Abstract
Background
Although bacterial antibiotic resistance is increasing, fewer new antibiotics are being developed to compensate. Localized delivery of synergistic antiseptics and antibiotics with a chitosan sponge device may offer an alternative infection treatment.
Questions/purposes
In this pilot study, we asked whether antiseptic and antibiotic combinations provided in vitro synergism against Staphylococcus aureus, whether synergism reduces cell viability, and whether their combination releases drugs at inhibitory levels.
Methods
To investigate the pharmacodynamics among three combinations of the antiseptic chlorhexidine digluconate (CHX) with the antibiotics amikacin, daptomycin, and vancomycin (VAN) (n = 1), we determined the fractional inhibitory concentration (FIC) index against S aureus Cowan I. The determined synergistic combination of CHX and VAN was evaluated for cell compatibility using NIH/3T3 fibroblasts (n = 3) and the drug release profile from a chitosan sponge device (n = 5).
Results
With an FIC index < 0.5, the combination of CHX + VAN exhibited synergism against S aureus. CHX concentrations ≥ 3.91 μg/mL resulted in fibroblast viability decrease, whereas the combination of CHX + VAN did not decrease fibroblast viability until their concentrations reached ≥ 7.81 μg/mL. The CHX and VAN release profile, both individually and in combination, was an initial bolus with no difference between eluate concentrations after Day 5.
Conclusions
CHX + VAN combination may be delivered locally by a chitosan sponge that synergistically inhibits S aureus growth.
Clinical Relevance
The use of synergism between combined antibiotic and antiseptics delivered at high local concentrations with an implanted chitosan sponge may provide a useful alternative infection treatment option.
Introduction
Bacterial resistance against current, first-line antibiotics continues to be a growing problem throughout the world [8, 27]. The shortage of novel antibiotics or antibiotic classes in development and the overuse of available antibiotics have made alternative therapies for resistant bacterial infections necessary. Proper antibiotic use is and will continue to be a critical factor in infection control [8]. Limitations of standard therapeutic options have led to increases in cost, toxicity, treatment time, and intensive care admissions. In the United States alone, the cost of treating antibiotic-resistant infections reportedly ranges from USD 18,588 to USD 29,069 per patient with extended hospital stays ranging from 6.4 to 12.7 days and with an estimated mortality rate of 6% [10, 33]. It is estimated that methicillin-resistant Staphylococcus aureus (MRSA) kills 19,000 people in the United States each year [22]. In the medical community, hospital-acquired infections occur primarily at surgical sites with an increased risk when using orthopaedic implants or other surgical devices [12]. Growth of antibiotic resistance threatens to negate sophisticated treatments like hip replacements, organ transplants, care of preterm infants in addition to routine surgical procedures [8, 9].
New approaches are needed to address the global threat of antibiotic resistance [7, 8]. One long-term goal to reduce antibiotic resistance is the worldwide, controlled use of antibiotics [7]. Additionally, alternative therapeutic approaches are being investigated for improving dose-response of commonly used antibiotics as well as the use of nonantibiotic therapies such as antiseptics [26]. One of these alternative approaches is the use of multidrug treatment strategies that exploit pharmacodynamic synergy between antiseptics and antibiotics to combat specific bacterial infections [17]. Pharmacodynamic synergism occurs when the antiseptic and antibiotic combination has a greater effect on the microbial target than the sum of the drugs’ effects individually [3]. However, just as important as selecting appropriate therapeutics is their efficient delivery to the infection site [24]. The local delivery of the selected antiseptics and antibiotics to the infection site reduces the effects of metabolism and degradation, thus providing high local drug concentrations while minimizing systemic toxicity [29]. We explored the use of chlorhexidine digluconate (CHX) as a model antiseptic that is commonly used in hospitals for topical and oral decolonization treatments and is bactericidal against a broad spectrum of bacteria [6, 23, 26, 32].
We asked the following questions in this pilot study: Do any of the combinations of the antiseptic CHX with daptomycin (DAP), vancomycin (VAN), or amikacin (AMK) result in synergism against S aureus? Does the synergistic combination reduce the viability of cells? Can this synergistic combination be delivered at bacteriostatic concentrations using a biocompatible and biodegradable chitosan sponge?
Materials and Methods
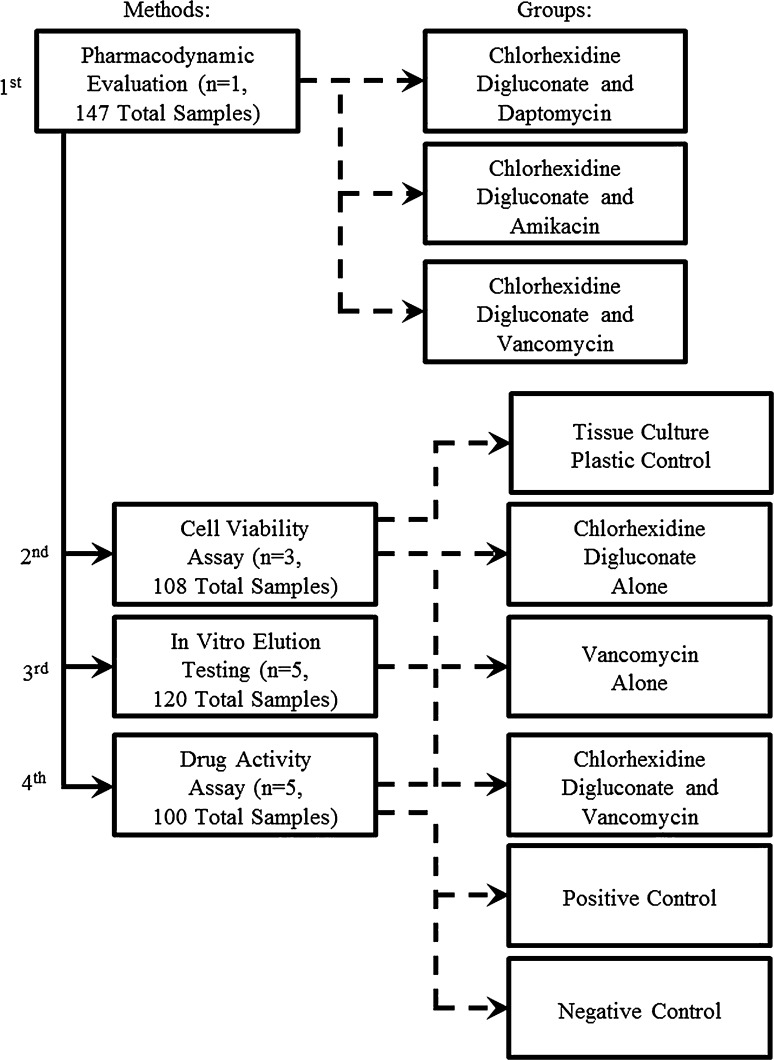
To answer these preliminary questions (Fig. 1), first we tested the ability of the combinations of CHX with AMK, DAP, or VAN to synergistically inhibit growth of S aureus (n = 1). The combination that produced synergism was then analyzed for its effects on NIH/3T3 fibroblast cell viability compared with controls grown on tissue culture plastic (n = 3). Finally, we evaluated the release profile of the drugs individually and in combination using a previously investigated chitosan sponge device (n = 5) and also tested the eluate samples for drug activity against S aureus (n = 5).
Fig. 1.
A flowchart summarizes the study design.
The underlying method used in synergism analysis was turbidity testing. In the turbidity assay, a solution containing a 1:10 dilution of the samples in 0.2 M calcium-supplemented Mueller Hinton II broth (BD, Franklin Lakes, NJ, USA) was inoculated with S aureus (Cowan I) and incubated overnight at 37° C. We quantified growth by measuring the absorbance at 530 nm [29]. Lack of growth was indicated by absorbance values equivalent to negative controls. The minimum inhibitory concentration (MIC) is the lowest concentration that completely inhibits growth. We determined the MIC for each individual drug, chlorhexidine digluconate (MP Biomedical, Salon, OH, USA), amikacin (MP Biomedical), daptomycin (Cubist Pharmaceuticals, Lexington, MA, USA), and vancomycin (MP Biomedical), by turbidity analysis. The pharmacodynamic interaction among each of the drug combinations, CHX + AMK (n = 1), CHX + DAP (n = 1), and CHX + VAN (n = 1), was determined by combining a range of concentrations of the drugs in a checkerboard method and testing a matrix of each combination at multiple concentrations (7 x 7 matrix per number) for their effect on the growth of S aureus [25]. We then calculated the fractional inhibitory concentration (FIC) for each drug by dividing its MIC when used in combination by its MIC when used alone [3]. The FIC index is the sum of the two drugs’ FICs given by the equation: FIC index = FICA + FICB = (MIC of drug A in combination/MIC of drug A alone) + (MIC of drug B in combination/MIC of drug B alone). When the FIC index is less than 0.5, then the combination is synergistic; if the index is greater than 0.5 or less than 2, then the combination is additive; if the index is greater than 2 or less than 4, then the combination is indifferent; and if the index is greater than 4, then the combination is antagonistic (Table 1) [3]. We determined the FIC index for combinations of CHX + AMK, CHX + DAP, and CHX + VAN.
Table 1.
Minimum inhibitory concentration (MIC), fractional inhibitory concentration (FIC) index, and the resulting effect for various antimicrobials against Staphylococcus aureus
| Antimicrobial | MIC (μg/mL) | FICI | Effect |
|---|---|---|---|
| AMK | 0.5 | – | – |
| DAP | 0.5 | – | – |
| VAN | 0.5 | – | – |
| CHX | 0.5 | – | – |
| CHX + AMK | – | 0.750 | Additivity |
| CHX + DAP | – | 1.500 | Additivity |
| CHX + VAN | – | 0.375 | Synergism |
FIC index < 0.5 indicates synergy, 0.5 ≤ FIC index < 2.0 indicates additivity, 2.0 ≤ FIC index < 4.0 indicates indifference. and FIC index ≥ 4.0 indicates antagonism (n = 1); AMK = amikacin; DAP = daptomycin; VAN = vancomycin; CHX = chlorhexidine digluconate.
We only evaluated the antiseptic and antibiotic that provided synergism for effects on host cell viability. We evaluated the effects of synergistic combinations of CHX + VAN (n = 3), CHX alone (n = 3), and VAN alone (n = 3) on host cell viability using NIH/3T3, mouse embryonic fibroblast cells ATCC #CRL-1658 (Manassas, VA, USA). The host NIH/3T3 fibroblasts grown on tissue culture plastic without treatment were used for a control. Cells were seeded at 2 x 104 cells/cm2 in 108 wells of 96-well plates in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and allowed to proliferate overnight in standard culture conditions (37° C, 5% CO2). We used twofold serial dilutions in media to create a range of nine treatment standards from 1000 μg/mL to 0.49 μg/mL for each drug group (four total groups with n = 3 and nine treatment dilutions). The group with the CHX + VAN combination contained each at the concentrations indicated. Cells were treated for 24 hours with the appropriate antiseptic and/or antibiotic of drug group standards. The CellTiter-Glo Luminescent Cell Viability assay (Promega, Madison, WI, USA) was then used to determine the number of viable, metabolically active NIH/3T3 fibroblast cells after treatment with standard concentrations. This assay quantifies ATP, which is correlated to the number of metabolically active and viable cells, based on a luminescent signal obtained using a 96-well plate reader at 590 nm (FLx800; Bio-Tek, Ontario, Canada).
We then used the same three antiseptic and antibiotic groups for in vitro elution testing to determine release profiles. Chitosan sponges for each group were manufactured using previously described methods [29] with five replicates used as the local delivery device for each group of antibiotic combinations. Briefly, the chitosan sponges were manufactured with 1% (w/v) of 71% deacetylated chitosan Primex (Siglufjordur, Iceland) dissolved in a 1% (v/v) blended lactic/acetic acid solvent (75:25, respectively). The solution was filtered through a 180-μm nylon mesh (Gilson, Lewis Center, OH, USA), frozen at −80 °C, and subsequently lyophilized (FreeZone 2.5; Labconco, Kansas City, MO, USA). The dehydrated sponge was then neutralized in 1 M (molar) NaOH (Fisher Scientific, Fair Lawn, NJ, USA), washed with distilled/deionized water, frozen at 80° C, and lyophilized (Fig. 2). We initiated elution testing by hydrating these twice lyophilized sponges, normalized by weight (n = 5), for 1 minute in 50 mL of drug solution comprised of 5 mg/mL VAN (n = 5), CHX (n = 5), or CHX + VAN (n = 5) with each drug in the combination at 5 mg/mL. The drug-loaded sponges were then transferred into a 125-mL Nalgene container with 20 mL of phosphate-buffered saline (PBS) and then incubated at 37° C. Solution samples were taken at 1, 2, 5, 10, 14, and 21 days with complete PBS solution replacement at each time point. We immediately froze samples after acquisition.
Fig. 2A–B.
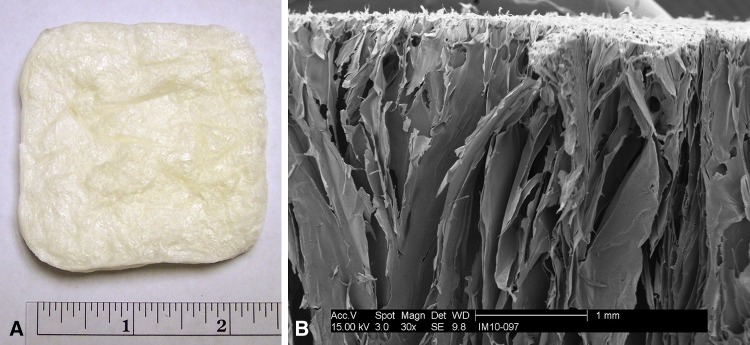
(A) The dehydrated chitosan sponge before testing and a (B) scanning electron micrograph at x30 of the same sponge show the high surface area, laminar morphology of the sponge.
We analyzed elution samples for drug concentration using high-performance liquid chromatography. The chemical separation was carried out using a reversed-phase C18 column (Varian) with a mobile phase consisting of 30% acetonitrile and 70% buffer with all chemicals purchased from Fisher Scientific. The buffered solution was 0.1 M phosphate buffer consisting of 0.08 M disodium phosphate and 0.013 M monosodium phosphate adjusted to pH 3 using phosphoric acid. Using a flow rate of 1.4 mL/min and UV detection at 250 nm, VAN had a 1.5-minute retention time, whereas CHX’s was 5 minutes. We also evaluated elution samples in a 10:1 dilution for activity to determine whether the elution process substantially altered or prevented the drug’s bacteriostatic ability. This evaluation used turbidity testing as described earlier with results indicated as either bacterial growth or inhibition.
We determined differences in host cell viability and drug release studies by using two-way analysis of variance with Tukey’s post hoc analysis followup. Statistical analysis was performed using SigmaPLOT Version 12 (San Jose, CA, USA).
Results
The MIC was 0.5 μg/mL for AMK, DAP, VAN, and CHX when tested against S aureus (Table 1). The FIC index was 0.75 for the combination of CHX and AMK indicating they were pharmacodynamically additive but not synergistic. The combination of CHX and DAP gave an FIC index of 1.5, also indicating additivity. However, the combination of CHX and VAN exhibited synergism as indicated by an FIC index of 0.375. This synergistic combination was the only one used in subsequent testing along with CHX and VAN individually.
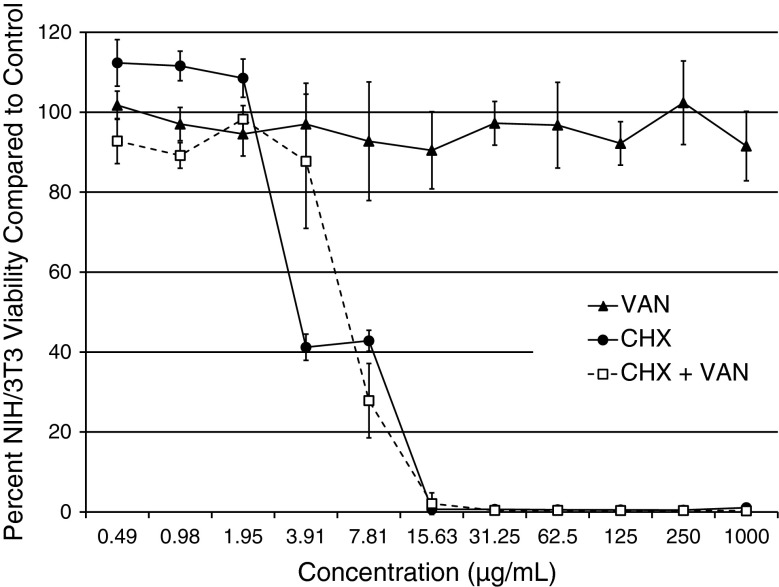
CHX reduced the percent fibroblast cell viability in comparison to the control group at concentrations ≥ 3.91 μg/mL, whereas the CHX + VAN combination decreased proliferation at ≥ 7.81 μg/mL (Fig. 3). In contrast, VAN-treated fibroblasts remained viable across the entire range of concentrations tested.
Fig. 3.
CHX, VAN, and CHX + VAN cell viability. Viability is reported as the percent of viable cells in comparison to cell proliferation controls (n = 3).
Both the antiseptic and antibiotic were released in an initial bolus elution. With the exception of VAN on Day 5, there were no differences between a singular drug’s release profiles when it was released individually or in combination with another drug (Fig. 4). At the Day 1 and 2 time points, VAN alone and in combination was released at higher concentrations than either CHX released alone or in combination. At the Day 5 time point, only individually released VAN was substantially higher than the other eluate concentrations and after the Day 5 time point, there were no major differences among any of the eluate data points. All test groups containing CHX, VAN, or CHX + VAN were inhibitory against S aureus, except for VAN released alone at later time points of 5, 10, 14, and 21 days (Table 2).
Fig. 4.
CHX, VAN, and CHX + VAN release from chitosan sponges. The CHX + VAN release profile is broken down to show each drug individually. Concentrations are given in micrograms per milliliter ± SD on a logarithmic scale (n = 5).
Table 2.
The average activity for eluate samples against Staphylococcus aureus
| Antimicrobial | Time (days) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 5 | 10 | 14 | 21 | |
| VAN | − | − | + | + | + | + |
| CHX | − | − | − | − | − | − |
| CHX + VAN | − | − | − | − | − | − |
“−” indicates S aureus inhibition and “+” indicates S aureus growth (n = 5); VAN = vancomycin; CHX = chlorhexidine digluconate.
Discussion
Increases in bacterial antibiotic resistance, both from the overuse and misuse of antibiotics, along with fewer new antibiotics, have all affected clinical treatment approaches [7, 17]. The severe impact of antibiotic-resistant infections has led to the search for new and more efficient therapeutic applications [19, 34, 36, 38]. Some of these methods include using pharmacodynamic synergism between two drugs against bacterial species. Additionally, a localized delivery device is able to provide high concentrations of drugs at the specific site of infection while limiting systemic concentrations and associated complications [28]. Combining synergistic dual-drug therapy with local drug delivery may provide more efficient use of antibiotics and antiseptics, thus decreasing the total concentration necessary for infection treatments. We therefore asked the following questions: Do any of the combinations of the antiseptic CHX with DAP, VAN, or AMK result in synergism against S aureus? Does the synergistic combination reduce the viability of cells? Can this synergistic combination be delivered at bacteriostatic concentrations using a biocompatible and biodegradable chitosan sponge?
Limitations on this pilot study include, first, that we studied only three antibiotics and one antiseptic was evaluated against one specific strain of bacteria in this preliminary screening approach. These drugs, all active against MRSA, were evaluated first against S aureus because it is one of the major concerns in orthopaedic infections [21]. The clinical application for the investigated therapy would be greatly enhanced by increasing the selection of synergistic drug combinations and by their evaluation against a large array of bacterial species. Additionally, knowing the therapeutics’ effects against human cell lines could prevent the possibility of increased toxicity of synergistic drug combinations. Second, the translation of in vitro evaluation to in vivo evaluation cannot be generalized and, therefore, it is not known yet whether our in vitro results can be generalized to in vivo application. Third, although our data suggest the CHX + VAN combination does not affect the elution of either drug out of the chitosan sponge in contrast to other dual-drug delivery therapies [4], we have not shown that the antiseptic and antibiotic will diffuse into an in vivo wound environment similarly. Fourth, the complex biological conditions found in the wound were not modeled in this investigation. Like in previous studies, this model is a simple screening approach to predict which therapeutic combinations should be evaluated in in vivo models [1, 15, 20]. Additionally, there are more detailed studies that could be performed such as a disc diffusion method for evaluating nondiluted eluate activity against bacteria as well as an enhanced elution study with more frequent sampling time points.
Using the standard calculation of FIC index for determining pharmacodynamic effects, we identified synergism in the CHX + VAN combination. Synergism was identified through techniques similar to other investigations using alternative synergistic, dual-drug therapies against bacteria [2]. The combination of CHX with protamine sulfate has in vitro synergism for combinatorial therapy against Escherichia coli [11]. CHX has synergism with hydrogen peroxide against Streptococcus sobrinus, Streptococcus faecalis, and Staphylococcus aureus [35]. VAN has a low degree of synergism with the antibacterial nisin against some S aureus strains [13]. Also, VAN has synergism with sulfamethoxazole/trimethoprim and rifampin against some Staphylococcus strains; however, when combined with imipenem and other drugs, VAN has synergistic effects against a broader range of methicillin-resistant, coagulase-negative, and biofilm forms of Staphylococcus [30, 34]. The synergism between CHX and VAN, like with the other examples mentioned, may ultimately allow for the reduced amount of total drug dosage clinically as well as greater antibiotic efficacy, coverage of multiple strains, and decreased incidence of resistance.
First used in the 1950s, CHX’s toxicity was quickly noted but was minimal when used in topical and oral applications [16]. CHX has been successfully used as a prophylactic treatment for surgical site infection prevention [6, 31]. When used in local drug delivery, although systemic concentrations remain low, there may be a transiently high concentration of local drug, which could cause a delay in the wound healing process. For this reason, the release profile of CHX needs to be well characterized to minimize possible interference with inflammatory and proliferative processes in musculoskeletal wound healing [14]. Reassessing the loading profile and modifying sponge parameters or the delivery device to minimize negative impacts on wound and bone healing processes can be pursued with more comprehensive in vivo work. In our study, CHX also exhibited toxicity toward NIH/3T3 cells at concentrations of average 3.91 and 7.81 μg/mL in comparison to the 0.5-μg/mL level needed to inhibit bacterial growth. Our CHX and VAN-loaded delivery device delivered concentrations that were above this toxic level at time points 1, 2, and 5 days but after this dropped below the toxic threshold. The possible advantages of preventing the growth of S aureus and the development of antibiotic-resistant strains may justify this potential delay in healing. Surprisingly, the VAN + CHX combination slightly reduced the toxic concentration’s effects. It is generally accepted that the local delivery of drugs provides results typically in a relatively low systemic drug concentration [28]. An in vivo study using chitosan sponges to locally deliver VAN loaded at 5 mg/mL to a S aureus-infected goat extremity musculoskeletal wound substantially decreased bacterial contamination with near negligible serum VAN concentrations [37].
In contrast to other local drug delivery systems, the highly open and laminar structure of the chitosan sponge (Fig. 2) allows for the initial bolus release of the CHX + VAN combination with a release profile similar to their individual elution [29]. The bolus release is contrasted with other local drug delivery systems such as bone cement, which exhibits slow and incomplete release of individually loaded antibiotics and unequal release profiles in dual-drug-loaded constructs [4, 18]. Bone cement’s unfavorable qualities reportedly result from its low porosity [5, 18]; however, the high porosity and surface area of a chitosan sponge does not inhibit the release of either CHX or VAN over the other drug. This property is expected to be beneficial for diffusion of dual-drug therapy in which both drugs will be released at active concentrations simultaneously, enabling their synergistic bacteriostatic activity. Furthermore, bolus release may be more appropriate than slow, extended release for delivery of CHX in that it minimizes tissue exposure to toxic levels of this antiseptic.
The major motivation for this study was the need for efficient use of currently available antiseptics and antibiotics to prevent orthopaedic surgical site infections by exploiting synergism between an antiseptic and antibiotic. We further identified and explored methods to deliver drug combinations locally at high concentrations without substantially altering the drugs’ release profile. Our data suggest the synergistic combination of CHX + VAN can be delivered at concentrations that are inhibitory to an S aureus strain. Although CHX has poor biocompatibility at high concentrations, local drug delivery of CHX is expected to release an initial bolus of CHX while keeping systemic concentrations at nontoxic levels. Over the course of 5 days, the local CHX release is expected to drop to biocompatible levels yet still be inhibitory to bacteria. Future research should include evaluation of expanded combinations of antiseptics and antibiotics in vitro and in vivo. Specifically, in vivo validation of this treatment method should be performed in comparison to standard systemic and local infection treatments in an infected animal model [5, 37]. The data also suggest the addition of local drug delivery to the efficient use of antiseptics and antibiotics agents through their synergic effects may provide alternative therapies for infection treatment in a world where the future of antibiotics is uncertain as a result of decreased novel antibiotics and increased antibiotic resistance.
Acknowledgments
We thank Cubist Pharmaceuticals (Lexington, MA, USA) for their donation of the daptomycin used in this investigation.
Footnotes
One or more of the authors (WOH) has received funding from the National Institutes of Health (R01 AI06907-02) and the NSF GRFP. One of the authors (WOH) receives royalties from Bionova Medical (Germantown, TN, USA). One of the authors (WOH) has received funding from US Army OETRP and Advanced Technology Grants, Cubist Pharmaceuticals (Lexington, MA, USA) material/research support.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
This work was performed at the University of Memphis and the Veterans Affairs Medical Center, Memphis, TN, USA.
References
- 1.Adams K, Couch L, Cierny G, Calhoun J, Mader JT. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop Relat Res. 1992;278:244–252. [PubMed] [Google Scholar]
- 2.Allahverdiyev AM, Kon KV, Abamor ES, Bagirova M, Rafailovich M. Coping with antibiotic resistance: combining nanoparticles with antibiotics and other antimicrobial agents. Expert Rev Anti Infect Ther. 2011;9:1035–1052. doi: 10.1586/eri.11.121. [DOI] [PubMed] [Google Scholar]
- 3.American Society for Microbiology Instructions to authors. Antimicrob Agents Chemother. 2004;48:i–xx. [Google Scholar]
- 4.Anagnostakos K, Kelm J. Enhancement of antibiotic elution from acrylic bone cement. J Biomed Mater Res B Appl Biomater. 2009;90:467–475. doi: 10.1002/jbm.b.31281. [DOI] [PubMed] [Google Scholar]
- 5.Beenken KE, Bradney L, Bellamy W, Skinner RA, McLaren SG, Gruenwald MJ, Spencer HJ, Smith JK, Haggard WO, Smeltzer MS. Use of xylitol to enhance the therapeutic efficacy of polymethylmethacrylate-based antibiotic therapy in treatment of chronic osteomyelitis. Antimicrob Agents Chemother. 2012;56:5839–5844. doi: 10.1128/AAC.01127-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buehlmann M, Frei R, Fenner L, Dangel M, Fluckiger U, Widmer AF. Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infect Control Hosp Epidemiol. 2008;29:510–516. doi: 10.1086/588201. [DOI] [PubMed] [Google Scholar]
- 7.Bush K, Courvalin P, Dantas G, Davies J, Eisenstein B, Huovinen P, Jacoby GA, Kishony R, Kreiswirth BN, Kutter E, Lerner SA, Levy S, Lewis K, Lomovskaya O, Miller JH, Mobashery S, Piddock LJ, Projan S, Thomas CM, Tomasz A, Tulkens PM, Walsh TR, Watson JD, Witkowski J, Witte W, Wright G, Yeh P, Zgurskaya HI. Tackling antibiotic resistance. Nat Rev Microbiol. 2011;9:894–896. doi: 10.1038/nrmicro2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan M. Antimicrobial resistance in the European Union and the world. Conference on Combating Antimicrobial Resistance. Copenhagen, Denmark: World Health Organization; 2012.
- 9.Darouiche RO. Antimicrobial approaches for preventing infections associated with surgical implants. Clin Infect Dis. 2003;36:1284–1289. doi: 10.1086/374842. [DOI] [PubMed] [Google Scholar]
- 10.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 11.Darouiche RO, Mansouri MD, Gawande PV, Madhyastha S. Efficacy of combination of chlorhexidine and protamine sulphate against device-associated pathogens. J Antimicrob Chemother. 2008;61:651–657. doi: 10.1093/jac/dkn006. [DOI] [PubMed] [Google Scholar]
- 12.Diefenbeck M, Muckley T, Hofmann GO. Prophylaxis and treatment of implant-related infections by local application of antibiotics. Injury. 2006;37(Suppl 2):S95–104. doi: 10.1016/j.injury.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Dosler S, Gerceker AA. In vitro activities of nisin alone or in combination with vancomycin and ciprofloxacin against methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. Chemotherapy. 2011;57:511–516. doi: 10.1159/000335598. [DOI] [PubMed] [Google Scholar]
- 14.Drosou A, Falabella A, Kirsner RS. Antiseptics on wounds: an area of controversy. Wounds. 2003;15:149–166. [Google Scholar]
- 15.Farhana SA, Shantakumar SM, Narasu L. Sustained release of diltiazem hydrochloride from chitosan micro-capsules. Curr Drug Deliv. 2009;6:238–248. doi: 10.2174/156720109788680840. [DOI] [PubMed] [Google Scholar]
- 16.Foulkes DM. Some toxicological observations on chlorhexidine. J Periodontal Res Suppl. 1973;12:55–60. doi: 10.1111/j.1600-0765.1973.tb02165.x. [DOI] [PubMed] [Google Scholar]
- 17.Guillemot D. Antibiotic use in humans and bacterial resistance. Curr Opin Microbiol. 1999;2:494–498. doi: 10.1016/S1369-5274(99)00006-5. [DOI] [PubMed] [Google Scholar]
- 18.Hiraishi N, Yiu CK, King NM, Tay FR. Chlorhexidine release and antibacterial properties of chlorhexidine-incorporated polymethyl methacrylate-based resin cement. J Biomed Mater Res B Appl Biomater. 2010;94:134–140. doi: 10.1002/jbm.b.31633. [DOI] [PubMed] [Google Scholar]
- 19.Itokazu M, Matsunaga T, Kumazawa S, Yang W. A novel drug delivery system for osteomyelitis using porous hydroxyapatite blocks loaded by centrifugation. J Appl Biomater. 1995;6:167–169. doi: 10.1002/jab.770060304. [DOI] [PubMed] [Google Scholar]
- 20.Jia WT, Luo SH, Zhang CQ, Wang JQ. In vitro and in vivo efficacies of teicoplanin-loaded calcium sulfate for treatment of chronic methicillin-resistant Staphylococcus aureus osteomyelitis. Antimicrob Agents Chemother. 2010;54:170–176. doi: 10.1128/AAC.01122-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalmeijer MD, van Nieuwland-Bollen E, Bogaers-Hofman D, de Baere GA. Nasal carriage of Staphylococcus aureus is a major risk factor for surgical-site infections in orthopedic surgery. Infect Control Hosp Epidemiol. 2000;21:319–323. doi: 10.1086/501763. [DOI] [PubMed] [Google Scholar]
- 22.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 23.Kuyyakanond T, Quesnel LB. The mechanism of action of chlorhexidine. FEMS Microbiol Lett. 1992;79:211–215. doi: 10.1111/j.1574-6968.1992.tb14042.x. [DOI] [PubMed] [Google Scholar]
- 24.Levy SB. Antibiotic resistance—the problem intensifies. Adv Drug Deliv Rev. 2005;57:1446–1450. doi: 10.1016/j.addr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Lorian V, editor. Antibiotics in Laboratory Medicine. 5. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 26.Muller G, Kramer A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother. 2008;61:1281–1287. doi: 10.1093/jac/dkn125. [DOI] [PubMed] [Google Scholar]
- 27.Nathan C. Antibiotics at the crossroads. Nature. 2004;431:899–902. doi: 10.1038/431899a. [DOI] [PubMed] [Google Scholar]
- 28.Nelson CL. The current status of material used for depot delivery of drugs. Clin Orthop Relat Res. 2004;427:72–78. doi: 10.1097/01.blo.0000143741.92384.18. [DOI] [PubMed] [Google Scholar]
- 29.Noel SP, Courtney HS, Bumgardner JD, Haggard WO. Chitosan sponges to locally deliver amikacin and vancomycin: a pilot in vitro evaluation. Clin Orthop Relat Res. 2010;468:2074–2080. doi: 10.1007/s11999-010-1324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pammi M, Liang R, Hicks JM, Barrish J, Versalovic J. Farnesol decreases biofilms of Staphylococcus epidermidis and exhibits synergy with nafcillin and vancomycin. Pediatr Res. 2011;70:578–583. doi: 10.1203/PDR.0b013e318232a984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos N, Skeete F, Haas JP, Hutzler L, Slover J, Phillips M, Bosco J. Surgical site infection prevention initiative - patient attitude and compliance. Bull NYU Hosp Jt Dis. 2011;69:312–315. [PubMed] [Google Scholar]
- 32.Ritz J, Pashnik B, Padula C, Simmons K. Effectiveness of 2 methods of chlorhexidine bathing. J Nurs Care Qual. 2012;27:171–175. doi: 10.1097/NCQ.0b013e3182398568. [DOI] [PubMed] [Google Scholar]
- 33.Roberts RR, Hota B, Ahmad I, Scott RD, 2nd, Foster SD, Abbasi F, Schabowski S, Kampe LM, Ciavarella GG, Supino M, Naples J, Cordell R, Levy SB, Weinstein RA. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis. 2009;49:1175–1184. doi: 10.1086/605630. [DOI] [PubMed] [Google Scholar]
- 34.Silva LV, Araujo MT, Santos KR, Nunes AP. Evaluation of the synergistic potential of vancomycin combined with other antimicrobial agents against methicillin-resistant Staphylococcus aureus and coagulase-negative Staphylococcus spp strains. Mem Inst Oswaldo Cruz. 2011;106:44–50. doi: 10.1590/S0074-02762011000100007. [DOI] [PubMed] [Google Scholar]
- 35.Steinberg D, Heling I, Daniel I, Ginsburg I. Antibacterial synergistic effect of chlorhexidine and hydrogen peroxide against Streptococcus sobrinus, Streptococcus faecalis and Staphylococcus aureus. J Oral Rehabil. 1999;26:151–156. doi: 10.1046/j.1365-2842.1999.00343.x. [DOI] [PubMed] [Google Scholar]
- 36.Stergiopoulou T, Meletiadis J, Sein T, Papaioannidou P, Walsh TJ, Roilides E. Synergistic interaction of the triple combination of amphotericin B, ciprofloxacin, and polymorphonuclear neutrophils against Aspergillus fumigatus. Antimicrob Agents Chemother. 2011;55:5923–5929. doi: 10.1128/AAC.00548-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stinner DJ, Noel SP, Haggard WO, Watson JT, Wenke JC. Local antibiotic delivery using tailorable chitosan sponges: the future of infection control? J Orthop Trauma. 2010;24:592–597. doi: 10.1097/BOT.0b013e3181ed296c. [DOI] [PubMed] [Google Scholar]
- 38.Traugott KA, Echevarria K, Maxwell P, Green K, Lewis JS., 2nd Monotherapy or combination therapy? The Pseudomonas aeruginosa conundrum. Pharmacotherapy. 2011;31:598–608. doi: 10.1592/phco.31.6.598. [DOI] [PubMed] [Google Scholar]