthroplasty population, which has been observed in other studies [7, 10, 11, 18]. In a previous study investigating AFB and fungal culture results, Wadey et al. [22] found a positive rate of 0% for AFB cultures and 0.6% for fungal cultures. Yet, their study involved atypical cultures sent during all orthopaedic procedures, including known infections. Our study is distinctive in that it explored cultures sent only in the setting of presumed aseptic hip and knee revision. Our results support the notion that positive atypical culture results are much more uncommon than what has been reported for bacterial cultures in this setting. In fact, three of the six positive AFB cultures were obtained from three different patients, from the same operating room, on the same day. Unfortunately, only one of these three patients achieved the minimum followup to determine whether they were indeed false-positive results. Nevertheless, if these three results were indeed contaminants, it would only serve to further highlight the rarity of these infections and the likelihood that they represent false-positive results in most cases. Lastly, it is important to note our cohort did not include known cases of infection. Currently, the MSIS recommends against the routine use of AFB and fungal cultures in cases of known or suspected infection unless the patient is at a higher risk of developing atypical infections [12].
In investigating the factors associated with positive AFB cultures, we found no associations with any patient demographics, surgical indications, or joint involved. In exploring factors associated with positive fungal cultures, we found, compared to all other surgical indications, patients receiving the second stage of a two-stage exchange had 5.3-fold increased odds of producing a positive culture. The cause and implication of this association are not completely clear, although we suggest several possibilities: (1) there may be an increased positive rate in patients undergoing reimplantation procedures because more cultures were sent in this group; (2) the increased risk of positive cultures in these cases may represent persistent subclinical infection; (3) patients undergoing two-stage exchange could be immunocompromised in a way that would make them susceptible to atypical infections; however, our cohort was insufficient to detect such an effect; and (4) the increased risk may be indicative of fungal contamination of the skin, which often occurs in the setting of antibiotic therapy.
We found, in most cases, positive AFB and fungal cultures likely represented false-positive results. We are unaware of previous literature investigating the true- and false-positive rates of atypical cultures sent in the setting of presumed aseptic revisions. We found a 100% false-positive rate for AFB cultures and a 73.3% false-positive rate for fungal cultures in this setting. Barrack et al. [3] found 5.9% of presumed aseptic knee revisions yielded unexpected positive bacteria cultures. Of those cultures that returned positive, 29% were classified as true positive. Thus, the authors found a Type I bacterial PJI rate of 1.7%. We found a much lower Type I fungal PJI rate of 0.1% and Type I AFB PJI rate of 0%.
Investigation of the charges associated with the practice of routinely sending specimens for atypical cultures in this setting showed our hospital charges were more than USD 1000 per case and USD 1,315,533 for all cases. This figure represents hospital charges and likely overestimates hospital collection and total healthcare cost of the practice. In their study, Wadey et al. [22] calculated, based on labor and materials, the charge for a single AFB culture as USD 41.07 and the charge for a single fungal culture as USD 24.20. This compares to our hospital charges of USD 84 per sample for AFB cultures and USD 339 per sample for fungal cultures. The discrepancy (USD 41.07 versus USD 84) in charge per AFB culture likely represents the difference between hospital charges and the cost of labor and materials. The greater discrepancy (USD 24.20 versus USD 339) in charge per culture for fungal cultures may be due to differences in materials used and number of days incubated between institutions. If the charges estimated by Wadey et al. [22] were applied to our cohort, the total charges for sending these cultures would have been USD 202,849. Our analysis shows the practice of routinely sending atypical cultures in the setting of atypical revisions poses substantial hospital chargers. In addition, inappropriately treated false-positive results pose additional costs associated with antimicrobial medication, including their potentially toxic effects [5, 16].
In summary, we found an exceptionally low rate of positive fungal and AFB cultures in the setting of aseptic hip and knee revision and an exceedingly low rate of true-positive results. In addition, the practice poses substantial costs to the healthcare system and may impose unnecessary risks to patients receiving toxic antimicrobials in cases of false-positive results. Based on our results, we believe it is reasonable practice to send specimens for AFB and fungal culture in cases of presumed aseptic revision only when the surgeon has a high suspicion for such infections, such as an immunocompromised host, a history of atypical infection, or living in an area with endemic atypical infection [2, 8, 10, 11].
Acknowledgments
The authors thank Mitchell Maltenfort PhD for his work in performing the statistical analyses for this study.
Footnotes
One or more of the authors (CAD, GKD) certifies that he, or a member of his or her immediate family, has or may receive payments or benefits, during the study period, an amount of more than USD 1,000,001 from CD Diagnostics (Wynnewood, PA, USA). One of the authors (CD) is the founder of CD Diagnostics, a company that investigates infection diagnostics not related to this study.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that our institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Altman DG. Practical Statistics For Medical Research. Boca Raton, FL: Chapman and Hall/CRC Press; 1991. [Google Scholar]
- 2.Azzam K, Parvizi J, Jungkind D, Hanssen A, Fehring T, Springer B, Bozic K, Della Valle C, Pulido L, Barrack R. Microbiological, clinical, and surgical features of fungal prosthetic joint infections: a multi-institutional experience. J Bone Joint Surg Am. 2009;91(suppl 6):142–149. doi: 10.2106/JBJS.I.00574. [DOI] [PubMed] [Google Scholar]
- 3.Barrack RL, Aggarwal A, Burnett RS, Clohisy JC, Ghanem E, Sharkey P, Parvizi J. The fate of the unexpected positive intraoperative cultures after revision total knee arthroplasty. J Arthroplasty. 2007;22:94–99. doi: 10.1016/j.arth.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 5.Cusini A, Rampini SK, Bansal V, Ledergerber B, Kuster SP, Ruef C, Weber R. Different patterns of inappropriate antimicrobial use in surgical and medical units at a tertiary care hospital in Switzerland: a prevalence survey. PLoS One. 2010;5:e14011. doi: 10.1371/journal.pone.0014011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Della Valle CJ, Sporer SM, Jacobs JJ, Berger RA, Rosenberg AG, Paprosky WG. Preoperative testing for sepsis before revision total knee arthroplasty. J Arthroplasty. 2007;22:90–93. doi: 10.1016/j.arth.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Eid AJ, Berbari EF, Sia IG, Wengenack NL, Osmon DR, Razonable RR. Prosthetic joint infection due to rapidly growing mycobacteria: report of 8 cases and review of the literature. Clin Infect Dis. 2007;45:687–694. doi: 10.1086/520982. [DOI] [PubMed] [Google Scholar]
- 8.Kao AS, Brandt ME, Pruitt WR, Conn LA, Perkins BA, Stephens DS, Baughman WS, Reingold AL, Rothrock GA, Pfaller MA, Pinner RW, Hajjeh RA. The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. Clin Infect Dis. 1999;29:1164–1170. doi: 10.1086/313450. [DOI] [PubMed] [Google Scholar]
- 9.Leone JM, Hanssen AD. Management of infection at the site of a total knee arthroplasty. J Bone Joint Surg Am. 2005;87:2335–2348. doi: 10.2106/00004623-200510000-00026. [DOI] [PubMed] [Google Scholar]
- 10.Marculescu CE, Berbari EF, Cockerill FR, 3rd, Osmon DR. Fungi, mycobacteria, zoonotic and other organisms in prosthetic joint infection. Clin Orthop Relat Res. 2006;451:64–72. doi: 10.1097/01.blo.0000229337.21653.f2. [DOI] [PubMed] [Google Scholar]
- 11.Marculescu CE, Berbari EF, Hanssen AD, Steckelberg JM, Osmon DR. Prosthetic joint infection diagnosed postoperatively by intraoperative culture. Clin Orthop Relat Res. 2005;439:38–42. doi: 10.1097/01.blo.0000183091.83509.d8. [DOI] [PubMed] [Google Scholar]
- 12.Parvizi J. New definition for periprosthetic joint infection. Am J Orthop. 2011;40:614–615. doi: 10.1007/s00132-011-1742-5. [DOI] [PubMed] [Google Scholar]
- 13.Parvizi J, Ghanem E, Menashe S, Barrack RL, Bauer TW. Periprosthetic infection: what are the diagnostic challenges? J Bone Joint Surg Am. 2006;88(suppl 4):138–147. doi: 10.2106/JBJS.F.00609. [DOI] [PubMed] [Google Scholar]
- 14.Parvizi J, Jacovides C, Zmistowski B, Jung KA. Definition of periprosthetic joint infection: is there a consensus? Clin Orthop Relat Res. 2011;469:3022–3030. doi: 10.1007/s11999-011-1971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parvizi J, Suh DH, Jafari SM, Mullan A, Purtill JJ. Aseptic loosening of total hip arthroplasty: infection always should be ruled out. Clin Orthop Relat Res. 2011;469:1401–1405. doi: 10.1007/s11999-011-1822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paterson DL. “Collateral damage” from cephalosporin or quinolone antibiotic therapy. Clin Infect Dis. 2004;38(suppl 4):S341–S345. doi: 10.1086/382690. [DOI] [PubMed] [Google Scholar]
- 17.Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty: a retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81:1434–1445. doi: 10.2106/00004623-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Shuman EK, Urquhart A, Malani PN. Management and prevention of prosthetic joint infection. Infect Dis Clin North Am. 2012;26:29–39. doi: 10.1016/j.idc.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Spangehl MJ, Masri BA, O’Connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81:672–683. doi: 10.2106/00004623-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Squire MW, Valle CJD, Parvizi J. Preoperative diagnosis of periprosthetic joint infection: role of aspiration. AJR Am J Roentgenol. 2011;196:875–879. doi: 10.2214/AJR.10.5160. [DOI] [PubMed] [Google Scholar]
- 21.Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty: a study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78:512–523. doi: 10.2106/00004623-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Wadey VM, Huddleston JI, Goodman SB, Schurman DJ, Maloney WJ, Baron EJ. Use and cost-effectiveness of intraoperative acid-fast bacilli and fungal cultures in assessing infection of joint arthroplasties. J Arthroplasty. 2010;25:1231–1234. doi: 10.1016/j.arth.2009.08.018. [DOI] [PubMed] [Google Scholar]