Abstract
Background
Benign lesions in the proximal femur can cause pathologic fractures. To avoid fracture, benign tumors and tumor-like lesions in this region often are treated surgically, yet there have been few reports regarding the decision-making processes or protocols for nonsurgical treatment of these lesions.
Questions/purposes
In this study, we asked (1) whether some benign lesions of the proximal femur can be managed safely using a conservative protocol, and (2) if observed according to such a protocol, what are the outcomes of such lesions at this anatomic site?
Methods
Fifty-four consecutive patients who had been followed for at least 12 months were enrolled in this study. The mean age of the patients at first visit was 38 years (range, 13–70 years), and the minimum followup was 12 months (mean, 25 months; range, 12–59 months). After ruling out malignancy, lesions were categorized as aggressive benign tumors or nonaggressive benign lesions using a standardized approach. We used conservative treatment for most patients with nonaggressive, benign lesions. Surgery was performed only for patients with nonaggressive lesions who met our fracture risk criteria: pain on initiating hip movement, progressively worsening pain, cortical thinning, and the absence of a sclerotic margin.
Results
Of the 47 patients with a nonaggressive, benign lesion without fracture at presentation, 83% were treated conservatively and only 10% of these patients had progression of the lesion. No new pathologic fractures developed during followup. In 88% of patients who presented with pain that was managed conservatively, pain improved either partially or completely at final followup.
Conclusions
Most nonaggressive, benign lesions in the proximal femur can be treated conservatively, and our protocol appears to be a useful outpatient guideline.
Level of Evidence
Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Benign tumors or tumor-like lesions that develop in the bone are most commonly detected incidentally and frequently are “leave-me-alone” lesions, which cause few clinical problems. In many cases, these silent lesions only require surveillance, not surgery [16]. Lesions in the proximal femur, however, raise a higher concern for fracture because of the stresses of weightbearing in this anatomic site; thus, surgical treatment often is preferred [4, 7]. There are several guidelines for prophylactic fixation of metastatic diseases such as Mirels’ [12] or Harrington’s classifications [5]. One of them puts additional weight on the peritrochanteric location, representing the vulnerability of this region to a pathologic fracture. However, these classifications of fracture risk may not apply to benign neoplastic lesions of the femur.
Surgical options have been reported for treating benign tumors or tumor-like lesions in the proximal femur, ranging from simple decompression to complex procedures [4]. Because these lesions are not life threatening and may at times spontaneously regress, the decision whether to operate often is more difficult than the actual method of surgical treatment. Snyder et al. addressed this concern by quantitatively evaluating the fracture risk with CT findings [17], and their predictive approach, which led to nonsurgical management of many patients, was found to be useful [9]. However, as their criteria were made from the analysis of the fractures at presentation and not during the followup, the question still remains: what if the surgically treated patients had been managed by watchful waiting for more?
In this study, we asked (1) whether some benign lesions of the proximal femur can be managed safely using a conservative protocol, (2) if observed according to such a protocol, what is the fracture rate of such lesions at this anatomic site, (3) how many of them progress if managed nonoperatively, and (4) how do the symptoms change with time?
Patients and Methods
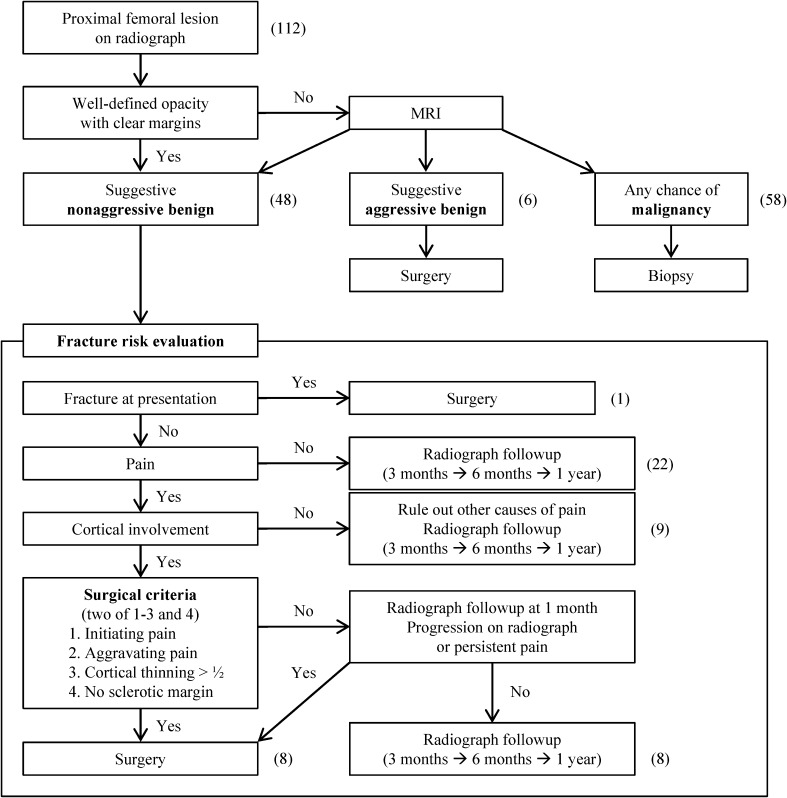
In 2008, we began managing benign tumors or tumor-like lesions of the proximal femur according to an established protocol (Fig. 1). When a patient presents with a proximal femoral lesion observed on radiography, in the absence of a pathologic fracture, the first step is to rule out malignancy. If the lesion does not show findings that strongly suggest that it is benign, such as a well-defined opacity and well defined bony margins [11], MRI is performed and the images are reviewed by a radiologist with musculoskeletal expertise and an orthopaedic oncology surgeon. MR images also may be obtained at any time during management if the lesion progresses or if surgery is planned. MRI findings are classified into three groups: nonaggressive benign lesions, aggressive benign tumors, and potential malignancy. If there is suspicion of malignancy after the MRI, a biopsy is performed. Aggressive benign tumors are defined as tumors that are known to grow progressively and thus in general are treated surgically, such as a giant cell tumor or chondroblastoma [1, 10, 13, 18]. These tumors are removed surgically and treated with an intraoperative local adjuvant, regardless of the fracture risk. The remaining lesions (nonaggressive benign lesions) are managed according to our approach to fracture risk evaluation.
Fig. 1.
The treatment protocol for benign tumors or tumor-like lesions in the proximal femur is shown. The numbers of patients are in parentheses.
If there is no apparent fracture, pain in the affected region is a significant factor in determining management. Simple followup with radiographs at intervals of 3 months initially is used for patients without pain. If the lesions remain stable, the intervals are increased until the lesion has healed or stabilized. When a patient has pain, cortical involvement is evaluated by either radiographs or MRI. Patients who have lesions without cortical involvement are followed with radiographs, ruling out pain sources other than the lesion. If the patient has hip pain and the lesion involves the cortex, the decision to perform surgery is determined by four major criteria: (1) initiating pain; (2) aggravating pain (symptomatic criteria); (3) cortex thinning greater than half the normal thickness; and (4) sclerotic margin greater than 2 mm in thickness (radiologic criteria). The presence of the first three criteria is used as an indication for surgical treatment, and a thick sclerotic margin is interpreted as an indication for nonoperative treatment. Initiating pain here was defined as pain caused by initiating hip motion. Aggravating pain was defined as pain that had increased in intensity in the preceding 2 weeks. Other types of pain, weightbearing pain and resting or positional pain, which we also analyzed for correlation with imaging findings or surgical decision, were defined as pain that developed when walking and pain that developed regardless of weightbearing or in a certain hip position, respectively.
During the study period, patients with two or three of the first three factors and no thick sclerotic margin underwent surgery. If the lesion has a thick sclerotic margin, the patient is followed for 1 month, and surgery is performed if there is no symptomatic improvement or the lesion progresses. Patients for whom surgery is not indicated using these criteria are followed with radiography at intervals increasing from 3 months. We advised against excessive activity in these patients, because in a previous report some patients sustained a pathologic fracture during sports activity even without preceding pain [3]. They were unaware of their bone lesions before the fracture, and the fracture could have been avoided if the patients had been aware of their lesions and had avoided excessive activity [3]. Otherwise, no aid (eg, crutches) was recommended for these patients, and no adjuvant therapies were used except for four patients with fibrous dysplasia who received alendronate for 1 to 8 months. When a patient presents with a pathologic fracture, surgery is performed to treat the lesion and provide the fixation needed to stabilize the proximal femur.
Between January 2008 and December 2011, 112 patients with a proximal femoral lesion were evaluated at our musculoskeletal oncology clinic. Of those, 58 were diagnosed with either a primary or a metastatic malignancy. The other 54 patients who presented with a benign tumor or tumor-like lesions in the proximal femur were managed according to our protocol and were retrospectively reviewed in this study. Patients with infectious lesions or lesions distal to the subtrochanteric area were excluded. All 54 patients were followed for at least 1 year, and no malignancies were found after surgery for a benign lesion. With the approval of the institutional review board (No. 2012-12-016-001), the demographic data, clinical characteristics including the nature of the pain, and tumor characteristics of the study patients were collected by reviewing medical records and imaging studies. Of the lesions evaluated and managed, giant cell tumors, chondroblastomas, and benign fibrous histiocytomas were classified as aggressive benign tumors.
Of the 54 patients, 48 (89%) were classified as having nonaggressive benign lesions and six (11%) had aggressive benign tumors (Table 1). The mean age of the patients at first visit was 38 years (range, 13–70 years), and the minimum followup was 12 months (mean, 25 months; range, 12–59 months). Only two patients (4%) initially presented with a pathologic fracture of the proximal femur, whereas 23 patients (43%) presented with an incidentally detected lesion. A total of 59% of patients experienced hip pain at least once during followup.
Table 1.
Patient characteristics
| Classification | Diagnosis | Number | Location H/N/T/S | Mean age (years; range) | Number of fractures at diagnosis | Number of incidental detections | Pain at presentation |
|---|---|---|---|---|---|---|---|
| Nonaggressive benign (n = 48) |
Fibrous dysplasia | 23 | 1/13/4/5 | 41 (14–70) | 0 | 11 | 14 |
| Intraosseous lipoma | 11 | 1/6/2/2 | 45 (25–50) | 0 | 7 | 2 | |
| Simple bone cyst | 9 | 0/3/2/4 | 30 (13–54) | 1 | 1 | 7 | |
| Intraosseous hemangioma | 2 | 0/2/0/0 | 27 (23–32) | 0 | 2 | 0 | |
| Osteoma | 2 | 0/0/2/0 | 54 (53–55) | 0 | 1 | 2 | |
| Osteochondroma | 1 | 0/0/1/0 | 26 | 0 | 1 | 1 | |
| Aggressive benign (n = 6) |
Chondroblastoma | 3 | 1/0/2/0 | 18 (14–21) | 0 | 0 | 3 |
| Giant cell tumor | 2 | 0/0/2/0 | 22 (19–24) | 1 | 0 | 2 | |
| Benign fibrous histiocytoma | 1 | 0/1/0/0 | 69 | 0 | 0 | 1 | |
| Total | 54 | 3/25/15/11 | 38 (13–70) | 2 (4%) | 23 (43%) | 32 (59%) |
H = head; N = neck; T = trochanteric; S = subtrochanteric.
The 48 patients with nonaggressive benign lesions were managed according to our fracture risk evaluation criteria (Fig. 1). The other six who were diagnosed with aggressive benign tumors were treated surgically by curettage and filling the defect with polyacrylamide cement, bone graft, or bone graft substitute. Four of them underwent prophylactic internal fixation with a dynamic hip screw. None of the six patients had a local recurrence or fracture develop after a minimum followup of 14 months (mean, 34 months; range, 14–55 months), with no loss to followup.
Pathologic fracture, lesion progression, symptom changes, and surgical results during followup were investigated as primary outcomes. In addition, correlations among imaging studies, symptoms, and surgery were evaluated using Pearson’s correlation. Statistical analyses were performed using SPSS Version 18.0 (SPSS, Inc, Chicago, IL, USA). The null hypotheses of no differences were rejected if p values were less than 0.05.
Results
Among the 47 patients who were diagnosed with a nonaggressive benign lesion and presented without a fracture, 39 (83%) were treated conservatively and eight (17%) were treated surgically, according to our protocol (Table 2). During followup, no new pathologic fractures developed in patients in the conservatively treated or surgically treated groups.
Table 2.
Treatment and outcomes of patients
| Treatment | Pain at presentation | Pain improvement | Lesion progression | Pathologic fracture during followup |
|---|---|---|---|---|
| Conservative (39 patients [83%]) | 17 patients (44%) | 15 patients (88%) | 4 patients (10%) | 0 patients |
| Surgery (8 patients [17%]) | 8 patients (100%) | 8 patients (100%) | 0 patients (0%) | 0 patients |
Four (10%) of the 39 conservatively treated patients had lesions that were observed to have progressed on followup radiography. Two of them presented with pain and the other two were pain-free. However, they did not meet our surgery criteria and were followed without surgery until the last visit.
Seventeen (44%) of the 39 conservatively treated patients presented with pain and 15 (88%) of them reported either partial or complete pain improvement during followup. The mean time to relief of pain was 7 months (range, 1–28 months) for these patients. All of the eight surgically treated patients presented with pain and they reported pain improvement at the last visit. The mean time to relief of pain was 8 months (range, 1–21 months) for these patients.
The two patients who initially presented with a pathologic fracture were treated surgically and both achieved union (Table 3). Of the eight surgically treated patients with a nonaggressive benign lesion, seven had two or three factors favoring surgery and no sclerotic margin in their lesion (Table 4). The other patient had two factors favoring surgery and had a sclerotic margin in the lesion but underwent surgery because of persistent pain. These eight patients walked with the assistance of crutches for 6 to 12 weeks postoperatively. Although all eight patients had no pain or lesion progression at final visit, one patient who had a simple bone cyst underwent repeat surgery because of remaining pain and lack of new bone formation.
Table 3.
Treatment and outcomes of two patients who initially presented with a pathologic fracture
| Age (years)/sex | Diagnosis | Location | Treatment | Union | Complication |
|---|---|---|---|---|---|
| 24/F | Giant cell tumor | Trochanteric | Curettage and allo-chip bone graft/DHS | + | − |
| 13/M | Simple bone cyst | Subtrochanteric | Curettage and calcium phosphate cement/DHS | + | − |
F = female; M = male; DHS = Dynamic Hip Screw (Synthes-Stratec, Oberdorf, Switzerland).
Table 4.
Characteristics of patients who underwent surgery
| Age (years)/ sex | Diagnosis | Site | Aggravating pain | Initiating pain | Cortex thinning > ½ | Sclerotic margin | Surgical procedure | Complication |
|---|---|---|---|---|---|---|---|---|
| 25/M | Simple bone cyst | N | + | + | + | − | CRT and allo-fibula graft | − |
| 26/F | Fibrous dysplasia | N | − | + | + | + | CRT and allo-fibula graft | − |
| 32/F | Simple bone cyst | S | + | + | − | − | Decompression and reaming | − |
| 17/F | Fibrous dysplasia | N | + | + | + | − | CRT and allo-fibula graft | − |
| 16/F | Simple bone cyst | T | + | + | − | − | Decompression and BM injection | Repeated surgery |
| 56/F | Fibrous dysplasia | N | + | + | − | − | CRT and allo-chip bone graft, DHS | − |
| 30/F | Fibrous dysplasia | H | + | + | + | − | CRT and allo-fibula graft | − |
| 25/M | Intraosseous lipoma | H | + | + | − | − | CRT and allo-chip bone graft | − |
M = male; F = female; N = neck; S = subtrochanteric; T = trochanteric; H = head; CRT = curettage; BM = bone marrow; DHS = Dynamic Hip Screw (Synthes-Stratec, Oberdorf, Switzerland).
Among the imaging findings, cortical thinning generally had a significant positive correlation with pain on presentation (Table 5). Among the types of pain, initiating pain had the strongest correlation with cortical thinning (R = 0.513, p < 0.001) followed by aggravating pain (R = 0.447, p = 0.002). A thick sclerotic margin had a significant negative correlation with aggravating pain (R = 0.443, p = 0.002) and initiating pain (R = 0.301, p = 0.040).
Table 5.
Correlations between pain and imaging findings (Pearson R and p value)
| Type of pain | Lesion size | Cortical thinning | Cortical circumference involved | Sclerotic margin |
|---|---|---|---|---|
| Pain, general | −0.151 (0.312) | 0.273 (0.064) | 0.015 (0.920) | −0.191 (0.199) |
| Aggravating pain | −0.236 (0.110) | 0.447 (0.002)* | −0.250 (0.090) | −0.443 (0.002)* |
| Initiating pain | −0.219 (0.139) | 0.513 (0.000)* | −0.165 (0.269) | −0.301 (0.040)* |
| Weightbearing pain | −0.060 (0.688) | 0.355 (0.014)* | −0.053 (0.725) | −0.214 (0.149) |
| Resting or positional pain | −0.152 (0.309) | 0.355 (0.014)* | −0.055 (0.713) | −0.122 (0.412) |
* p < 0.05.
Regarding the surgical decision, in general symptoms correlated more with surgery than imaging findings (Table 6). Initiating pain had the highest positive correlation with surgery among the symptoms, followed by aggravating pain. Among imaging findings, cortical thinning had a significant positive correlation with surgery and a thick sclerotic margin had a significant negative correlation with surgery.
Table 6.
Affect of each type of pain and imaging findings on the surgical decision
| Category | Type | Pearson R | p value |
|---|---|---|---|
| Pain | Pain, general | 0.425 | 0.003* |
| Aggravating pain | 0.849 | 0.000* | |
| Initiating pain | 0.871 | 0.000* | |
| Weightbearing pain | 0.540 | 0.000* | |
| Resting or positional pain | 0.176 | 0.237 | |
| Image finding | Lesion size | −0.123 | 0.411 |
| Cortical thickness involved | 0.447 | 0.002* | |
| Cortical circumference involved | −0.117 | 0.435 | |
| Sclerotic margin | −0.330 | 0.023* |
* p < 0.05.
Discussion
The femur is a common site of pathologic fractures most commonly caused by metastatic disease. The proximal femur is the bone most frequently affected by metastatic disease in the appendicular skeleton [6, 14]. However, benign tumors in this region are less common, therefore, our series of cases are instructive. There are few estimates of pathologic fracture incidence or the frequency of surgical treatment. In the current study, 4% of patients with benign lesions in the proximal femur who presented with a pathologic fracture and 17% with a nonaggressive benign lesion without a fracture received surgical intervention. Considering that the lesions were detected incidentally in 43% of patients, the actual incidence of pathologic fracture or surgical intervention may be even lower.
Although our study is the first of a consecutive series of patients with benign proximal femoral lesions treated by a novel protocol, it has some limitations. First, an aggressive or malignant tumor must be ruled out, which sometimes is difficult. A biopsy would be helpful for lesions with progression or indecisive imaging findings. Second, some lesions such as osteoid osteomas may cause severe pain without an actual risk of fracture and pediatric fibrous dysplasia may result in coxa vara deformity even without significant pain. Individualized management is indispensable for those cases. Third, although a fracture during conservative management might require more invasive procedures than prophylactic surgery, surgery has its own shortcomings; we believe that it is preferable to avoid surgery if possible. Fourth, our study is retrospective, deals with small study population with short-term followup, and it lacks a biomechanical basis. In addition, we cannot validate the predictive value of each criterion because no fractures developed during followup. Although a randomized prospective design is not realistic for treatment decisions, future studies incorporating biomechanical end points, a larger study population with long-term followup, or an analysis of fractures may further evaluate our protocol and allow for a detailed scoring system.
Managing patients according to this protocol, we observed no pathologic fractures even though more than 80% of the nonaggressive benign lesions were treated conservatively. To date, most articles on benign tumors or tumor-like lesions of the proximal femur have reported surgical treatments either for fractured or fracture-prone femurs [2, 4, 7, 8, 19]. However, there have been no specific guidelines for surgical decision making of these benign lesions in the proximal femur. In the current study, 11 (28%) of 39 conservatively treated patients had a Mirels’ score greater than seven (data not shown), which might be an indication for prophylactic fixation if the underlying condition were to have been metastatic disease. However, none of the patients in our series actually had a fracture develop, and thus we believe that the benign lesions are different in fracture risk and we need different criteria.
What makes these benign lesions different may be their behavior. They often progress slowly and even may regress [20]. In our results, only 10% of conservatively treated nonaggressive lesions showed radiologic progression. This provides support for watchful waiting, and the necessity of a guideline different from those for metastatic disease in the proximal femur.
One of the important results of this study is that 88% of conservatively treated patients reported pain improvement. It suggests immediate surgery may not be necessary in all cases, even for patients who have some pain associated with a proximal femoral lesion, as long as malignancy or aggressive tumors are ruled out. In addition, the radiologic progression rate was similar in patients who had pain or were pain free. The pain may be present for months as patients are managed conservatively; however, one must weigh this against the risks, postoperative pain, and limitations during the recuperative period associated with surgery. Moreover, lesions may recur after surgical treatment, making them more difficult to manage. Therefore, a conservative approach limiting surgery only to patients with a fracture or who are fracture prone seems to be a better choice.
Our protocol uses symptomatic and radiologic criteria for metastatic disease. Based on experience, we chose three criteria that favor prophylactic surgery and a surgery-avoidance criterion, sclerotic margin, which is unique for benign lesions. A sclerotic margin indicates a slow-growing lesion with a bony shell [15], which may reduce the risk of fracture. The correlation analyses among these criteria showed certain symptoms may be associated with the imaging findings, which represents the actual bony structure. Although there are more concrete ways to evaluate the fracture risk such as Snyder’s criteria [17], we found it was safe to follow patients who met our criteria and it is unnecessary to obtain CT scans at every followup.
Benign lesions of the proximal femur can be managed safely using the decision-making protocol based on the fracture risk evaluation. Considering the low progression rate and high pain relief rate after observation according to the protocol, we believe that most such lesions at this anatomic site can be managed conservatively in the outpatient setting.
Acknowledgments
We thank Hyoung Tae Kim MD, of Samsung Medical Center, Seoul, Korea, for assistance in revising the manuscript.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Bertoni F, Calderoni P, Bacchini P, Sudanese A, Baldini N, Present D, Campanacci M. Benign fibrous histiocytoma of bone. J Bone Joint Surg Am. 1986;68:1225–1230. [PubMed] [Google Scholar]
- 2.Durand S, Hamcha H, Pannier S, Padovani JP, Finidori G, Glorion C. [Fibrous dysplasia of the proximal femur in children and teenagers: surgical results in 22 cases][in French] Rev Chir Orthop Reparatrice Appar Mot. 2007;93:17–22. doi: 10.1016/S0035-1040(07)90199-3. [DOI] [PubMed] [Google Scholar]
- 3.Easley ME, Kneisl JS. Pathologic fractures through nonossifying fibromas: is prophylactic treatment warranted? J Pediatr Orthop. 1997;17:808–813. [PubMed] [Google Scholar]
- 4.George B, Abudu A, Grimer RJ, Carter SR, Tillman RM. The treatment of benign lesions of the proximal femur with non-vascularised autologous fibular strut grafts. J Bone Joint Surg Br. 2008;90:648–651. doi: 10.1302/0301-620X.90B5.20330. [DOI] [PubMed] [Google Scholar]
- 5.Harrington KD. Impending pathologic fractures from metastatic malignancy: evaluation and management. Instr Course Lect. 1986;35:357–381. [PubMed] [Google Scholar]
- 6.Jacofsky DJ, Haidukewych GJ. Management of pathologic fractures of the proximal femur: state of the art. J Orthop Trauma. 2004;18:459–469. doi: 10.1097/00005131-200408000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Jaffe KA, Dunham WK. Treatment of benign lesions of the femoral head and neck. Clin Orthop Relat Res. 1990;257:134–137. [PubMed] [Google Scholar]
- 8.Jaffe KA, Launer EP, Scholl BM. Use of a fibular allograft strut in the treatment of benign lesions of the proximal femur. Am J Orthop (Belle Mead NJ). 2002;31:575–578. [PubMed] [Google Scholar]
- 9.Leong NL, Anderson ME, Gebhardt MC, Snyder BD. Computed tomography-based structural analysis for predicting fracture risk in children with benign skeletal neoplasms: comparison of specificity with that of plain radiographs. J Bone Joint Surg Am. 2010;92:1827–1833. doi: 10.2106/JBJS.I.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin PP, Thenappan A, Deavers MT, Lewis VO, Yasko AW. Treatment and prognosis of chondroblastoma. Clin Orthop Relat Res. 2005;438:103–109. doi: 10.1097/01.blo.0000179591.72844.c3. [DOI] [PubMed] [Google Scholar]
- 11.Miller TT. Bone tumors and tumorlike conditions: analysis with conventional radiography. Radiology. 2008;246:662–674. doi: 10.1148/radiol.2463061038. [DOI] [PubMed] [Google Scholar]
- 12.Mirels H. Metastatic disease in long bones: a proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256–264. [PubMed] [Google Scholar]
- 13.Muramatsu K, Ihara K, Taguchi T. Treatment of giant cell tumor of long bones: clinical outcome and reconstructive strategy for lower and upper limbs. Orthopedics. 2009;32:491. doi: 10.3928/01477447-20090527-08. [DOI] [PubMed] [Google Scholar]
- 14.Narazaki DK, de Alverga Neto CC, Baptista AM, Caiero MT, de Camargo OP. Prognostic factors in pathologic fractures secondary to metastatic tumors. Clinics (Sao Paulo). 2006;61:313–320. [DOI] [PubMed]
- 15.Osborne RL. The differential radiologic diagnosis of bone tumors. CA Cancer J Clin. 1974;24:194–211. doi: 10.3322/canjclin.24.4.194. [DOI] [PubMed] [Google Scholar]
- 16.Schaser KD, Bail HJ, Haas NP, Melcher I. [Treatment concepts of benign bone tumors and tumor-like bone lesions][in german] Chirurg. 2002;73:1181–1190. doi: 10.1007/s00104-002-0584-4. [DOI] [PubMed] [Google Scholar]
- 17.Snyder BD, Hauser-Kara DA, Hipp JA, Zurakowski D, Hecht AC, Gebhardt MC. Predicting fracture through benign skeletal lesions with quantitative computed tomography. J Bone Joint Surg Am. 2006;88:55–70. doi: 10.2106/JBJS.D.02600. [DOI] [PubMed] [Google Scholar]
- 18.Stacy GS, Mahal RS, Peabody TD. Staging of bone tumors: a review with illustrative examples. AJR Am J Roentgenol. 2006;186:967–976. doi: 10.2214/AJR.05.0654. [DOI] [PubMed] [Google Scholar]
- 19.Wai EK, Davis AM, Griffin A, Bell RS, Wunder JS. Pathologic fractures of the proximal femur secondary to benign bone tumors. Clin Orthop Relat Res. 2001;393:279–286. doi: 10.1097/00003086-200112000-00032. [DOI] [PubMed] [Google Scholar]
- 20.Yanagawa T, Watanabe H, Shinozaki T, Ahmed AR, Shirakura K, Takagishi K. The natural history of disappearing bone tumours and tumour-like conditions. Clin Radiol. 2001;56:877–886. doi: 10.1053/crad.2001.0795. [DOI] [PubMed] [Google Scholar]