Abstract
Study Objectives:
To date, cognitive probe paradigms have been used in different guises to obtain reaction time measurements suggestive of an attention bias towards sleep in insomnia. This study adopts a methodology which is novel to sleep research to obtain a continual record of where the eyes—and therefore attention—are being allocated with regard to sleep and neutral stimuli.
Design:
A head mounted eye tracker (Eyelink II,SR Research, Ontario, Canada) was used to monitor eye movements in respect to two words presented on a computer screen, with one word being a sleep positive, sleep negative, or neutral word above or below a second distracter pseudoword. Probability and reaction times were the outcome measures.
Participants:
Sleep group classification was determined by screening interview and PSQI (> 8 = insomnia, < 3 = good sleeper) score.
Measurements and Results:
Those individuals with insomnia took longer to fixate on the target word and remained fixated for less time than the good sleep controls. Word saliency had an effect with longer first fixations on positive and negative sleep words in both sleep groups, with largest effect sizes seen with the insomnia group.
Conclusions:
This overall delay in those with insomnia with regard to vigilance and maintaining attention on the target words moves away from previous attention bias work showing a bias towards sleep, particularly negative, stimuli but is suggestive of a neurocognitive deficit in line with recent research.
Citation:
Woods; HC; Scheepers C; Ross KA; Espie CA; Biello SM. What are you looking at? Moving toward an attentional timeline in insomnia: a novel semantic eye tracking study. SLEEP 2013;36(10):1491-1499.
Keywords: Insomnia, attention, eye-tracking, neurocognitive deficits, fixations
INTRODUCTION
With revisions of diagnostic criteria currently being made for DSM5 (http://www.dsm5.org), understanding the etiology of insomnia has never been more relevant. Daytime impairment, which may include cognitive impairment, is necessary for an insomnia diagnosis and a reported impairment in attention would fit this criteria.
A number of studies have now been carried out establishing the selective attention to sleep of those with insomnia (PI). An attention bias is said to have developed when disproportionate processing resources appear to be automatically allocated to exemplars, as compared with otherwise equivalent stimuli, producing a disproportionate impact on current cognitions. Taylor et al.1 investigated the role of attention bias in the development of persistent insomnia by using an emotional Stroop task with two groups of people with cancer who developed sleep-onset difficulties. Both acute and persistent insomnia groups demonstrated attention bias for cancer-related words, but only the persistent insomnia group demonstrated attention bias for sleep-related words. Using images, Marchetti et al.2 demonstrated that PI selectively attended to sleep related objects using a computerized ICB flicker paradigm by comparing the change detection latencies for sleep and neutral stimuli. PI were significantly faster to respond to sleep object changes compared with good sleepers (GS) and those with delayed sleep phase syndrome as well as significantly slower with neutral changes. Using words, MacPhee Marchetti et al.3 demonstrated that PI were delayed in disengaging from negative sleep words compared to GS and delayed sleep phase syndrome, but no such effect was found for positive sleep or neutral words. This is relevant to the underlying mechanism of the attention bias such as threat and craving proposed by Espie et al.4
With regard to craving, Spiegelhalder et al.5 raised the question of whether sleep related attention bias is due to sleepiness or sleeplessness. Again, using the emotional Stroop presenting sleep and neutral words, the authors found that sleepiness had an impact on bias for sleep words as well as sleep quality. Similarities are then made with the substance dependence literature, where an attention bias has been attributed to craving. In this context, an attention bias to sleep may be due to a greater need for sleep being associated with an attention preference for sleep related stimuli.
The studies outlined above, alongside others, have used both semantic and pictorial stimuli for different presentation times within a number of cognitive probe paradigms. For example, MacMahon et al.6 presented sleep and neutral words in a dot probe paradigm, and Woods et al.7 presented sleep times shown on an alarm clock in a modified Posner paradigm. It therefore feels timely to progress from simple “snapshots” of attention allocation toward establishing the timeline of attention allocation to sleep salient stimuli. The method employed in this study to achieve this continuous timeline is eye tracking. Eye tracking allows us to gather data about eye movements, fixation direction, and duration.8 Information on where an individual is looking and for how long is obtained by cameras monitoring the movement of the darkest part of the eye, the pupil. Comparisons can be made on eye, and overtly attention, movements toward and away from stimuli, as well as how long stimuli are fixated on. In keeping with other psychopathology research, it would be appropriate to do so using stimuli which are positive, negative, and neutral. We intended to investigate the pattern of attention allocation over time that reflects selective attentional processing in insomnia and its association with craving and threat. It was expected that PI will show higher vigilance for sleep related words than GS and maintain attention on negative sleep words for longer than positive sleep and neutral words.
METHODS
Participants
Participants were recruited through advertising online within the University of Glasgow and through advertising on the psychology department's undergraduate student portal for students to obtain course credits. The title of the study as displayed on the departmental website was “People with insomnia wanted,” and the study description read as follows:
“My research interests are sleep and particularly those who have difficulty getting and/or staying asleep. If you:
* have difficulty getting to sleep or
* have difficulty staying asleep and
* one/both of these problems occur 3 or more times per week please get in touch”
If an individual selected the study, an email was sent to the first author notifying their interest and providing a contact email address. An email was then sent to the potential participant requesting a telephone number so they may be contacted to answer questions about their sleep. During this subsequent telephone conversation, the University of Glasgow Sleep Centre Preliminary Screening Interview was completed. This tool allows participants to be prospectively assigned to the PI or GS group, and screened for other sleep disorders as well as physical and/or mental health issues which could possibly affect their sleep. On successfully completing the phone interview and confirming that the individual met the criteria for inclusion in the study, an appointment was made to complete the experiment in the School of Psychology.
Sleep Quality Assessment
The sleep quality assessment begins with the initial recruitment phone call and completion of the University of Glasgow Sleep Centre Preliminary Screening Interview based on a template set out by Morin and Espie.9 This interview presents questions such as “Do you have difficulty sleeping at the moment?” “How long have you had a sleep problem?” and “Do you work shifts, night shifts?” which enables a very accurate, personal account of an individual's sleep quality and factors influencing it to be obtained. It also enables individuals presenting with extreme sleep misperception, idiopathic problems with sleep, and physical or mental health issues to be excluded. Individuals meeting DSM-IV criteria for primary insomnia were recruited into the study. Participants, therefore, reported difficulties with initiation and/or maintenance of sleep lasting ≥ 1 month, and reported the sleep disturbance (or associated daytime fatigue) causing clinically significant distress or impairment in social, occupational, or other important areas of functioning. There was also an absence of another medical or mental disorder to account for sleep disturbance.
On completion of the experimental task, the assessment of sleep quality continued, and the participants completed the Pittsburgh Sleep Quality Index (PSQI)10 as well as the Insomnia Severity Index (ISI).11
In addition to measures of sleep quality, the following measures were administered post-experimental task to assess the daytime impact and cognitive factors which manifest themselves in this 24-h disorder.
The Daytime Functioning and Sleep Attribution Scale (DFSAS)12 is a new insomnia-specific measure to probe daytime impairment and poor sleep attributions. It is a 2-part measure designed to assess impairment in daytime domains commonly reported by individuals with insomnia (part 1), and, importantly, sleep-related attributions in accounting for such reported daytime impairment (part 2). Parts 1 and 2 successfully discriminated PI individuals and normal sleepers (both P < 0.001). Both parts 1 & 2 had high sensitivity and specificity (> 87%). Cronbach α was 0.81 for part 1 and 0.89 for part 2. DFSAS scores (part 1) were positively associated with insomnia severity (ISI; rho = 0.49) and occupational impairment (OISQ; rho = 0.76), and negatively associated with several SF-36 dimensions, including vitality (rho = -0.51), general health (rho = -0.54) and emotional role limitations (rho = -0.043).
The Dysfunctional Beliefs and Attitudes about Sleep Scale (DBAS)13 helps to identify particular salient, irrational, and affect-laden thoughts intruding prior to sleep onset. This 10-item scale is reported as having a satisfactory internal consistency of 0.69 and is sensitive to treatment related improvements in sleep related cognitions as well as a means of descriptive quantification of maladaptive cognitions in insomnia.14
The Sleep Preoccupation Scale (SPS)15 is a 20-item scale which assesses the frequency of sleep-related thoughts, feelings, or behaviors during the day, with higher scores indicating more sleep preoccupation. Items include “I cannot stop thinking about the sleep during the day” and “I keep checking to see if I look tired.” The SPS has been shown to have a high internal reliability in both student and older adult samples (α = 0.79 and 0.82, respectively) and to discriminate late-life insomniacs from normal sleepers.15
Both the DBAS and SPS provide measures of how much participant's thoughts are influencing their sleep or inability to sleep and how much of their cognitive space is being taken up by thinking about their sleep. This is relevant within the context of this study as the concern here is to provide an objective measure of saliency and attraction to/avoidance of attention to sleep.
Inclusion/Exclusion Criteria
PI participants met combined DSM-IV criteria for primary insomnia and ICSD-2 criteria for psychophysiological insomnia as well as scoring > 8 on the PSQI. Buysse et al.10 reported the global PSQI score > 5 yielded a diagnostic sensitivity of 89.6% and specificity of 86.5% (κ = 0.75, P > 0.001) in distinguishing good and poor sleepers. The decision was made to create clinical poles using the PSQI by increasing the score cutoff for PI. Exclusion criteria for PI included active psychological or drug interventions for sleep problems, and suspicion of a sleep disorder other than insomnia. GS were required to score < 3 on the PSQI, report no problems with their sleep, and have no history of sleep problems. Polysomnography to rule out the presence of other sleep disorders such as sleep apnea was not conducted, as the initial clinical interview was thorough enough to identify the presence of sleep disorders beyond insomnia.
Circadian Preference
The Horne and Ostberg Morningness Eveningness Questionnaire (MEQ)16 provides a measure of an individual's circadian preference, i.e., morning-type or evening type. This measure was included as a precautionary measure to ensure differences in circadian phenotype were not accountable for performance variation.
Psychopathology Assessment
In previous studies2,3 on attention bias in insomnia, the Beck Depression Inventory (BDI) and Spielberger Trait Anxiety Inventory (STAI) were used to provide measures of anxiety and depression. The Hospital Anxiety and Depression Scale (HADS)17 provides a clinical diagnostic element as opposed to a predispositional measurement and allows for cutoffs of within normal range, mild, moderate, and severe disordered state; it was used as a measure of psychopathology in this study. HADSA refers to the anxiety subcomponent, and HADSD refers to the depression subcomponent of this measure.
Apparatus and Stimuli
Two words were presented on a computer screen for 3,000 ms, one being a target word and the other a distracter pseudoword (Figure 1). Participants were asked to ignore the distracter and focus on the actual word. The target words were 26 sleep positive, 26 sleep negative, and 26 neutral words, which were paired with pseudowords, resulting in 78 word pairs in total. Each word pair was randomly presented once per participant. Eye movements were tracked throughout the stimulus presentation. Recordings were made using an Eyelink II (SR Research, Ontario, Canada. http://www.sr-research.com/EL_II.html) eye-tracking system set at 500 Hz. The stimuli were presented to participants on a 22-inch computer monitor (total viewing area 20 inches × 51 cm) connected to a Dell Windows XP-based PC, with all words presented in 28-point Courier font. The eye-tracking system consisted of 2 head-mounted cameras in addition to 2 infrared LEDs to illuminate each eye. A camera was used to track the movement of the darkest part of the eye, the pupil, which provided information on where the individual was looking in relation to target and non-target words presented on the screen. A head-tracking camera, mounted to the center of the headband, was used to measure the head position of each participant, and 4 LEDs attached to each corner of the computer screen were viewed by the head-tracking camera while the participant was facing the computer screen. The eye-tracking system could compensate automatically for possible head motions by detecting movements of the 4 LEDs, and the compensation was better than 1 degree over the acceptable range of head motion. For each trial, areas of interest were identified for the area occupied by target and non-target words on each trial, enabling accuracy, approach, and fixation parameters to be determined with respect to the areas of interest. A DOS-based PC was used to record eye movement data. Although binocular registration of eye movement is possible, in this study monocular registration of eye movement was conducted by tracking the dominant eye of the participant, as determined by a subjective test carried out at time of participation.
Figure 1.
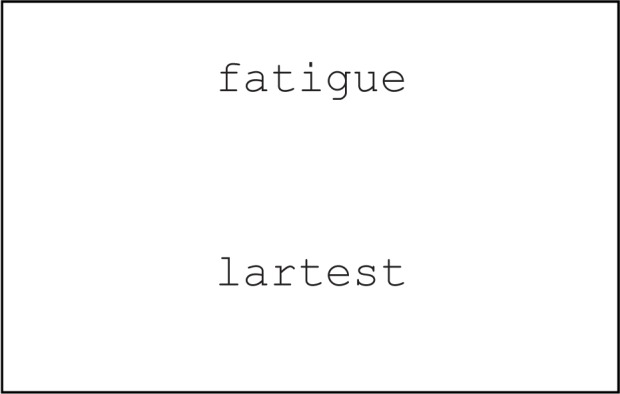
Example of stimulus presented during semantic eye tracking experiment. The position of the target word was counterbalanced across trials. “Fatigue” was the negative target word and “lartest” was the pseudoword.
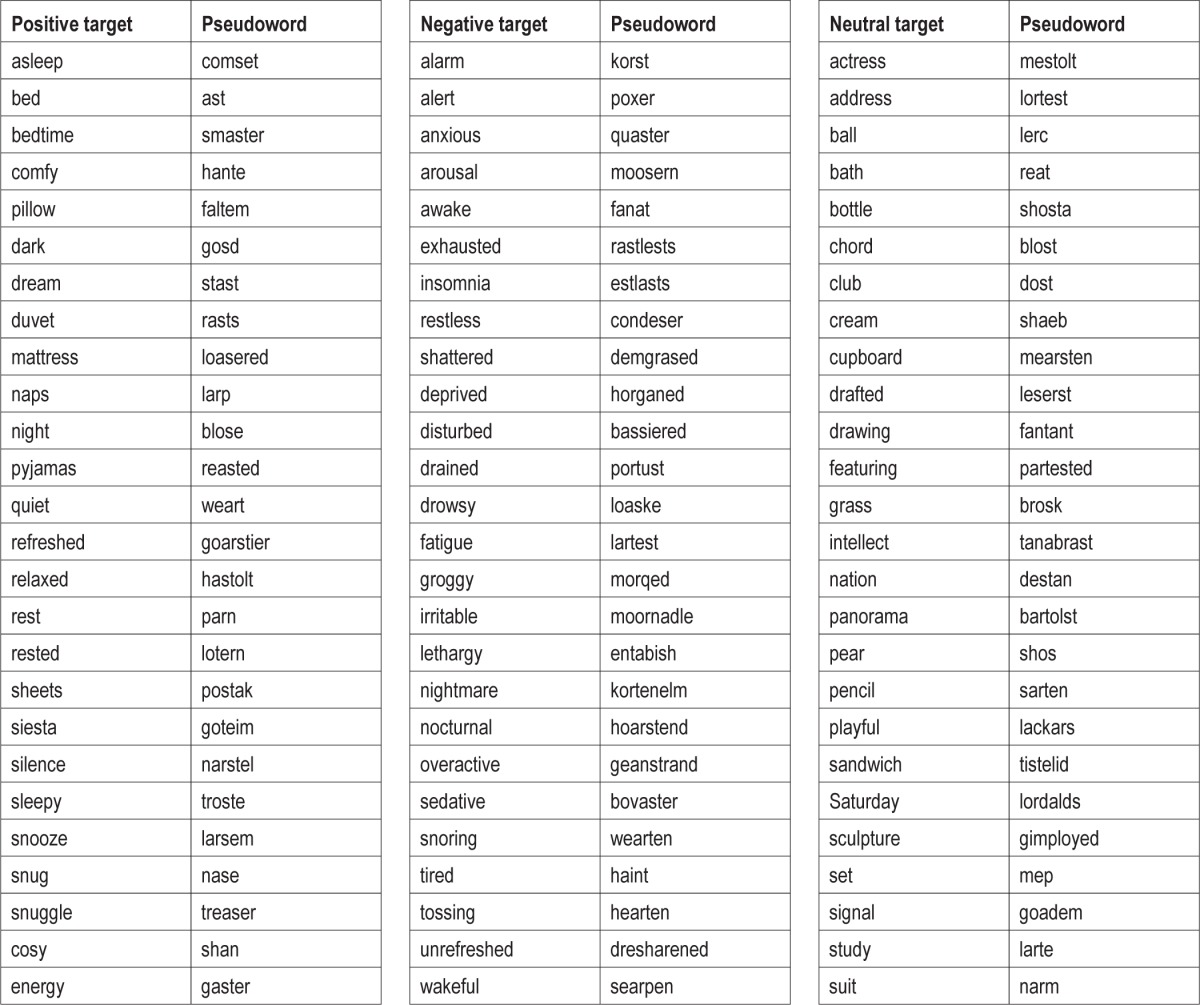
The nature of this experiment required that the saliency of the words presented be as high as possible. The words were therefore taken from previous sleep research on selective attention1,6 having been generated from a study on the content of pre-sleep cognitions in insomnia.18 Each word pair was presented only once per participant, decreasing the number of trials compared to other studies, but the source of the words ensured saliency was high and participants were presented with clinically relevant stimuli. The words used in this experiment can found in Table 1.
Table 1.
Positive sleep-related, negative sleep-related, and neutral target words used as stimuli in semantic eye tracking experiment with matched pseudo words
Procedure
All participants were tested in a quiet room with controlled lighting and underwent a simple test to determine their dominant eye. Participants were then seated in a height-adjustable chair in front of the eye-tracker and asked to rest their chin on a fixed chin rest placed approximately 50 cm from the computer monitor. The eye-tracking cameras were adjusted to best capture the measurements of each participant's dominant eye, and a 9-point calibration cycle was then completed to ensure that the recording of eye movements fell within < 1 degree of visual angle for each calibration point. A 9-point validation cycle was then completed to verify the calibration cycle, and if necessary, both of these cycles were repeated until accurate measurements were obtained.
At the start of the experiment, participants were instructed to focus on the actual word presented to them and to completely ignore the pseudoword. Prior to the start of each trial, a fixation cross was displayed centrally to ensure that the starting location of their gaze was standardized, and the calibration and validation cycles were repeated randomly to confirm that the eye-tracking system was accurately recording eye movements. The experimenter made as little noise as possible while monitoring both the stimulus presentation and eye-tracking measurements. Following the computer task, all the questionnaires were completed and the experiment explained.
Eye Tracking Analysis
In order to prepare the eye-tracking data for subsequent statistical analysis, standard EyeLink acceleration and velocity criteria were used to distinguish between fixations and saccades. Saccades and fixations were defined in terms of motion, acceleration, and velocity thresholds, using the default criteria implemented in the EyeLink software.
Bitmap templates were created for each experimental display, which identified the distracter word, the target word, and the background. The regions of interest were defined by color with red for distracter and green for target words. Fixations occurring within these regions were attributed toward that word. The 3,000-ms time period between the onset of the word pair and the end of the trial was divided into 100-ms time slots, with the number of fixations on the target word counted and converted into fixation probabilities.
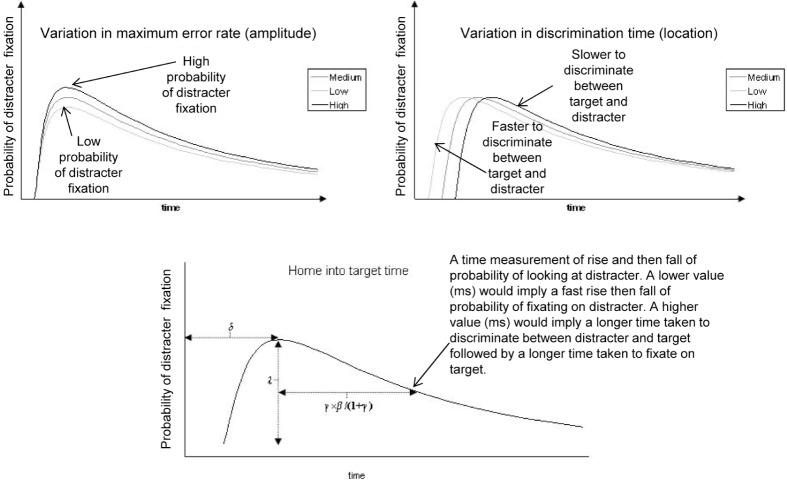
The data produced were filtered to extract the factors of interest, i.e. fixations on target and distracter words for analysis. In the present analyses, we used parameters based on Scheepers et al.,19 which provide information beyond basic fixations and eye movements (Figure 2). Scheepers et al. used the logistic power peak (LPP) function as the best description of the variance both between and within conditions. The measures describe different characteristics of how probabilities of eye movements to the distracter change over time: Amplitude, Location, and Home in to target time (with the latter being derived from the width and symmetry parameter estimates from the 4-parameter log-normal function). As nonlinear estimation of eye-tracking time series data is novel in this research area, more descriptive names have been adopted for ease of understanding.
Figure 2.
Illustration of the logistic power peak (LPP) parameters used to fit the probability difference distributions. The peak of each graph is the point in time where discrimination between target and distracter occurs and probability of fixation on target word increases thereafter.
Maximum error rate (Amplitude)
This is where the probability of the individual looking at the distracter word is at its highest. The probability of fixation on distracter increases to peak and then decreases. This is represented by the peak in the graph and indicates the point at which the probability of fixation on distracter reaches its highest point and then decreases and fixation on target increases. As it represents the point where fixation on distracter is at its highest, it represents where the individual is making the most errors (looking in the wrong place).
Discrimination time (Location)
This is the location in time of the peak probability of distracter fixation described above. It is a measure of time taken for the discrimination between target and distracter to occur.
Home in to target time
This is a measure of fixation on target. As fixation on target never reaches 100%, a measure in time is taken when the right side of the peak (target fixation probability increasing) reaches half of the left side of the peak (distracter fixation increasing). It is a measure of when target fixation considerably outweighs distracter fixation.
Parameters also analyzed in relation to first fixation
Onset: time that elapses between the onset of the visual stimulus presentation until the eye has landed on the target word for the first time.
Duration: onset of first fixation – offset of first fixation (time between start of the first fixation on target until fixation moves or trial finishes).
Parameters
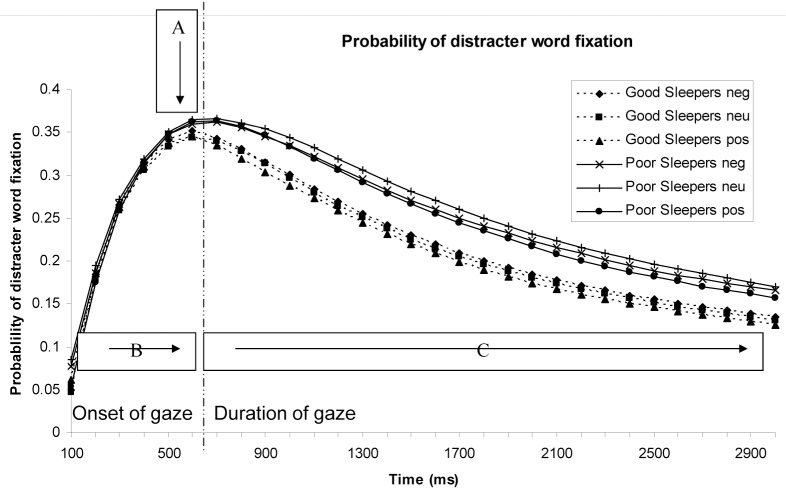
Five parameters were analyzed in total; 3 with regard to vigilance and 2 measuring maintenance of attention. Figure 3 shows the position in time for each parameter. The vigilance parameters were: Onset (ms), Discrimination time (ms), and Maximum error rate (probability). The following 2 parameters were also analyzed in relation to maintenance of attention: Duration of first fixation (ms), and Home in on target time (ms). The effects of interest are participant group (PI and GS) and word condition (sleep positive, sleep negative, and neutral). With regard to target position, the graphical representations of the target word above the distracter were presented for the onset of fixation and duration of fixation parameters, as reading from top to bottom is the natural state (just as from left to right), and therefore produced the largest effect sizes.
Figure 3.
Probability of fixating on distracter word with parameters of interest highlighted; A = maximum error rate (probability), B = discrimination time (location of parameter A in time; ms) and C = home in on target time (ms). Parameters on left of dotted line are used to address the vigilance question and those on right are to address maintenance of gaze.
RESULTS
All data obtained was entered into and analysis was carried out in the statistical program SPSS for Windows, version 15 (Release 15.0.0, 6th Sept. 2006). Mean reaction times were calculated followed by analysis of variance (ANOVA) and Bonferroni corrected post hoc analysis to determine whether manipulating the independent variables (IV) statistically significantly affected the variance within and between the PI and GS groups. Effect sizes were also calculated, which enabled any effects of sleep group or the IVs to be quantified.
The experimental population as a whole consisted of 20 GS and 21 PI. The GS participant group consisted of 49% females and had a mean age of 22 years. The PI group had a mean age of 24 years; 51% were female. The length of disorder ranged from 1 year to 10 years, with a mean duration of 3 years.
To confirm correct allocation to participant group and substantiate the profile of each group, comparisons were made on the questionnaire measures. As expected from the selection criteria, significant differences were found on the PSQI (P < 0.0005) and ISI (P < 0.0005), with PI scoring higher on both (see Table 2 for mean and SD scores for all subjective measures). PI also scored higher than GS on both the HADSA (P < 0.0005) and HADSD (P < 0.0005). With research highlighting the bidirectional relationship of anxiety and depression with insomnia, this was not controlled for but was taken as a characteristic of the insomnia disorder.20 The DBAS (P < 0.00005), DFSAS (P < 0.0005), and SPS (P < 0.0005) all showed the same pattern of higher scores by PI. These measures highlight the cognitive nature of the insomnia disorder present in this PI participant group.
Table 2.
The mean scores and standard deviations of PI and GS on 8 subjective measures of sleep, anxiety, depression, circadian preference, sleep related cognitions, and sleep effort
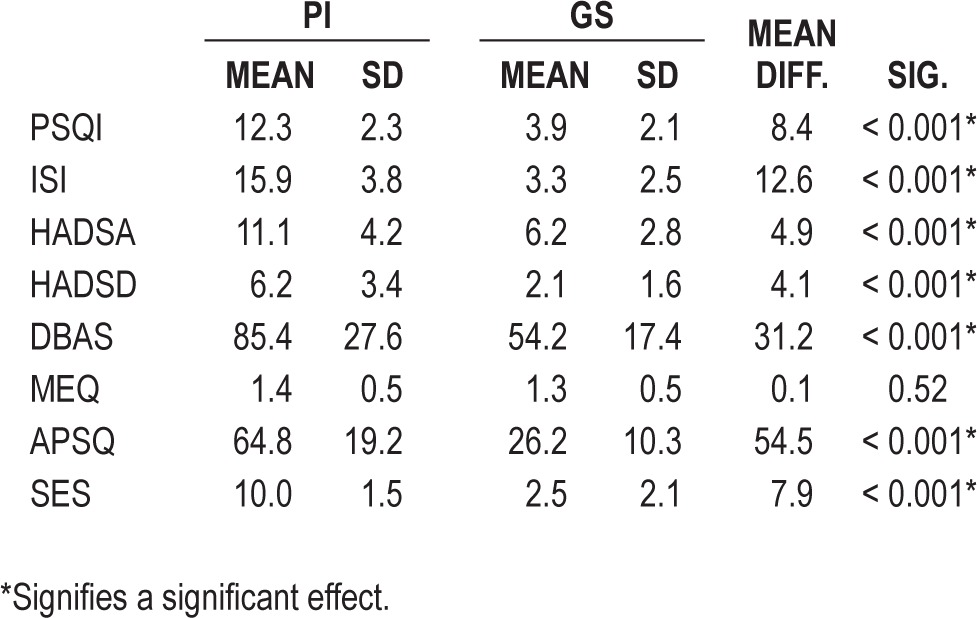
The only nonsignificant difference between the participant groups was found in the MEQ (P = 0.56), suggesting that PI had no significant circadian preference compared to GS.
Vigilance measures as shown in Figure 3 will be considered first: onset of first fixation, discrimination time, and maximum error rate.
Onset
Figure 4A suggests that PI started to first fixate on the target word later than GS irrespective of word salience. This observation was confirmed by formal analysis (P < 0.005), including the expected target position effect (P < 0.005). Between participant effect sizes were small; sleep negative d = 0.06, neutral d = 0.13, and sleep positive d = 0.08. There was no significant effect of word condition or interactions between sleep quality and condition, sleep quality × word condition × target position, word condition × target position, or sleep quality × target position (all P > 0.05).
Figure 4.
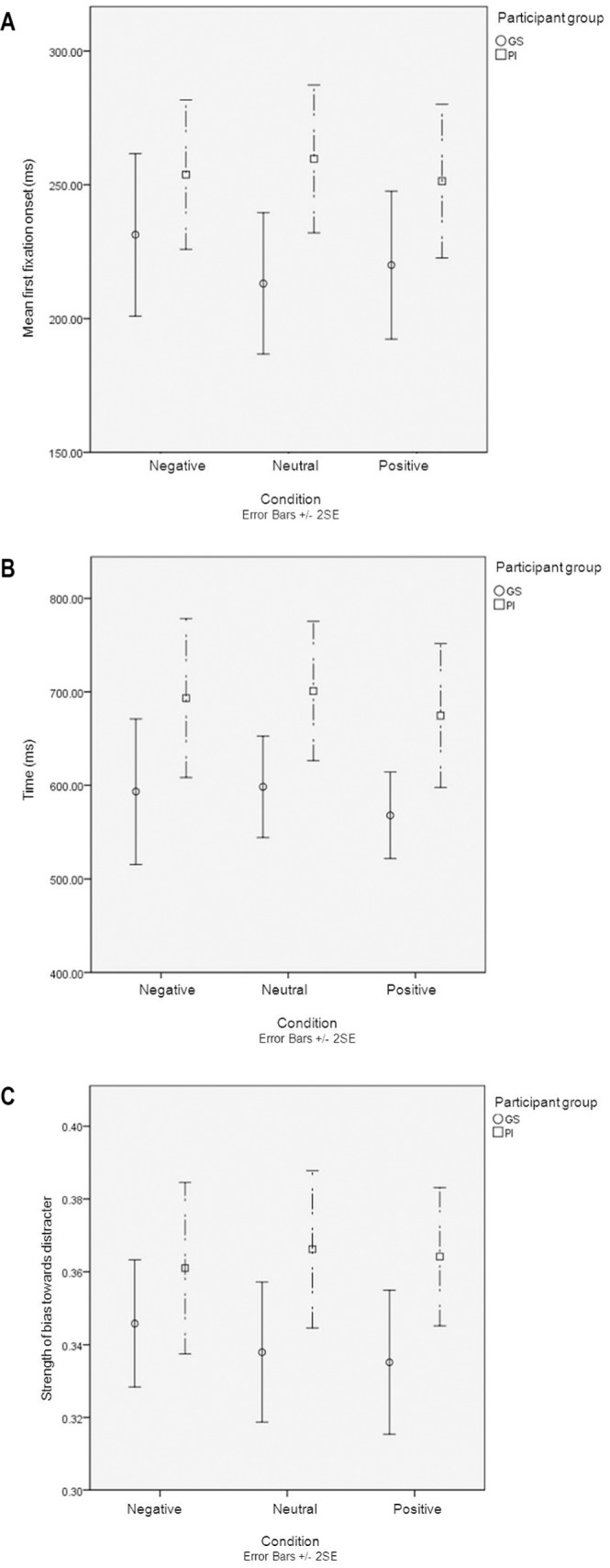
Mean performance (SE bars) of PI and GS on three vigilance parameters. (A) Mean first fixation onset (ms). (B) Discrimination time (ms). (C) Maximum error rate. Negative, negative sleep words presented as target words; positive, positive sleep words presented as target words; neutral, neutral words presented as target words.
Discrimination Time
Figure 4B suggests that the time taken for target and distracter words to be discriminated remained constant across word conditions; however, PI took consistently longer to achieve this discrimination. This observation was again confirmed by the formal analysis with a significant main effect of participant group (P < 0.05). Of note also were the larger effect sizes in relative terms between PI and GS on all 3 word conditions when compared to the effect sizes obtained for onset of first fixation. Between group effect sizes here were sleep negative d = 0.56, neutral d = 0.71, and sleep positive d = 0.62. No significant effect was found for word condition or a word condition × participant group interaction (both P > 0.05).
Maximum Error Rate
With regard to the strength of bias towards distracter word, Figure 4C shows that PI had a stronger bias than GS toward the distracter. This was borne out in the formal analysis with a significant effect of participant group (P < 0.05). The effect of word condition remained stable over the 3 conditions. There was a suggestion of variance of performance with negative sleep words and a smaller effect size within this condition, d = 0.23, but no main effect of word condition or interaction between participant group and word condition was seen (both P > 0.05). Between group effect sizes for neutral and sleep positive words were d = 0.68 and 0.51, respectively.
Our data suggest that PI show delayed onset of first fixation, discrimination time, and maximum error rate when fixating on target word, as compared to GS. In addition, they were more likely to look at distracter irrespective of word condition/salience.
Duration
In this analysis, an effect of word condition was observed (P < 0.05). Figure 5A suggests that this is due to longer gaze duration on sleep positive and negative words compared to neutral, particularly with the PI group. The between group effect size was most pronounced with neutral words, d = 0.5 (with sleep negative d = 0.37 and sleep positive d = 0.37). The analysis also revealed a main effect of participant group (P < 0.005), target position (P < 0.005), and the participant group × target position interaction (P < 0.005). This interaction is illustrated in Figure 5A, with the larger effect sizes between neutral and sleep negative and sleep positive words in PI (sleep negative and neutral d = 0.24 and neutral and sleep positive d = 0.19. With the GS the same effect sizes were d = 0.1 and 0.06, respectively) There were no significant interactions between participant group and word condition, word condition × target position, or participant group × word condition × target position (all P > 0.05).
Figure 5.
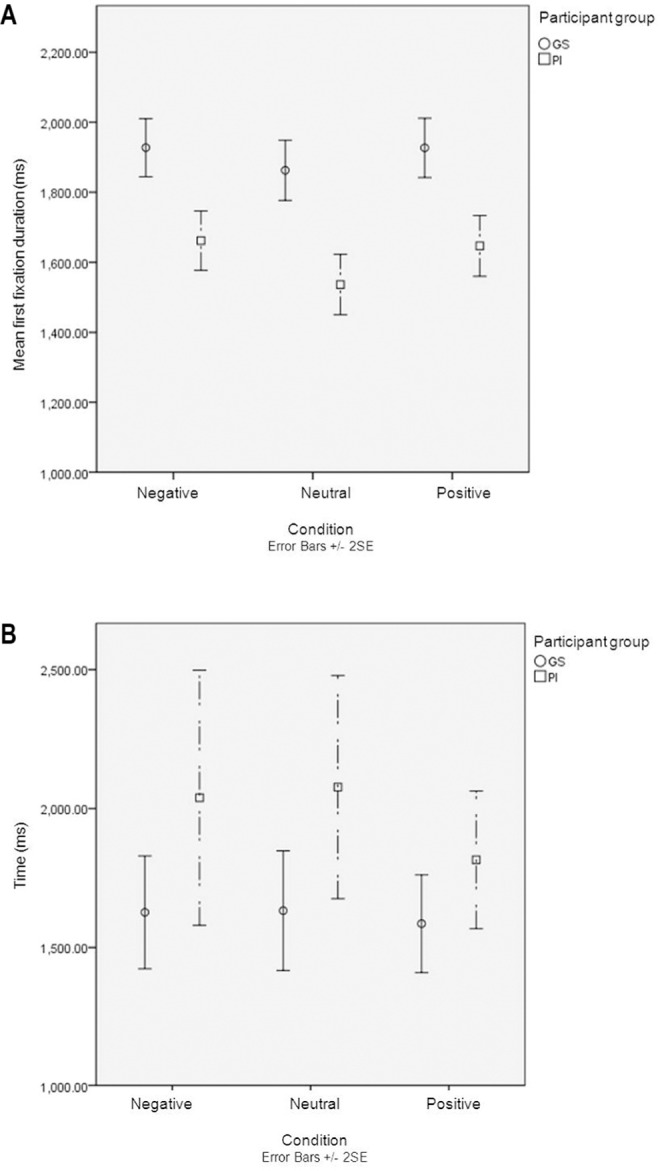
(A) Mean first fixation duration (ms) and (B) home in to target time (ms). Negative, negative sleep words presented as target words; positive, positive sleep words presented as target words; neutral, neutral words presented as target words.
Home in to Target Time
Figure 5B again demonstrated, a delay in PI response compared to GS—in this case, to home in on target word. With GS, the home in time was stable across the 3 conditions, whereas it was less stable with PI who appeared to be faster on sleep positive words. However, formal analysis was nonsignificant; likewise, there was no significant interaction with participant group (both P > 0.05). There was a significant simple main effect of participant group (P < 0.05). Effect sizes were d = 0.52 for sleep negative, d = 0.62 with neutral words, and d = 0.48 with sleep positive.
Duration of first fixation and time to home in on target word showed us the delay found with initially looking at the target word persisted with maintenance of fixation and continued over the period of the trial. PI maintained their first fixation on the target word for less time than GS; they also took longer to home in on the target word.
Regression Analysis
Five stepwise regression analyses were carried out, using the 5 parameters as separate dependent variables, and the DFSAS parts 1 and 2, PSQI, ISI, HADS Anxiety and Depression measures, SPS, and MEQ as predictor variables. Our hypotheses were that sleep quality as measured by the PSQI and ISI as well as the effects of poor sleep as measured by the remaining subjective measures listed above would significantly predict performance on this task.
Three models came out as significant. Firstly, with maximum error rate, when the SPS was entered into the model, it deviated from the null (P < 0.005), and the incremental variance explained on the basis of adjusted R2 was 4.7 (P < 0.05) with a beta value of 0.23 (P < 0.05). Secondly, with discrimination time, when ISI were entered, the model deviated from the null (P < 0.005), and the incremental variance explained on the basis of adjusted R2 was 7.6 with a beta of 0.29 (P < 0.05). ISI was also a significant predictor with the home in to target time parameter (P < 0.05) and the incremental variance explained on the basis of adjusted R2 was 4.9 (P < 0.05) with a beta value of 0.24 (P < 0.05). No other significant models were found.
DISCUSSION
The aim of the present study was the construction of a continuous timeline of attention allocation to positive sleep, negative sleep, and neutral words. The data produced suggest that PI are less vigilant for a target word irrespective of saliency, take longer to discriminate between the target and distracter words, and maintain their attention for less time once they have discriminated between the words displayed.
Sleep preoccupation and insomnia severity have been shown to account for variance in performance over three of the five parameters, although the variance explained by these measures accounts for between approximately 5% and 8%. As this is a small but significant relationship, the task is now to account for the remainder.
Previous work on attention in insomnia has used a variety of cognitive probe paradigms, stimuli, and presentation times to establish selective attention to sleep in insomnia. This study's aim was to move away from these “snapshots” and establish whether selective attention was found in initial vigilance and/ or maintained attention towards sleep. Semantic studies, such as MacPhee Marchetti et al.,3 who used a modified semantic Posner paradigm with the aim of assessing attentional engagement/disengagement to sleep-related words, found that PI took significantly longer than GS to disengage from negative sleep-related stimuli, suggesting that negative sleep stimuli are more salient to PI. Using eye tracking and a longer presentation time, PI were found to take longer to engage with the target word and sustain attention on that word for less time than GS. MacPhee Marchetti et al.3 would suggest that PI engaged then had difficulty disengaging their attention from negative sleep words, whereas the current study suggests that PI had difficulty in engaging in a sustained manner with all words.
The low predictive power of the preoccupation with sleep/ sleep-related cognitive dysfunction measures in the current study suggest that an attention bias or excessive sleep focus is not an adequate explanation for these findings. As has been found in previous attention bias work,21 sleep-related measures significantly predicted performance. In this study, the ISI accounted for a small amount of variance on discrimination and home in to target time parameters which relates our findings to the insomnia disorder but raises the question of what else could account for the impairment in performance in PI.
There are two possible explanations: avoidance or a more general performance deficit in PI. If avoidance was a possible underlying cause for this differing pattern between PI and GS, it would be seen particularly with the positive and negative sleep words, and there would be no difference between the groups on neutral words. Word condition influences first fixation only, particularly with PI, who showed a larger effect of sleep word compared to neutral than GS. This could be due to the ecological validity of sleep stimuli and is not consistent with avoidance, as PI fixated on both negative and positive sleep words longer than neutral, although these fixations were shorter than GS. This is more suggestive of a delay or disruption in processing in PI.
Altena et al.22 undertook a study of vigilance to investigate whether PI show performance deficits compared to healthy GS controls. Participants were administered both a simple and complex vigilance task, with the simple task involving a response to the appearance of an asterisk and the complex involving participants reacting to a target letter and ignoring a distracter. An interaction was found between task and sleep status, in that PI performed faster than GS on the simple task but slower on the complex task, which the authors attribute to a larger “complexity cost” in PI. With this variable reaction time seen in PI, an explanation of reduced awareness is suggested, but, the authors conclude, the data points to a disturbance of brain processes involved in higher aspects of information processing.23
With regard to the current study, it had been thought the task prescribed to our participants was simple, in that they were not measured in terms of reaction time but only where they were looking over the 3-second presentation time. However, Altena et al.22 classed discriminating between a target and distracter as complex, which is what the participants were being instructed to do with regard to the words in the current study. This would offer a possible explanation for PI being less vigilant and engaging for less time in the current study.
Edinger and colleagues24 conducted a study looking at performance of PI and GS on psychomotor tasks and their relationship with subjective and objective sleep measures. PI were found to have longer response latencies and more variability in their reaction times across several tasks—specifically, PI took longer to respond on three of the four switching attention tasks and showed significantly more variability in reaction times than GS on a simple reaction time task and all of the switching attention tasks. The above authors discuss these findings within the context of daytime complaints of PI, including difficulties in concentrating and a general lack of mental sharpness, which would appear to be most challenged in the switching attention tasks. Also, as Edinger et al.24 had participants undergo MSLT, a measure of physiological alertness and sleepiness is available that suggests the PI participants in this study had a longer mean MSLT latency than GS, although they rated themselves as more sleepy on the Stanford Sleepiness Scale. Therefore, despite objectively and subjectively reporting poorer sleep, the PI group would appear to be less able to initiate sleep when given the opportunity, which is attributed to hyperarousal.
Hyperarousal would suggest vigilance as seen in Marchetti et al.2 but does not fit as well with the performance deficits seen in this study. Shekleton et al.,25 in their clinical review paper, summarize that generally, when performance on a task is measured by accuracy rather than speed, PI were more likely to perform poorer than control groups. The authors also note that studies which showed a performance deficit in PI included distracter stimuli and required participants to make a response choice.
The conclusions which can be drawn from this study, within the context of the contemporary research discussed above, is that by using eye tracking over a longer than previously used presentation time, the difficulty that PI have in discriminating between categories and maintaining their attention on the correct word becomes evident. This is suggestive of more than an attention bias that may be seen at shorter presentation times, but moving toward impairment in discrimination and maintaining attention, which has been linked to functional impairment of prefrontal cortex as proposed in the wider insomnia literature.
One of the challenges in this area of insomnia research is to develop a reliable and substantial collection of insomnia relevant stimuli, particularly along the positive and negative sleep continuum, as this would strengthen experimental paradigms.26 Another experimental improvement would be to include a recall or recognition task for participants and the words presented. This would allow processing of the words presented to be measured and related to performance on task.
Classification into the PI and GS groups did not include an objective measure of sleep quality. With regard to developing this experiment and due to the interesting findings it produced, an ideal step forward would be to subjectively and objectively define the participant groups as well as take measures of daytime impairment and sleepiness. This could be done using polysomnography and physiological measures of arousal such as heart rate as objective measures and more in-depth clinical interview to understand the individual perspective on sleep.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by a studentship from the Economic and Social Research Council (ESRC) in the UK.
REFERENCES
- 1.Taylor L, Espie CA, White CA. Attentional bias in people with acute versus persistent insomnia secondary to cancer. Behav Sleep Med. 2003;1:200–12. doi: 10.1207/S15402010BSM0104_3. [DOI] [PubMed] [Google Scholar]
- 2.Marchetti LM, Biello SM, Broomfield NM, MacMahon KMA, Espie CA. Who is preoccupied with sleep? A comparison of attention bias in people with psychophysiological insomnia, delayed sleep phase syndrome and good sleepers using the induced change blindness paradigm. J Sleep Res. 2006;15:212–21. doi: 10.1111/j.1365-2869.2006.00510.x. [DOI] [PubMed] [Google Scholar]
- 3.MacPhee Marchetti LM. Glasgow: University of Glasgow; 2006. Let me draw your attention to sleep [dissertation] [Google Scholar]
- 4.Espie CA, Broomfield NM, MacMahon KM, Macphee LM, Taylor LM. The attention-intention-effort pathway in the development of psychophysiological insomnia: a theoretical review. Sleep Med Rev. 2006;10:215–45. doi: 10.1016/j.smrv.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Spiegelhalder K, Espie CA, Riemann D. Is sleep-related attentional bias due to sleepiness or sleeplessness? Cogn Emot. 2009;23:541–50. [Google Scholar]
- 6.MacMahon K, Broomfield N, Macphee L, Espie CA. attention bias for sleep related stimuli in primary insomnia and delayed sleep phase syndrome using the dot-probe task. Sleep. 2006;29:1420–7. doi: 10.1093/sleep/29.11.1420. [DOI] [PubMed] [Google Scholar]
- 7.Woods H, Marchetti LM, Biello SM, Espie CA. The clock as a focus of selective attention in those with primary insomnia: an experimental study using the modified Posner paradigm. Behav Res Ther. 2009;47:231–6. doi: 10.1016/j.brat.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Schütz AC, Braun DI, Gegenfurtner KR. Eye movements and perception: A selective review. J Vis. 2011;11:1–30. doi: 10.1167/11.5.9. [DOI] [PubMed] [Google Scholar]
- 9.Morin CM, Espie CA. Kluwer Academic/Plenum Publishers; 2003. Insomnia: a clinical guide to assessment and treatment. [Google Scholar]
- 10.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 11.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 12.Kyle SD, Morgan K, Espie CA. Insomnia and health-related quality of life. Sleep Med Rev. 2010;14:69–82. doi: 10.1016/j.smrv.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Morin CM. Insomnia: psychological assessment and management. New York, NY: Guilford Press; 1993. [Google Scholar]
- 14.Espie CA, Inglis SJ, Harvey L, Tessier S. Insomniacs' attributions: psychometric properties of the dysfunctional beliefs and attitudes about sleep scale and the sleep disturbance questionnaire. J Psychosom Res. 2000;48:141–8. doi: 10.1016/s0022-3999(99)00090-2. [DOI] [PubMed] [Google Scholar]
- 15.Ellis J, Mitchell K, Hogh H. Sleep preoccupation in poor sleep: Psycho-metric properties of the Sleep Preoccupation Scale. J Psychosom Res. 2007;63:579–85. doi: 10.1016/j.jpsychores.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 17.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 18.Wicklow A, Espie CA. Intrusive thoughts and their relationship to acti-graphic measurement of sleep: towards a cognitive model of insomnia. Behav Res Ther. 2000;38:679–93. doi: 10.1016/s0005-7967(99)00136-9. [DOI] [PubMed] [Google Scholar]
- 19.Scheepers C, Keller F, Lapata M. Evidence for serial coercion: A time course analysis using the visual-world paradigm. Cogn Emot. 2008;56:1–29. doi: 10.1016/j.cogpsych.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Jansson-Fröjmark M, Lindblom K. A bidirectional relationship between anxiety and depression and insomnia? A prospective study in the general population. J Psychosom Res. 2008;64:443–9. doi: 10.1016/j.jpsychores.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Jones BT, Macphee LM, Broomfield NM, Jones BC, Espie CA. Sleep related attentional bias in good, moderate and poor (primary insomnia) sleepers. J Abnorm Psychol. 2005;114:249–58. doi: 10.1037/0021-843X.114.2.249. [DOI] [PubMed] [Google Scholar]
- 22.Altena E, Van Der Werf YD, Strijers RL, Van Someren EJ. Sleep loss affects vigilance: effects of chronic insomnia and sleep therapy. J Sleep Res. 2008;17:335–43. doi: 10.1111/j.1365-2869.2008.00671.x. [DOI] [PubMed] [Google Scholar]
- 23.Bastien CH, Fortier-Brochu E, Rioux I, LeBlanc M, Daley M, Morin CM. Cognitive performance and sleep quality in the elderly suffering from chronic insomnia. Relationship between objective and subjective measures. J Psychosom Res. 2003;54:39–49. doi: 10.1016/s0022-3999(02)00544-5. [DOI] [PubMed] [Google Scholar]
- 24.Edinger JD, Means MK, Carney CE, Krystal AD. Psychomotor performance deficits and their relation to prior nights' sleep among individuals with primary insomnia. Sleep. 2008;31:599–607. doi: 10.1093/sleep/31.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shekleton JA, Rogers NL, Rajaratnam SM. Searching for the daytime impairments of primary insomnia. Sleep Med Rev. 2010;14:47–60. doi: 10.1016/j.smrv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Mogg K, Bradley BP, Field M, De Houwer J. Eye movements to smoking-related pictures in smokers: relationship between attentional biases and implicit and explicit measures of stimulus valence. Addiction. 2003;98:825–36. doi: 10.1046/j.1360-0443.2003.00392.x. [DOI] [PubMed] [Google Scholar]