Abstract
Study Objectives:
To assess whether the frequency of impulse control disorders (ICDs), addictive behaviors, impulsivity, and impairment of decision-making task performance under ambiguous and risky conditions were present in patients with restless legs syndrome (RLS) and whether changes could be related to dopaminergic medications.
Design:
Case-control prospective study.
Setting:
Academic Sleep Disorders Center.
Participants:
Of the 149 participants, there were 39 who were drug free with primary RLS, 50 who were taking dopamine agonists (DA), and 60 control subjects. Participants were assessed with a clinical interview screening for ICDs, augmentation syndrome, impulsivity, depression, and addictive behaviors. All participants completed two decision-making tasks, one under an ambiguous condition (Iowa Gambling Task) and the other under a risky condition (Game of Dice Task). Drug-free patients with RLS underwent 1 night of polysomnography recording.
Measurements and Results:
Seventy percent of patients were treated with pramipexole (median dose, 0.36 mg), and 30% with ropinirole (median dose, 0.75 mg). Median duration of DA intake was 11 mo (range, 1-72 mo). No differences were found on impulsivity scores, ICDs, and substance addiction between drug-free patients or those taking DA, or control subjects. Patients with RLS reported more depressive symptoms than control subjects, but without differences between patients taking or not taking DA. Drug-free and treated patients demonstrated reduced performances on the Iowa Gambling Task but not on the Game of Dice Task compared to control subjects, with no differences between patients taking medications and those who were not. No association was found between decision-making task performances, or polysomnographic and clinical variables.
Conclusion:
Impulse control disorders, impulsivity, and substance addiction were infrequent in drug-free patients with restless legs syndrome or those treated with a low dose of dopamine agonists. However, patients with restless legs syndrome, either drug free or taking dopamine agonists, had preferences toward risky choices on the Iowa Gambling Task, which led to negative consequences in the long run, a condition potentially leading to further development of impulse control disorders.
Citation:
Bayard S; Langenier MC; Dauvilliers Y. Decision-making, reward-seeking behaviors and dopamine agonist therapy in restless legs syndrome. SLEEP 2013;36(10):1501-1507.
Keywords: Restless legs syndrome, dopamine agonist, impulsive compulsive disorders, decision-making, Iowa Gambling Task, Game of Dice Task
INTRODUCTION
Restless legs syndrome (RLS) is a common sleep related movement disorder associated with mood and cognitive disorders.1–4 Non-ergot-derived dopamine agonists (DA) such as ropinirole, pramipexole, and rotigotine are the first-line treatment of RLS with rapid efficacy, generally at low doses.5 DA used in RLS have high selective affinity for the D2/D3 receptor subtypes, which are expressed predominantly in the brain limbic areas,6 regions that are implicated in addiction and impulse control disorders (ICDs).7
Abnormal reward-mediated processing is frequently observed in human disorders. In Parkinson disease (PD), use of the D2/D3 selective receptor agonists is associated with an increased risk of ICD including pathological gambling, compulsive buying, compulsive sexual behavior, and binge or compulsive eating. Prevalence rates of ICDs in DA-treated patients with PD is 13.6%, compared to 0.5-1% in the general population.8,9 Vigilance for ICDs is essential because of their potentially devastating financial, social, and marital consequences.
As opposed to PD, the relationship between ICDs and DA in RLS is not as well established, with prevalence estimates for ICDs and/or addictive behaviors ranging from 5-17%.10–14 Recently, we reported the absence of any difference in impulsivity, ICDs, or addictive behaviors in drug-free patients with RLS compared with sex- and age-matched control subjects, but we reported impairments in decision-making in ambiguous situations using the Iowa Gambling Task (IGT), with normal decision-making performance in risky conditions using the Game of Dice Task (GDT).15 The IGT is thought to simulate decision-making in real-life situations where premises, outcomes, rewards, or punishments are initially uncertain.16 In contrast, in the GDT, the potential consequences of different options and their subsequent probabilities rely on explicit information.17
In recent years, the relevance of the frontostriatal loops for decision-making in the IGT was demonstrated by neuro-imaging studies.18,19 Low IGT performances were associated with abnormal emotional and cognitive feedback processing that point to dysfunction in the orbitofrontal/ventromedial prefrontal cortex linked to the ventral striatum.18,19 In contrast, results on the GDT were linked to performance in tasks measuring executive functions involving ventromedial and dorsolateral prefrontal cortices.18 The performance discrepancies found between both tasks suggested a dysfunction in the orbitofrontal/ventromedial prefrontal cortex, possibly related to impaired activation by ventral striatum secondary to alterations in dopamine signaling.15
We hypothesize that stimulation of mesolimbic dopamine receptors during DA treatment results in abnormal responses in patients with RLS who already are susceptible to disadvantageous choices in decision-making. In the current study, we proposed to explore the frequency of ICDs, impulsivity, and addictive behaviors in drug-free patients with RLS, patients with RLS on DA medication, and control subjects. In contrast to previous works, we used a standardized face-to-face clinical interview and assessed decision-making in ambiguous and risky situations with two validated laboratory tasks (i.e., IGT and GDT) in all subjects. Moreover, decision-making tasks were performed the day after polysomnography (PSG) in drug-free patients with RLS to assess for potential interaction with nighttime sleep variables.
METHODS
Participants
One hundred forty-nine consecutive subjects were included, with 89 patients (39 untreated and 50 DA-treated patients) with primary RLS investigated and conditions diagnosed according to the four established standard criteria.5 Age at onset was determined by clinical interview. Patients with associated comorbidities potentially inducing secondary RLS (i.e., anemia due to iron deficiency, renal disease, neuropathy, PD, or diabetes mellitus) were excluded based on clinical and chemical examinations. In the untreated group, 29 patients were never treated and 10 had received medication, but were no longer receiving any RLS medication, hypnotic agents, or antidepressants for at least 2 weeks prior to entry in the study. All patients from the treated group (n = 50) were being treated with a single DA (pramipexole or ropinirole) once per day, 2-4 h before bedtime. Total DA medication was calculated as a median of each DA and using standard criteria. The presence of RLS augmentation syndrome was also screened on previous established criteria including higher intensity of symptoms, shorter latency to symptoms when at rest, shorter duration of relief from treatment, earlier onset of symptoms, and spreading of symptoms to other body parts.20
We also included 60 normal control subjects who were community-dwelling adults recruited from a local association network. Exclusion criteria were a positive history of neurologic disease including RLS and the presence of medication known to influence sleep and cognition. Neither patients nor controls received money for participation. The study was performed after obtaining permission of the hospital ethics committee (Comité de Protection des Personnes Sud Méditérranée IV, Montpellier, France) and all subjects provided written informed consent according to the ethical standards.
Outcome Assessment
All subjects participated in a face-to-face standard clinical interview and completed questionnaires and decision-making tasks.
Clinical Interview and Questionnaires
Current (in the past 12 mo) and past substance (alcohol and drug) use disorders, obsessive-compulsive disorder (OCD), and hypomania/mania not being classified as ICDs, and ICDs including pathological gambling, compulsive eating, trichotillomania, kleptomania, intermittent explosive disorder, compulsive shopping, and hypersexuality were diagnosed in all patients and controls using a structured clinical interview in according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DMS-IV-TR) Axis I Disorders,21 and to recent criteria for compulsive shopping and hypersexuality.22 Depressive symptoms were evaluated with the Beck Depression Inventory.23 Impulsivity was assessed with the Urgency Premeditation Perseverance Impulsive Behavior Scale (UPPS) evaluating four facets of impulsivity: urgency, (lack of) premeditation, (lack of) perseverance, and sensation seeking.24 RLS severity was determined by the validated International Restless Legs Syndrome Severity Scale (IRLS).25
Iowa Gambling Task
We used the computerized version of IGT for all subjects to assess decision-making under ambiguity.15,16 The goal of the game is to win as much money as possible. Four decks of cards are presented that are labeled in a row A, B, C, and D. Participants are required to select one card at the time through a mouse click for a total of 100 card selections (the total number of card selections is unknown to the participants). They are also told that some decks are better than others and that, to win, they have to avoid the disadvantageous decks. The selection of a card from decks A and B results in large gains of money but followed by a large penalty at certain unpredictable times, with accumulated penalties being larger than the accumulated gains. Decks A and B are therefore disadvantageous in the long run. The selection of a card from decks C and D produces small, immediate gains of money, but the unpredictable losses are small, with accumulated penalties being smaller than the accumulated gains. Decks C and D are the advantageous decks in the long run. We calculated an overall net score to analyze IGT performance (advantageous minus disadvantageous card selections). Additionally, task performance was divided into five blocks of 20 card selections: early blocks are of little significance and the latter blocks are where subjects are expected to improve. Failure of the scores to improve indicates poor learning or greater impulsive choices. Net scores were calculated for each block to determine the overall task performance profile.
Game of Dice Task
We used the computerized GDT in all subjects to measure decision-making under risky conditions. Participants were asked to maximize their fictive starting capital of 1,000 € within 18 dice throws, according to the standard protocol.15,17 Before each throw, subjects had to choose between the different single numbers or a combination of two, three, or four numbers. Each choice was associated with a gain or loss, depending on the probability of occurrence: 1,000 € gain/loss for the choice of a single number (winning probability 1:6), 500 € for 2 numbers (winning probability 2:6), 200 € for 3 numbers (winning probability 3:6), and 100 € for 4 numbers (winning probability 4:6). To determine the decision-making risk, we classified the choice of three or four number combinations as “nonrisky” (≥ 50% winning probability) and the choice of one or two numbers as “risky” (< 50% winning probability). We calculated a net score by subtracting the number of risky choices from the number of nonrisky choices.
PSG Recordings
Drug-free patients with RLS underwent 1 night of PSG recording. Lights-out time was based on the patient's routine sleeping hours, mainly 23:00 to 06:00. PSG investigation included measures of sleep, respiratory events, cortical arousals, and periodic limb movements, scored according to standard criteria. Face-to-face standardized, questionnaires, and decision-making tasks were completed from 09:00 to 12:00, the day after PSG. DA-treated patients were encountered the day of their routine follow-up medical consultation.
Statistical Analysis
Data were examined for normal distribution and homogeneity of variance. Group differences in demographic variables, clinical variables, and rating scale scores were analyzed with one-way between-groups analysis of variance (ANOVA) or independent-samples t-test for parametric variables, and the χ2 or Mann-Whitney U tests for nonparametric variables. Performance on the IGT was analyzed using a repeated-measure ANOVA with group as the between-subject factor and blocks as the within-subjects factor. Bravais Pearson correlations were used for correlation analyses. The level of significance was α < 0.05. All statistical analyses were performed with IBM SPSS software version 19 for Windows.
RESULTS
Demographic and Clinical Data
Results of demographic and clinical variables are shown in Table 1. Two main-group effects were obtained regarding age (P = 0.02) and education (P = 0.02); DA-treated patients with RLS were older than drug-free patients with RLS and control subjects, and were less educated than healthy control subjects. Age at onset and duration of illness did not differ between patient groups. The proportion of patients with an IRLS score > 20 (severe symptoms) was significantly higher in drug-free patients than in the DA-treated group (P = 0.04). Median duration of DA intake was 11 mo (range = 1-72), including 70% of patients treated with pramipexole (median dose at 0.36 mg) and 30% with ropinirole (median dose at 0.75 mg; Table 1).
Table 1.
Demographic and clinical data (clinical interview and questionnaires) of drug-free and DA-treated patients with RLS and healthy control subjects
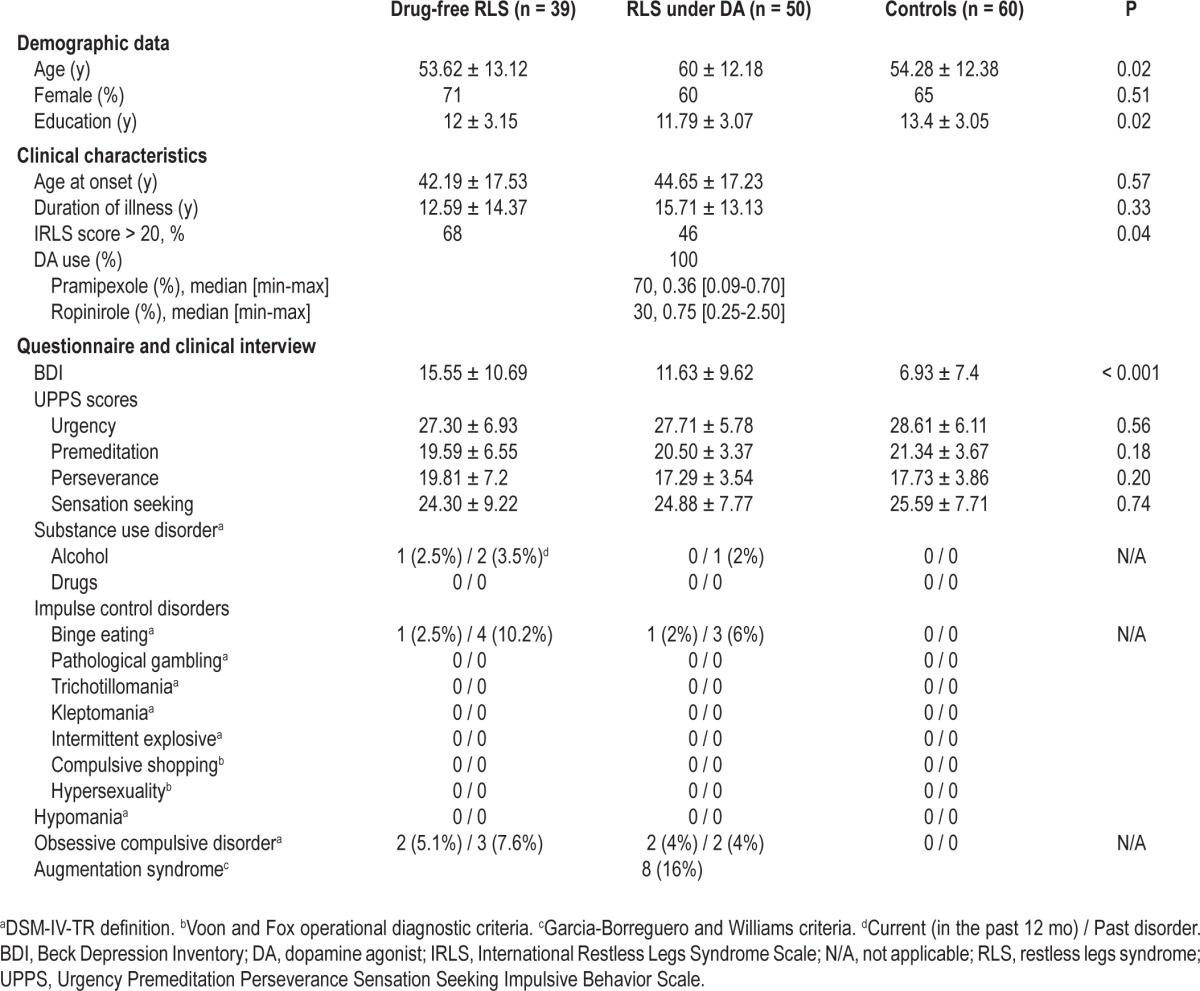
Clinical Interview and Questionnaires
Depressive symptoms were higher in RLS groups than in control subjects without any significant difference between drug-free and treated patients (Table 1). No differences were found between RLS groups and control subjects for urgency, premeditation, perseverance, and impulsivity UPPS subscales (Table 1). Among drug-free patients with RLS, those never treated (n = 29) and those previously treated (n = 10) did not differ on clinical or questionnaires variables. Past and current substance abuse, and OCD and ICD spectrum disorders were relatively rare in both drug-free and DA-treated patients and without any between-group differences (Table 1). Among the 39 drug-free patients with RLS, 4 patients (10.2%) were affected with current substance abuse, OCD, or ICDs: one patient with alcohol dependence, two with OCD, and one with compulsive eating disorder. In addition, one patient had past alcohol dependence, three had past binge eating, and one had past OCD but these disorders were absent at the time of the study (Table 1). Substance abuse, OCD, or ICD were present only during the daytime, were not used by the patients as a way to alleviate RLS symptoms, began before RLS onset, and did not increase with the onset or evolution of RLS. Demographic and clinical characteristics including age, age at onset, duration of disease, severity of depressive symptoms and RLS, and impulsivity did not differ between patients with RLS plus a current ICD, substance addiction, or OCD diagnosis than those without. In the 50 patients with RLS treated with DA, three patients (6%) were currently affected with OCD (two patients) or ICD (one patient with current and past compulsive eating; Table 1). These three patients developed these problems before DA introduction. Another patient had past alcohol dependence and two others had past compulsive eating disorder but were without these conditions at the time of study (Table 1). DA-treated patients with RLS experiencing these problems had no differences among demographic and clinical characteristics, and treatment intake than those without. None of the patients with RLS taking DA (or not) participated in pathological gambling, compulsive buying, or compulsive sexual behavior. RLS augmentation syndrome was diagnosed in eight patients treated with DA (three received pramipexole and five received ropinirole; 16%; males n = 6) without any current or past history of ICD or OCD.
Iowa Gambling Task
A group effect was observed for the IGT net win (P = 0.006) with lower final outcome in both drug-free and DA-treated patients with RLS compared to control subjects (Figure 1). With respect to the net scores for each block of 20 cards, a 3 (group) × 5 (blocks) repeated-measures ANOVA with age and education at covariates showed a significant group effect (P = 0.013) and an interaction group × block interaction (P = 0.001). Hence, control subjects performed better than both groups of patients with RLS with more advantageous choices selected on blocks four and five (all P < 0.01; Figure 2). No difference was observed between drug-free and DA-treated patients with RLS (Figure 2). Patients with RLS with substance addiction, OCD/ICD, or with augmentation syndrome had similar IGT performances as those without (all P > 0.15). We found no correlation between IGT scores, drug doses, and depressive symptom severity. In drug-free patients, IGT performances were not associated with nighttime sleep variables (sleep efficiency, percentage of non-rapid eye movement and rapid eye movement sleep, and apnea/hypopnea, microarousal, and periodic limb movements during sleep indexes).
Figure 1.
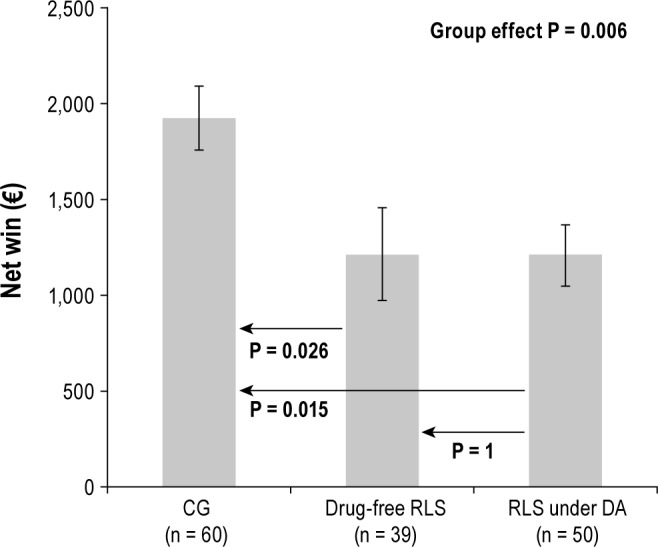
Iowa Gambling Task net win (€) for patients with RLS (drug-free and DA-treated) and the control group (CG). Mean (± SEM) are given.
Figure 2.
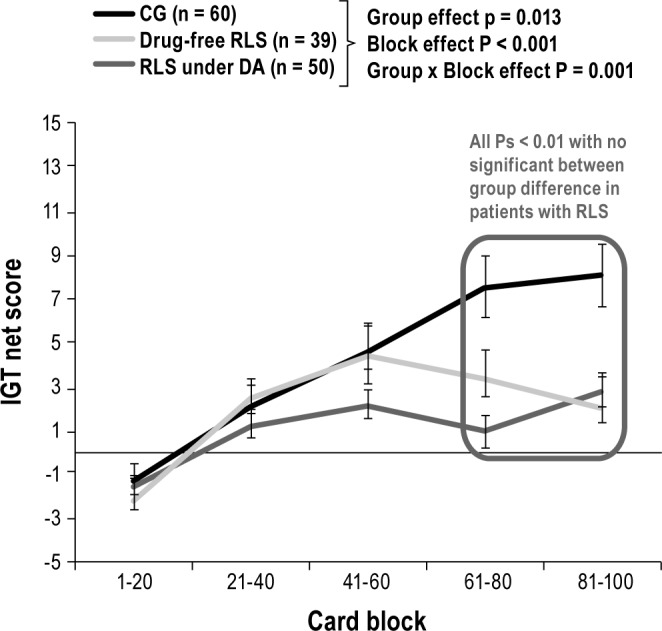
Iowa Gambling Task net scores of the 5 blocks, consisting of 20 cards selections for patients with RLS (drug-free and DA-treated) and the control group (CG).
Game of Dice Task
Analysis of covariance with age and education at covariates did not show group effects regarding GDT net score [healthy control subjects, mean = 6.73 ± 9.24; drug-free patients with RLS, mean = 4.79 ± 11.46; DA-treated patients with RLS, mean = 4.18 ± 10.48; (F = 0.92, P = 0.39)] and net win (€) [healthy control subjects, mean = -555 ± 2,661; drug-free patients with RLS, mean = -330 ± 2,195; DA-treated patients with RLS, mean = -1,004 ± 2,473; (F = 0.88, P = 0.41)]. Patients with OCD/ICD or with augmentation syndrome had GDT performances similar to those without (all P > 0.15). No correlation was found between drug doses, depressive symptom severity, and GDT scores. Performances on the GDT were not related to PSG characteristics in drug-free patients.
DISCUSSION
Our study was the first to explore with a standardized face-to-face clinical interview the ICDs, OCD, and substance addiction occurrence in drug-free and in DA-treated patients with RLS. Furthermore, we evaluated decision-making performances under risk (GDT) and under ambiguity (IGT) in drug-free and in DA-treated patients with RLS compared to control subjects. Reduced decision-making performances were observed in drug-free and in DA-treated patients when the outcome probabilities are unknown (IGT), but not for decisions under risk (GDT), where outcome probabilities are known or calculable. Neuropsychological tests were performed the day after PSG in drug-free patients with RLS; however, decision-making performances were not associated with nighttime sleep variables. No significant differences on IGT scores were noted between patients on or off medication. No differences in impulsivity, ICDs, OCD, substance addiction occurrence, and GDT scores were found between patients with RLS taking DA compared to drug-free patients with RLS and a control group. We may emphasize that none of the patients with RLS either taking or not taking a low dose of DA participated in pathological gambling or compulsive buying, or exhibited hypersexuality, all behaviors with potential devastating financial, social, and marital consequences.
Several studies have explored the frequency of ICDs in patients with RLS; some were limited by nonstandardized assessment and results have been contradictory. By asking about any increased impulsivity in various behaviors on a simple three-point scale, 10% of patients with RLS taking DA reported increased spending behavior or gambling in contrast to 24.6% in patients taking DA for PD.11 A prospective study using a personal screening questionnaire for ICD plus follow-up phone interviews reported prevalence of 17% for any ICD, being higher than in patients with obstructive sleep apnea (6%) and drug-free patients with RLS (8%).10 Another questionnaire study reported the development of abnormal behaviors in 12.4% of patients with RLS after 1 year of DA treatment, but again without using any published criteria for ICD diagnosis.12 A questionnaire for high impulsivity sent to 423 patients followed by a structured interview found only 12 patients treated for RLS with ICD; however, the response rate was only 17.3%.13 A recent study reported the results of validated questionnaires from 140 patients treated for RLS with a ICD diagnosis in 7.1%.14 However, in all these studies questionnaires were first mailed to patients with variable response rates, and therefore would be subject to important responder bias, making accurate assessment of ICD prevalence difficult.
Our study investigated the occurrence of impulsivity, ICDs, substance use disorders in the past and at the time of study, and OCD spectrum due to a potential overlap on different levels including phenomenology, comorbidity, neurocircuitry, neuro-cognition, neurochemistry, and family history.26 We found that these disorders were relatively uncommon in patients with RLS, without any difference between those taking or not taking DA. Moreover, when OCD, ICDs, or substance addiction were detected, they occurred before RLS onset and apparently were not modified by DA. No differences were found on impulsivity UPPS scores between drug-free, DA-treated patients with RLS and control subjects. Several methodological and clinical factors may explain discrepancies between our results and those from literature. We used a face-to-face structured diagnostic interview validated in PD that relies on a categorical approach based on stringent criteria.21,22 Deciding when ICD really started is challenging by the imperfect recollection of the events, the variable self-insight into one's behaviors, and the diagnostic criteria use to detect “pathologic” ICD and not subsyndromal ICD symptoms related to behavioral changes. Cultural differences in the tendency to express pathologic behaviors such as pathological gambling may also exist. Finally, we do believe that the most important reason for the differences between studies relate to the DA dose, with three to five times lower doses in our population than other RLS populations.10–14 Clinical practice differences exist, and our group has a ‘lower the better’ dose strategy with DA to avoid adverse effects and particularly augmentation.
Standardized tests of the propensity toward risk behavior are a unique opportunity to study the effect of DA on the neurological substrate of risk evaluation in susceptible individuals with RLS. We reported for the first time the decision-making patterns in drug-free and DA-treated patients with RLS compared to control subjects. Hence, patients with RLS selected greater disadvantageous and impulsive choices in a decision-making task in an ambiguous situation (IGT) in comparison with control subjects, without any differences between patient groups with or without DA, and with or without augmentation syndrome. In contrast, no differences were found between patients with RLS taking DA compared to drug-free patients with RLS and a control group in performances in decision-making in a gambling situation with explicit and stable rules for gains and losses (GDT).
Decision-making and reward-based learning behaviors are sensitive to change in dopaminergic activity.16,18,27,28 In the absence of executive dysfunction, altered IGT performances were frequently related to dysfunction in the orbitofrontal/ ventromedial prefrontal cortex that links to the ventral striatum.18 As altered striatum dopamine profile was demonstrated in RLS with a decrease in D2 receptors in the putamen,29 patients with RLS may be predisposed to perform greater impulsive choices in a decision-making task under ambiguity (IGT) in the presence of DA or not. Despite its extensive application in the neuropsychological research field, and the phenomenological evidence of deficient decision-making in ICDs, studies of the ecological validity of the IGT remained rare. The relationship between performances on a decision-making task and performances in a real-world assessment of decision-making lacks validated clinical studies. Some studies suggest a link between IGT and clinically relevant risky behaviors, including substance use disorders and ICDs.18,27,30,31 A recent study reported some trends toward a worse performance (greater loss of money and more risky choices) on the IGT in patients with PD and ICD, as compared to matched patients without ICD.31 In the context of our current study, one question remains as to whether propensity toward risk behavior on the IGT constitutes a potential risk factor to further develop ICDs. Longitudinal studies of treatment-naïve patients with RLS may answer such questions of whether or not reduced IGT performances at baseline predispose to further development of ICD/OCDs and addictive behaviors, especially in patients taking DA.
Our study has several limitations. The lack of significant differences regarding impulsivity, substance addiction, and OCD/ICD occurrence among drug-free, DA-treated patients with RLS and control groups may be related to the small sample size, the limited duration of DA exposure, and the low doses of DA intake. The absence of between drug-free and DA-treated RLS group differences on the number of risky choices in a gambling situation may also be related to the effect size. To show a significant difference of -3.7 (standard deviation = 19.59, even if nonclinically significant) on the total IGT score between drug-free and DA-treated patients with RLS, 222 subjects would be necessary with a power of 0.80 and a type I error α = 0.05. In our study, we decided to use a dichotomic approach of the presence of ICD/OCDs. The disadvantage of this approach is that subsyndromal behaviors, which can have a substantial effect on patient's quality of life, could go undetected. In the future, it may be of interest to use validated rating tools such as the recently published Questionnaire for Impulsive-Compulsive Disorders in Parkinson Disease-Rating Scale32 to assess and detect both severity and frequency of subsyndromal behaviors and their evolution over time. Finally, our study did not focus on caregiver responses, and some compulsive behaviors such as punding or mental compulsions were not screened.
In conclusion, this study demonstrated that ICD/OCD, impulsivity, and substance addiction when explored through a standardized face-to-face clinical interview were infrequent in drug-free patients with RLS or treated with a low dose of DA. However, patients with RLS either drug-free or taking DA had preferences toward disadvantageous and impulsive choices in a decision-making task under ambiguity compared to controls. Further prospective studies investigating decision-making processes and reward-based behaviors in patients with RLS are needed to clarify whether reduced IGT performances at baseline predispose to further development of ICD/OCDs and addictive behaviors particularly after dopaminergic agonist intake.
DISCLOSURE STATEMENT
This was not an industry supported study. Professor Dauvilliers has received speaker's honoraria and funding for travel to conferences from UCB Pharma, Cephalon, Novartis, Jazz, and Bioprojet. Prof. Dauvilliers has participated in advisory boards of UCB and Bioprojet. Doctor Bayard and Ms Croisier Lang-enier report no disclosures.
REFERENCES
- 1.Picchietti D, Winkelman JW. Restless legs syndrome, periodic limb movements in sleep, and depression. Sleep. 2005;1:891–8. [PubMed] [Google Scholar]
- 2.Pearson VE, Allen RP, Dean T, Gamaldo CE, Lesage SR, Earley CJ. Cognitive deficits associated with restless legs syndrome (RLS) Sleep Med. 2006;7:25–30. doi: 10.1016/j.sleep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Gamaldo CE, Benbrook AR, Allen RP, Oguntimein O, Earley CJ. A further evaluation of the cognitive deficits associated with restless legs syndrome (RLS) Sleep Med. 2008;9:500–5. doi: 10.1016/j.sleep.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fulda S, Beitinger ME, Reppermund S, Winkelmann J, Wetter TC. Short-term attention and verbal fluency is decreased in restless legs syndrome patients. Mov Disord. 2010;25:2641–8. doi: 10.1002/mds.23353. [DOI] [PubMed] [Google Scholar]
- 5.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 6.Manconi M, Ferri R, Zucconi M, et al. Preferential D2 or preferential D3 dopamine agonists in restless legs syndrome. Neurology. 2011;77:110–7. doi: 10.1212/WNL.0b013e3182242d91. [DOI] [PubMed] [Google Scholar]
- 7.Brewer JA, Potenza MN. The neurobiology and genetics of impulse control disorders: relationships to drug addictions. Biochem Pharmacol. 2008;75:63–75. doi: 10.1016/j.bcp.2007.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petry NM, Stinson FS, Grant BF. Comorbidity of DSM-IV pathological gambling and other psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2005;66:564–74. doi: 10.4088/jcp.v66n0504. [DOI] [PubMed] [Google Scholar]
- 9.Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67:589–95. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 10.Cornelius JR, Tippmann-Peikert M, Slocumb NL, Frerichs CF, Silber MH. Impulse control disorders with the use of dopaminergic agents in restless legs syndrome: a case-control study. Sleep. 2010;33:81–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Ondo WG, Lai D. Predictors of impulsivity and reward seeking behavior with dopamine agonists. Parkinsonism Relat Disord. 2008;14:28–32. doi: 10.1016/j.parkreldis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Pourcher E, Rémillard S, Cohen H. Compulsive habits in restless legs syndrome patients under dopaminergic treatment. J Neurol Sci. 2010;290:52–6. doi: 10.1016/j.jns.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Dang D, Cunnington D, Swieca J. The emergence of devastating impulse control disorders during dopamine agonist therapy of the restless legs syndrome. Clin Neuropharmacol. 2011;34:66–70. doi: 10.1097/WNF.0b013e31820d6699. [DOI] [PubMed] [Google Scholar]
- 14.Voon V, Schoerling A, Wenzel S, et al. Frequency of impulse control behaviours associated with dopaminergic therapy in restless legs syndrome. BMC Neurol. 2011;11:117. doi: 10.1186/1471-2377-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayard S, Yu H, Langenier MC, Carlander B, Dauvilliers Y. Decision making in restless legs syndrome. Mov Disord. 2010;25:2634–40. doi: 10.1002/mds.23326. [DOI] [PubMed] [Google Scholar]
- 16.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 17.Brand M, Kalbe E, Labudda K, Fujiwara E, Kessler J, Markowitsch HJ. Decision-making impairments in patients with pathological gambling. Psychiatry Res. 2005;133:91–9. doi: 10.1016/j.psychres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Brand M, Labudda K, Markowitsch HJ. Neuropsychological correlates of decision making in ambiguous and risky situations. Neural Netw. 2006;19:1266–76. doi: 10.1016/j.neunet.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Bolla KI, Eldreth DA, London ED, et al. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–94. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García-Borreguero D, Williams AM. Dopaminergic augmentation of restless legs syndrome. Sleep Med Rev. 2010;14:339–46. doi: 10.1016/j.smrv.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Washington DC: American Psychiatric Association; 2004. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 22.Voon V, Fox SH. Medication-related impulse control and repetitive behaviors in Parkinson disease. Arch Neurol. 2007;64:1089–96. doi: 10.1001/archneur.64.8.1089. [DOI] [PubMed] [Google Scholar]
- 23.Beck AT, Steer RA, Brown GK. Inventaire de dépression de Beck. Paris, France: Editions du Centre de Psychologie Appliquée; 1998. [Google Scholar]
- 24.Van der Linden M, d'Acremont M, Zermatten A, et al. A French adaptation of the UPPS Impulsive Behavior Scale: Confirmatory factor analysis in a sample of undergraduate students. Eur J Psy. 2006;22:38–42. [Google Scholar]
- 25.IRLS. International Restless Legs Syndrome Study Group. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 26.Fontenelle LF, Oostermeijer S, Harrison BJ, Pantelis C, Yücel M. Obsessive-compulsive disorder, impulse control disorders and drug addiction: common features and potential treatments. Drugs. 2011;7:827–40. doi: 10.2165/11591790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Decision making in pathological gambling: a comparison between pathological gamblers, alcohol dependents, persons with Tourette syndrome, and normal controls. Brain Res Cogn Brain Res. 2005;23:137–51. doi: 10.1016/j.cogbrainres.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Voon V, Pessiglione M, Brezing C, et al. Mechanisms underlying dopamine-mediated reward bias in compulsive behaviors. Neuron. 2010;65:135–42. doi: 10.1016/j.neuron.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Connor JR, Wang XS, Allen RP, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009;132:2403–12. doi: 10.1093/brain/awp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavedini P, Riboldi G, Keller R, D'Annucci A, Bellodi L. Frontal lobe dysfunction in pathological gambling patients. Biol Psychiatry. 2002;51:334–41. doi: 10.1016/s0006-3223(01)01227-6. [DOI] [PubMed] [Google Scholar]
- 31.Bentivoglio AR, Baldonero E, Ricciardi L, De Nigris F, Daniele A. Neuropsychological features of patients with Parkinson's disease and impulse control disorders. Neurol Sci. 2012 Nov 18; doi: 10.1007/s10072-012-1224-5. [DOI] [PubMed] [Google Scholar]
- 32.Weintraub D, Mamikonyan E, Papay K, Shea JA, Xie SX, Siderowf A. Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease-Rating Scale. Mov Disord. 2012;27:242–7. doi: 10.1002/mds.24023. [DOI] [PMC free article] [PubMed] [Google Scholar]


