Abstract
Study Objectives:
To determine whether the duration of sleep, sleep quality, insomnia, or sleep disturbance was associated with incident breast cancer in the Women's Health Initiative (WHI).
Design:
Prospective cohort study.
Setting:
Women enrolled in one of the Clinical Trial (CT) arms or the Observational Study (OS) from the WHI conducted in the United States.
Participants:
This study included 110,011 women age 50 to 79 years with no history of cancer.
Measurements and Results:
Typical sleep duration, sleep quality, and other self-reported sleep measures over the past 4 weeks were assessed during the screening visits for both the CT and OS participants. The presence of insomnia and level of sleep disturbance was calculated from an index of the WHI Insomnia Rating Scale. The outcome for this study was primary, invasive breast cancer. A total of 5,149 incident cases of breast cancer were identified in this study. No statistically significant associations were found between sleep duration, sleep quality, insomnia, or level of sleep disturbance with the risk of breast cancer after multivariable adjustment. A positive trend was observed for increasing sleeping duration with the risk of estrogen receptor positive breast cancer, but the association estimates for each sleep duration category were weak and nonsignificant.
Conclusions:
This study does not provide strong support for an association between self-reported sleep duration, sleep quality, insomnia, or sleep disturbance with the risk of breast cancer.
Citation:
Vogtmann E; Levitan EB; Hale L; Shikany JM; Shah NA; Endeshaw Y; Lewis CE; Manson JE; Chlebowski RT. Association between sleep and breast cancer incidence among postmenopausal women in the Women's Health Initiative. SLEEP 2013;36(10):1437-1444.
Keywords: Sleep, insomnia, breast cancer, women
INTRODUCTION
Studies conducted among rotating shift workers have suggested that circadian sleep disorders may play a role in the development of breast cancer,1–5 possibly through melatonin suppression or inflammation. Melatonin is mainly produced by the pineal gland with a typical peak in production during the night, but production of melatonin can be inhibited through exposure to light at night.5 Increased exposure to light at night due to shorter sleep duration may lead to a lower production of melatonin, which in turn increases circulating estrogen.6,7 Increased lifetime exposure to endogenous and exogenous estrogen has been previously observed to increase the risk of both premenopausal and postmenopausal breast cancer and may be a link between sleep and breast cancer.8–10 Sleep disturbance has also been observed to induce inflammatory cytokines,11 and inflammation has long been linked to cancer incidence.12 However, the observed association between typical sleep duration, sleep quality, or sleep disturbance and breast cancer has not yielded consistent findings.
One case-control study, including premenopausal and post-menopausal breast cancers, found that longer sleep duration slightly increased the risk of breast cancer so that for each hour increase in self-reported recent sleep, the odds of breast cancer increased by 6%.13 In contrast, the authors of two cohort studies among women in Asia observed inverse associations between sleep duration and breast cancer incidence, but these inverse associations were restricted to postmenopausal women.14,15 There was no association between sleep duration and risk of breast cancer in the Nurses' Health Study in the United States, a Finnish cohort, or an Australian case-control study.16–18 To our knowledge, two published studies investigated the association between sleep quality and breast cancer and no association was detected.17,18 One study considered the association between sleep disturbance and breast cancer and no association was detected.19 Because the associations between sleep measures and the incidence of breast cancer have been inconsistent, the aim of this study was to determine whether the duration of sleep, sleep quality, insomnia, or sleep disturbance was associated with incident breast cancer among participants in the Women's Health Initiative (WHI), a large cohort of postmenopausal women.
METHODS
Study Population
The design and recruitment for the WHI have been previously described in detail.20 Briefly, the WHI consists of four Clinical Trials (CTs) and an Observational Study (OS) with 68,132 and 93,676 women enrolled in the CTs and OS, respectively. For this study, we used data from both the CTs and the OS. The CTs were a Dietary Modification (DM) trial, two Hormone Therapy (HT) trials, and a Calcium and Vitamin D (CaD) trial that recruited participants already enrolled in the DM or HT trials. Post-menopausal women age 50 to 79 years who gave written informed consent and planned to remain in the area for at least 3 years were eligible for the WHI. Exclusion criteria included predicted survival of less than 3 years due to comorbid conditions, medical conditions that precluded adherence and participation, and participation in other CTs. Each of the CTs had specific exclusion criteria that largely included safety concerns. The WHI study protocol was approved by the Institutional Review Boards at all participating institutions. For this study, we excluded any woman with a prior cancer other than nonmelanoma skin cancer (N = 14,849), missing information on prior cancer (N = 1,406), and women with no information on last contact or last follow-up (N = 772). Women who did not provide data on the primary sleep exposures of interest were also excluded (N = 3,117). For multivariable analyses, 31,653 women were missing data on at least one of the covariates of interest, which left 110,011 women for analysis. The distribution of the sleep variables were similar for participants who were included and not included. Being excluded was also not associated with the risk of invasive breast cancer.
Sleep Duration, Sleep Quality, and Insomnia
Typical sleep duration, sleep quality, and other sleep questions from the WHI Insomnia rating scale for sleep over the past 4 weeks were assessed during the screening visits for both the CT and OS participants and have been used in prior WHI analyses.21–23 Sleep variables were reassessed after 1 year for all CT participants and for some randomly selected women every 2 years thereafter. For OS participants, typical sleep duration, sleep quality, and sleep disturbance were reassessed 3 years after baseline. For sleep duration, participants were asked, “about how many hours of sleep did you get in a typical night during the past 4 weeks?” and could respond as 5 h or less, 6 h, 7 h, 8 h, 9 h, or 10 h or more. Because only a small proportion of participants (0.44%) responded with 10 h or more, we combined the groups with 9 h and 10 h or more. For the sleep quality variable, participants were asked, “overall, was your typical night's sleep during the past 4 weeks: very restless, restless, average quality, sound or restful, or very sound or restful.” The presence of insomnia was calculated by combining the responses from the WHI Insomnia Rating Scale, which included five questions assessing whether participants had difficulty falling asleep, woke up several times, woke up early, had difficulty returning to sleep, and the participant's overall sleep quality with variable coding from 0 to 4. The Insomnia Rating scale had a range from 0 to 20, whereas a higher score signified increased sleep disturbance. A score of 9 or greater on the WHI Insomnia Rating Scale indicated problematic insomnia.24 We also created four categories for the sleep disturbance variable (0-3, 4-6, 7-10, and ≥ 11) based on the score distribution as was recently used for a study in the WHI.23
Breast Cancer Ascertainment
Primary invasive breast cancer was the outcome for this study. Participants were considered to have invasive breast cancer if the first invasive cancer identified was breast cancer (99.3%) or if breast cancer was listed as a cause of death on the death certificate (0.7%). Clinical outcome information was collected annually in the OS and every 6 months in the CT. Initial breast cancer reports were confirmed by trained physician adjudicators at the local clinical centers after medical record and pathology report review. Final verification and staging using the Surveillance Epidemiology and End Results (SEER) registry criteria were conducted by coders at the Clinical Coordinating Center. Estrogen receptor (ER) status was available for 92.1% of the breast cancer cases. Data were unavailable for 409 women who had borderline test results (2.7%), tests that were ordered but results unavailable (25.9%), and missing information for ER status (71.4%). For time-to-event analyses, we considered time from baseline to the date of diagnosis of incident breast cancer or censoring at the date of diagnosis of another cancer, loss to follow-up, nonbreast cancer death, or end of study period (September 30, 2010). All of the incident invasive breast cancer outcomes were centrally adjudicated for the CT and OS; however, the CT participants had mammograms at least every 2 years (every year for HT trial participants) throughout the study, whereas the OS participants had mammograms at their personal physicians' discretion. In all participants, information on mammography was collected annually. During the core WHI study (1993-2005), death was adjudicated centrally for the CT and locally for the OS.
Other Covariates of Interest
Data on additional covariates of interest were obtained at baseline for both the CT and OS participants. Demographic variables included age, race/ethnicity, education, family income, and marital status. Reproductive factors included number of live births, age at first birth, age at menarche, age at meno-pause, and postmenopausal hormone use (unopposed estrogen or combined estrogen and progesterone). Medical history variables assessed at baseline were history of benign breast disease and family history of breast cancer. Body mass index (BMI) was calculated using the height and weight measured at the baseline visit (kg/m2). If data on BMI from the baseline visit were missing, we used the first calculated BMI available during follow-up. Behavioral variables, including alcohol intake, smoking status, and total energy expenditure per week from physical activity (metabolic equivalent-hours per week), were also included in analyses.
Statistical Analyses
Because WHI CT and OS participants had slightly different follow-up procedures and breast cancer assessments, we initially tested whether an interaction was present between sleep duration, sleep quality, insomnia, or sleep disturbance score and CT enrollment on the risk of incident breast cancer by comparing models with and without the interaction using the likelihood ratio test. Because none of the likelihood ratio test comparisons was statistically significant at the P < 0.05 level, we conducted all analyses with the CT and OS participants combined with adjustment for CT arm.
Descriptive statistics of the population were presented with demographic, clinical, and sleep quality characteristics of the women stratified by baseline sleep duration categories. Differences by sleep duration category were evaluated with analysis of variance for continuous variables and Pearson chi-square tests for categorical variables. A Cox proportional hazards model was used to estimate hazard ratios (HR) and 95% confidence intervals (CI) between the baseline levels of categorical sleep duration (7 h as a reference), sleep quality (average quality as a reference), insomnia (no insomnia as a reference), and sleep disturbance score (0-3 as a reference) separately, with the incidence of breast cancer, in addition to ER positive and ER negative breast cancers. All initial models included age and CT arm adjustment. Multivariable-adjusted HRs and 95% CIs adjusting for additional potential confounders were calculated for each of the sleep measures separately. We estimated a model including sleep duration and sleep quality and another model including sleep duration and insomnia with all potential confounders to determine whether the two sleep measures were independently associated with breast cancer incidence. We conducted a test for trend across the categories of sleep duration, sleep quality, and sleep disturbance score by entering the categories as a continuous variable. We tested the proportional hazards assumption by including an interaction between the sleep exposure variables and the log of time in the Cox proportional hazards models. The interactions were not statistically significant; therefore, we assumed that the proportional hazards assumption had not been violated.
Several secondary analyses were conducted. First, we conducted the same analyses as mentioned previously, but excluding women in whom breast cancer was diagnosed in the first 3 years after baseline in order to explore whether the HRs were affected by potentially undiagnosed breast cancer cases. Also, we conducted the analyses excluding women who reported taking medication or alcohol in order to promote sleep at any time throughout the study to determine the effect of natural sleep on the risk of breast cancer. We also evaluated effect modification between sleep and breast cancer by BMI category. To evaluate effect modification, we calculated adjusted HRs for the association between the sleep measures and incident breast cancer stratified by category of BMI. A Cox proportional hazards model with time-dependent variables for sleep duration, sleep quality and insomnia was used to evaluate the association between the sleep measures and incident breast cancer allowing for the sleep measures to change over time.
All statistical analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, NC). A two-sided P < 0.05 was considered statistically significant.
RESULTS
Table 1 presents the descriptive statistics for the population by the number of hours of sleep reported by participants at baseline. Participants with fewer hours of sleep were more likely to have very restless sleep, have greater sleep disturbances, have less than a high school education, have lower family incomes, to be non-Hispanic black, Hispanic or another race/ethnicity, be divorced, separated or widowed, and have lower energy expenditures than those with more hours of sleep. Differences were also observed for age at first birth, postmenopausal hormone use, BMI, family history of breast cancer, alcohol intake, and smoking status.
Table 1.
Descriptive statistics for the clinical trial and observational study arms of the Women's Health Initiative by self-reported sleep duration at baseline (N = 110,011)
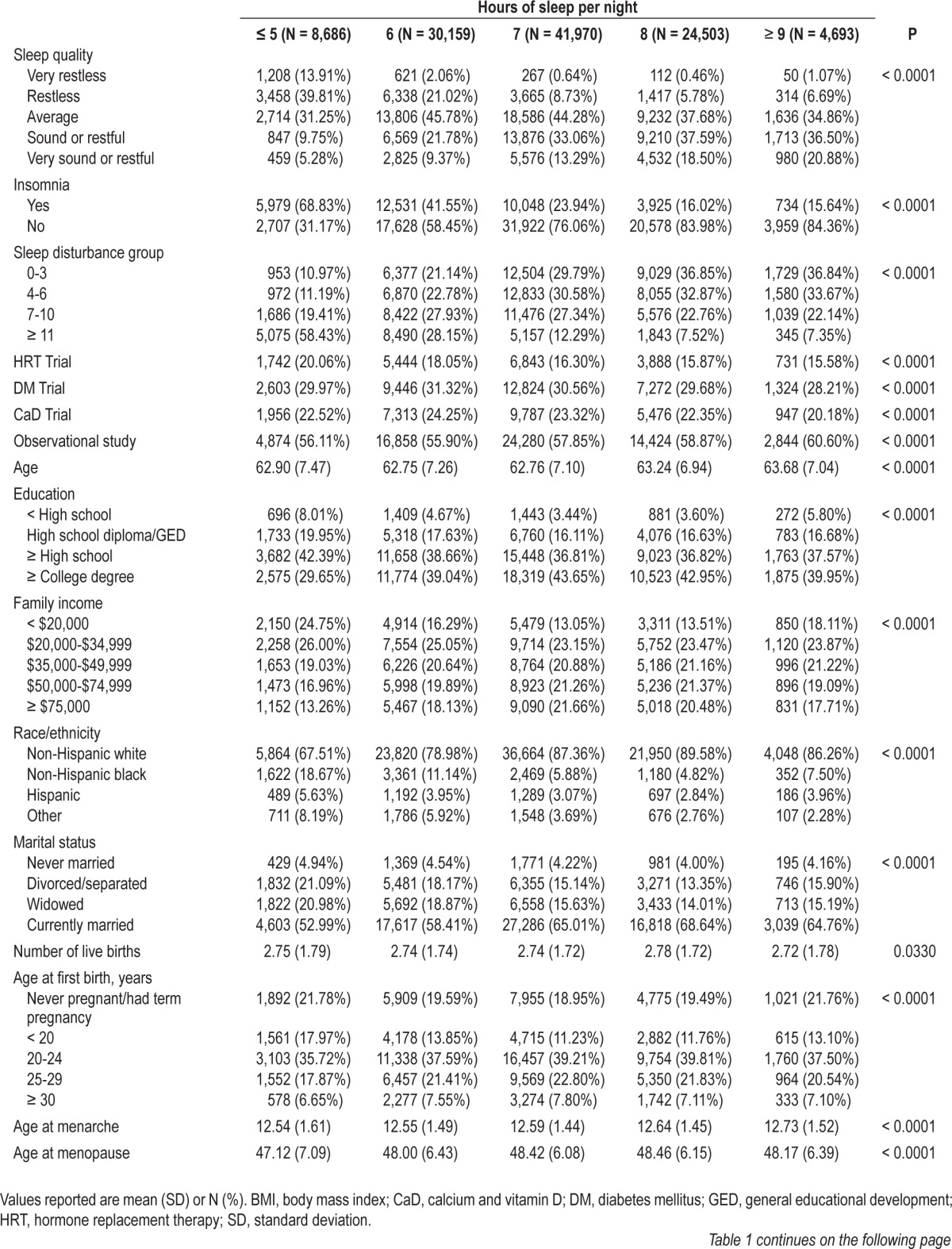
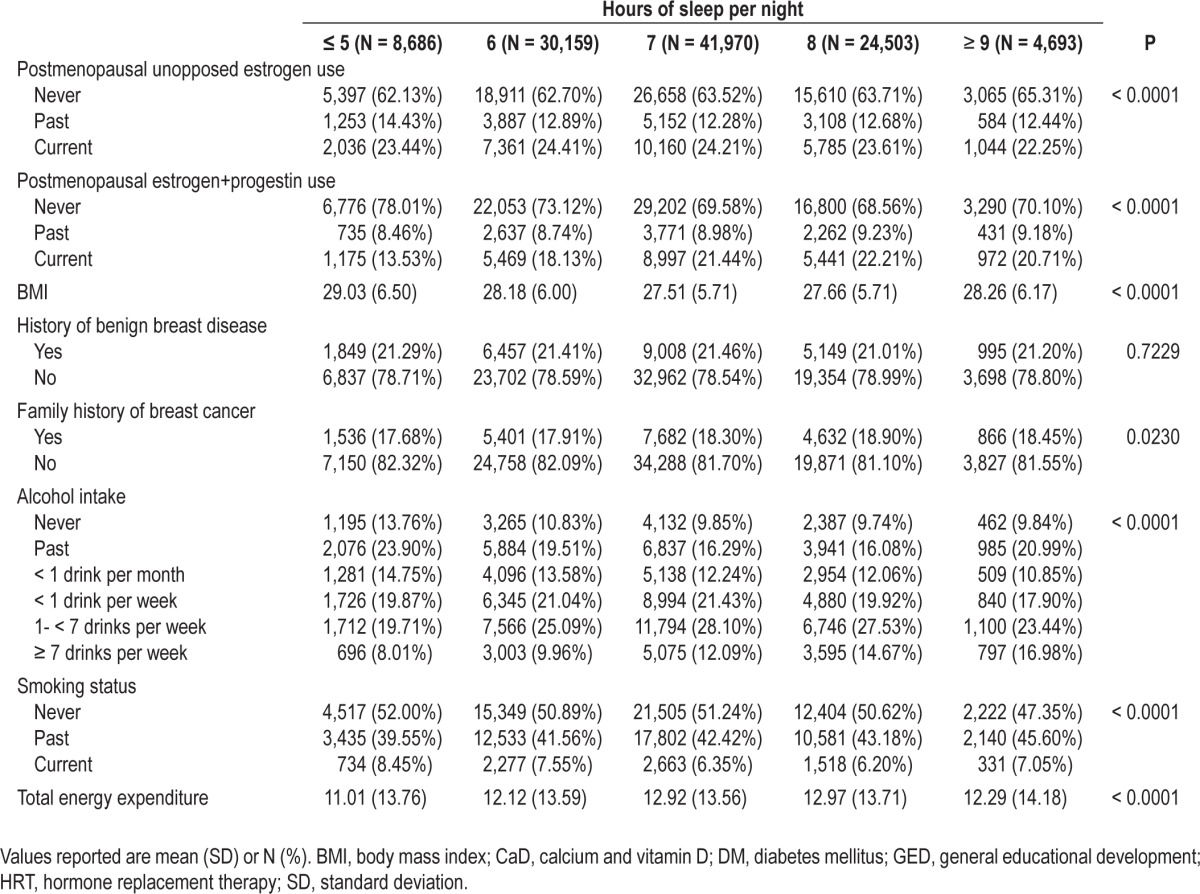
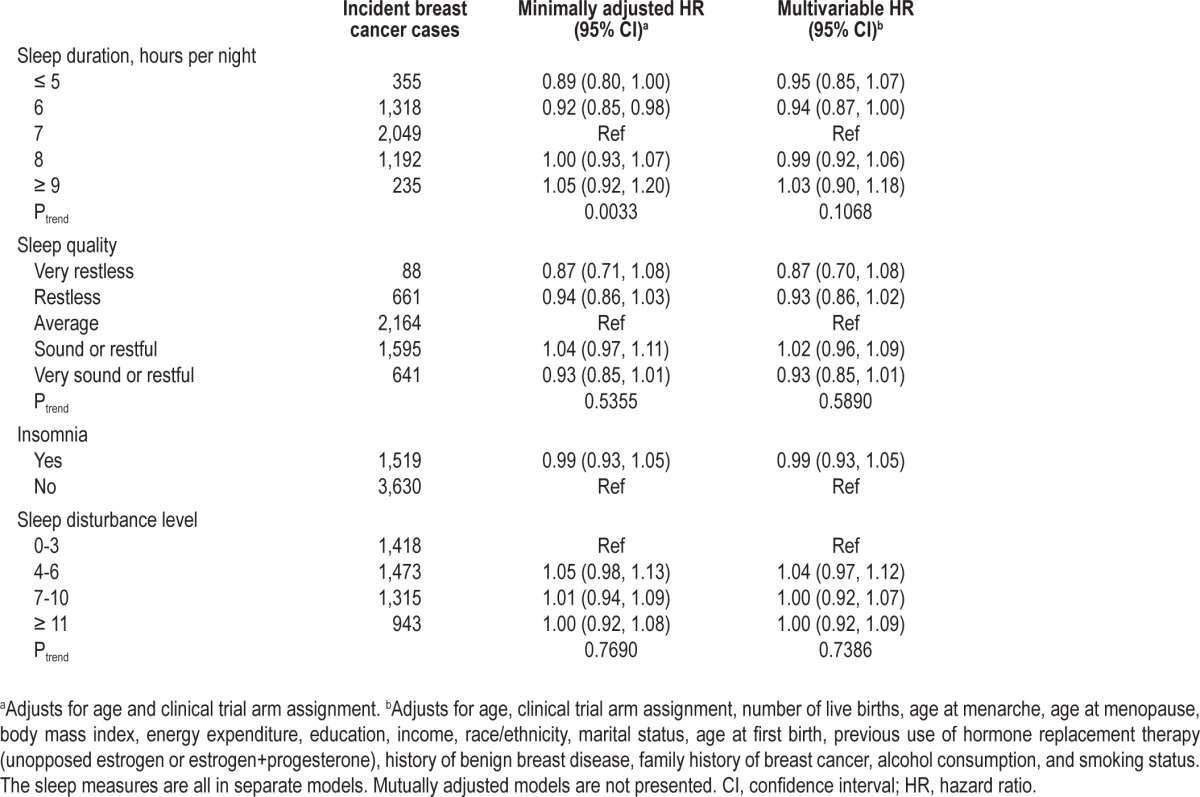
After 1,190,565 cumulative person-years of follow-up in the WHI, 5,149 incident cases of breast cancer were identified. In the minimally adjusted models for sleep duration, compared with 7 h of sleep per night, sleeping 5 h or less (HR 0.89; 95% CI: 0.80, 1.00) or 6 h per night (HR 0.92; 95% CI: 0.85, 0.98) were both inversely associated with breast cancer and a significant trend was identified (Ptrend = 0.0033). However, after adjustment for potential confounders, the associations were no longer significant (Ptrend = 0.1068). No statistically significant associations in minimally adjusted or multivariable analyses were observed between sleep quality, insomnia, or sleep disturbance level and breast cancer risk (Table 2), and no association was observed for the sleep disturbance variable when treated continuously (results not shown). The models that mutually adjusted for both sleep duration and sleep quality yielded similar results and no statistical interaction was observed between sleep duration and sleep quality (results not shown).
Table 2.
Hazard ratios and 95% confidence intervals for the associations between sleep and incident breast cancer in the Women's Health Initiative population (N = 110,011)
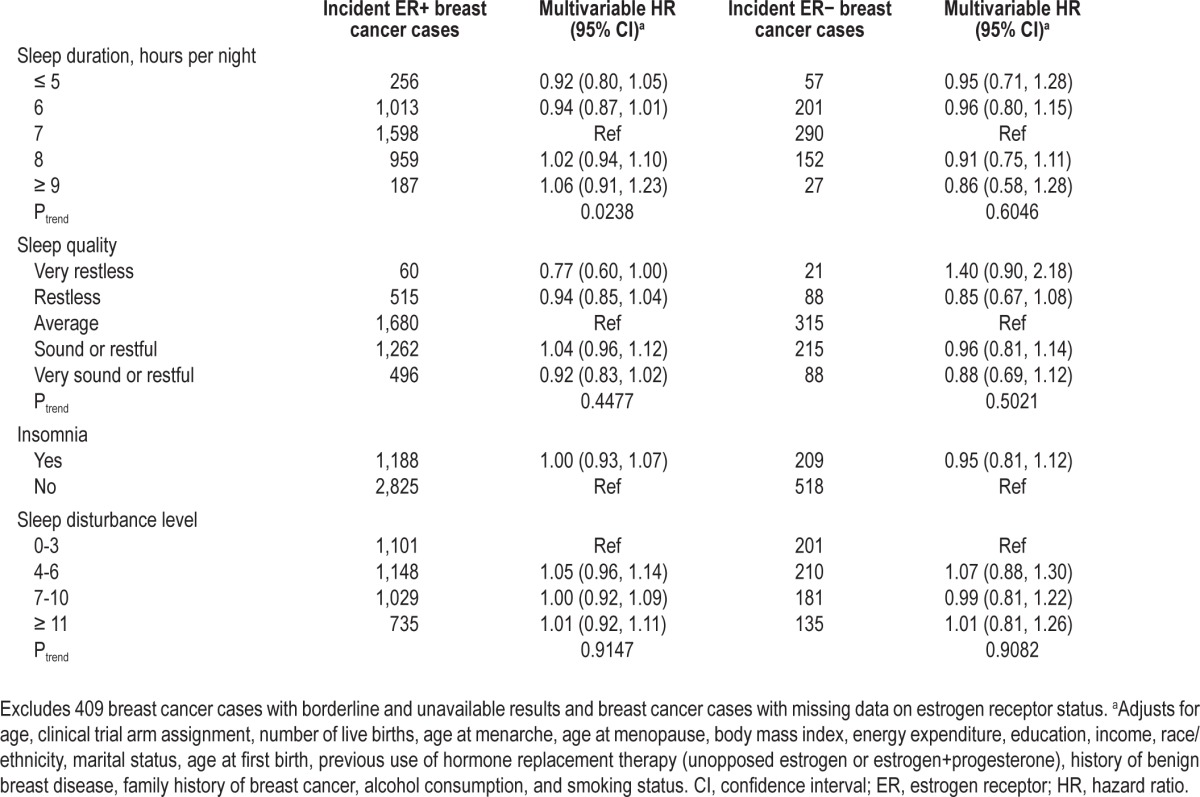
When ER status for incident breast cancers was considered, a positive trend was observed between sleep duration and ER positive breast cancer (Ptrend = 0.0238); however, the individual association estimates each included the null value of 1.0. Compared with 7 h of sleep, women who slept 5 h or less a night had an HR of 0.92 (95% CI: 0.80, 1.05) whereas women who slept 9 h or more a night had an HR of 1.06 (95% CI: 0.91, 1.23) for ER-positive breast cancer. No other trends or associations were observed for sleep quality, insomnia or sleep disturbance with ER-positive breast cancers and no associations were observed for any of the sleep measures with ER-negative breast cancers (Table 3).
Table 3.
Hazard ratios and 95% confidence intervals for the associations between sleep and incident breast cancer by estrogen receptor status in the Women's Health Initiative population (N = 109,602)
Findings were similar after exclusion of participants who developed breast cancer within 3 years of baseline interview (results not shown). In general, the results were similar after stratification by BMI (results not shown), although there was some indication of a positive association between sleep duration and breast cancer risk among the 38,401 women of normal weight (Ptrend = 0.0764). In the multivariable model among normal weight women, compared with 7 h of sleep, the HRs were 1.01 (95% CI: 0.82, 1.26), 0.92 (95% CI: 0.81, 1.04), 1.05 (95% CI: 0.93, 1.19), and 1.12 (95% CI: 0.88, 1.42) for sleeping 5 h or less, and 6, 8, and 9 h or more, respectively. For sleep duration, sleep quality, and sleep disturbance level, the exclusion of participants who reported ever using sleep aids at baseline or follow-up did not materially change results. However, after multivariable adjustment, insomnia appeared to have an inverse relationship with breast cancer (HR 0.92; 95% CI: 0.84, 1.00). Inclusion of the sleep variables as time-varying covariates did not affect results and insomnia was not associated with the risk of breast cancer (HR 1.00; 95% CI: 0.95, 1.07) (results not shown).
DISCUSSION
In this study including 110,011 postmenopausal women and 5,149 cases of breast cancer, we hypothesized that there would be an inverse association between sleep duration and sleep quality with breast cancer incidence. However, no statistically significant associations were observed between sleep duration, sleep quality, insomnia, or sleep disturbance with breast cancer risk in multivariable adjusted models. Although, a positive trend was observed between sleep duration and ER-positive breast cancer incidence. Various secondary analyses were conducted, such as excluding participants who developed breast cancer within 3 years of baseline interview, stratification by BMI category, and exclusion of participants who reported ever using sleep aids, which included taking medication or alcohol in order to promote sleep, but these secondary analyses generally did not affect our findings.
These null findings are in agreement with the results of two previous cohort studies, the Nurses' Health Study in the United States and the Finnish Twin Cohort, and one case-control study conducted in Australia.16–18 In the Nurses' Health Study, among postmenopausal women, compared with 7 h of sleep per night, the HR for breast cancer was 0.97 (95% CI: 0.82, 1.14) for 5 h or less and 0.96 (95% CI: 0.81, 1.13) for 9 h or more of sleep per night.16 In the Finnish Twin Cohort, which included mainly younger women at baseline, although the principal analyses showed no significant associations between sleep and breast cancer, when the analyses were restricted to women who reported the same average sleep duration per night in 1975 and 1981, women who reported sleeping 9 h or more per night had a multivariable HR of 0.28 for incident breast cancer (95% CI: 0.09, 0.88) compared with those with 7 or 8 h of sleep per night.17 Because these restrictions can lead to survival bias, we instead conducted additional analyses treating self-reported sleep duration as a time-varying covariate and did not find any statistically significant associations. In the Australian case-control study, no association was observed between sleep duration and breast cancer case status, irrespective of ER status.18 Other previous research has found associations between sleep duration and breast cancer incidence that are in contrast with our findings. A previous case-control study conducted in the United States identified a positive trend for breast cancer with a multivariable OR of 1.06 (95% CI: 1.01, 1.11) for every hour increase in recently reported sleep duration.13 In contrast, two cohort studies in Asia observed inverse associations between sleep duration and breast cancer incidence with a risk ratio of 0.67 (95% CI: 0.4, 1.1) for 9 h or more of sleep compared with 6 h or less among postmenopausal women in Singapore and a HR of 0.74 (95% CI: 0.35, 1.59) for 9 h or more compared with 7 h among postmenopausal women in Japan.14,15 The weak positive association between sleep duration and risk of ER positive breast cancer in our study was unexpected because lower melatonin levels are correlated with higher estrogen levels,7 and increased estrogen exposure has been strongly linked to ER positive breast cancer.8,25
To our knowledge, only two studies have investigated the association between sleep quality and breast cancer and, similar to the current study, did not observe a significant association.17,18 Similarly, no association has been previously observed between sleep disturbance and breast cancer risk.19 However, the association between insomnia and breast cancer has, to our knowledge, not been previously assessed. Our finding that problematic insomnia may be inversely associated with breast cancer among women who never took sleep aids was surprising, but a previous study found that insomnia decreased the odds of ovarian cancer, which shares similar risk factors with breast cancer including a number of reproductive factors and exogenous hormone exposure, by 50% (95% CI for the OR: 0.3, 0.8).26 It is possible that insomnia and breast cancer share a common cause that increases the risk of insomnia and decreases the risk of breast cancer. For example, estrogen replacement therapy was found to improve subjective sleep quality,27 so it is possible that women with lower lifetime estrogen exposure were more likely to experience insomnia and also less likely to develop breast cancer. However, this finding should be interpreted with caution because a number of comparisons were conducted and sleep aids were defined by self-reported use of medication or alcohol to promote sleep. But future studies could consider this potential association with breast cancer, particularly among women who do not take medications for sleep, in order to determine whether this was a spurious finding.
Strengths of this study should be noted. First, the WHI is a rigorously designed clinical trial and observational study with carefully assessed outcomes, so incident breast cancer has a low likelihood to be misclassified.28 Similarly, the women in the WHI were well characterized with comprehensive baseline and follow-up information. Second, the results of this study were generally consistent throughout sensitivity analyses, which suggest that in this study population, there was no strong association between the different aspects of sleep and the risk of breast cancer. Finally, this analysis included a large number of breast cancer cases from a large population with a long follow-up period.
However, this study has limitations. For instance, a large proportion of women (22.3%) in this study were excluded due to missing data on one or more covariates; however, it is unlikely that the weak, nonsignificant associations observed in this study would be greatly affected by the exclusion of these participants because, in a sensitivity analysis, age-adjusted association estimates for sleep duration, sleep quality, insomnia, and sleep disturbance score were similar for the sample prior to exclusions based on missing confounders (N = 141,664) compared with the complete case analysis (N = 110,011; results not shown). In addition, all of the sleep data and data on many of the other covariates of interest were obtained by self-report, so participants may have been misclassified on the primary exposure of interest. Direct assessment of sleep through actigraphy or polysomnography were not available for this sample and self-reported sleep duration has poor agreement with sleep measured with actigraphy.29 But self-reported sleep duration has been observed to be correlated with urinary melatonin levels,15 although another study found a slightly increased level of urinary melatonin for women who reported shorter sleep duration.30 However, because interviews were conducted prior to breast cancer development, any potential misclassification of sleep should be nondifferentially related to our outcome of interest. Information on the timing of sleep (daytime or nighttime) was not recorded in this study and therefore the hypothesis for exposure to light at night could not be assessed in this study. We also did not assess the effect of shift work on the risk of breast cancer in this population, but future work could address this association. Finally, although we adjusted for a number of potentially important confounders, residual confounding due to unmeasured confounders could have affected our association estimates.
In conclusion, this large study from the WHI does not provide support for an association between self-reported sleep duration, sleep quality, insomnia, or sleep disturbance with the risk of breast cancer in postmenopausal women. The observed association between insomnia and the risk of breast cancer among women who do not use sleep aides warrants further investigation. Consideration of the association between sleep measures and different breast cancer subtypes could also be considered.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Levitan is a co-investigator on a project supported by Amgen. Dr. Vogtmann was previously employed as a part-time employee of Thomson Reuters Healthcare (now Truven Analytics). Dr. Lewis has received research support from Novo Nordisk. Dr. Chlebowski has received grant support from Amgen and serves as a consultant from Novartis, Astra-Zeneca, Pfizer, and Amgen. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller. Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg. Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker. Women's Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker. This analytic work was conducted at the University of Alabama at Birmingham. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN 268201100003C, HHSN-268201100004C, and HHSN271201100004C. Dr. Vogtmann was funded by the Cancer Prevention and Control Training Program at the University of Alabama at Birmingham through the National Institutes of Health (5R25 CA047888).
REFERENCES
- 1.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93:1557–62. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 2.Pukkala E, Auvinen A, Wahlberg G. Incidence of cancer among Finnish airline cabin attendants, 1967-92. BMJ. 1995;311:649–52. doi: 10.1136/bmj.311.7006.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009;13:257–64. doi: 10.1016/j.smrv.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES. Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer. 2005;41:2023–32. doi: 10.1016/j.ejca.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336:186–95. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- 6.Voordouw BC, Euser R, Verdonk RE, et al. Melatonin and melatoninprogestin combinations alter pituitary-ovarian function in women and can inhibit ovulation. J Clin Endocrinol Metab. 1992;74:108–17. doi: 10.1210/jcem.74.1.1727807. [DOI] [PubMed] [Google Scholar]
- 7.Schernhammer ES, Rosner B, Willett WC, Laden F, Colditz GA, Hankinson SE. Epidemiology of urinary melatonin in women and its relation to other hormones and night work. Cancer Epidemiol Biomarkers Prev. 2004;13:936–43. [PubMed] [Google Scholar]
- 8.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276–85. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 9.Chavez-MacGregor M, Elias SG, Onland-Moret NC, et al. Postmenopausal breast cancer risk and cumulative number of menstrual cycles. Cancer Epidemiol Biomarkers Prev. 2005;14:799–804. doi: 10.1158/1055-9965.EPI-04-0465. [DOI] [PubMed] [Google Scholar]
- 10.Haus EL, Smolensky MH. Shift work and cancer risk: Potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev. 2013;17:273–84. doi: 10.1016/j.smrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 12.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 13.McElroy JA, Newcomb PA, Titus-Ernstoff L, Trentham-Dietz A, Hampton JM, Egan KM. Duration of sleep and breast cancer risk in a large population-based case-control study. J Sleep Res. 2006;15:241–9. doi: 10.1111/j.1365-2869.2006.00523.x. [DOI] [PubMed] [Google Scholar]
- 14.Kakizaki M, Kuriyama S, Sone T, et al. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br J Cancer. 2008;99:1502–5. doi: 10.1038/sj.bjc.6604684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu AH, Wang R, Koh WP, Stanczyk FZ, Lee HP, Yu MC. Sleep duration, melatonin and breast cancer among Chinese women in Singapore. Carcinogenesis. 2008;29:1244–8. doi: 10.1093/carcin/bgn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinheiro SP, Schernhammer ES, Tworoger SS, Michels KB. A prospective study on habitual duration of sleep and incidence of breast cancer in a large cohort of women. Cancer Res. 2006;66:5521–5. doi: 10.1158/0008-5472.CAN-05-4652. [DOI] [PubMed] [Google Scholar]
- 17.Verkasalo PK, Lillberg K, Stevens RG, et al. Sleep duration and breast cancer: a prospective cohort study. Cancer Res. 2005;65:9595–600. doi: 10.1158/0008-5472.CAN-05-2138. [DOI] [PubMed] [Google Scholar]
- 18.Girschik J, Heyworth J, Fritschi L. Self-reported sleep duration, sleep quality, and breast cancer risk in a population-based case-control study. Am J Epidemiol. 2013;177:316–27. doi: 10.1093/aje/kws422. [DOI] [PubMed] [Google Scholar]
- 19.Girschik J, Fritschi L, Erren TC, Heyworth J. Quantitative exposure metrics for sleep disturbance and their association with breast cancer risk. Cancer Causes Control. 2013;24:919–28. doi: 10.1007/s10552-013-0168-2. [DOI] [PubMed] [Google Scholar]
- 20.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 21.Chen JC, Brunner RL, Ren H, et al. Sleep duration and risk of ischemic stroke in postmenopausal women. Stroke. 2008;39:3185–92. doi: 10.1161/STROKEAHA.108.521773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sturgeon SR, Luisi N, Balasubramanian R, Reeves KW. Sleep duration and endometrial cancer risk. Cancer Causes Control. 2012;23:547–53. doi: 10.1007/s10552-012-9912-2. [DOI] [PubMed] [Google Scholar]
- 23.Luo J, Sands M, Wactawski-Wende J, Song Y, Margolis KL. Sleep Disturbance and Incidence of Thyroid Cancer in Postmenopausal Women The Women's Health Initiative. Am J Epidemiol. 2013;177:42–9. doi: 10.1093/aje/kws193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine DW, Kripke DF, Kaplan RM, et al. Reliability and validity of the Women's Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15:137–48. doi: 10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- 25.Huang WY, Newman B, Millikan RC, Schell MJ, Hulka BS, Moorman PG. Hormone-related factors and risk of breast cancer in relation to estrogen receptor and progesterone receptor status. Am J Epidemiol. 2000;151:703–14. doi: 10.1093/oxfordjournals.aje.a010265. [DOI] [PubMed] [Google Scholar]
- 26.Titus-Ernstoff L, Perez K, Cramer DW, Harlow BL, Baron JA, Greenberg ER. Menstrual and reproductive factors in relation to ovarian cancer risk. Br J Cancer. 2001;84:714–21. doi: 10.1054/bjoc.2000.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saletu-Zyhlarz G, Anderer P, Gruber G, et al. Insomnia related to postmenopausal syndrome and hormone replacement therapy: sleep laboratory studies on baseline differences between patients and controls and double-blind, placebo-controlled investigations on the effects of a novel estrogen-progestogen combination (Climodien, Lafamme) versus estrogen alone. J Sleep Res. 2003;12:239–54. doi: 10.1046/j.1365-2869.2003.00356.x. [DOI] [PubMed] [Google Scholar]
- 28.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13:S122–8. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 29.Girschik J, Fritschi L, Heyworth J, Waters F. Validation of self-reported sleep against actigraphy. J Epidemiol. 2012;22:462–8. doi: 10.2188/jea.JE20120012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schernhammer ES, Hankinson SE. Urinary melatonin levels and postmenopausal breast cancer risk in the Nurses' Health Study cohort. Cancer Epidemiol Biomarkers Prev. 2009;18:74–9. doi: 10.1158/1055-9965.EPI-08-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]


