Abstract
Hybrid agarose hydrogels embedded with pH-responsive diblock copolymers micelles were developed to achieve functional hydrogels capable of stimulus-triggered drug release. Specifically, a well-defined poly(ethylene oxide) (PEO)-based diblock copolymer, PEO-bpoly(2-(N,N-diisopropylamino)ethyl methacrylate) (PEO113-b-PDPAEMA31, where the subscripts represent the degrees of polymerization of two blocks), was synthesized by atom transfer radical polymerization. PDPAEMA is a pH-responsive polymer with a pKa value of 6.3. The PEO113-b-PDPAEMA31 micelles were formed by a solvent-switching method, and their pH-dependent dissociation behavior was investigated by dynamic light scattering and fluorescence spectroscopy. Both studies indicated that the micelles were completely disassembled at pH = 6.40.
The biocompatibility of PEO113-b-PDPAEMA31 micelles was demonstrated by in vitro primary cortical neural culture. Hybrid agarose hydrogels were made by cooling agarose solutions that contained various amounts of PEO113-b-PDPAEMA31 micelles at either 2 or 4 °C. Rheological measurements showed that the mechanical properties of gels were not significantly adversely affected by the incorporation of diblock copolymer micelles with a concentration as high as 5.0 mg/g. Using Nile Red as a model hydrophobic drug, its incorporation into the core of diblock copolymer micelles was demonstrated. Characterized by fluorescent spectroscopy, the release of Nile Red from the hybrid hydrogel was shown to be controllable by pH due to the responsiveness of the block copolymer micelles. Based on the prominent use of agarose gels as scaffolds for cell transplantation for neural repair, the hybrid hydrogels embedded with stimuli-responsive block copolymer micelles could allow the controlled delivery of hydrophobic neuroprotective agents to improve survival of transplanted cells in tune with signals from the surrounding pathological environment.
Keywords: hydrogels, agarose, block copolymers, pH responsive, micelles, neurons
Introduction
Hydrogels, both natural and synthetic, have been extensively explored as scaffolds for tissue repair.1-5 The high level of interest is fueled by the structural resemblance of hydrogels to that of extracellular matrix, hence providing a niche environment that is favorable for both survival and functions of transplanted cells. Hydrogels are especially attractive for neural repair due to their unique properties.6-8 In particular, hydrogels can be easily tailored to be mechanically compliant with host neural tissues, an important aspect to consider given the increasing knowledge on the critical influence of scaffold stiffness on cell differentiation and functions.9,10 Furthermore, the well-hydrated macroporous network exhibited by hydrogels permits mass transports and cellular outgrowth.
Among various types of hydrogels reported for neural repair to date, agarose has found applications both in the central nervous system11-16 and the peripheral nervous system.12,17 Agarose is a biocompatible polysaccharide derived from red algae.18 It displays thermo-reversible gelation, a feature favorable for minimally invasive injection and in situ gelation at the site of injury.11 The mechanical properties of agarose gels are easily tunable to be comparable with those of neural tissue simply by varying the solution concentrations.19-21 To further expand the functional role of agarose in neural repair, researchers have studied a number of strategies to address different needs. For example, cell adhesion motifs can be introduced into agarose hydrogels either by physical blending20 or chemical immobilization22-24 to improve cell adhesion. Another strategy is to incorporate soluble biomolecules such as growth factors and other proteins to improve cell survival and differentiation.25 The delivery of biomolecules can be achieved by direct inclusion within the hydrogel matrix,17 or encapsulation inside some sort of carriers such as lipid microtubules followed by blending with the hydrogel.11,14,26
While the aforementioned treatments provide viable options to further improve the potential of agarose hydrogels for neural repair, it is important to take into consideration the hostility of the local environment where the hydrogels will be implanted and design strategies accordingly. A damaged neural tissue region is known to present a very aggressive pathological microenvironment with some of the common characteristics of neuroinflammation, that is, the presence of various neurotoxic factors.27 Therefore, integrating a protective mechanism into the hydrogel scaffold to achieve localized delivery of therapeutic agents against neuroinflammation could be beneficial. Considering the hydrophobic nature of commonly used anti-inflammatory drugs and anti-oxidants, it is desirable to utilize a carrier to encapsulate the hydrophobic drugs and incorporate it into the hydrophilic agarose hydrogel matrix. For example, anti-inflammatory drug methylprednisolone was loaded into nanoparticles made from synthetic biodegradable copolymer poly(lactide-co-glycolide) (PLGA) and used in conjunction with agarose hydrogels to treat spinal cord injury in a rat model.28,29 Therapeutic effect was achieved as the polymers degraded and released drugs locally. To gain more active control of the release, it is advantageous to effectively capitalize on the pathological tissue environment and use it to trigger the release. Such an approach allows the intended protective therapeutic actions to be exerted in tune with signals from the surrounding tissue environment, thus maximizing the efficiency. In this regard, pH-responsive carriers can be engineered to utilize the fact of inflamed pathological tissue being more acidic than healthy tissue.30-33 By incorporating pH-responsive carriers into the hydrogel matrix to enable triggered drug release, we can potentially extend the functional role of agarose hydrogels in supporting neural repair.
Here we focus on developing pH-responsive block copolymer micelles as drug carriers for use in hydrogel scaffolds for neural repair. These micelles are formed from block copolymers in aqueous solution,34-39 allowing encapsulation of hydrophobic molecules in the core. By carefully selecting the constituents of the block copolymers, the formed micelles can undergo dissociation upon pH changes, causing drug release. Specifically, a poly(ethylene oxide) (PEO) based diblock copolymer, PEO-b-poly(2-(N,N-diisopropylamino)ethyl methacrylate) (PEO-b-PDPAEMA),38,39 was used in this study, with tertiary amine groups introduced to the second block to impart pH sensitivity. The degree of protonation of PDPAEMA, and thus the hydrophilicity/hydrophobicity balance of the PDPAEMA block, can be precisely controlled by changing the solution pH. One attractive feature of tertiary amine-containing polymers is that at acidic pH values they become hydrophilic. This change will induce the dissociation of block copolymer micelles and subsequently the release of the payload.
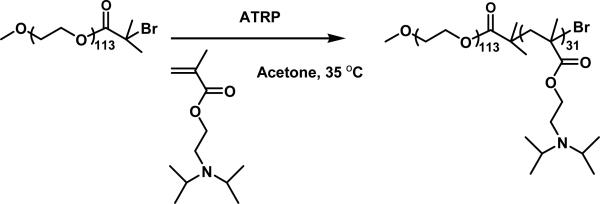
In the present work, PEO-b-PDPAEMA was synthesized by atom transfer radical polymerization of DPAEMA from a PEO macroinitiator (Scheme 1). The solution behavior of PEO-b-PDPAEMA was first studied in order to determine the critical pH value of micelle dissociation. The biocompatibility of the PEO-b-PDPAEMA micelles was demonstrated through in vitro primary cortical neural culture. Hybrid hydrogels embedded with PEO-b-PDPAEMA micelles were made by cooling 1.0 wt% agarose solutions that contained various concentrations of block copolymer micelles at lower temperatures. It was found that the mechanical properties of the hydrogels were not significantly adversely affected even when the concentration of PEO-b-PDPAEMA was as high as 5.0 mg/g. Nile Red was used as a model hydrophobic drug and loaded into the core of micelles. The controlled release behavior of Nile Red in a hybrid hydrogel was studied by fluorescence spectroscopy. Note that hydrogels containing block copolymer micelles have been reported.40,41 For example, Gong et al. reported a novel composite delivery system composed of drug-loaded PEO-b-poly(ε-caprolactone)-b-PEO micelles in synthetic physical gels.40 The novelty of our present work lies in the use of stimuli-responsive block copolymer micelles to expand the functions of agarose hydrogels which possess many desired properties for biomedical engineering, and thus this work could open a new avenue for the applications of agarose hydrogels.
Scheme 1.
Synthesis of Poly(ethylene oxide)-b-poly(2-(N,N-diisopropylamino)ethyl methacrylate) (PEO-b-PDPAEMA) by atom transfer radical polymerization (ATRP) of DPAEMA from a PEO macroinitiator in acetone.
Experimental Section
Materials
Poly(ethylene oxide) monomethyl ether (PEO-OH, MW = 5000 g/mol) was purchased from Sigma Aldrich. CuBr (98%, Aldrich) was stirred in glacial acetic acid, filtered, and washed sequentially with absolute ethanol and diethyl ether. The purified CuBr was then dried in vacuum and stored in a desiccator. 2-(N,N-Diisopropylamino)ethyl methacrylate (DPAEMA) was synthesized by a one-step reaction between 2-(N,N-diisopropylamino)ethanol (99%, Aldrich) and methacryloyl chloride (Fisher Scientific, 97%); The molecular structure of DPAEMA was confirmed by 1H and 13C NMR spectroscopy. 1,1,4,7,10,10-Hexamethyltriethylenetetramine (HMTETA, 97%) was purchased from Aldrich and used as is.N,N-dimethylformamide (DMF, 99.8%, extra dry), potassium hydrogen phthalate (KHP, primary standard, p.a.), acetone (99.8%, HPLC grade), and Nile Red (99%) were obtained from Acros and used as received. Diethyl ether, 1.0 M KOH solution (volumetric standard solution) and 1.0 M HCl solution (volumetric standard solution) were obtained from Fisher Scientific. SeaPrep® Agarose was purchased from Lonza. A 20 mM aqueous KHP buffer with pH of 3.30 was made by dissolving KHP in Milli-Q water and the pH value was adjusted by adding a 1.0 M HCl solution. The pH values in this work were measured with a pH meter (Accumet AB15 pH meter from Fisher Scientific, calibrated with pH = 4.01, 7.00, and 10.01 standard buffer solutions) at room temperature. All other chemicals and solvents were purchased from either Aldrich or Fisher/Acros and used without further purification.
General Characterization
Size exclusion chromatography (SEC) was conducted at room temperature using PL-GPC 50 Plus (an integrated GPC system from Polymer Laboratories, Inc.). The instrument was equipped with a refractive index detector, one GRAL guard column (8 × 50 mm, 10 micron particles), and two GRAL linear columns (each 8 × 300 mm, 10 micron particles, molecular weight range from 500 to 1,000,000 Da according to Polymer Standards Service-USA, Inc.). N,N-Dimethylformamide was used as the carrier solvent at a flow rate of 1.0 mL/min. The system was calibrated by employing polystyrene standards (Polymer Laboratories, Inc.). The data were processed using Cirrus™ GPC/SEC software (Polymer Laboratories, Inc.). The 1H NMR (300 MHz) spectra were recorded on a Varian Mercury 300 NMR spectrometer with CDCl3 as solvent.
Synthesis of pH-Responsive Diblock Copolymer PEO-b-poly(2-(N,N-diisopropylamino)ethyl methacrylate) (PEO-b-PDPAEMA)
The monofunctionalized PEO macroinitiator (PEO-Br) was prepared by the reaction of poly(ethylene oxide) monomethyl ether (PEO-OH, MW = 5000 g/mol, degree of polymerization (DP) = 113) with 2-bromoisobutyryl bromide as described in previous publications.42,43 The following is the procedure for the synthesis of pH-responsive diblock copolymer PEO-b-PDPAEMA used in the present work.
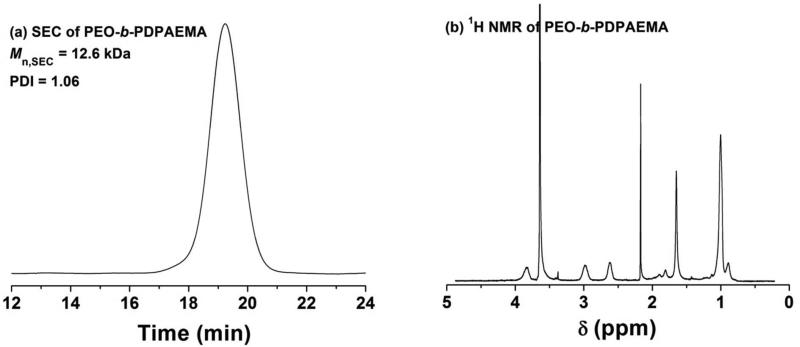
PEO-Br (1.010 g, 0.197 mmol), CuBr (40.9 mg, 0.285 mmol), 1,1,4,7,10,10-hexamethyltriethylenetetramine (HMTETA, 63.5 mg, 0.276 mmol), DPAEMA (1.624 g, 7.62 mmol), and acetone (10.000 g) were added into a 25 mL two-necked flask. The mixture was degassed by three freeze-pump-thaw cycles. The flask was then placed in a 35 °C oil bath; the polymerization was monitored by SEC. After the reaction proceeded for 440 min, the flask was removed from the oil bath and opened to air. The copper complex was removed by passing the reaction mixture through a basic aluminum oxide column using tetrahydrofuran (THF) as solvent. The solution was concentrated by using a rotary evaporator. The polymer was then dissolved in acetone at room temperature, and precipitated in acetone that was cooled in an acetone/dry ice bath. This purification process was repeated additional four times. The diblock copolymer was then dried under high vacuum at 55 °C overnight and analyzed by SEC and 1H NMR spectroscopy analysis. The Mn,SEC and PDI were 12.6 kDa and 1.06, respectively. The DP of the PDPAEMA block was 31, calculated from the 1H NMR spectrum. The diblock copolymer was denoted as PEO113-b-PDPAEMA31, where the subscripts represent the degrees of polymerization of two blocks.
Preparation of PEO113-b-PDPAEMA31 Micelles in Aqueous Buffer Solution
The so-called co-solvent or solvent-switching method was used for the preparation of PEO113-b-PDPAEMA31 micelles in aqueous buffer solutions.44,45 A typical procedure is described below. PEO113-b-PDPAEMA31 (88.3 mg) was dissolved in DMF (0.531 g) and stirred overnight at room temperature. A 1× phosphate buffered saline (PBS buffer) with pH of 7.4 (5.009 g) was added into the DMF solution in a dropwise fashion under the stirring condition to induce the formation of micelles. The polymer solution was then dialyzed against the 1× PBS buffer with pH of 7.4. The buffer solution was changed every hour in the first 8 h and then every 8 h for the next 48 h. A certain amount of a 1× PBS buffer (pH 7.4) was added to the dialyzed solution to adjust the final concentration of PEO113-b-PDPAEMA31 to 10 mg/g. This stock solution was used for other experiments in this work.
Dynamic Light Scattering Study of pH-Induced Dissociation of PEO113-b-PDPAEMA31 Micelles in Aqueous Buffer Solutions
The dynamic light scattering (DLS) study of pH-induced dissociation of PEO113-b-PDPAEMA31 micelles in aqueous buffer solutions was conducted using a Brookhaven Instruments BI-200SM goniometer equipped with a PCI BI-9000AT digital correlator, a solid-state laser (model 25-LHP-928-249, λ = 633 nm), and a temperature controller at a scattering angle of 90°.
The DLS samples were made by first diluting the 10 mg/g polymer stock solution to 1.0 mg/g with a 1× PBS buffer (pH 7.4). A series of micelle solutions with different pH values were prepared by injecting 1.0 M HCl into the 1.0 mg/g PEO113-b-PDPAEMA31 solution via a microsyringe in a stepwise fashion. When a desired pH value was reached, a portion of the solution was taken out for the DLS measurement. The solutions were filtered through a Millipore hydrophilic PTFE filter (0.2 μm pore size) into borosilicate glass tubes with an inner diameter of 7.5 mm. The tubes were sealed with a PE stopper and a Teflon tape. The glass tube was then placed into the cell holder of the DLS instrument. The solution was equilibrated for 30 min at 25 °C prior to the collection of data. The correlation functions were analyzed by CONTIN program. The pH value at which block copolymer micelles were completely dissociated was determined by analyzing the plots of hydrodynamic diameter (Dh) and scattering intensity versus pH.
Fluorescence Spectroscopy Study of pH-Induced Dissociation of PEO113-b-PDPAEMA31 Micelles in Aqueous Buffer Solutions
The pH-induced dissociation of PEO113-b-PDPAEMA31 micelles in aqueous buffer solutions was studied by fluorescence spectroscopy using Nile Red as fluorescence probe. A stock solution of Nile Red in acetone with a concentration of 0.71 mg/g (100 μL) was added into a pre-weighed vial using a microsyringe. The weight of the vial plus the solution was measured immediately. The vial was then dried under high vacuum for 3 h at 55 °C, followed by the addition of a 1 mg/g PEO113-b-PDPAEMA31 solution with pH of 7.4 (20.000 g). The mixture was then sonicated for 30 min and kept in the fridge overnight prior to use. The nominal concentration of Nile Red was 6.7 × 10−6 M. The pH of the Nile Red-loaded micelle solution was adjusted by the injection of 1 M HCl solution in a step-wise fashion. Samples were taken out at different pH values for fluorescence measurements. The fluorescence emission spectra of Nile Red in these solutions were recorded using a PerkinElmer LS 55 fluorescence spectrometer equipped with a 20 kW xenon discharge lamp at room temperature. The slit width was 4 nm. The excitation wavelength was set at 543 nm and the fluorescence emission spectra were collected in the range from 550 to 720 nm.
Primary Cortical Neuron Culture
Cortical neurons were obtained from 10-day-old chicken embryo following previously reported protocols.20 Forebrains of the embryo were dissected, minced into small pieces, and enzymatically dissociated with 0.25% trypsin in PBS for 20 min at 37 °C, followed by inactivation with a medium containing 10% fetal bovine serum (FBS, Invitrogen). A cell pellet was obtained after a brief centrifugation, and mechanical trituration using a fire-polished Pasteur pipet was applied to further dissociate the cells. Cells were then preplated on a collagen-coated Petri dish and incubated for 1 h at 37 °C in a 5% CO2 atmosphere. It has been reported that this process provides the culture with 97% of neuron composition.46 The purified neuronal cells were then collected and resuspended for seeding.
In Vitro Cytotoxicity Study
The cytotoxicity of the PEO113-b-PDPAEMA31 micelles toward neurons was determined. Cortical neurons were seeded at a density of 250,000 cells/cm2 into wells of 96-well tissue culture plates precoated with poly-L-lysine and cultured in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% L-glutamine at 37 °C in a 5% CO2 atmosphere for 2 days. Cells were then incubated for another 24 h in the presence of the micelles at various concentrations and cell viability was determined using a WST-1 assay (Roche) according to the manufacturer's instruction. Cell viability was investigated by comparing the absorbance to that of the controls without treatment of micelles. Statistical analysis was conducted using Student's t-test. Cell morphology was also examined using a Zeiss Observer A1 inverted optical microscope.
Preparation of 1.0 wt% Agarose Solutions with Different Amounts of PEO113-b-PDPAEMA31 Micelles
A 2.0 wt% agarose solution was first prepared by following the procedure described below. Agarose (0.308 g) and a 1× PBS buffer with pH of 7.4 (15.086 g) were added into a vial. The mixture was stirred vigorously in a 65 °C oil bath until a homogeneous solution was obtained. The vial was then taken out from the oil bath and cooled to room temperature. To prepare a sample with 1.0 wt% agarose and 1.0 mg/g PEO113-b-PDPAEMA31 micelles, a portion of the 2.0 wt% agarose stock solution (0.274 g) was transferred into a vial immediately. A stock solution of PEO113-b-PDPAEMA31 micelles with a concentration of 10 mg/g (0.0547 g) and a 1× PBS buffer with pH of 7.4 (0.220 g) were added into the same vial. The mixture was then stirred vigorously at 65 °C to form a homogeneous solution. The solution was then taken out from the oil bath, cooled to room temperature, and used immediately. The final concentration of agarose and PEO113-b-PDPAEMA31 micelles were 1.0 wt% and 1.0 mg/g, respectively. For other samples with the same concentration of agarose (1.0 wt%) and different amounts of PEO113-b-PDPAEMA31 micelles (0.5, 2.0, 5.0 mg/g), the same procedure was employed. The samples and the unused 2.0 wt% pure agarose stock solution were stored at room temperature. Prior to use, they were reheated and stirred at 65 °C for 15 min, and cooled to room temperature. The solutions were always used immediately after cooling to room temperature. Note that the gelation will not occur within several hours at room temperature for hybrid samples, which is long enough for most of measurements to be completed.
Preparation of a Pure 1.0 wt% Agarose Solution
To prepare a 1.0 wt% agarose solution, the 2.0 wt% agarose stock solution was first heated and stirred at 65 °C for 15 min and then cooled to room temperature. A portion of the solution (0.358 g) was transferred into a vial immediately, and a 1× PBS buffer solution with pH of 7.4 (0.354 g) was added into the vial. The mixture was then stirred vigorously at 65 °C to form a homogeneous solution. The solution was then taken out from the oil bath, cooled to room temperature, and used immediately.
Study of Pure and Hybrid Agarose Hydrogels by Rheological Measurements and Scanning Electron Microscopy
Mechanical characteristics of hydrogels were analyzed through rheological experiments conducted on a TA Instruments rheometer (TA AR 2000ex). A cone-plate geometry with a cone diameter of 20 mm and an angle of 2° (truncation 52 μm) was used; the temperature was controlled by the bottom Peltier plate. Agarose hydrogels are usually prepared by cooling hot aqueous agarose solutions. To mimic this process on the rheometer, a hot solution was first prepared by dissolving agarose in a 1× PBS buffer (pH 7.4) at 65 °C and then cooled to room temperature. No gelation occurred at this stage. 90 μL of the solution was loaded onto the plate of the rheometer. The solvent trap was filled with water and the solvent trap cover was used to minimize water evaporation. The gel was formed on the plate by lowering the temperature. The dynamic viscoelastic properties (dynamic storage modulus G′ and loss modulus G″) were first measured at 2 °C as a function of time by oscillatory shear experiments to determine a proper cooling time. Dynamic strain sweep experiments from strain amplitude of 0.01% to 80% at frequencies of 0.5, 1.0, 2.5, and 5.0 Hz were conducted to determine the linear viscoelastic regime. The dynamic viscoelastic properties of agarose gels and gels embedded with block copolymer micelles were measured by oscillatory shear experiments performed at a fixed frequency of 1 Hz in cooling/heating ramps at a rate of 3 °C/min and a strain amplitude of 1 %. The frequency dependences of G′ and G″ of a sample at selected temperatures were obtained by frequency sweep tests from 0.1 to 100 Hz at a strain amplitude of 1 %.
Hydrogel microstructures were characterized using scanning electron microscopy (SEM). Samples were prepared by lyophilizing frozen agarose hydrogels with or without incorporation of PEO113-b-PDPAEMA31 micelles. The dried samples were then mounted on aluminum studs, sputter-coated with gold, and imaged on a LEO 1525 scanning electron microscope at an accelerating voltage of 3 keV.
Triggered Release of Nile Red from a Hybrid Agarose Hydrogel Embedded with Nile Red-Loaded PEO113-b-PDPAEMA31 Micelles
A stock solution of Nile Red in acetone with a concentration of 0.71 mg/g (25 μL) was added into a pre-weighed vial using a microsyringe. The weight of the vial plus the solution was measured immediately. The vial was then dried under high vacuum at 55 °C for 3 h. The stock solution of PEO113-b-PDPAEMA31 micelles (1.005 g, 10 mg/g) was added into the vial. The solution was diluted to a polymer concentration of 2.0 mg/g by adding a 1× PBS buffer with pH of 7.4. The nominal concentration of Nile Red was 8.5 × 10−6 M. The mixture was then sonicated for 30 min and kept in the fridge overnight prior to use. The previously prepared 2.0 wt% agarose stock solution was reheated and stirred at 65 °C for 15 min. The solution was then cooled to room temperature. A portion of the solution (2.539 g) was transferred into a vial with inner diameter of 20 mm immediately. The Nile Red-loaded 2.0 mg/g PEO113-b-PDPAEMA31 solution (2.561 g) was added into the same vial at room temperature. The mixture was then stirred vigorously at 65 °C to form a homogeneous solution. The solution was then taken out from the oil bath, cooled to room temperature, and used immediately. The final concentrations of PEO113-b-PDPAEMA31 micelles and agarose were 1.0 mg/g and 1.0 wt%, respectively. A portion of the mixture (2.000 g) was transferred into a glass tube and stored in a refrigerator (~ 4 °C) overnight to obtain the Nile Red-loaded hybrid hydrogel. A 20 mM KHP buffer with a pH of 3.30 (3.000 g) was added onto the top of the hybrid agarose hydrogel. In a control experiment, a 1× PBS buffer with pH of 7.4 was added onto the top of another hybrid gel prepared by the same procedure. The fluorescence emission intensity of Nile Red from the gel zone was monitored as a function of time for both samples.
Results and Discussion
Synthesis of PEO-b-PDPAEMA
The pH-responsive block copolymer PEO-b-poly(2-(N,N-diisopropylamino)ethyl methacrylate) (PEO-b-PDPAEMA) was synthesized by atom transfer radical polymerization (ATRP)47 of DPAEMA at 35 °C from a PEO macroinitiator, PEO-Br, using CuBr/1,1,4,7,10,10-hexamethyltriethylenetetramine as catalyst (Scheme 1). The polymer was purified by precipitation of its acetone solution in acetone that was cooled in a dry ice/acetone bath (–78 °C), taking advantage of the solubility difference of PEO-b-PDPAEMA in the same solvent at different temperatures. Figure 1 shows the SEC trace and 1H NMR spectrum of the obtained diblock copolymer. The Mn,SEC and PDI were 12.6 kDa and 1.06 (with respect to polystyrene calibration), respectively, indicating that the diblock copolymer was well-defined. The degree of polymerization (DP) of the PDPAEMA block was calculated by using the DP of PEO (DP = 113) and the integral values of the peaks from 4.10 to 3.40 ppm (-COOCH2- of DPAEMA units and CH2CH2O- of PEO) and the peak from 3.20 to 2.85 ppm (-N(CHCH3CH3)2 of DPAEMA units) in the NMR spectrum. The calculated DP of PDPAEMA was 31. The DP of PEO was 113. The diblock copolymer was denoted as PEO113-b-PDPAEMA31, where the subscripts represent the degrees of polymerization of two blocks.
Figure 1.
(a) SEC trace of PEO113-b-PDPAEMA31. DMF was used as solvent in the SEC analysis. (b) 1H NMR spectrum of PEO113-b-PDPAEMA31. CDCl3 was used as solvent in the 1H NMR spectroscopy analysis.
Dynamic Light Scattering Study of pH-Induced Dissociation of PEO113-b-PDPAEMA31 Micelles
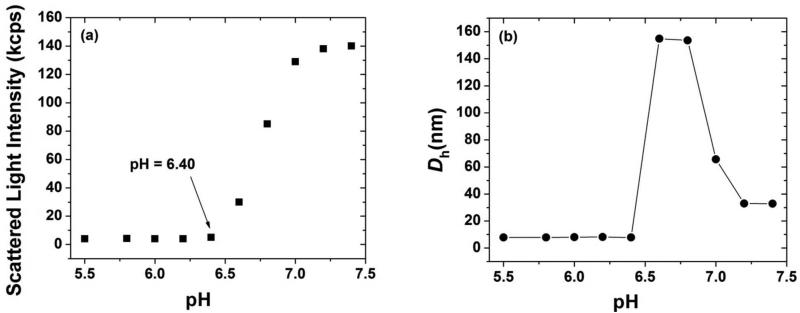
We first studied the dissociation of PEO113-b-PDPAEMA31 micelles in response to pH changes by dynamic light scattering (DLS). A 1.0 mg/g PEO113-b-PDPAEMA31 micelle solution with a pH of 7.40 was prepared by a solvent-switching method. The purified diblock copolymer was dissolved in DMF, followed by the gradual addition of a 1× phosphate buffered saline (PBS buffer) to induce the formation of micelles. The micellar solution was then dialyzed against the 1× PBS buffer. A DLS study showed that the micelles had an apparent hydrodynamic diameter (Dh) of 33 nm and a single size distribution and the scattering intensity was high (~140 kcps). To change the pH, a 1.0 M HCl solution was injected into the solution via a microsyringe in a stepwise fashion. At each desired pH, a portion of the solution was taken out and filtered for DLS measurements. As shown in Figure 2, upon decreasing the pH to 7.20, while the size of micelles remained the same, the scattering intensity decreased slightly. At pH = 7.00, the average Dh increased to 65 nm, and two size distributions were observed. The scattering intensity decreased appreciably. At pH = 6.80 and 6.60, the apparent hydrodynamic size jumped to 150 nm, and there was only one size distribution. Meanwhile, the scattering intensity dropped sharply. When the solution pH was decreased to 6.40 and below, the scattering intensity dropped to below 5 kcps and an average size of 7.8 nm was observed. The values of these two parameters indicated that a unimer state was reached. It was clear from Figure 2 that the micelle-to-unimer transition was complete at pH = 6.40.
Figure 2.
Scattered light intensity at scattering angle of 90° (a) and apparent hydrodynamic size Dh (b), obtained from CONTIN analysis, as a function of pH in a dynamic light scattering study of a 1.0 mg/g solution of PEO113-b-PDPAEMA31 in a 1× PBS buffer.
PDPAEMA is a pH-responsive polymer.38 Using ATRP, we also synthesized a homopolymer of DPAEMA with DP of 38, which was similar to the DP of PDPAEMA in the block copolymer PEO113-b-PDPAEMA31. A titration experiment showed that the pKa of the homopolymer PDPAEMA in water was 6.3 (see Supporting Information), which is the same as the pKa value reported by Zhou et al.38 PDPAEMA undergoes an insoluble-to-soluble transition upon decreasing the solution pH from above to below the pKa. At high pH, the tertiary amine groups in PEO113-b-PDPAEMA31 were not protonated and therefore the PDPAEMA block was insoluble in water. Consequently, micelles were formed with PDPAEMA forming the core and the hydrophilic PEO block constituting the corona. With the decrease of pH, the PDPAEMA block became soluble in water because of the protonation. As a result, the micelles were dissociated. DLS studies showed that with the decrease of pH from 7.40 to 6.40 the scattering intensity of a 1.0 mg/g solution of PEO113-b-PDPAEMA31 decreased and the micelles were completely disassembled at pH of 6.40. The change in Dh is likely due to the reorganization of micelles caused by the gradual introduction of charges onto the PDPAEMA block. Further lowering the pH had no effect on both scattering intensity and Dh, confirming that the block copolymer was in the unimer state.
Fluorescence Spectroscopy Study of pH-Induced Dissociation of PEO113-b-PDPAEMA31 Micelles
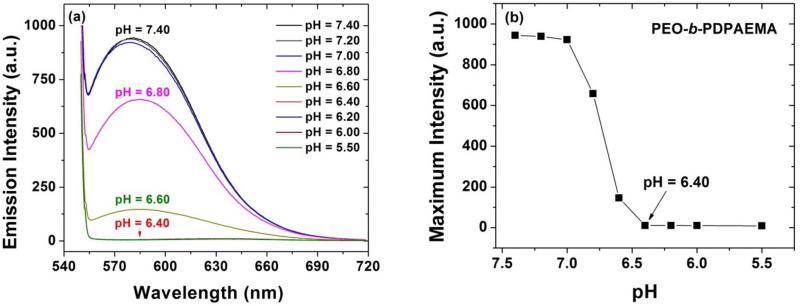
We also studied the pH-triggered dissociation of PEO113-b-PDPAEMA31 micelles by fluorescence spectroscopy. Nile Red, which is hydrophobic, was used as the fluorescent probe 42 and loaded into the micelles of PEO113-b-PDPAEMA31. The pH of the 1.0 mg/g micelle solution was adjusted by gradual addition of a 1.0 M HCl solution, and the emission spectra of Nile Red at various pH values were recorded. Figure 3 shows the fluorescence emission spectrum of Nile Red and the maximum emission intensity as a function of pH. From pH 7.40 to 7.00, the emission spectra almost overlapped and the maximum intensity decreased only very slightly.
Figure 3.
(a) Fluorescence emission spectrum of Nile Red in aqueous buffer solutions of PEO113-b-PDPAEMA31 with a concentration of 1.0 mg/g at various pH values, and (b) plot of maximum fluorescence emission intensity of Nile Red from (a) versus pH.
These results were in agreement with the DLS data that there was little change in the scattering intensity from pH = 7.40, to 7.20, and 7.00 shown in Figure 2a, suggesting that the micelles were stable. When the pH was decreased to 6.80 and 6.60, the maximum intensity dropped sharply (Figure 3). At pH of 6.40 and below, the fluorescence intensity was nearly zero, indicating that the PEO113-b-PDPAEMA31 micelles were dissociated and Nile Red was completely released. Thus, the results from fluorescence spectroscopy further confirmed that the pH value for the complete dissociation of PEO113-b-PDPAEMA31 micelles was 6.40.
In vitro Biocompatibility of PEO113-b-PDPAEMA31 Micelles
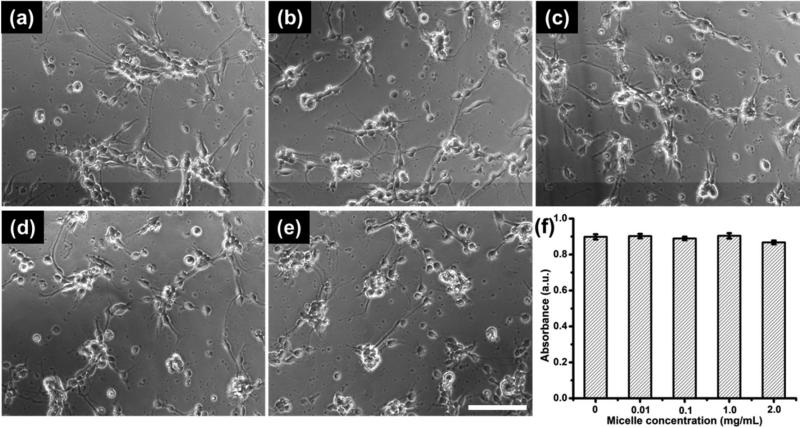
The toxicity of PEO113-b-PDPAEMA31 micelles was studied both qualitatively and quantitatively. Qualitative analysis was achieved by comparing neuron morphology from cultures with or without micelle treatment. As shown from the phase contrast images (Figure 4a-e), cortical neurons were able to extend neurites in the presence of various concentrations of micelles (Figure 4b-e), and no distinct morphological difference was observed when compared with non-treated neurons (Figure 4a). It indicates that within the concentrations studied (0.01 to 2.0 mg/mL), the PEO113-b-PDPAEMA31 micelles did not hinder neurons developing neurites, an important function to consider for neural repair. Furthermore, cytotoxicity was quantitatively examined with a WST-1 assay. This standard assay measures the reduction of a tetrazolium salt by cellular mitochondrial dehydrogenases to a water-soluble salt, which can be photometrically quantified at 450 nm. The absorbance values directly correlate with the number of viable cells. As shown in Figure 4f, no statistical difference was observed in the absorbance results, suggesting that the number of viable cells were comparable among various experimental groups and the control, thus confirming the micelles being non-cytotoxic toward primary neurons in vitro.
Figure 4.
Phase contrast images show morphology of cortical neurons following 24 h treatment with (a) 0 mg/mL, (b) 0.01 mg/mL, (c) 0.10 mg/mL, (d) 1.0 mg/mL, and (e) 2.0 mg/mL micelles respectively. Scale bar = 100 μm. (f) Neural cytotoxicity of the micelles was also quantified using WST-1 assay. The data shown (f) are average ± STD (n = 3).
Rheological Properties and Microstructures of Pure and Hybrid Agarose Gels
We then studied whether the incorporation of PEO113-b-PDPAEMA31 micelles would affect the rheological properties of agarose hydrogels. Of particular interest is the stiffness of the hybrid agarose gels, considering the known close mechanical compliance exhibited by plain agarose gels favoring the use for neural repair. Four agarose solutions that contained different concentrations of PEO113-b-PDPAEMA31 micelles were prepared by mixing a 2.0 wt% solution of agarose in a 1× PBS buffer (pH 7.4) with calculated amounts of a PEO113-b-PDPAEMA31 micelle solution and the 1× PBS buffer (pH 7.4) at room temperature. The final concentrations of agarose in these samples were the same, 1.0 wt%, and the polymer micelle concentration ranged from 0.5 to 1.0, to 2.0, and 5.0 mg/g. For comparison, a pure 1.0 wt% agarose solution was also made. Dynamic time sweep and strain sweep experiments were first performed to determine the appropriate gelation time and the linear viscoelastic regimes of the gels, respectively. Afterwards, cooling and heating ramps as well as frequency sweep experiments were conducted to investigate the effect of the incorporation of PEO113-b-PDPAEMA31 micelles on the gel properties. Note that a DLS study showed that the micelles of a similar diblock copolymer, PEO113-b-PDPAEMA36, in a 1× PBS buffer (pH 7.4) were thermally stable in the studied temperature range from 25 to 65 °C (see the details in Supporting Information).
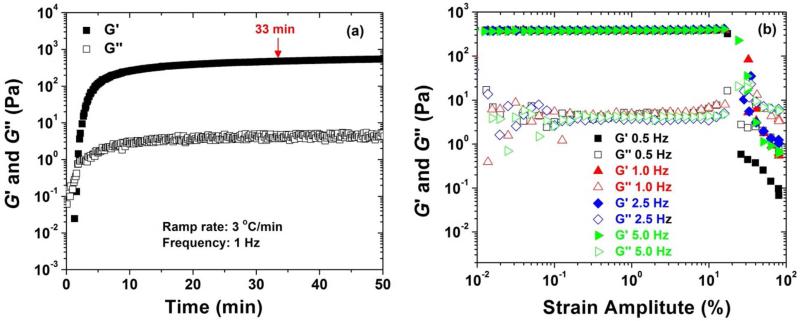
As described in the experimental section, an agarose solution was loaded onto the bottom plate of the rheometer in a liquid form at room temperature and cooled to 2 °C. Figure 5a shows the dynamic storage modulus G′ and G″ loss modulus of a 1.0 wt% agarose solution in the absence of block copolymer micelles as a function of time when the sample was cooled from 25 °C to 2 °C and kept at 2 °C. A strain amplitude of 1.0 % and a frequency of 1 Hz were used. The ramp rate was 3 °C/min, and thus it took ~ 8 min for the temperature to decrease from 25 to 2 °C. In the first several minutes, both G′ and G″ increased sharply with the increase of time. G′ quickly became larger than G″, indicating the formation of a gel. After 5 min, both G′ and G″ increased only slightly with time, and a plateau was reached for each of them. It was found that there was essentially no change for G′ and G″ after 33 min. Therefore, we chose 33 min as the cooling time for gelation. To determine the linear viscoelastic regime, we conducted dynamic strain sweep experiments at 0.5 Hz, 1.0 Hz, 2.5 Hz, and 5.0 Hz.48,49 The data are shown in Figure 5b. Clearly, the linear range was up to 15 % strain. Therefore, a 1 % strain amplitude was employed in other dynamic experiments.
Figure 5.
(a) Dynamic storage modulus (G′) and dynamic loss modulus (G″) as a function of time for a 1.0 wt% agarose solution in a 1× PBS buffer (pH 7.4) upon cooling the sample from 25 to 2 °C and maintaining at 2 °C. A strain amplitude of 1.0 % and a frequency of 1 Hz were used. The cooling rate was 3 °C /min. (b) Dynamic strain amplitude sweeps at frequencies of 0.5, 1.0, 2.5, and 5.0 Hz for a 1 wt% agarose hydrogel. The experiments were conducted at 2 °C after the agarose gel was formed on the bottom plate of the rheometer.
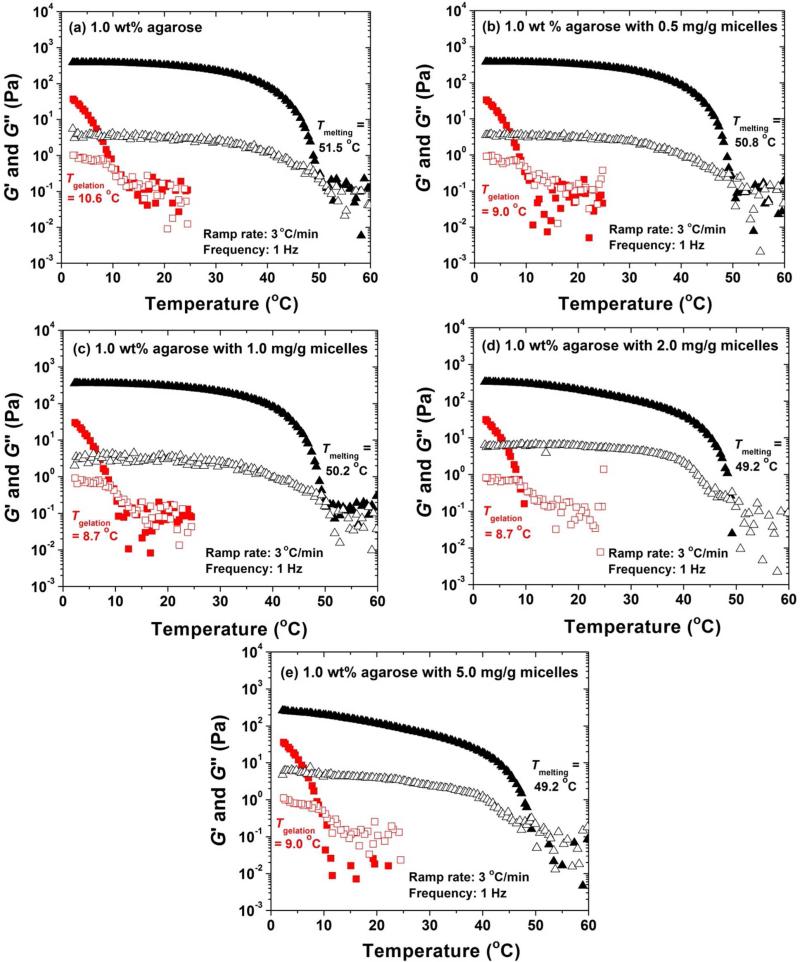
Figure 6 shows the cooling and heating ramps of a pure 1.0 wt% agarose solution and four mixed 1.0 wt% agarose solutions that contained different concentrations of PEO113-b-PDPAEMA31 micelles (0.5, 1.0, 2.0, and 5.0 mg/g), which were performed at a frequency of 1 Hz and a strain amplitude of 1 %. For each sample, 90 μL of the solution was loaded onto the bottom plate of the rheometer at 25 °C. After a short equilibration at 25 °C, the sample was cooled to 2 °C at a cooling rate of 3 °C/min and then maintained at 2 °C for 25 min (the total cooling time: 33 min). A heating ramp was then started from 2 to 60 °C at a heating rate of 3 °C/min. As can be seen from Figure 6, the shapes of cooling and heating ramps of five samples are similar. If the temperature at which G′ = G″ in the cooling curve is taken as the gelation temperature (Tgelation),50 then it is 10.6, 9.0, 8.7, 8.7, and 9.0 °C for the gels containing 0, 0.5, 1.0, 2.0, and 5.0 mg/g PEO113-b-PDPAEMA31 micelles, respectively. The calculated gelation times were 4.8, 5.3, 5.4, 5.4, and 5.3 min for the samples containing 0, 0.5, 1.0, 2.0, and 5.0 mg/g micelles, respectively. This observation suggests that the addition of diblock copolymer micelles in the studied polymer concentration range has a negligible effect on the gelation temperature and the gelation time. If the temperature at which G′ = G″ in the heating curve is taken as the melting temperature (Tmelting), then it is 51.5 °C for the 1.0 wt% pure agarose gel, 50.8 °C for the gel containing 0.5 mg/g PEO113-b-PDPAEMA31 micelles, 50.2 °C for the gel with 1.0 mg/g micelles, and 49.2 °C for the gel with 2.0 and 5.0 mg/g of micelles. The melting temperature decreased very slightly, indicating that the incorporation of micelles did not significantly affect the Tmelting. The G′ values of five samples at various temperatures from heating curves are summarized in Table 1. With the concentration of PEO113-b-PDPAEMA31 micelles increasing from 0, to 0.5, and to 1.0 mg/g, the G′ values at 2, 25, and 37 °C remained essentially the same. When the micelle concentration in the gel was further increased to 2.0 mg/g and 5.0 mg/g, while G′ values at 2 °C were comparable, the values at 25 and 37 °C decreased. This is likely due to the increased interactions between the PEO corona of PEO113-b-PDPAEMA31 micelles and agarose molecules with the increase of polymer concentration as it is known that PEO can form hydrogen bonds with agarose.51,52
Figure 6.
Dynamic storage modulus G′ (solid triangle and solid square) and loss modulus G″ (hollow symbols) of a 1.0 wt% agarose sample containing a concentration of PEO113-b-PDPAEMA31 micelles of (a) 0, (b) 0.5, (c) 1.0, (d) 2.0, and (e) 5.0 mg/g. The samples were first cooled from 25 to 2 °C (shown in red square) and then equilibrated at 2 °C for 25 min. The whole process took about 33 min. The samples were then heated to 60 °C (shown in black triangle). A cooling/heating rate of 3 °C/min, a strain amplitude of 1.0 %, and a frequency of 1 Hz were used.
Table 1.
G′ Values at Different Temperatures for a Pure Agarose Hydrogel and Four Micelle-Embedded Agarose Hydrogels from Figure 6
| Micelle Concentration (mg/g) | G′ at 2 °C (Pa) | G′ at 25 °C (Pa) | G′ at 37 °C (Pa) |
|---|---|---|---|
| 0 | 389.6 | 284.5 | 131.2 |
| 0.5 | 384.9 | 281.2 | 130.6 |
| 1.0 | 360.5 | 261.0 | 122.2 |
| 2.0 | 336.2 | 150.8 | 58.7 |
| 5.0 | 262.5 | 83.0 | 29.2 |
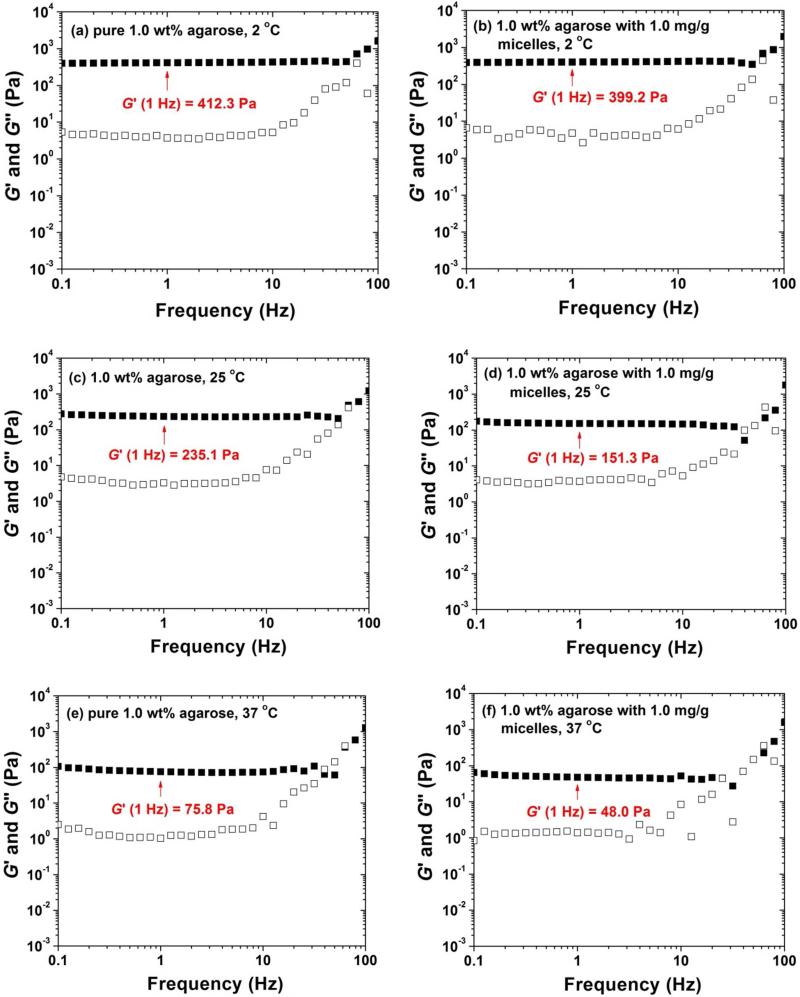
To further investigate the effect of the incorporation of block copolymer micelles on rheological properties of agarose gels, we conducted frequency sweep experiments for five samples at 2 °C, 25 °C, and 37 °C using a strain amplitude of 1 %. Figure 7 shows two sets of representative frequency sweep data of the pure 1.0 wt% agarose gel and the gel embedded with 1 mg/g micelles. The frequency sweep data of other samples can be found in Supporting Information. The shapes of the curves of all samples at the same temperatures were similar.51 The G′ values of five gels at 1 Hz at 2, 25, and 37 °C are summarized in Table 2. Similar to the observations from heating ramps, G′ decreased with increasing the concentration of PEO113-b-PDPAEMA31. However, the values at the same temperature were still comparable. These data indicated that the rheological properties of agarose hydrogels were not significantly affected by the incorporation of PEO113-b-PDPAEMA31 with a concentration up to 5.0 mg/g.
Figure 7.
Frequency dependences of dynamic storage modulus G′ and loss modulus G″ experiments of a 1.0 wt% pure agarose hydrogel at (a) 2, (c) 25, and (e) 37 °C, and of a 1.0 wt% hybrid agarose hydrogel embedded with 1.0 mg/g PEO113-b-PDPAEMA31 micelles at (b) 2, (d) 25, and (f) 37 °C. A strain amplitude of 1 % was used.
Table 2.
G′ Values from Frequency Sweep Experiments at Different Temperatures for a Pure Agarose Hydrogel and Four Hybrid Agarose Gels Embedded With Different Concentrations of PEO113-b-PDPAEMA31 Micelles.
| Micelle Concentration (mg/g) | G′ at 2 °C, 1 Hz (Pa) | G′ at 25 °C, 1 Hz (Pa) | G′ at 37 °C, 1 Hz (Pa) |
|---|---|---|---|
| 0 | 412.3 | 235.1 | 75.8 |
| 0.5 | 400.6 | 198.3 | 63.2 |
| 1.0 | 399.2 | 151.3 | 48.0 |
| 2.0 | 383.7 | 140.9 | 45.4 |
| 5.0 | 353.2 | 112.0 | 33.7 |
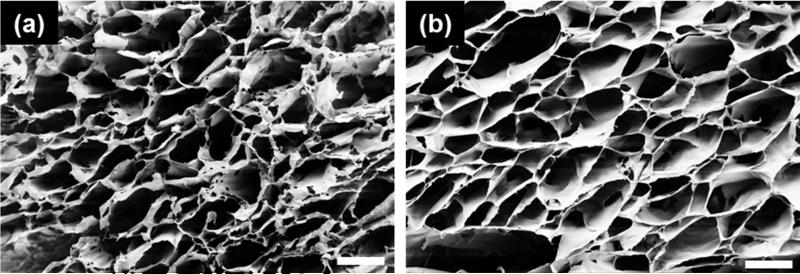
The effect of micelle incorporation on the agarose hydrogel microstructure was studied by scanning electron microscopy (SEM). Since agarose hydrogels are known to have porosities allowing neurite extension, it is imperative that such feature is not compromised by the addition of micelles. Figure 8 shows the SEM images of the cross section of the plain 1.0 wt% agarose hydrogel and 1.0 wt% hybrid agarose gel containing 1.0 mg/g PEO113-b-PDPAEMA31 micelles. Both samples present an open and interconnected porous structure. The pore size was comparable between the two samples, suggesting that the microstructure of agarose was preserved in the presence of micelles, and the hybrid agarose gel could provide structural support of cell growth in a similar manner as agarose could.
Figure 8.
SEM images of the cross sections of 1.0 wt% agarose hydrogels (a) without and (b) with 1.0 mg/g of PEO113-b-PDPAEMA31 micelles. Scale bar = 200 μm.
pH-Induced Release of Nile Red in a Hybrid Agarose Hydrogel Embedded with 1.0 mg/g PEO113-b-PDPAEMA31 Micelles
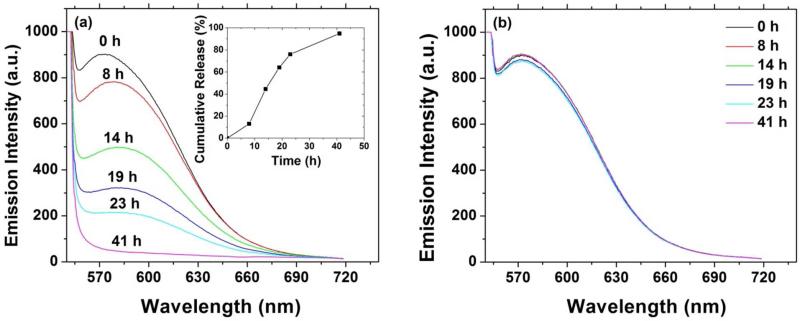
As a demonstration, Nile Red was used as a model substance to study the pH-induced release in a hybrid agarose hydrogel embedded with PEO113-b-PDPAEMA31 micelles. A solution of Nile Red-loaded PEO113-b-PDPAEMA31 micelles in a 1× pH = 7.4 PBS buffer was mixed with a solution of agarose in the same buffer. The final solution contained 1.0 mg/g of block copolymer micelles and 1.0 wt% agarose. The sample was pink, similar to the Nile Red-loaded micelle solution. This indicated that Nile Red was still encapsulated in the core of micelles and the micelles were well dispersed in the final solution. The hybrid hydrogel was formed by cooling the sample to 4 °C and keeping it at 4 °C overnight. The gel was pink, suggesting that the Nile Red-loaded PEO113-b-PDPAEMA31 micelles were stable in the hybrid agarose hydrogel.
To demonstrate the pH-induced release of Nile Red, we designed and conducted the following experiments. Two identical Nile Red-loaded hybrid agarose hydrogels, each 2.000 g, were prepared. A 20 mM KHP buffer with pH of 3.30 (3.000 g) was added onto the top of one gel, and the same amount of a 1× PBS buffer with pH of 7.4 was loaded onto the top of another gel as a control experiment. The fluorescence emission spectra of both gels at various times were recorded and are shown in Figure 9. For the experiment with a pH = 3.30 buffer placed on top of the hydrogel, the maximum emission intensity decreased gradually over the time (Figure 9a). The complete release of Nile Red occurred at ~ 41 h, evidencing that the process was controlled. It was observed that the gel at the beginning of the experiment was pink, but became transparent after 41 h. The inset in Figure 9a shows the cumulative release of Nile red as a function of time from the portion of the hydrogel where fluorescence spectra were collected. For the control experiment, no significant decrease in fluorescent emission intensity was observed (Figure 9b), indicating that the Nile Red-loaded PEO113-b-PDPAEMA31 micelles were stable in the absence of pH trigger during the period of study. This set of release studies demonstrated the pH responsiveness of the newly developed hybrid gel, and its advantage over those embedding degradable polymer (e.g. PLGA) based carriers to gain more active control over release of therapeutic molecules by integrating triggers relevant to the local tissue environment.
Figure 9.
Fluorescence emission spectrum of Nile Red as a function of time for a 1.0 wt% agarose hybrid hydrogel embedded with Nile Red-loaded, 1.0 mg/g PEO113-b-PDPAEMA31 micelles with (a) a 20 mM pH = 3.30 KHP buffer solution and (b) a 1× pH = 7.4 PBS buffer solution placed on top. The inset in Figure 9a shows the cumulative release of Nile Red as a function of time.
Conclusions
A well-defined pH-responsive block copolymer PEO113-b-PDPAEMA31 was synthesized by atom transfer radical polymerization from a PEO macroinitiator. The PEO113-b-PDPAEMA31 micelles were formed by adding a 1× pH = 7.4 PBS buffer into the solution of the block copolymer in DMF followed by dialysis. Dynamic light scattering and fluorescence spectroscopy studies showed that the PEO113-b-PDPAEMA31 micelles were completely dissociated when the pH decreased to 6.40. The biocompatibility of PEO113-b-PDPAEMA31 micelles was demonstrated with cortical neuron cultures. This block copolymer was used to make micelle-embedded hybrid agarose gels. Rheological measurements showed that the gel properties were not significantly affected with the incorporation of PEO113-b-PDPAEMA31 micelles up to 5.0 mg/g. SEM studies showed that the microstructures of a pure agarose gel and a hybrid gel embedded with 1.0 mg/g micelles were similar and the pore sizes were comparable. To demonstrate the pH-induced dissociation of block copolymer micelles and the controlled release in the hybrid hydrogel, Nile Red was used as a model compound and was loaded into the core of PEO113-b-PDPAEMA31 micelles. It was found that when the gel was in contact with a pH 3.30 buffer, the fluorescence emission intensity gradually decreased with the increase of time and the release of Nile Red was complete after 41 h. In contrast, the Nile Red-loaded micelles were stable in the hybrid gel when a pH 7.4 buffer was placed on the top. Using this system, various substances, such as anti-inflammatory agents and neuroprotective agents, can be integrated into the agarose hydrogels to improve their functions as scaffolds for transplantation of regenerative cells for neural repair.
Supplementary Material
Acknowledgements
This work was supported in part by National Science Foundation from awards DMR-1055208 (W.H.) and DMR-1206385 (B.Z.), National Institute of Health Training Grant NIGMS-IMSD R25 GM086761 (E.M.), and by the Joint Institute of Advanced Materials at the University of Tennessee, Knoxville (W.H. and B.Z.). The authors thank Professor Mingjun Zhang and Dr. Yongzhong Wang for allowing us to use their Malvern Zetasizer Nano ZS to study the thermal stability of block copolymer micelles.
Footnotes
Supporting Information Available: Synthesis and pKa of homopolymer PDPAEMA; thermal stability of PEO-b-PDPAEMA micelles; frequency dependences of G′ and G″ of 1.0 wt% agarose hydrogels embedded with 0.5, 2.0, and 5.0 mg/g PEO113-b-PDPAEMA31 micelles, respectively, at 2, 25, and 37 °C. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Lee KY, Mooney DJ. Chem. Rev. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 2.Ratner BD, Bryant SJ. Annu. Rev. Biomed. Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 3.Lutolf MP, Hubbell JA. Nat. Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 4.Yu L, Ding JD. Chem. Soc. Rev. 2008;37:1473–1481. doi: 10.1039/b713009k. [DOI] [PubMed] [Google Scholar]
- 5.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Adv. Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhong Y, Bellamkonda RV. J. R. Soc., Interface. 2008;5:957–975. doi: 10.1098/rsif.2008.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nisbet DR, Crompton KE, Horne MK, Finkelstein DI, Forsythe JS. J. Biomed. Mater. Res., Part B. 2008;87B:251–263. doi: 10.1002/jbm.b.31000. [DOI] [PubMed] [Google Scholar]
- 8.Li XW, Katsanevakis E, Liu XY, Zhang N, Wen XJ. Prog. Polym. Sci. 2012;37:1105–1129. [Google Scholar]
- 9.Engler AJ, Sen S, Sweeney HL, Discher DE. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 10.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Biophys. J. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain A, Kim YT, McKeon RJ, Bellamkonda RV. Biomaterials. 2006;27:497–504. doi: 10.1016/j.biomaterials.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Stokols S, Sakamoto J, Breckon C, Holt T, Weiss J, Tuszynski MH. Tissue Eng. 2006;12:2777–2787. doi: 10.1089/ten.2006.12.2777. [DOI] [PubMed] [Google Scholar]
- 13.Gros T, Sakamoto JS, Blesch A, Havton LA, Tuszynski MH. Biomaterials. 2010;31:6719–6729. doi: 10.1016/j.biomaterials.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 14.Lee H, McKeon RJ, Bellamkonda RV. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3340–3345. doi: 10.1073/pnas.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynam D, Bednark B, Peterson C, Welker D, Gao MY, Sakamoto JS. J. Mater. Sci.: Mater. Med. 2011;22:2119–2130. doi: 10.1007/s10856-011-4387-3. [DOI] [PubMed] [Google Scholar]
- 16.Gao MY, Lu P, Bednark B, Lynam D, Conner JM, Sakamoto JS, Tuszynski MH. Biomaterials. 2013;34:1529–1536. doi: 10.1016/j.biomaterials.2012.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodla MC, Bellamkonda RV. Biomaterials. 2008;29:33–46. doi: 10.1016/j.biomaterials.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rinaudo M. Polym. Int. 2008;57:397–430. [Google Scholar]
- 19.Balgude AP, Yu X, Szymanski A, Bellamkonda RV. Biomaterials. 2001;22:1077–1084. doi: 10.1016/s0142-9612(00)00350-1. [DOI] [PubMed] [Google Scholar]
- 20.Cao Z, Gilbert RJ, He W. Biomacromolecules. 2009;10:2954–2959. doi: 10.1021/bm900670n. [DOI] [PubMed] [Google Scholar]
- 21.Zuidema JM, Pap MM, Jaroch DB, Morrison FA, Gilbert RJ. Acta Biomater. 2011;7:1634–1643. doi: 10.1016/j.actbio.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 22.Bellamkonda R, Ranieri JP, Aebischer P. J. Neurosci. Res. 1995;41:501–509. doi: 10.1002/jnr.490410409. [DOI] [PubMed] [Google Scholar]
- 23.Dillon GP, Yu XJ, Sridharan A, Ranieri JP, Bellamkonda RV. J. Biomater. Sci., Polym. Ed. 1998;9:1049–1069. doi: 10.1163/156856298x00325. [DOI] [PubMed] [Google Scholar]
- 24.Luo Y, Shoichet MS. Nat. Mater. 2004;3:249–253. doi: 10.1038/nmat1092. [DOI] [PubMed] [Google Scholar]
- 25.Willerth SM, Sakiyama-Elbert SE. Adv. Drug Delivery Rev. 2007;59:325–338. doi: 10.1016/j.addr.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu XJ, Dillon GP, Bellamkonda RV. Tissue Eng. 1995;5:291–304. doi: 10.1089/ten.1999.5.291. [DOI] [PubMed] [Google Scholar]
- 27.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chvatal SA, Kim YT, Bratt-Leal AM, Lee HJ, Bellamkonda RV. Biomaterials. 2008;29:1967–1975. doi: 10.1016/j.biomaterials.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YT, Caldwell JM, Bellamkonda RV. Biomaterials. 2009;30:2582–2590. doi: 10.1016/j.biomaterials.2008.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, Vincent A, Fugger L. Nat. Med. 2007;13:1483–1489. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- 31.Gupta AK, Zygun DA, Johnston AJ, Steiner LA, Al-Rawi PG, Chatfield D, Shepherd E, Kirkpatrick PJ, Hutchinson PJ, Menon DK. J. Neurotrauma. 2004;21:678–684. doi: 10.1089/0897715041269722. [DOI] [PubMed] [Google Scholar]
- 32.Clausen T, Khaldi A, Zauner A, Reinert M, Doppenberg E, Menzel M, Soukup J, Alves OL, Bullock MR. J. Neurosurg. 2005;103:597–607. doi: 10.3171/jns.2005.103.4.0597. [DOI] [PubMed] [Google Scholar]
- 33.Cao Y, He W. Biomacromolecules. 2010;11:1298–1307. doi: 10.1021/bm100095t. [DOI] [PubMed] [Google Scholar]
- 34.Gil ES, Hudson SM. Prog. Polym. Sci. 2004;29:1173–1222. [Google Scholar]
- 35.Mai Y, Eisenberg A. Chem. Soc. Rev. 2012;41:5969–5985. doi: 10.1039/c2cs35115c. [DOI] [PubMed] [Google Scholar]
- 36.He CL, Kim SW, Lee DS. J. Controlled Release. 2008;127:189–207. doi: 10.1016/j.jconrel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Bae Y, Fukushima S, Harada A, Kataoka K. Angew. Chem., Int. Ed. 2003;42:4640–4643. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]
- 38.Zhou K, Wang Y, Huang X, Luby-Phelps K, Sumer BD, Gao J. Angew. Chem., Int. Ed. 2011;50:6109–6114. doi: 10.1002/anie.201100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giacomelli FC, Stepánek P, Giacomelli C, Schmidt V, Jäger E, Jäger A, Ulbrich K. Soft Matter. 2011;7:9316–9325. [Google Scholar]
- 40.Gong C, Shi S, Wang X, Wang Y, Fu S, Dong P, Chen L, Zhao X, Wei Y, Qian Z. J. Phys. Chem. B. 2009;113:10183–10188. doi: 10.1021/jp902697d. [DOI] [PubMed] [Google Scholar]
- 41.Gou M, Li X, Dai M, Gong C, Wang X, Xie Y, Deng H, Chen L, Zhao X, Qian Z, Wei Y. Int. J. Pharm. 2008;359:228–233. doi: 10.1016/j.ijpharm.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 42.Jiang XG, Zhao B. Macromolecules. 2008;41:9366–9375. [Google Scholar]
- 43.Jiang XG, Jin S, Zhong QX, Dadmun MD, Zhao B. Macromolecules. 2009;42:8468–8476. [Google Scholar]
- 44.Zhang L, Eisenberg A. J. Am. Chem. Soc. 1996;118:3168–3181. [Google Scholar]
- 45.Zhang L, Eisenberg A. Polym. Adv. Technol. 1998;9:677–699. [Google Scholar]
- 46.Hanson GR, Iversen PL, Partlow LM. Dev. Brain Res. 1982;3:529–545. doi: 10.1016/0165-3806(82)90052-9. [DOI] [PubMed] [Google Scholar]
- 47.Xia JH, Matyjaszewski K. Chem. Rev. 2001;101:2921–2990. doi: 10.1021/cr940534g. [DOI] [PubMed] [Google Scholar]
- 48.Jin NX, Zhang H, Jin S, Dadmun MD, Zhao B. Macromolecules. 2012;45:4790–4800. [Google Scholar]
- 49.Jin NX, Zhang H, Jin S, Dadmun MD, Zhao B. J. Phys. Chem. B. 2012;116:3125–3137. doi: 10.1021/jp300298a. [DOI] [PubMed] [Google Scholar]
- 50.O'Lenick TG, Jin NX, Woodcock JW, Zhao B. J. Phys. Chem. B. 2011;115:2870–2881. doi: 10.1021/jp2001332. [DOI] [PubMed] [Google Scholar]
- 51.The Agarose Monograph, Marine Colloids Division . In: Womer MC, editor. FMC Corporation; Rockland, ME: 1982. [Google Scholar]
- 52.Charlionet R, Levasseur L, Malandain J. J. Electrophoresis. 1996;17:58–66. doi: 10.1002/elps.1150170111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.