Abstract
Background
Fungal infections are rare but major problems when they involve orthopaedic implants. Preferred treatment in North America is two-staged: resection and then delayed reconstruction, with local delivery of an antifungal between stages. The effect of voriconazole, a hydrophobic antifungal, on local tissues and wound healing is unclear.
Questions/purposes
We asked: (1) Is voriconazole cytotoxic to fibroblasts or osteoblasts at target concentrations for local delivery? And (2) if cytotoxic, can fibroblasts or osteoblasts resume proliferation after voriconazole is removed?
Methods
We exposed 5000 fibroblasts or osteoblasts/well to voriconazole concentrations of 0, 1, 5, 10, 25, 100, 500, 1000, 5000, 10,000, and 20,000 μg/mL (n = 4 wells/concentration) in 24-well plates. At 3 and 7 days, cell growth was assessed with alamarBlue® and light microscopy. After Day 7, exposure to voriconazole was stopped and incubation continued for 4 days in medium with no voriconazole. On Day 11, cell growth (recovery) was assessed with alamarBlue® and light microscopy.
Results
Increasing voriconazole concentration to more than 100 μg/mL decreased osteoblast and fibroblast growth. Cell growth recovered after 7 days’ exposure to 1000 μg/mL or less.
Conclusions
Voriconazole is cytotoxic to osteoblasts and fibroblasts, but cell growth recovers over 4 days after exposure to 1000 μg/mL or less.
Clinical Relevance
Cytotoxicity seen from voriconazole to mouse osteoblasts and fibroblasts occurs at concentrations achievable clinically from local delivery. It may be prudent to limit the dose of voriconazole in antibiotic-loaded bone cement.
Introduction
Infection of orthopaedic implants is a devastating complication that can lead to considerable morbidity. There is limited clinical experience managing these infections when they are caused by fungi and a paucity of data to guide local antifungal delivery. A 2009 multiinstitutional report concluded resection arthroplasty should be performed in confirmed fungal prosthetic joint infections because of poor results from other treatment methods [3]. This opinion is consistent with the Infectious Disease Society of America’s guidelines [3, 18]. Anagnostakos et al. [2] reported control of fungal infections in seven patients with four hip arthroplasties and three knee arthroplasties using a two-stage exchange protocol and systemic antifungal agents. Two single-case reports described infection control after reimplantation of a TKA [8] and after a resection arthroplasty of the hip [9]. The very low incidence of fungal implant infections precludes adequately powered studies needed for high levels of evidence.
Similar to bacterial implant infections, fungal implant infections are difficult to treat because fungi also form biofilms [5]. The concentration of antifungals required to kill fungi in biofilm is much higher than that required to treat planktonic fungi [16, 17], by as much as 1000 times [4, 7]. Systemic toxicity is a major problem with antifungals, which have low therapeutic indexes. Antifungal-loaded bone cement (ALBC) has been used clinically to provide high local drug levels and to reduce the systemic toxicity [14]. Anecdotally, amphotericin B is the antifungal that has typically been used to formulate ALBC. Even with limited amphotericin B release from ALBC, there is concern about the potential for local toxicity, based on in vitro cell culture data [11]. Also anecdotally, the azole antifungals have been used in ALBC. Voriconazole, a triazole antifungal that acts by inhibition of cytochrome P 450 14α-demethylase [19], is hydrophobic and has a systemic toxicity profile similar to that of amphotericin B. Multiple organ systems are affected, but the severity of the toxic effects from systemic administration is less than that encountered with amphotericin B [13]. Fungi are considered susceptible to voriconazole if their minimum lethal concentration is 4 μg/mL or less [6]. Voriconazole reportedly elutes from bone cement [15] in large amounts, but whether there are cytotoxic effects from local delivery is unclear.
We therefore asked the following questions: (1) Is voriconazole cytotoxic to fibroblasts or osteoblasts at concentrations targeted by local delivery? And (2) if cytotoxic, can fibroblasts or osteoblasts resume growth after voriconazole is removed?
Materials and Methods
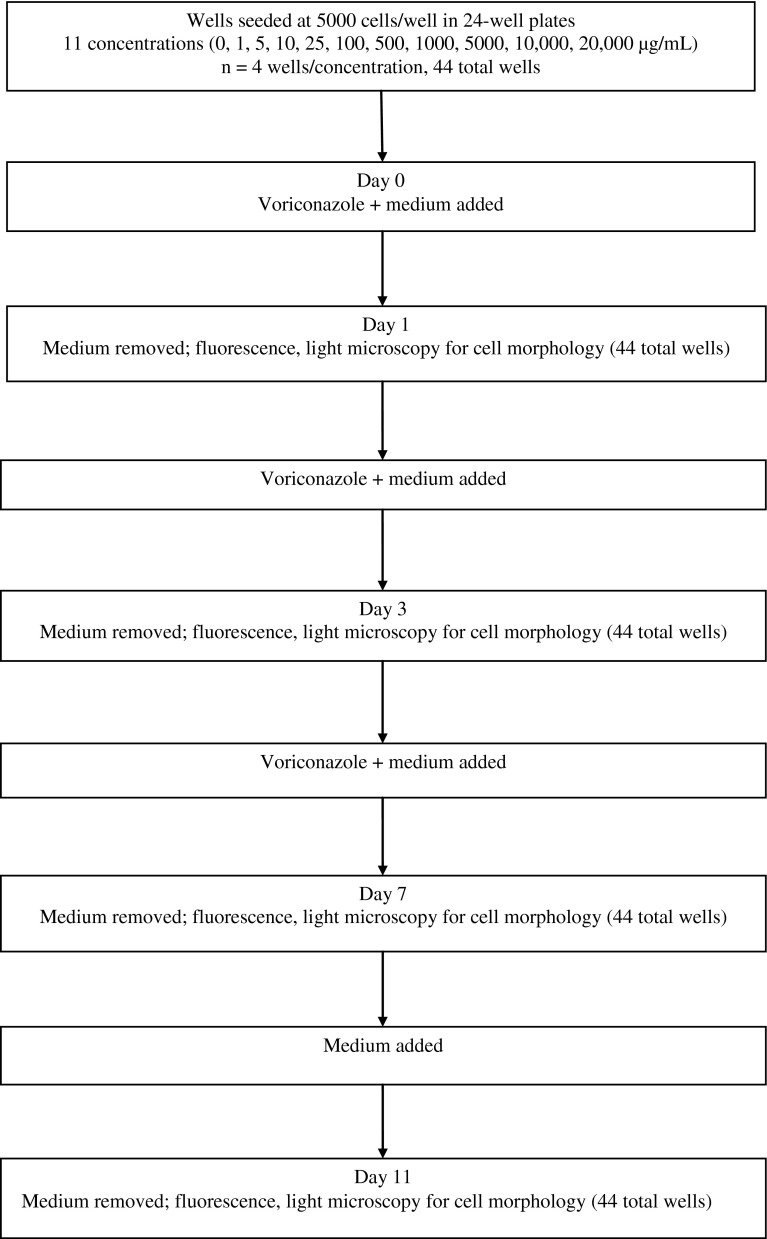
This study was designed to assess the susceptibility of osteoblasts and fibroblasts to toxic effects from a range of voriconazole concentrations, including levels exceeding the therapeutic target for local delivery of 1000 times the levels usually lethal to planktonic fungi (4000 μg/mL) (Fig. 1). Exposure to various voriconazole concentrations was maintained for 7 days, over which the cellular response was evaluated. The voriconazole was then removed from the culture medium, and the culture was continued for 4 more days with fresh medium. The cells were monitored for recovery of morphology and replication. This study was a benchtop pilot study intended to provide baseline data for effect size and was conducted on an in vitro model. No a priori power analysis was conducted for this study.
Fig. 1.
A flowchart shows the experimental design.
Mouse fibroblasts (BALB/3T3 A31) and mouse osteoblasts (MC3T3) (both from American Type Culture Collection, Manassas, VA, USA) were grown in Dulbecco’s Modified Eagle’s Medium with glutamine (2 mmol/L), 10% fetal bovine serum, penicillin (10 IU/mL), and streptomycin (10 μg/mL) (all from Fisher Scientific, Pittsburgh, PA, USA). Each cell line was of moderate cell passage number. Neither of these lines is present on the lists of likely contaminated cells [12]. These cultures do not involve feeder layers or genetic modification. The cells behaved as typical fibroblasts and osteoblasts, but cell type was not independently verified. Cell morphology was monitored daily using light microscopy.
The fibroblasts and osteoblasts were plated at 5000 cells/well in 24-well plates in medium containing voriconazole at concentrations of 0, 1, 5, 10, 25, 100, 500, 1000, 5000, 10,000, or 20,000 μg/mL for 7 days (n = 4 wells/concentration × 11 concentrations = 44 wells). At 1, 3, 7, and 11 days, the medium was removed and replaced with 0.5 mL fresh medium containing 50 μL alamarBlue® (Invitrogen, Carlsbad, CA, USA) after washing with sterile phosphate-buffered saline (PBS). Fluorescence from alamarBlue® was measured on a FLUOstar Omega Multiplate Reader (BMG LABTECH GmbH, Ortenburg, Germany) to determine cell number (excitation, 540 nm; emission, 590 nm). Cell growth was reported in relative fluorescence units (RFU) not cell count. Relative fluorescence is nonlinear with cell count for large cell numbers [20]. After completion of the fluorescence measurements on each of Days 1 and 3, the alamarBlue®-containing medium was replaced with medium containing voriconazole at the concentration each respective well had previously contained. On Day 7, the medium was replaced with fresh medium containing no voriconazole.
We used repeated-measures ANOVA to determine at what concentrations voriconazole inhibited cell growth and recovery. Standard normal plots of residuals were employed to confirm the normality of the data evaluated with ANOVA [1]. Data were analyzed using MINITAB® (Minitab Inc, State College, PA, USA).
Results
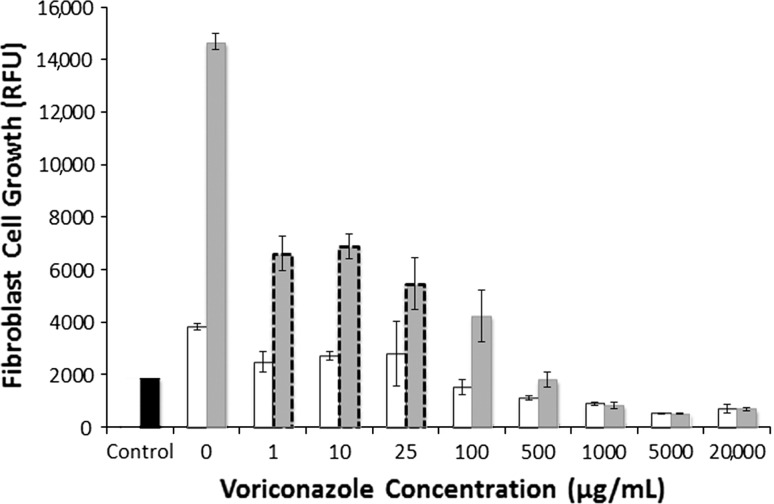
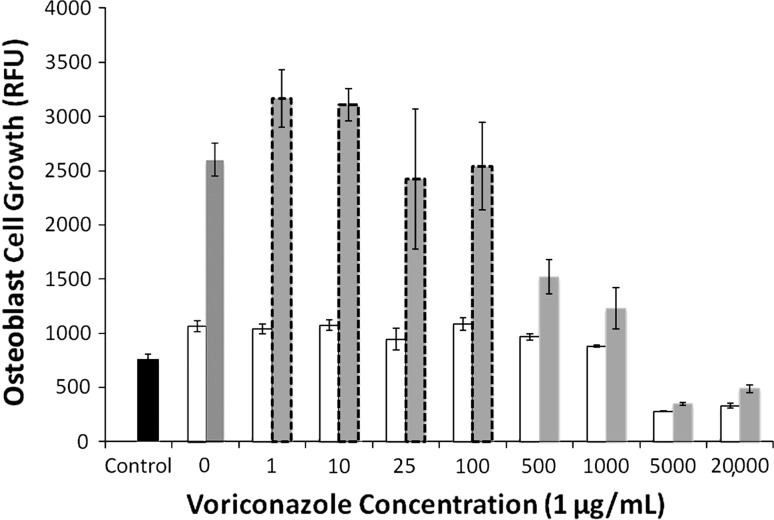
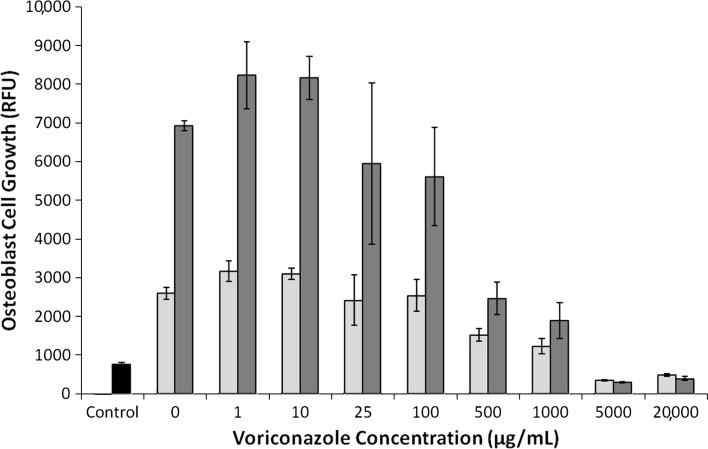
Increasing voriconazole concentration decreased (p < 0.001) fibroblast and osteoblast growth on Days 3 and 7. Fibroblasts exposed to voriconazole concentrations of between 1 and 25 μg/mL exhibited reduced (p < 0.001) growth at Day 3 (Fig. 2) (3820 RFU versus 2483 RFU) but appeared morphologically normal. Osteoblasts exposed to 1 to 100 μg/mL did not decrease growth (Fig. 3) and morphology remained normal (Fig. 4). For concentrations of 500 μg/mL and greater, both cell lines exhibited increasingly rounded morphology and floating cells (Fig. 4). After 7 days of exposure to 500 and 1000 μg/mL, cell growth was reduced in both cell lines, fibroblasts (Fig. 2) more than osteoblasts (Fig. 3). Neither fibroblasts (Fig. 2) nor osteoblasts (Fig. 3) survived 3 days of exposure to voriconazole concentrations of 5000 μg/mL and greater.
Fig. 2.
A graph plots fibroblast cell growth versus voriconazole concentration. Cell growth is quantified by RFU at 3 days (white bars) and 7 days (gray bars). Cells exposed to 1 to 25 μg/mL had decreased growth (p < 0.001) but normal morphology (gray bars with dashed borders). Cells exposed to 100 μg/mL or more had decreased growth and abnormal morphology. Control (black bar) is the RFU for the 5000 cells initially seeded in each well. Data are shown as mean ± SD, for n = 4 wells/concentration.
Fig. 3.
A graph plots osteoblast cell growth versus voriconazole concentration. Cell growth is quantified by RFU at 3 days (white bars) and 7 days (gray bars). Cells exposed to 1 to 100 μg/mL had normal growth and normal morphology (gray bars with dashed borders). Cells exposed to 500 μg/mL or more had decreased growth and abnormal morphology. Control (black bar) is the RFU for the 5000 cells initially seeded in each well. Data are shown as mean ± SD, for n = 4 wells/concentration.
Fig. 4A–B.
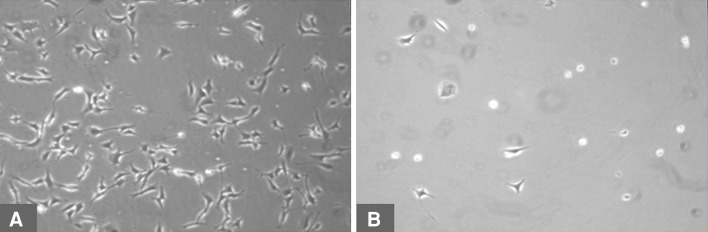
Cell morphology is shown for (A) healthy osteoblasts and (B) rounded, floating, dead osteoblasts.
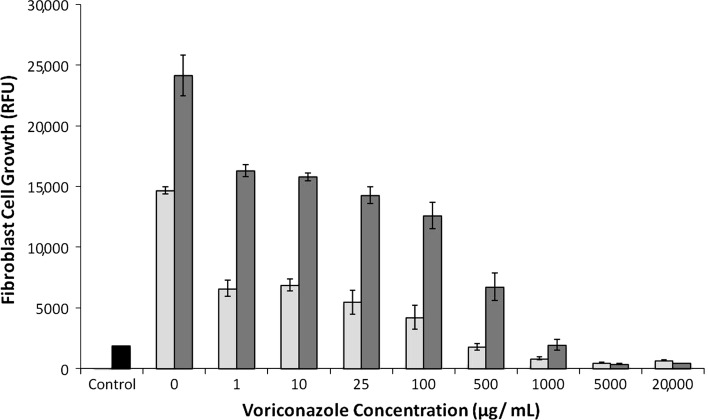
After removal of voriconazole from the medium, both fibroblasts (Fig. 5) and osteoblasts (Fig. 6) exposed to 1000 μg/mL or less for 7 days returned to a more normal spreading morphology on light microscopy and resumed growth, as indicated by increased RFU.
Fig. 5.
A graph plots fibroblast cell growth versus initial voriconazole concentration after removal of voriconazole. Cell growth is quantified by RFU at 7 days (light gray bars) and 11 days (dark gray bars). In cells initially exposed to 1 to 500 μg/mL, growth and morphology recovered. Control (black bar) is the RFU for the 5000 cells initially seeded in each well. Data are shown as mean ± SD, for n = 4 wells/concentration.
Fig. 6.
A graph plots osteoblast cell growth versus initial voriconazole concentration after removal of voriconazole. Cell growth is quantified by RFU at 7 days (light gray bars) and 11 days (dark gray bars). In cells exposed to 1 to 500 μg/mL, growth and morphology recovered. Control (black bar) is the RFU for the 5000 cells initially seeded in each well. Data are shown as mean ± SD, for n = 4 wells/concentration.
Discussion
Voriconazole is a hydrophobic antifungal with a known systemic toxicity profile. The large amounts of cyclodextrin used as a carrier to achieve solubility in the parenteral formulation of voriconazole functions as a poragen, leading to high release from ALBC [15]. Cytotoxicity to concentrations targeted by local delivery has not been studied. We therefore asked the following questions: (1) Is voriconazole cytotoxic to fibroblasts or osteoblasts at concentrations targeted by local delivery? And (2) if cytotoxic, can fibroblasts or osteoblasts resume growth after voriconazole is removed?
There are limitations to this study. First, in vitro cytotoxicity studies do not replicate the barriers to drug distribution, tissue architecture, or fluid flow found in surgical wounds. Second, mouse cells may not accurately indicate the susceptibility of human cells to voriconazole; however, the selected cell lines are commonly used for preclinical toxicity testing [11]. Third, specialized cellular functions such as osteoid production or fibroblast extracellular matrix production were not studied. Cellular morphology and proliferation are established indicators of cytotoxicity, which are appropriate for preliminary studies. Further work is needed to determine the effect of voriconazole on specific cell functions. Fourth, guidance for clinical dosing in ALBC cannot be determined directly from cell culture data. Although 5000 μg/mL was lethal in cell culture and that is realistically achievable in clinical practice by local delivery, it is unknown what load will cause that concentration in any specific delivery site. Until in vivo data are available, it may be prudent to limit the dose of voriconazole loaded into ALBC. A guide that has been used to limit systemic exposure of hydrophilic antibacterials in ALBC is to use up to the equivalent of a 24-hour parenteral dose per batch of porous ALBC. For voriconazole, this is 12 mg/kg, 840 mg for a 70-kg patient, per batch of ALBC. However, there is no such guide for antimicrobial loads to limit local toxicity. Anecdotally, 300 mg voriconazole per batch of ALBC has been used without wound-healing problems. Miller et al. [15] reported 300 mg voriconazole per batch of ALBC as a balance point between high release and excessive loss of mechanical strength. The large volume of cyclodextrin added to commercially available voriconazole provides sufficient porosity in the ALBC such that additional poragens are not needed.
We found mouse fibroblasts and osteoblasts sustained reversible, sublethal toxicity at voriconazole concentrations of up to 1000 μg/mL. Cytotoxicity to hydrophobic antifungals has been studied previously. Harmsen et al. [11] reported concentrations of amphotericin B as low as 5 μg/mL alter morphology and decrease proliferation in mouse fibroblasts and osteoblasts. Recovery occurred after exposure to 10 μg/mL of amphotericin B or less for 7 days. In their experiment, no cells survived at 100 μg/mL or greater [6]. This is 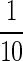 of the target concentration of 1000 times the lethal level for planktonic fungi and less than 10 times greater than targeted systemic levels. Han et al. [10] reported voriconazole is cytotoxic and alters morphology in endothelial cells at concentrations of more than 100 μg/mL at 24 hours. Some cells survived after exposure to concentrations of up to 1000 μg/mL. Toxicity to cell lines from musculoskeletal tissues has not been reported. Our cytotoxicity data for mouse fibroblasts and osteoblasts exposed to voriconazole are consistent with cytotoxicity to endothelial cells: decreased replication in cells exposed to voriconazole concentrations of up to 1000 μg/mL. The toxic effects from voriconazole in our study occurred at 50 to 100 times higher concentrations than similar effects reported by Harmsen et al. [11] for amphotericin B deoxycholate. Recoverable morphology and replication changes occurred in our study for concentrations of voriconazole of 1000 μg/mL or less, for both cell lines. There are no published data on recovery of the toxic effects caused by voriconazole after exposure to voriconazole is removed; however, there are published data on the recovery of cell morphology and replication when exposure to amphotericin B is removed. Voriconazole and amphotericin B have similar therapeutic concentrations [11], but similar toxicity takes 100 times more voriconazole than amphotericin B. In clinical applications, locally delivered concentrations are transient, decreasing as the local depot is depleted. Recovery from sublethal toxicity would be important to allow wound healing to progress at postresection surgical sites where local delivery is used. In the absence of in vivo data, anecdotal experience is the best available guide. Local depots of ALBC with 300 mg voriconazole per batch have high delivery, acceptable loss of mechanical strength of the ALBC, and no documented wound-healing problems.
of the target concentration of 1000 times the lethal level for planktonic fungi and less than 10 times greater than targeted systemic levels. Han et al. [10] reported voriconazole is cytotoxic and alters morphology in endothelial cells at concentrations of more than 100 μg/mL at 24 hours. Some cells survived after exposure to concentrations of up to 1000 μg/mL. Toxicity to cell lines from musculoskeletal tissues has not been reported. Our cytotoxicity data for mouse fibroblasts and osteoblasts exposed to voriconazole are consistent with cytotoxicity to endothelial cells: decreased replication in cells exposed to voriconazole concentrations of up to 1000 μg/mL. The toxic effects from voriconazole in our study occurred at 50 to 100 times higher concentrations than similar effects reported by Harmsen et al. [11] for amphotericin B deoxycholate. Recoverable morphology and replication changes occurred in our study for concentrations of voriconazole of 1000 μg/mL or less, for both cell lines. There are no published data on recovery of the toxic effects caused by voriconazole after exposure to voriconazole is removed; however, there are published data on the recovery of cell morphology and replication when exposure to amphotericin B is removed. Voriconazole and amphotericin B have similar therapeutic concentrations [11], but similar toxicity takes 100 times more voriconazole than amphotericin B. In clinical applications, locally delivered concentrations are transient, decreasing as the local depot is depleted. Recovery from sublethal toxicity would be important to allow wound healing to progress at postresection surgical sites where local delivery is used. In the absence of in vivo data, anecdotal experience is the best available guide. Local depots of ALBC with 300 mg voriconazole per batch have high delivery, acceptable loss of mechanical strength of the ALBC, and no documented wound-healing problems.
In conclusion, voriconazole caused reversible morphologic changes and decreased growth in mouse fibroblasts and osteoblasts at concentrations of up to 1000 μg/mL. Voriconazole was lethal at concentrations of 5000 μg/mL or more. Although clinical studies are needed to evaluate any potential cytotoxicity that may occur in surgical wounds, toxicity to mouse osteoblasts and fibroblasts occurred at concentrations achievable clinically from local delivery. Based on empiric anecdotal experience, it may be prudent to limit the dose of voriconazole in ALBC to 300 mg per batch of ALBC.
Footnotes
One or more of the authors have received, during the study period, funding from the Herbert J. Lewis fund at Orthopedic Research and Education Foundation (Rosemont, IL, USA) (AM, RM) and from the Southwest Orthopaedic Trauma Association (Albuquerque, NM, USA) (KS, AM).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
This work was performed at Arizona State University (Tempe, AZ, USA) and Banner Good Samaritan Medical Center (Phoenix, AZ, USA).
References
- 1.Altman DG. Practical Statistics for Medical Research. 2. Boca Raton, FL: Chapman & Hall/CRC Press; 2006. [Google Scholar]
- 2.Anagnostakos K, Kelm J, Schmitt E, Jung J. Fungal periprosthetic hip and knee joint infections clinical experience with a 2-stage treatment protocol. J Arthroplasty. 2012;27:293–298. doi: 10.1016/j.arth.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 3.Azzam K, Parvizi J, Jungkind D, Hanssen A, Fehring T, Springer B, Bozic K, Della Valle C, Pulido L, Barrack R. Microbiological, clinical, and surgical features of fungal prosthetic joint infections: a multi-institutional experience. J Bone Joint Surg Am. 2009;91(suppl 6):142–149. doi: 10.2106/JBJS.I.00574. [DOI] [PubMed] [Google Scholar]
- 4.Bink A, Pellens K, Cammue BP, Thevissen K. Anti-biofilm strategies: how to eradicate Candida biofilms? Open Mycol J. 2011;511:29–38. doi: 10.2174/1874437001105010029. [DOI] [Google Scholar]
- 5.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clancy CJ, Nguyen MH. In vitro efficacy and fungicidal activity of voriconazole against Aspergillus and Fusarium species. Eur J Clin Microbiol Infect Dis. 1998;17:573–575. doi: 10.1007/BF01708622. [DOI] [PubMed] [Google Scholar]
- 7.Diefenbeck M, Mückley T, Hofmann GO. Prophylaxis and treatment of implant-related infections by local application of antibiotics. Injury. 2006;37(suppl 2):S95–S104. doi: 10.1016/j.injury.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Graw B, Woolson S, Huddleston JI. Candida infection in total knee arthroplasty with successful reimplantation. J Knee Surg. 2010;23:169–174. doi: 10.1055/s-0030-1267470. [DOI] [PubMed] [Google Scholar]
- 9.Hall RL, Frost RM, Vasukutty NL, Minhas H. Candida glabrata: an unusual fungal infection following a total hip replacement. BMJ Case Rep. 2012;2012. pii: bcr-2012-006491. [DOI] [PMC free article] [PubMed]
- 10.Han SB, Shin YJ, Hyon JY, Wee WR. Cytotoxicity of voriconazole on cultured human corneal endothelial cells. Antimicrob Agents Chemother. 2011;55:4519–4523. doi: 10.1128/AAC.00569-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harmsen S, McLaren AC, Pauken C, McLemore R. Amphotericin B is cytotoxic at locally delivered concentrations. Clin Orthop Relat Res. 2011;469:3016–3021. doi: 10.1007/s11999-011-1890-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Healey JH. Editorial: the imperative to authenticate cell lines. Clin Orthop Relat Res. 2010;468:3413–3414. doi: 10.1007/s11999-010-1585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kullberg BJ, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, Rex JH, Cleary JD, Rubinstein E, Church LW, Brown JM, Schlamm HT, Oborska IT, Hilton F, Hodges MR. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet. 2005;366:1435–1442. doi: 10.1016/S0140-6736(05)67490-9. [DOI] [PubMed] [Google Scholar]
- 14.Marra F, Robbins GM, Masri BA, Duncan C, Wasan KM, Kwong EH, Jewesson PJ. Amphotericin B-loaded bone cement to treat osteomyelitis caused by Candida albicans. Can J Surg. 2001;44:383–386. [PMC free article] [PubMed] [Google Scholar]
- 15.Miller RB, McLaren AC, Pauken C, Clarke HD, McLemore R. Voriconazole is delivered from antifungal-loaded bone cement. Clin Orthop Relat Res. 2012;471:195–200. doi: 10.1007/s11999-012-2463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramage G, Saville SP, Thomas DP, López-Ribot JL. Candida biofilms: an update. Eukaryot Cell. 2005;4:633–638. doi: 10.1128/EC.4.4.633-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramage G, Walle KV, Wickes BL, López-Ribot JL. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001;45:2475–2479. doi: 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rex JH, Walsh TJ, Sobel JD, Filler SG, Pappas PG, Dismukes WE, Edwards JE. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:662–678. doi: 10.1086/313749. [DOI] [PubMed] [Google Scholar]
- 19.Sionov E, Chang YC, Garraffo HM, Dolan MA, Ghannoum MA, Kwon-Chung KJ. Identification of a Cryptococcus neoformans cytochrome P450 lanosterol 14α-demethylase (Erg11) residue critical for differential susceptibility between fluconazole/voriconazole and itraconazole/posaconazole. Antimicrob Agents Chemother. 2012;56:1162–1169. doi: 10.1128/AAC.05502-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voytik-Harbin SL, Brightman AO, Waisner B, Lamar CH, Badylak SF. Application and evaluation of the alamarBlue assay for cell growth and survival of fibroblasts. In Vitro Cell Dev Biol Anim. 1998;34:239–246. doi: 10.1007/s11626-998-0130-x. [DOI] [PubMed] [Google Scholar]