Abstract
Background
Regular screening of cirrhotic patients for hepatocellular carcinoma (HCC) has been suboptimal, but there is little data regarding specific risk factors for reduced screening.
Methods
From 1996-2010, patients with cirrhosis were retrospectively identified from outpatient gastroenterology and primary care practices. Data was obtained from the diagnosis of cirrhosis until the time of elevated alpha-fetoprotein (AFP) or lesion suspicious for HCC, death, liver transplantation, or end of the data collection period. Recommended screening was defined as abdominal imaging (ultrasound, contrast-enhanced CT, or MRI) with or without serum alpha-fetoprotein (AFP) at least once every 12 months based on professional guidelines.
Results
One hundred fifty-six patients with cirrhosis were identified. The etiologies of cirrhosis were viral hepatitis (n=65), alcohol (n=40), non-alcoholic steatohepatitis (NASH) (n=27) and non-viral, non-alcoholic, non-NASH cirrhosis (n=24). Of the 156 patients, 51% received recommended screening for HCC. Patients with NASH cirrhosis received recommended screening significantly less (P=0.016) than cirrhotics with viral hepatitis, alcoholic cirrhosis, or non-viral, non-alcoholic, non-NASH cirrhosis and were less likely to receive gastroenterology referral (P<0.001). Additionally, 20 patients were diagnosed with cirrhosis incidentally during a surgical procedure. These patients were significantly less likely to receive recommended HCC screening than those diagnosed nonsurgicaly (10.0% vs. 56.6%; P<0.001). Screening was significantly more likely to occur in patients seen regularly by a gastrointestinal subspecialist (66.7% vs. 22.8%; P<0.001).
Conclusions
Patients with NASH cirrhosis and surgically discovered cirrhosis have low rates of HCC screening and are referred less to gastroenterologists. These data suggest a need for increased education about NASH cirrhosis and better systems of communication among general practitioners, surgeons, and gastroenterologists.
Keywords: cirrhosis, hepatocellular carcinoma, screening
Introduction
Hepatocellular carcinoma (HCC) is the third most common cause of cancer mortality worldwide.1 Modern cohort studies have shown that HCC is now the leading cause of death in patients with cirrhosis.2-4 Historically, HCC has been most associated with hepatitis B (HBV) and C infection as well as chronic alcohol use, and in the past 30 years, the incidence of HCC in the United States has almost doubled owing mostly to increases in hepatitis C infection (HCV).5 However, in recent years and particularly in the United States, the number of patients with non-alcoholic fatty liver disease (NAFLD) leading to non-alcoholic steatohepatitis (NASH) and cirrhosis has also contributed to the increasing incidence of HCC.6
Survival for symptomatic HCC is very poor with a mean survival at 1 year of ~20% and 5 year survival <5%.7 However, when diagnosed early and treated with surgical or ablative therapy, prognosis is improved, with 3 year survival rates of 60-80% and 5 year survival rates of 40-70%.8 For this reason, HCC screening is recommended and has been shown to reduce mortality, as much as 37% in one study.9
Screening for HCC in patients with cirrhosis using annual abdominal imaging with or without annual alpha-fetoprotein (AFP) measurement has been recommended by the American Association for the Study of Liver Disease (AASLD).10 Despite these recommendations, however, screening rates remain low, with rates varying from ~50% to lower than 20%.11-13 Low screening rates have been linked to delayed tumor detection, larger tumors at diagnosis, and reduced therapeutic options.14
Although a number of studies document low rates of screening, it is unclear whether or how HCC screening varies by etiology of cirrhosis, practice type, or involvement of specialists. Therefore, we performed a retrospective cohort study designed to examine patient and provider characteristics affecting HCC screening in cirrhotics followed in primary care and gastroenterology outpatient practices.
Methods
Patients diagnosed with cirrhosis between the years of 1996-2010 were retrospectively identified from gastroenterology and primary care practices at our institution and affiliated sites. Patients were selected using an ICD-9 code diagnosis of cirrhosis from an institutional research database and from primary care and gastroenterology practices. A Research Patient Data Registry, which is a registry of all patients in the Partners Healthcare system, was searched using the search terms “liver biopsy” and “cirrhosis”, from which all patients from our institution and affiliated sites with cirrhosis were selected. Similarly, primary care practices were searched specifically using a research database with the search term “cirrhosis,” from which all patients with a cirrhosis diagnosis were selected. Patients from gastroenterology practices with an ICD-9 code diagnosis of cirrhosis were selected using medical record numbers, which were not tied to other diagnoses. Cirrhosis diagnoses were verified by review of biopsy histology, imaging studies consistent with a cirrhotic liver, or review of the medical record for evidence of portal hypertension and liver synthetic dysfunction. Patients were eligible if they were older than 18 years old at time of cirrhosis diagnosis.
Data was collected from the first diagnosis of cirrhosis or first evidence of cirrhosis in the medical record until the time of (1) a lesion suspicious for HCC noted on imaging or elevated AFP (AFP greater than 20 ng/ml), (2) death, (3) liver transplantation, or (4) end of the data collection period. If patients were lost to follow up, data collection ended at the date of the last patient encounter. Patients were excluded if they carried a pre-existing diagnosis of HCC, had a lesion suspicious for HCC or elevated AFP on initial screening for HCC, or if they had already undergone liver transplantation. The following patient data were also obtained from the medical record: age, sex, race, etiology of cirrhosis as noted in biopsy reports or physician notes, documentation of gastroenterology or hepatology clinic visits, and insurance status. Patients were considered to have regular gastroenterology/hepatology follow up if they had at least 1 documented clinic visit per year.
Recommended screening was defined as abdominal imaging (ultrasound or contrast-enhanced CT or MRI) with or without serum AFP measurement at least once every 12 months as indicated by the most recent AASLD guidelines.10 All radiographic imaging was reviewed and reported by an attending radiologist. A diagnosis of HCC required biopsy confirmation or lesions on radiology that prompted empiric treatment with either transcatheter arterial chemoembolization or percutaneous radiofrequency ablation.
Statistical analysis was performed using Microsoft Excel 2004 v11.6. Categorical variables were analyzed using the Chi-square test. P values <0.05 were considered statistically significant. This study was approved by the Partners Healthcare Human Research Committee.
Results
Overall Rates of HCC Screening Vary by Etiology of Cirrhosis
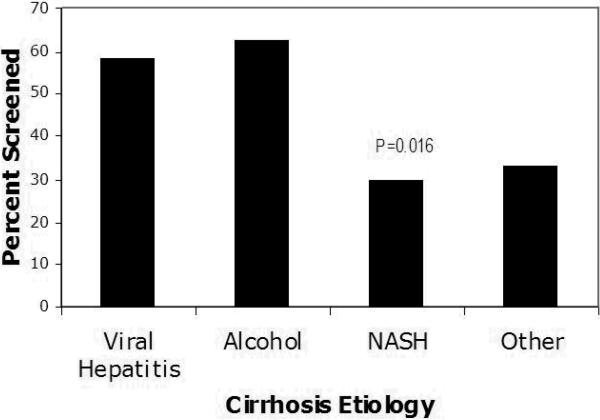
A total of 156 patients with cirrhosis were identified from June, 1996 through January 2010, with a mean follow up time of 43 months (range 4-150). Cirrhosis was diagnosed based on biopsy data in 50% of patients, imaging consistent with cirrhosis in 40%, and solely on clinical features in 1%. Cirrhosis etiologies were viral hepatitis (n=65 with 17 HBV, 45 HCV, and 3 HBV and HCV), alcohol (n=40), non-alcoholic steatohepatitis (NASH) (n=27), and non-viral, non-alcoholic, non-NASH cirrhosis (n=24). (see Table 1) Sixty-three percent of patients were seen regularly by gastroenterologists or hepatologists and 37 % by internists or surgeons alone. Of the 156 patients, 51% received the recommended screening for HCC with at least yearly AFP and abdominal imaging. Rates of HCC screening varied dramatically by etiology of cirrhosis. HCC screening was highest in patients with alcoholic or viral cirrhosis. In contrast, patients with NASH cirrhosis had significantly lower rates of screening (29.7%; p=0.016) than cirrhotics with viral hepatitis (58.4%), alcoholic cirrhosis (62.5%), or non-viral, non-alcoholic, non-NASH cirrhosis (33.3%). (Figure 1)
Table 1.
Baseline Demographic and Clinical Characteristics (N=156)
| Age (years) | 56.2 ± 13.5 |
| Male (%) | 66 |
| Etiology of Cirrhosis (%) | |
| Viral* | 41.6 |
| Alcohol | 25.6 |
| NASH | 17.3 |
| Other† | 15.3 |
| Race‡ (%) | |
| White | 81 |
| Black | 3 |
| Asian | 5 |
| Hispanic | 11 |
| Gastroenterologist/Hepatologist Involvement (%) | 63 |
| Insurance (%) | |
| Medicaid | 14 |
| Medicare | 40 |
| Private | 46 |
| Cirrhosis Diagnosed during Surgery (%) | 13 |
Viral etiologies include n=17 HBV, 45 HCV, 3 both HBV and HCV
Includes hemachromatosis, primary biliary cirrhosis, primary sclerosing cholangitis, autoimmune hepatitis, cryptogenic cirrhosis without evidence of NASH, and sarcoidosis
Race was self reported
Figure 1.
Association between Cirrhosis Etiology and HCC Screening
HCC Screening Rates Vary by Practitioner Type
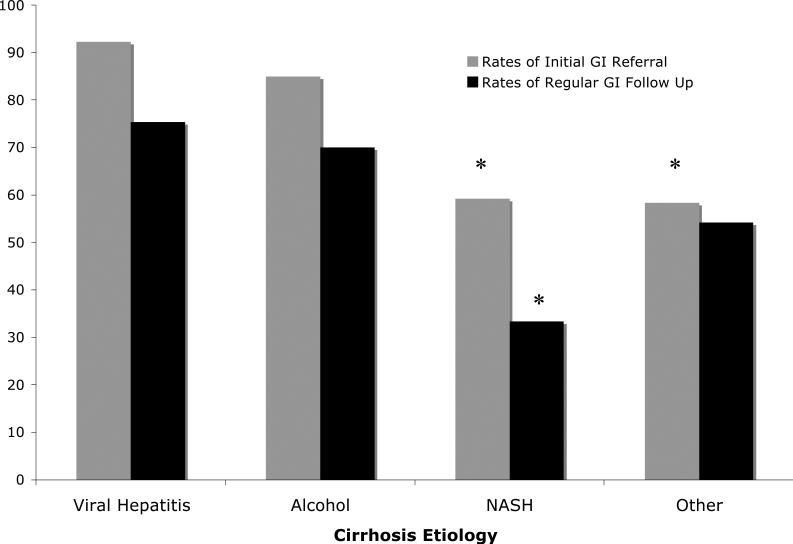
We found significant differences in rates of HCC screening based on the type of practitioner involved. Compared to patients seen solely by their primary care physician or surgeon alone, screening was significantly higher in patients referred to a gastroenterologist or hepatologist (58.8% vs, 18.8%; P<0.001). The rate of screening increased to 66.7% for those patients who were seen at least once per year by their gastroenterologist compared to those who did not receive regular gastroenterology follow-up (22.8%; P<0.001). (see Table 2) Of the patients who were referred for an initial gastroenterology or hepatology consultation, 80% continued to follow up at least annually.
Table 2.
Determinants of Regular HCC Screening with yearly AFP and abdominal imaging
| Screened (%) | P value | |
|---|---|---|
| Gastroenterologist/Hepatologist Involvement | <0.00001 | |
| ≥1 visit per year (n=99) | 67 | |
| < 1 vist per year (n=57) | 23 | |
| Cirrhosis Diagnosed during Surgery | <0.0001 | |
| Yes (n=20) | 10 | |
| No (n=136) | 57 | |
| Sex | 0.10 | |
| Male (n=103) | 55 | |
| Female (n=53) | 42 | |
| Race* | 0.27 | |
| White (n=126) | 48 | |
| Black (n=5) | 80 | |
| Asian (n=8) | 75 | |
| Hispanic (n=17) | 47 | |
| Insurance Type+ | 0.32 | |
| Medicaid (n=22) | 41 | |
| Medicare (n=62) | 50 | |
| Private (n=72) | 54 |
Race was self reported
No patients were uninsured since Massachusetts has mandatory health coverage. All patients were enrolled in state sponsored health insurance following their initial visit
In addition, patients with NASH cirrhosis were statistically less likely to have received regular gastroenterology or hepatology follow up (33%) compared to those with viral hepatitis (70%), alcoholic (75%), or non-viral, non-alcoholic, non-NASH cirrhosis (54%; P<0.001). They were also less likely to be referred for an initial evaluation by a gastroenterologist or hepatologist (59%) compared to those with viral hepatitis (92.3%) and alcoholic cirrhosis (85%; P<0.001).
A subset of 20 patients (13%) was diagnosed with cirrhosis incidentally during a surgical procedure. Patients with incidentally discovered cirrhosis underwent surgery for the following indications: gastric bypass (10), cholecystectomy (5), diverticulitis (1), nephrectomy (1), and Whipple procedure (3). The etiologies of cirrhosis in these specific patients included NASH (n=11), viral hepatitis (n=2), alcohol (n=1), and non-alcoholic, non-viral, non-NASH related liver disease (n=6). These patients were significantly less likely to receive recommended HCC screening (10.0% vs 56.6%; P<0.001) and were less likely to have either an initial consultation with a gastroenterologist or hepatologist (25% vs 94%) or regular gastrointestinal subspecialty follow up (5% vs 72%) compared to those diagnosed non-surgically (P<0.001 for both). Importantly, while we found that practitioner type influenced screening rates, we found no significant difference in screening based on insurance coverage, race, or gender.
When the definition of HCC screening was broadened to include only abdominal imaging, not requiring AFP measurements, the overall screening rate rose to 66%, but there continued to be a trend towards reduced screening in patients with NASH cirrhosis (51%; P=0.087) compared to viral hepatitis (73.8%), alcoholic cirrhosis (65%), and non-viral, non-alcoholic, non-NASH cirrhosis (62.5%). Significantly fewer patients with cirrhosis diagnosed incidentally during surgery and those not regularly seen by a gastroenterologist underwent screening regardless of whether AFP was included as part of a screening protocol (P<0.001 for all groups).
Although this study was not powered to detect differences in the rates of hepatocellular carcinoma, of the156 patients screened over a period of 563 person-years, hepatocellular carcinoma was diagnosed in 14 (2.5%). This incidence was similar to the rates of HCC seen in other studies.10,15,16
Discussion
This study confirms previous findings that HCC screening practices in cirrhotic patients are suboptimal, with rates comparable to those in previous studies.9,12-13 This study is unique, however, in its evaluation of screening rates by cirrhosis etiology and highlights the suboptimal HCC screening rates in patients with NASH cirrhosis. In addition, to our knowledge, it is one of the first studies to also demonstrate reduced screening in patients whose cirrhosis was diagnosed incidentally during surgery, thus identifying another high risk group.
Although previously thought not to be a significant risk factor for HCC, NASH cirrhosis has recently been shown to have risks for HCC that are comparable to other etiologies of cirrhosis. In a study by Ascha et al.15 the yearly cumulative incidence of HCC in NASH cirrhotics was 2.6% compared to 4% in cirrhotics with HCV. Similarly, in a study by Marrero et al.14 approximately 29% of cases with HCC were found to be attributable to NAFLD. HCC survival in NASH cirrhosis also appears to be similar to HCC in other forms of cirrhosis.17 However, despite recent data identifying NASH cirrhosis as an important risk factor for developing HCC, the reduced rate of screening in these patients suggests that awareness of this risk is still lagging. This contention is supported by the fact that patients with NASH cirrhosis in our study were less likely to have gastroenterology or hepatology referral.
However, our study underscores the importance of gastroenterology and/or hepatology involvement in screening for HCC. Similar to a recent study by Davila et al,13 which found higher rates of HCC screening in patients seen by a gastroenterologist or hepatologist, our study showed a 3-fold increase in the rate of HCC screening when patients were referred to a gastroenterologist or hepatologist. The precise mechanism by which gastroenterology or hepatology involvement improves screening is still unclear, but it may simply reflect a greater awareness of cirrhosis complications by the patient's primary care provider rather than solely a direct sub-specialist effect. Additionally, even though gastroenterology and hepatology involvement was associated with improved screening, not all patients seen regularly by gastroenterologists and hepatologists received screening. This suggests that more robust screening of cirrhotic patients for HCC by gastrointestinal specialists may require not only recommending screening to primary care providers, but also personally directing it.
Systems based practices may prove useful in improving HCC screening rates. In our study, 13% of patients had their cirrhosis diagnosed incidentally by biopsy during a surgical procedure unrelated to their liver disease. That these patients with surgically discovered disease were not referred to a gastroenterologist or hepatologist and did not receive the recommended screening for HCC suggests that primary care physicians may not be aware of cirrhosis diagnoses made during surgery. This finding is especially important because it may represent a communication gap between multidisciplinary providers. Patients at risk for NASH cirrhosis are often cared for by a multidisciplinary team comprised of an internist, a nutritionist, an endocrinologist managing diabetes, and a surgeon managing obesity related complications. With more patients receiving care in a multidisciplinary team, effective communication takes on even greater importance.
Because of this potential gap, systems-based interventions such as automated gastroenterology and primary care physician notifications of all cirrhosis diagnoses made at biopsy, either computer-generated or by other means, may prove beneficial. Automated specialist referral, as seen with infectious disease consultation for inpatients with positive blood cultures has also shown benefit and improved outcomes.18, 19 Systems-based notifications and reminders have similarly been shown to increase patient compliance in screening for colon cancer and breast cancer and may be applicable to hepatocellular carcinoma.20, 21
As the number of obese patients rises, there will likely be even greater numbers of undiagnosed cirrhotic patients with NASH who will require HCC screening. Furthermore, with increasing data showing the benefits of weight reduction surgery, more cirrhotic patients are likely to be discovered incidentally during surgery and will be at risk for failure to screen.22, 23 Systems to track information and methods of facilitating communication between primary care physicians, surgeons, gastroenterologists, and hepatologist will therefore be vitally important to take optimal care of this vulnerable population.
Since this study is retrospective, it has limitations. These include a potential selection bias, since those patients who were not seen by gastroenterologists or hepatologists and did not receive recommended HCC screening may represent a group that is less likely to seek subspecialty care or adhere to prescribed therapies than other patients. However, this type of selection bias is likely to be a less significant contributor to our results since patients with NASH were less likely to be seen by gastroenterologists than patients with viral hepatitis or alcoholic cirrhosis, who historically have had more social risk factors associated with poor follow up.
Additionally, our study sample was not large enough to assess outcomes of patients who had inadequate screening compared to those who received the recommended screening. However, compared to prior case-control studies investigating HCC screening in patients with diagnosed HCC, this cohort study should be generalizable to cirrhotic patients of many different etiologies who are cared for in variety of practice settings. Lastly, because of limitations of our database, we were unable to differentiate between provider efforts and patient compliance. Are providers not appropriately screening for HCC or are patients not following up regularly despite a provider's best efforts? Further studies are required to determine the role of specific barriers to patient compliance.
In conclusion, overall rates of HCC screening remain suboptimal and are related to the involvement of gastroenterologists and hepatologists. Importantly, patients with NASH cirrhosis and those diagnosed with cirrhosis incidentally during surgery likely represent a vulnerable group of cirrhotics at even greater risk for failure to screen. Although further prospective study is required, these findings imply that screening awareness remains limited in primary care and surgical practices and that systems-based measures to reduce communication gaps between providers are needed. Future studies to evaluate barriers to screening as well as interventions to improve screening in primary care settings are clearly warranted.
Figure 2.
Rates of Specialist Involvement by Cirrhosis Etiology
*P<0.05
Acknowledgements
We thank Dr. Kathleen Finn and Dr. Daniel Hunt for their critical reading of this manuscript and their suggestions. Part of this work was presented at a poster session at the 61st Annual Meeting of the American Association for the Study of Liver Diseases; 2010 November 2; Boston, MA.
Grant Support:
RTC was supported by NIH DK078772
List of Abbreviations
- HCC
hepatocellular carcinoma
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- NAFLD
non-alcoholic fatty liver disease
- NASH
non=alcoholic steatohepatitis
Footnotes
Financial Disclosures:
None
Conflicts of Interest:
None
References
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Sangiovanni A, Del Ninno E, Fasani P, et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005–1014. doi: 10.1053/j.gastro.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 3.Degos F, Christidis C, Ganne-Carrie N, et al. Hepatitis C virus related cirrhosis: time to occurrence of hepatocellular carcinoma and death. Gut. 2000;47:131–136. doi: 10.1136/gut.47.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benvegnu L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53:744–749. doi: 10.1136/gut.2003.020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Serag HB, Davila JA, Peteren NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 6.Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26:2183–2191. doi: 10.1185/03007995.2010.506375. [DOI] [PubMed] [Google Scholar]
- 7.Liu JH, Chen PW, Asch SM, Busutill RW, Ko CY. Surgery for hepatocellular carcinoma: does it improve survival? Ann Surg Oncol. 2004;11:298–303. doi: 10.1245/aso.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 9.Zhang BH, Yang BH, Tang Zy. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PubMed] [Google Scholar]
- 10.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 11.Davila JA, Weston A, Smalley W, El-Serag HB. Utilization of screening for hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2007;41:777–782. doi: 10.1097/MCG.0b013e3180381560. [DOI] [PubMed] [Google Scholar]
- 12.Leykum LK, El-Serag HB, Cornell J, Papadopoulos KP. Screening for hepatocellular carcinoma among veterans with hepatitis C on disease stage, treatment received, and survival. Clin Gastroenterol Hepatol. 2007;5:508–512. doi: 10.1016/j.cgh.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–141. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 15.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 16.Degos F, Christidis C, Ganne-Carrie N, et al. Hepatitis C virus related cirrhosis: time to occurrence of hepatocellular carcinoma and death. Gut. 2000;47:131–136. doi: 10.1136/gut.47.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tokushige K, Hashimoto E, Yatsuji, et al. Prospective study of hepatocellular carcinoma in nonalcoholic steatohepatitis in comparison with hepatocellular carcinoma caused by chronic hepatitis C. J Gastroenterol. 2010 doi: 10.1007/s00535-010-0237-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins TC, Price CS, Sabel AL, Mehler PS, Burman WJ. Impact of Routine Infectious Diseases Service Consultation on the Evaluation, Management, and Outcomes of Staphylococcus aureus Bacteremia. Clin Infect Dis. 2008;46:1000–1008. doi: 10.1086/529190. [DOI] [PubMed] [Google Scholar]
- 19.Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med. 2010;123:631–637. doi: 10.1016/j.amjmed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leffler DA, Neeman N, Rabb JM, et al. An alerting system improves adherence to follow-up recommendations from colonoscopy examinations. Gastroenterology. 2011;140:116–1173. e3. doi: 10.1053/j.gastro.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Feldstein AC, Perrin N, Rosales AG, et al. Effect of a multimodal reminder program on repeat mammogram screening. Am J Prev Med. 2009;37:94–101. doi: 10.1016/j.amepre.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Brien PE. Bariatric surgery: mechanisms, indications and outcomes. J Gastroenterol Hepatol. 2010;25:1358–1365. doi: 10.1111/j.1440-1746.2010.06391.x. [DOI] [PubMed] [Google Scholar]
- 23.Pannain S, Mokhlesi B. Bariatric surgery and its impact on sleep architecture, sleep-disordered breathing, and metabolism. Best Pract Res Clin Endocrinol Metab. 2010;24:745–761. doi: 10.1016/j.beem.2010.07.007. [DOI] [PubMed] [Google Scholar]