Abstract
Study Objectives:
The present study addresses the need for a validated tool that prospectively identifies favorable candidates for oral appliance therapy in treatment of obstructive sleep apnea. The objective of the study was to evaluate the ability of a mandibular titration study, performed with a remotely controlled mandibular positioner (RCMP), to predict treatment outcome with a mandibular repositioning appliance (MRA) and to predict an effective target protrusive position (ETPP).
Design:
A prospective, blinded, outcome study.
Setting:
Standard clinical care with tests performed in the polysomnographic laboratory.
Participants:
Consecutive patients (n = 67) recruited from a sleep center or a dental practice using broad inclusion criteria (age 21-80 years; AHI > 10/h; BMI < 40 kg/m2).
Interventions:
Therapeutic outcome with a mandibular protruding oral appliance was predicted following a mandibular protrusive titration study in the polysomnographic laboratory using a remotely controlled positioner and prospectively established predictive rules. An ETPP was also prospectively determined for participants predicted to be therapeutically successful with MRA therapy. All participants were blindly treated with a MRA, at either the predicted ETPP or a sham position, and therapeutic outcome was compared against prediction.
Measurements and Results:
At the final protrusive position, standard predictive parameters (sensitivity, specificity, positive and negative predictive values) showed statistically significant predictive accuracy (P < 0.05) in the range of 83% to 94%. The predicted ETPP provided an efficacious protrusive position in 87% of participants predicted to be therapeutically successful with MRA therapy (P < 0.05).
Conclusions:
Using prospectively established rules for interpreting the polysomnographic data, the mandibular titration study predicted mandibular repositioning appliance therapeutic outcome with significant accuracy, particularly with regard to accurately predicting therapeutic success. As well, among the participants predicted to be therapeutically successful with mandibular repositioning appliance therapy, the effective target protrusive position provided efficacious mandibular protrusion in the majority.
Citation:
Remmers J; Charkhandeh S; Grosse J; Topor Z; Brant R; Santosham P; Bruehlmann S. Remotely controlled mandibular protrusion during sleep predicts therapeutic success with oral appliances in patients with obstructive sleep apnea. SLEEP 2013;36(10):1517-1525.
Keywords: Obstructive sleep apnea, sleep disordered breathing, oral appliance therapy, mandibular repositioning titration
INTRODUCTION
Obstructive sleep apnea (OSA) poses a significant medical challenge to industrialized societies because it is common, carries cardiovascular and safety risks, and compromises quality of life.1–5 The predominant therapy for OSA is nasal continuous positive airway pressure (CPAP), a highly efficacious and benign treatment. However, adherence to this therapy is reported to be low, raising the need for alternative treatments.6,7 Surgical procedures, other than those involving bony reconstruction, have not proven to provide long-term benefit.8 Pharmaceutical and neuro-stimulatory approaches have been the subject of research, but no therapies in these categories are, as yet, generally available. Mandibular repositioning appliances (MRAs), which hold the mandible anteriorly and inferiorly during sleep, have been shown to open the passive pharynx in a dose-dependent fashion.9 Even though they convey therapeutic efficacy in approximately 50% of unselected patients,10,11 are preferred by most patients,12 and are recommended as a CPAP alternative for mild-to-moderate OSA,13 MRAs are not widely used to treat OSA. The reason for this is probably multifactorial, but the comparatively low efficaciousness rate, together with a lack of reliable selection methods, would appear to play an important role. Thus, accurate selection of patients for MRA therapy may have clinical utility in managing patients with OSA.
Several studies have examined clinical features that predict outcome with MRA therapy. Females sleeping on the side and males with positionally dependent OSA are likely to respond, as are younger patients with lower BMI, neck circumference, and apnea-hypopnea index (AHI).13–16 While excess body weight and severe sleep apnea have been shown to have negative implications for therapeutic success with oral appliance therapy, neither BMI nor AHI have been found to be predictive of therapeutic success or failure.14 Cephalometric features that have been associated with treatment response include anterior-posterior distance, mandibular length, and mandibular plane-hyoid distance.17,18
Two studies have reported associations between test results and MRA therapeutic response. Chan et al.19 reported that patients showing closure of the velo-pharynx by naso-pharyngoscopy during a Mueller maneuver are less likely to experience a positive response to MRA therapy. Tsuiki et al.20 correlated the nasal CPAP treatment pressures with MRA treatment outcome and found that patients having a prescribed pressure greater than 10.5 cm H2O were significantly less likely to experience a successful therapeutic outcome. Both of these studies provide interesting leads; however, as they enrolled relatively small numbers of patients and were correlational in design, formal predictive conclusions are not warranted at present. As well, neither of these methods predicts an effective protrusive position for the appliance.
The present study addresses the need for a validated tool that prospectively identifies favorable candidates for MRA therapy and accurately determines an effective target protrusive position (ETPP) for the appliance. One method of predicting an OSA patient's airway response to mandibular advancement is to experimentally simulate the mechanical action of a MRA by progressively protruding the mandible during sleep while examining the effects on respiratory status. If respiratory events associated with OSA are eliminated by such a mandibular protrusion “titration,” one could predict that a custom-fitted MRA would be an effective therapy and that the observed minimum effective protrusive distance might constitute a useful ETPP for the appliance. Such an approach was employed in three previous studies using a temporary dental appliance connected to a remotely controlled mandibular positioner (RCMP) so that the mandible could be progressively protruded, under polysomno-graphic observation, without disturbing the patient.21–23 Using a prototype device, Petelle et al.23 showed, in a limited number of participants, a correlation between AHI during titration and with the oral appliance in place. Dort et al.21 and Tsai et al.,22 also using prototype devices, showed that elimination of airway obstruction by such a mandibular titration predicted the eventual MRA therapeutic outcome with reasonable accuracy.
A commercially available RCMP device (MATRx, Zephyr Sleep Technologies Inc., Calgary, Canada) incorporates several improvements over the prototype devices. Specifically, it allows small, precise movements in the anterior-posterior dimension, while restricting movements in other dimensions. The purpose of the present study was to evaluate the ability of mandibular titration studies performed with this device, using prospectively and explicitly defined criteria for interpreting the polysomno-graphic results, to predict outcome with MRA therapy and to provide an adequate ETPP for the appliance.
METHODS
Research participants were recruited from the Sleep Centre of the Foothills Medical Centre (Calgary, Canada), which receives the majority of referrals from primary care physicians, respirologists, internists, otolaryngologists, psychiatrists, and dentists practicing in southern Alberta. In addition, a minority of participants (40.2%) were recruited from the Sierra Centre for Dental Wellness (Calgary, Canada), a large and diverse dental practice. Consecutive patients satisfying the inclusion (aged: 21 to 80 years; AHI > 10/h; BMI < 40 kg/m2; neck circumference < 50 cm; mean oxygen saturation > 90%; mandibular range of motion > 5 mm; adequate dentition: ≥ 10 upper and 10 lower teeth) and the exclusion criteria (inability to breathe comfortably through the nose; inability to tolerate overnight polysomnography in the sleep laboratory; > 50% of observed sleep apneas being central; anticipated change in medical therapy that could alter the severity of OSA during the protocol; anticipated change in body weight by ≥ 5% during the protocol; symptomatic, non-respiratory sleep disorder, e.g., restless leg syndrome, chronic insomnia) were invited to participate in the study at no expense. Prior treatment with CPAP or oral appliance therapy was not an exclusionary criterion.
The mandibular protrusion titration study was performed using the RCMP device, MATRx, during a standard polysomno-graphic (PSG) study. The intent of the titration was to progressively protrude the mandible, without disturbing sleep, until obstructive apneas and hypopneas were eliminated, particularly in REM sleep while supine. The device consists of a controller, located in a patient monitoring room that receives commands from the device software installed on the PSG computer and, in turn, activates a mandibular positioner attached to dental trays in the patient's mouth (Figure 1). The positioner (50 g; 62 mm × 41 mm × 20 mm) has two movable rods that connect to brackets extending anteriorly from the dental trays. The upper rod is driven by an internal linear actuator and attaches to the upper bracket. The lower rod is driven by a manually adjustable screw and connects to the lower bracket.
Figure 1.
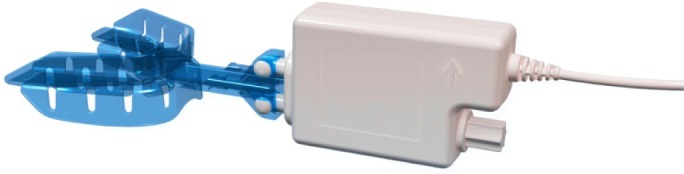
Remotely controlled mandibular positioner (MATRx) attached to disposable upper and lower dental trays that are fit to a patient's teeth with impression material.
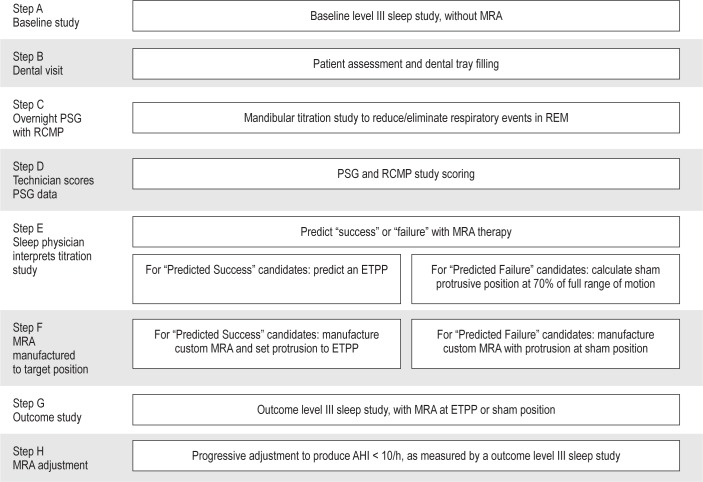
Participants followed the research protocol outlined in Figure 2.
Figure 2.
The research protocol. MRA, mandibular repositioning appliance; PSG, polysomnogram; ETPP, effective target protrusive position; AHI, apneahypopnea index; RCMP, remotely controlled mandibular positioner.
STEP A comprised a 2-night baseline study in the home with a portable monitor that has been validated in the laboratory setting23 (SnoreSat, SagaTech Electronics, Calgary, Canada) to assess the apnea-hypopnea index (AHI). The monitor recorded oxygen saturation, heart rate, airflow, snoring sound, and body position, and automatically calculated AHI using a > 4% oxygen desaturation criterion.
STEP B comprised a dental visit for fitting of disposable dental titration trays, which consist of close fitting upper and lower trays that are filled with impression material to grip the teeth. The brackets have guide rails that maintain the anterior-posterior alignment of the trays, thereby minimizing relative motion in the lateral and cranial-caudal dimensions (Figure 1). With the trays in position, the upper and lower teeth are separated by 3 mm. As well, a pointer on the guide rails overlays a millimeter scale on the upper surface of the upper bracket, allowing quantification of the relative position of the upper and lower trays. After fitting the trays to the participant's upper and lower teeth, the dentist noted the scale readings for maximum voluntary retrusion and protrusion, and rest position (i.e., habitual bite position). These readings, which characterize the participant's mandibular range of motion for the overnight study, were provided to the PSG technologist.
STEP C comprised the overnight titration study carried out under PSG monitoring by a computerized system (Sandman, Nellcor Puritan Bennett [Melville] Ltd., Ottawa, Canada) that also ran the device software (OATRx TS, Zephyr Sleep Technologies Inc., Calgary, Canada). The standard montage of bioelectric signals consisted of: electrocardiogram, electrooculogram, submental electromyogram, electroencephalogram, anterior tibialis electromyogram, nasal airflow, respiratory movements of the rib cage and abdomen, arterial oxyhemoglobin saturation, and snoring sound. These signals, together with mandibular position (i.e., relative position of the dental trays), were displayed on the PSG monitor. At the start of the study, the participant's dental trays were attached to the mandibular positioner of the RCMP device (Figure 1), and the positioner was calibrated to the PSG system using the device software. The values for range of motion and rest position were entered in the device software. Using the manual adjustor screw, the trays were set at rest position, minus 2 mm, and inserted into the participant's mouth. Once asleep, the participant's mandible was protruded remotely by the PSG technologist in 0.2-0.6 mm steps in response to evidence of respiratory events (i.e., apneas and hypopneas). If an arousal occurred, the mandible was not moved until stable sleep had resumed. The technician was provided no specific protocol regarding assessment of the participant's respiratory status during the study. Rather, the technician was instructed to protrude the mandible in order to eliminate sleep apnea, using experienced judgement, similar to a CPAP titration. Stepwise protrusion of the mandible was continued over the range of mandibular protrusion until respiratory events were unequivocally eliminated in REM and NREM sleep in both the supine and lateral decubitus positions, or until maximum protrusion was reached (as determined in Step B).
STEP D involved the PSG data being scored by a technologist using the 2007 American Academy of Sleep Medicine (AASM) Manual for Scoring of Sleep and Associated Events (respiratory events scored by criterion 4a).25
STEP E began with the review of the scored data from Step D, together with body position and mandibular position signals, by a sleep physician (investigator J.R.) who was blinded to all knowledge of the participant. The reviewer applied the predetermined interpretative rules depicted in Figure 3 to predict success or failure with MRA therapy. To constitute conclusive predictive data, the participant must have undergone REM sleep for ≥ 5 min in the supine position or, if REM supine was not observed, in the lateral decubitus position so long as the baseline study indicated that the participant slept for the majority of the night in the lateral position. All REM cycles were examined to identify a minimum 5-min interval, continuous or fragmented, where a frequency of ≤ 1 respiratory events occurred. If this was found, the study was deemed to predict MRA therapeutic success (“predicted success” group, PS) and, if not, it was judged to predict therapeutic failure (“predicted failure” group, PF). For all participants predicted to experience therapeutic success, the minimum protrusive position that was associated with ≤ 1 respiratory event per 5-min REM interval was provided as the predicted effective target protrusive position (ETPP).
Figure 3.
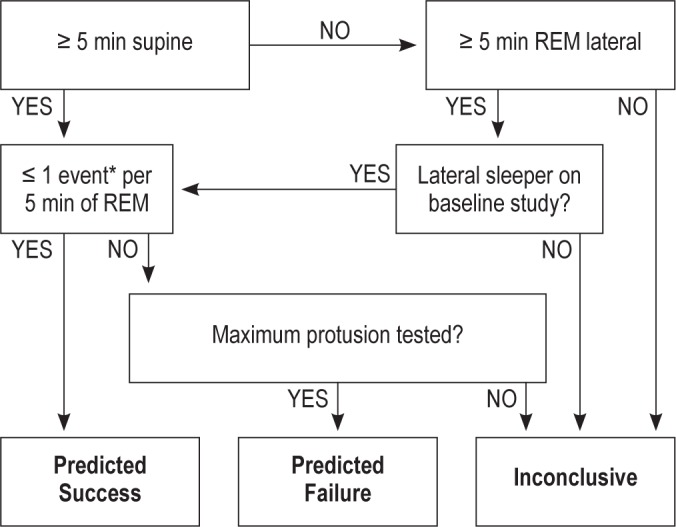
Predictive criteria used to blindly predict therapeutic outcome with MRA. *Event defined as apnea and/or hypopnea.
STEP F involved relaying a target protrusion for oral appliance therapy to the dentist (investigator S.C.) who was blinded with regard to the prediction of therapeutic outcome established from the titration study. For PS participants, the predicted ETPP was provided. For PF participants a sham position equal to 70% of anterior-posterior range of motion was provided, thus maintaining the blinded status of the dentist. The dentist then completed the bite registration and records process, and arranged for the appliance manufacturer (SomnoMed Ltd., Australia) to fabricate an appropriate MRA (SomnoDent) with 3-mm separation of the occlusal planes at the target protrusive setting.
STEP G comprised a 2-night outcome study at the target position, with the portable monitor23 as described in STEP A, which was completed within 3 weeks of receiving the MRA. These data were used to calculate the accuracy of the ETPP for the PS group. If therapeutic success was not achieved at this first setting (ETPP for PS group, sham setting for PF and the inconclusive group), participants continued on to STEP H.
STEP H provided progressive adjustment of the protrusive position at 1- to 2-mm steps, as per the clinical judgment of the dentist, until either therapeutic success was attained, or the clinical limits of maximum protrusion were reached. The AHI in this step was evaluated with the portable monitor23 as described in Step A. The final protrusive position, attained in either Step E or Step H, was used in the evaluation of final therapeutic outcome as presented in the results.
Therapeutic success was defined as an AHI < 10/h and ≥ 50% reduction less than baseline AHI. Descriptive summary statistics were performed and non-normally distributed variables were logarithmically transformed. Paired ANOVA compared baseline AHI with final AHI for participants predicted to be therapeutic successes and those predicted to be therapeutic failures. Two-by-two frequency tables were used to examine the association between the test's predictions and outcome AHI; sensitivity, specificity, and positive and negative predictive values were also calculated. Treatment outcome at final position, was used as the dependent variable in a multiple logistic regression analysis of clinical predictors that included baseline AHI, age, BMI, positional dependence (the ratio: supine AHI/ lateral AHI), neck circumference and adjusted neck circumference (ANC)26; potential interactions were also examined. Receiver operator characteristic (ROC) displaying the sensitivities and specificities over the continuous range of decision cut-points as well as the overall summary of the area under the curve (AUC) were also provided.
This study was approved by the Conjoint Health Research Ethics Board of the University of Calgary (Ethics ID: E-22389) and informed consent was obtained from all participants.
RESULTS
A total of 67 participants meeting the study criteria were recruited. All participants completed the study protocol up to Step G (i.e., all underwent an outcome study with the MRA set at the either the ETPP or sham position). Three participants failed to complete Step H, as they declined to have further adjustment of their appliance (1 after the results of the outcome study indicated that the AHI was < 10/h at ETPP; 2 after experiencing discomfort with the oral appliance). The titration study was unable to make a prediction for 6 participants (4 owing to inadequate REM sleep; 2 owing to having not achieved maximum protrusion during the test). One predicted success participant was incorrectly assigned and tested at a sham position of 70%, instead of the predicted ETPP. The results for this participant are not included in the ETPP analysis.
The baseline characteristics of the study population are presented in Table 1. On average, the participants were obese and had moderate sleep apnea and mild hypoxemia during sleep. The overall therapeutic success rate for the study population was 58.2%. The mean age of participants in the therapeutic failure group was significantly greater than that of the therapeutic success group (P = 0.007). Results of the multiple regression analysis indicated that age (P = 0.006) and ANC (P = 0.034) were jointly significant in predicting therapeutic success or failure. Neither BMI, neck circumference nor AHI were independently or jointly predictive.
Table 1.
Baseline characteristics of the study population

The AHI response to MRA therapy for the two predictive groups (PS and PF) is shown in Figure 4. Thirty of 32 participants in the predicted success group had final therapy AHI values < 10/h and a 50% reduction from baseline, whereas only 5 of the 29 predicted failures had final therapy AHI values that met the criteria for therapeutic success. Four participants in the predicted failure group showed an increased AHI over baseline values with MRA therapy. The mean baseline AHI values (PS = 23.6/h; PF = 27.6/h; P = 0.36) and BMI values (PS = 29.4 kg/m2; PF = 31.5 kg/m2; P = 0.116) for each predictive group were not significantly different. By contrast, mean values for age (P = 0.031) and ANC (P = 0.036) differed significantly between the two groups.
Figure 4.
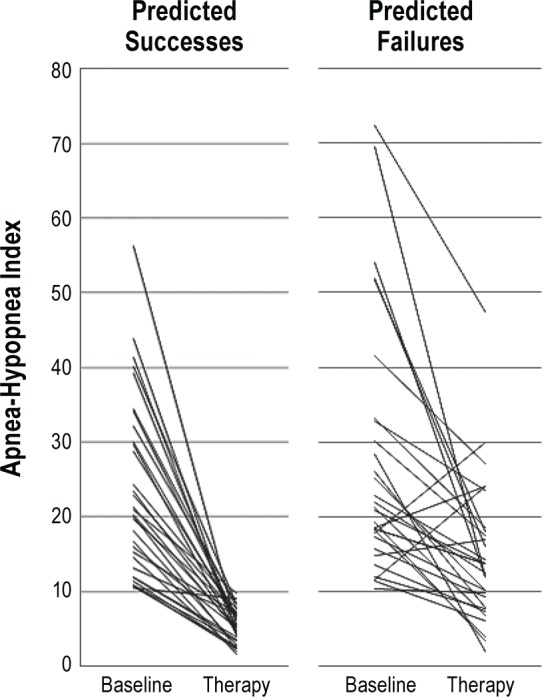
Response to therapy at final protrusive position for the two predictive groups, predicted success and predicted failure.
Figure 5 illustrates the test's predictions and therapeutic outcome in relation to baseline AHI and BMI values. Overall, the AHI and BMI values for participants who experienced therapeutic success and for those who experienced therapeutic failure overlap in distribution. Participants who were correctly predicted to experience therapeutic success had BMI values ranging from 18.6 to 38.1 kg/m2 and baseline AHI values ranging from 10.7/h to 56.3/h (top panel). Of the 32 participants in the predicted success group, 20 (62.5%) had BMI and/ or baseline AHI values that exceeded those usually recommended for selecting candidates for oral appliance therapy13 (BMI < 30 kg/m2 and AHI < 30/h; indicated by shaded area in Figure 5), and all of these were therapeutically successful. Similarly, 24.1% of participants in the predicted failure group lying within the recommended guidelines were not therapeutically successfully. A cluster of predicted failures with high values of BMI (> 30 kg/m2) and AHI (> 40 events/h) is apparent in Figure 5 (and Figure S1 in the supplemental material), but formal analysis based on logistic regression and AUC did not indicate clinical utility.
Figure 5.
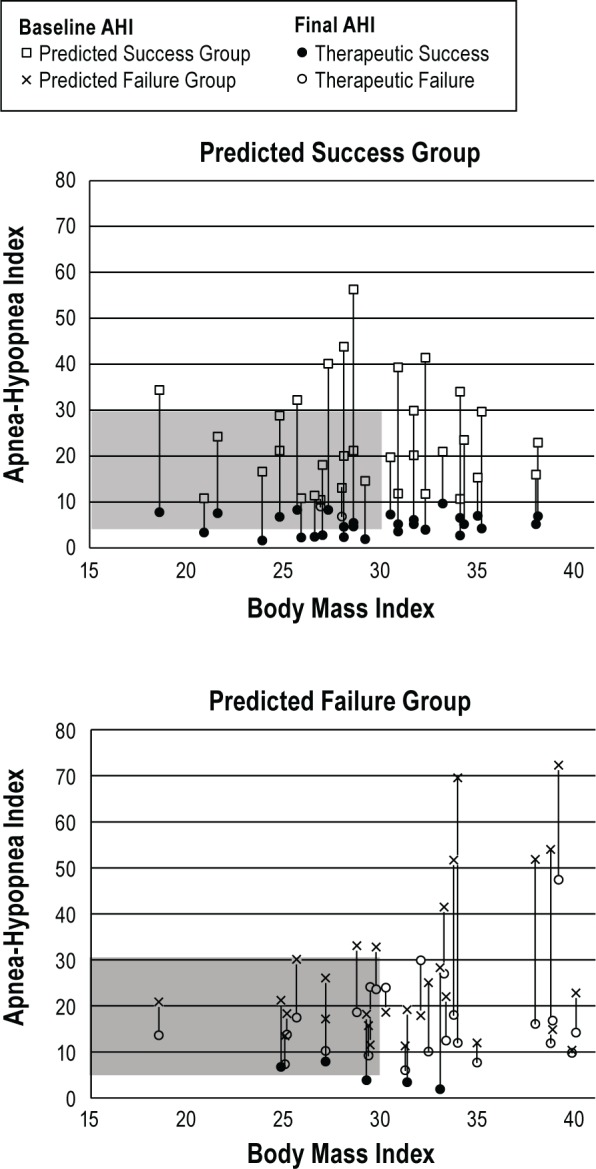
The effect of MRA therapy at the final protrusive position for each participant in relation to their body mass index (BMI, kg/m2). Therapeutic success defined as AHI < 10/h and at least a 50% reduction from baseline. Shaded area denotes guidelines of recommended oral appliance therapy based on AHI and BMI.
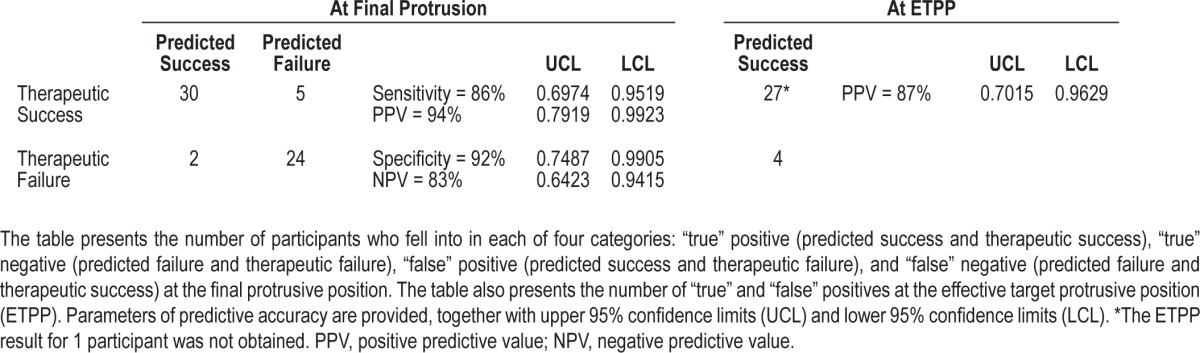
A numerical description of predictive accuracy is presented in Table 2, where prediction of therapeutic success or failure is cross-referenced with observed therapeutic outcome at the final protrusive position and the predicted ETPP. At the final protrusive position, all 4 predictive parameters showed statistically significant predictive accuracy (P < 0.05) in the range of 83% to 94%. The two participants who were incorrectly predicted to be successful had baseline AHI between 10 and 15, and, while their final therapeutic AHI values were less than 10/h, they exceeded 50% of baseline AHI. The prediction of therapeutic failure was correct in 82.8% of participants. All incorrect predictions of therapeutic failure (false negatives) had a baseline AHI < 30/h (Figure 5). Predictive accuracy, taking into account inconclusive tests, is described by an ROC curve (Figure S2 in the supplemental material) and corresponding AUC of 0.89 (95% CI 0.81-0.97). For comparison, the ROC curve corresponding to predictions based on age and ANC yielded an AUC of 0.74 (95% CI 0.62-0.86).
Table 2.
Numerical matrix describing the predictive accuracy of MATRx
Of the 31 participants in the predicted success group that were tested at the ETPP (see note in legend of Table 2), 87.1% were successfully treated at this first position (P < 0.05). The 4 participants who did not achieve therapeutic success at the ETPP were prescribed further protrusion beyond the ETPP by the dentist. Two participants achieved therapeutic success with further protrusions of 2.5 mm; one participant achieved an AHI < 10/h without the 50% reduction from baseline with 1 mm of further protrusion; and the one remaining participant declined further adjustment as the AHI value at ETPP was < 10/h. Figure S3 in the supplemental material displays the relationship between ETPP and baseline AHI and BMI. Both reveal considerable scatter, indicating that ETPP cannot be predicted from either variable.
Figure 6 shows the final protrusive position at which successful MRA therapy was achieved for all participants, using the full A-P range of motion (i.e., maximum voluntary retrusion to maximum voluntary protrusion). The final protrusive position using full protrusion (i.e., habitual bite position to maximum voluntary protrusion) is provided in Figure S4 in the supplemental material. Therapeutic success was achieved with protrusion ranging from as little as 0.5 mm to 10 mm from habitual bite. The median values for successful therapeutic position was 68% for full protrusion and 79% for full range of motion.
Figure 6.
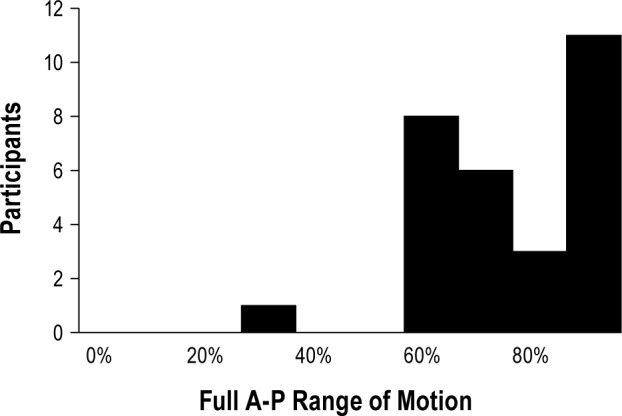
The protrusive position at which successful treatment was achieved for all PS group participants using the effective target protrusive position (ETPP) as the starting position for therapy using the full anterior-posterior (A-P) range of motion, i.e., maximum retrusion to maximum protrusion.
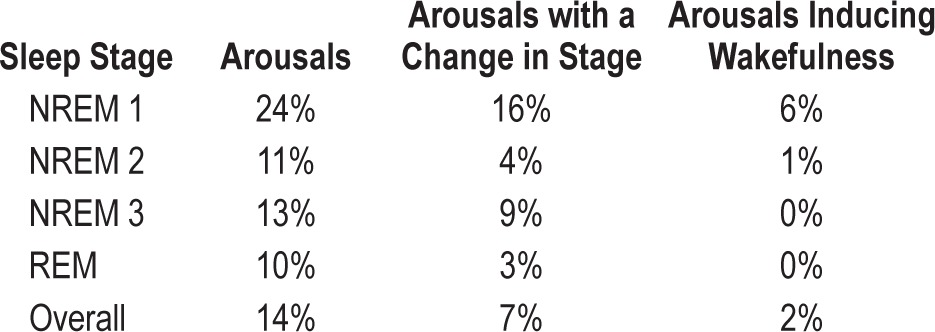
Table 3 shows the incidence of arousals by sleep stage associated with mandibular movement. The occurrences of spontaneous arousals that may have been coincidental with device movement were not separately considered. As might be expected, arousal occurrence (expressed as a fraction of actuator movements), was highest in stage 1 NREM and lowest in REM. A similar distribution was applied to movement related arousals associated with a change in sleep stage or a change to wakefulness. Overall, 14% of mandibular movements were associated with arousals; half of these involved a change in sleep stage and only rarely involved the appearance of wakefulness. No wakefulness inducing arousals occurred when the mandibular movements were made during REM.
Table 3.
The results of an analysis, by sleep state, of the incidence of arousals associated with mandibular movement by the MATRx device
DISCUSSION
The primary purpose of this study was to assess the accuracy of a remotely operated and polysomnographically monitored mandibular protrusion titration in predicting therapeutic outcome with an oral appliance in patients with obstructive sleep apnea. We also sought to evaluate the utility of a effective target protrusive position (ETPP), determined during the mandibular titration study, as a target setting for the MRA in participants predicted to experience success with this therapy. Using prospectively determined rules for scoring and interpreting the polysomnographic data, the mandibular titration study predicted therapeutic outcome with MRAs with significant accuracy, particularly with regard to predicting therapeutic success. Also, in those participants who were predicted to be a therapeutic success, the ETPP provided efficacious mandibular protrusion in the majority.
A strength of the present study is its prospective design, where prediction rules and outcome criteria were explicitly established in advance and rigorously applied. An additional strength is the blinded design, where no one—interpreter, therapist, nor patient—had information that might bias the results. Together, these study design features suggest that this titration method can be replicated in other polysomnographic settings and that the results will yield predictive accuracies comparable to those reported here.
An important potential use of a mandibular titration study is to identify patients who will be successfully treated with MRA therapy. The high efficaciousness rate of the relatively benign alternative management, CPAP, means that to be a reasonable alternative, oral appliance therapy must also be highly efficacious. While a somewhat elevated “false negative” prediction rate may be undesirable, a high “false positive” rate would not be clinically acceptable, as it would create an efficaciousness rate lower than that for CPAP. Accordingly, we prospectively established a conservative polysomnographic prediction rule (i.e., one or fewer respiratory events in a 5-min interval of REM sleep while supine) postulating that if sleep apnea is eliminated in this condition, MRA therapy would very likely be successful. This postulate is supported by the relatively high positive prediction value and specificity observed using a reasonably stringent criterion of therapeutic success (AHI < 10/h and ≥ 50% reduction from baseline AHI). Had we used a more relaxed criterion of therapeutic success (AHI < 10/h without the associated fractional reduction), both predictive statistics would have been 100%, since both “false positive” participants had outcome AHI values less than 10/hour (baseline: 13.1 and 10.5/h; outcome: 6.9 and 9.0/h, respectively). Thus, the present results indicate that MATRx titration can select patients with OSA who will experience successful treatment with MRAs.
As noted above, our methods for predicting success meant that we failed to identify some candidates who were successfully treated with MRA. On the other hand, the titration test identified a substantial number of therapeutically successful participants who would not have been offered oral appliance therapy as a front-line treatment if the commonly used criteria based on AHI and BMI were used. Noteworthy in this regard, age and ANC were the only pre-test variables that were jointly significant in predicting therapeutic outcome. While age has previously been shown to be a factor in correlating of therapeutic outcome15, the present study appears to be the first to examine the predictive accuracy of the ANC. That this variable, which includes the neck circumference plus three historical features indicative of OSA, has predictive accuracy is somewhat unexpected, and the full implications of the finding await further study. The positional dependency of AHI, shown to correlate with MRA outcome in a previous study,14 failed to reach statistical significance in the present study. Obese patients with severe sleep apnea generally begin management with CPAP therapy and only explore MRA therapy after failing CPAP. Thus, one of the contributions of the RCMP titration study may be to provide a direct avenue to successful MRA therapy for some patients who might otherwise not be considered for MRA as a first-line or even second-line therapy. In this way, RCMP titration might increase efficiency, reduce the time to successful treatment, and improve the overall therapeutic outcome.
The present results confirm those reported in two previous comparable studies21,22 with remotely controlled mandibular positioners. Participant characteristics and overall therapeutic success rate in these studies are comparable to the present study. However, the MRA differed, being Klearway in the previous studies and SomnoDent in the present study. Both of these appliances share the same basic protrusive mechanism, causing protrusion of the mandible with minimal separation of the teeth. This suggests that the predictive accuracy in the present study will apply to other appliances that use a similar protrusive mechanism. The present study differs importantly from our previous studies in that a more refined, commercially available device was used and the predictive criteria were explicitly and prospectively defined. In addition, the positive predictive statistics are somewhat greater in the present study than in the previous studies, which may relate to MRA differences or improved design of the dental trays that allow more precise measurement of mandibular position and less lateral or cranial-caudal movement of the mandible. A limitation of all of these studies, including the present one, is the portable monitor used to measure the baseline and therapeutic outcome AHI has been validated only in the PSG lab rather than in the home setting. Presumably, the predictive accuracy of the PSG test would have been greater had we used AHI values derived from baseline and outcome PSG studies.
Another important result of the present study is that the ETPP provided an efficacious protrusive position for 87% of participants predicted to be therapeutically successful with MRA therapy. In three participants, the ETPP was insufficient to provide the 50% or less reduction in AHI from the baseline value and in one participant it did not provide an AHI less than 10/hour. This predictive capability of the mandibular titration study may have considerable clinical utility, as no validated method for predicting an efficacious mandibular protrusive position is currently available. An interesting related finding is that the ETPP values derived from our tests were surprisingly low, with the lowest being 6% and the median being 68% of their protrusive range. All studies at the ETPP were also conducted within 3 weeks of fitting the appliance. This suggests that use of the device will likely decrease the time and the number of dental visits needed to arrive at a therapeutically successful protrusive position, potentially providing immediate efficacious treatment from night one, and may reduce the risk of over-protruding the patient's mandible.
The efficiency of the MATRx titration in the present study was compromised somewhat by the 9% rate of inconclusive tests, with 3% owing to an inadequate test range and 6% owing to a lack of REM sleep. The former might be reduced as technicians become more experienced with the titration protocol. The titration procedures probably did not compromise the efficiency of the titration test as arousals caused by movement of the mandible only rarely (3% of movements) terminated a REM cycle. While observations made during the NREM periods may prove useful in patient management, assessment of the predictive accuracy of such information was beyond the scope of this study. The predictive rules did, however, consider instances where insufficient data in REM supine was observed; in such cases, the results in REM lateral were used, provided the baseline study indicated that the participant was a side-sleeper.
To our knowledge, the remotely controlled mandibular positioner is the only device for which the ability to positively identify candidates for oral appliance therapy and to predict an effective target protrusive position has been demonstrated. A reason for this may be that such devices move the mandible by remote control with little disturbance of sleep. This means that arousals are infrequently induced, that sleep architecture is minimally disturbed, and that the entire range of motion of the mandible can be explored. These advantageous features of the RCMP study are lost in the other approach to protruding the mandible in a polysomnographic study, namely: manual manipulation of an oral appliance. Kuna et al.,27 using temporary dental trays, moved the mandible by extracting and adjusting the appliance. The results showed no predictive accuracy. Similar problems are likely to arise in “titration” studies performed using manual adjustment of a screw device in a custom fitted appliance,28,29 where sleep disturbances are likely to occur and the range of mandibular motion available for study is limited. Unfortunately, the accuracy of such manual adjustment of a custom fitted MRA have not been reported. However, even if this approach were shown to be useful in determining a therapeutically efficacious protrusive position, it is not suitable for prospective patient selection as the study uses a custom fitted MRA. Another approach may be the use of a temporary oral appliance to evaluate a number of protrusive positions using repeated home studies.30 However, the predictive accuracy of this approach has similarly not been demonstrated.
Remotely controlled mandibular positioners, such as the one studied here, may convey clinical capability that is comparable to remotely controlled CPAP, in that both enable the PSG lab to carry out titration studies using a potential therapy for OSA. The goal of each study is the same, namely: to determine an optimal therapeutic “dose” that eliminates sleep disordered breathing. For CPAP, this is the lowest pressure that eliminates sleep apnea; for RCMP, it is the minimum mandibular protrusion that eliminates OSA. In the latter instance, maximal protrusion may not prove adequate, indicating that MRA therapy is not suitable. With either CPAP or RCMP titration studies, the clinician observes the dose effect of the therapeutic intervention during sleep and makes appropriate management decisions.
DISCLOSURE STATEMENT
This study was funded by Zephyr Sleep Technologies Inc. SagaTech Electronics provided the home monitoring equipment for data collection. Dr. Charkhandeh serves as a consultant for Zephyr Sleep Technologies Inc. Dr. Bruehlmann, Mr. Grosse, Mr. Santosham, Dr. Topor, and Dr. Brant are affiliated with Zephyr Sleep Technologies. Dr. Remmers is employed by Zephyr Sleep Technologies and is a shareholder in SagaTech Electronics.
ACKNOWLEDGMENTS
The authors thank the participants, the staff at the Sleep Centre of the Foothills Medical Centre and the Centre for Human Performance in Sleep, SagaTech Electronics for the loan of Remmers Sleep Recorders, SomnoMed Ltd. for the complementary provision of SomnoDent appliances and Zephyr Sleep Technologies for the provision of the MATRx device.
Footnotes
A commentary on this article appears in this issue on page 1417.
SUPPLEMENTAL MATERIAL
The body mass index (kg/m2) and baseline apnea-hypopnea index (events/h) values for each participant by outcome with oral appliance therapy (●: therapeutic success; ○: therapeutic failure).
Comparison of MATRx test receiver operator characteristic (ROC) curve to ROC curve for age and ANC pre-test predictors. AUC: area under curve; CI: confidence interval; ANC: adjusted neck circumference.
The effective target protrusive position (ETPP) plotted against baseline apnea-hypopnea index (AHI) (top) and body mass index (bottom) for participants in the predicted success group.
The number of participants in the predicted success (PS) group plotted as a function of the percent of protrusive position at which successful treatment was achieved. Full protrusion is the distance from resting position to maximum protrusion.
REFERENCES
- 1.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea - implications for cardiac and vascular disease. JAMA. 2003;290:1906–19. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin Sleep Cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573–81. [PMC free article] [PubMed] [Google Scholar]
- 4.Horstmann S, Hess CW, Bassetti C, Gugger M, Mathis J. Sleepiness-related accidents in sleep apnea patients. Sleep. 2000;23:383–9. [PubMed] [Google Scholar]
- 5.D'Ambrosio C, Bowman T, Mohsenin V. Quality of life in patients with obstructive sleep apnea. Chest. 1999;115:123–9. doi: 10.1378/chest.115.1.123. [DOI] [PubMed] [Google Scholar]
- 6.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS) Sleep Med Rev. 2003;7:81–9. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 7.McArdle N, Devereux G, Heidarnejad H, Mackay T, Douglas N. Long term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:1108–14. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 8.Aurora RN, Casey KR, Kristo D, Auerbach S, et al. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep. 2010;33:1408–13. doi: 10.1093/sleep/33.10.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isono S, Tanaka A, Sho Y, Konno A, Nishino T. Advancement of the mandible improves velopharyngeal airway patency. J Appl Physiol. 1995;79:2132–8. doi: 10.1152/jappl.1995.79.6.2132. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson KA, Cartwright R, Rogers R, et al. Oral Appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29:244–62. doi: 10.1093/sleep/29.2.244. [DOI] [PubMed] [Google Scholar]
- 11.Clark GT, Blumenfeld I, Yoffe N, Peled E, Lavie P. A crossover study comparing the efficacy of continuous positive airway pressure with anterior mandibular positioning devices on patients with obstructive sleep apnea. Chest. 1996;109:1477–83. doi: 10.1378/chest.109.6.1477. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson KA, Ono T, Lowe AA, Keenan SP, Fleetham JA. A randomized crossover study of an oral appliance vs. nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest. 1996;109:1269–75. doi: 10.1378/chest.109.5.1269. [DOI] [PubMed] [Google Scholar]
- 13.Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: An update for 2005. Sleep. 2006;29:240–3. doi: 10.1093/sleep/29.2.240. [DOI] [PubMed] [Google Scholar]
- 14.Marklund M, Persson M, Franklin KA. Treatment success with a mandibular advancement device is related to supine-dependent sleep apnea. Chest. 2000;17:1065–72. doi: 10.1378/chest.114.6.1630. [DOI] [PubMed] [Google Scholar]
- 15.Cartwright RD. Predicting response to the tongue retaining device for sleep apnea syndrome. Arch Otolaryngol. 1985;111:385–8. doi: 10.1001/archotol.1985.00800080071008. [DOI] [PubMed] [Google Scholar]
- 16.Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1457–61. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Lowe AA, Fleetham JA, Park YC. Cephalometric and physiologic predictors of the efficacy of an adjustable oral appliance for treating obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 2001;120:639–47. doi: 10.1067/mod.2001.118782. [DOI] [PubMed] [Google Scholar]
- 18.Otsuka R, Almeida FR, Lowe AA, Ryan F. A comparison of responders and non-responders to oral appliance therapy for the treatment of obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 2006;129:222–9. doi: 10.1016/j.ajodo.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 19.Chan AS, Lee RW, Srinivasan VK, Darendeliler MA, Grunstein RR, Cistulli PA. Nasopharyngoscopic evaluation of oral appliance therapy for obstructive sleep apnoea. Eur Respir J. 2010;35:836–42. doi: 10.1183/09031936.00077409. [DOI] [PubMed] [Google Scholar]
- 20.Tsuiki S, Kobayashi M, Namba K, Oka Y, et al. Optimal positive airway pressure predicts oral appliance response to sleep apnea. Eur Respir J. 2010;35:1098–105. doi: 10.1183/09031936.00121608. [DOI] [PubMed] [Google Scholar]
- 21.Dort LC, Hadjuk E, Remmers JE. Mandibular advancement and obstructive sleep apnoea: a method for determining effective mandibular protrusion. Eur Respir J. 2006;27:1003–9. doi: 10.1183/09031936.06.00077804. [DOI] [PubMed] [Google Scholar]
- 22.Tsai WH, Vazquez JC, Oshima T, et al. Remotely controlled mandibular positioner predicts efficacy of oral appliances in sleep apnea. Am J Respir Crit Care Med. 2004;170:366–70. doi: 10.1164/rccm.200310-1446OC. [DOI] [PubMed] [Google Scholar]
- 23.Vazquez JC, Tsai WH, Flemons WW, et al. Automated analysis of digital oximetry in the diagnosis of obstructive sleep apnoea. Thorax. 2000;55:302–7. doi: 10.1136/thorax.55.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petelle B, Vincent G, Gagnadoux F, et al. One-night mandibular advancement titration for obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2002;165:1150–3. doi: 10.1164/ajrccm.165.8.2108056. [DOI] [PubMed] [Google Scholar]
- 25.Iber C, Ancoli-Israel S, Chesson A, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 26.Flemons WW. Clinical practice. Obstructive sleep apnea. N Engl J Med. 2002;347:498–504. doi: 10.1056/NEJMcp012849. [DOI] [PubMed] [Google Scholar]
- 27.Kuna ST, Giarraputo PC, Stanton DC, Levin LM. Evaluation of an oral mandibular advancement titration appliance. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:593–603. doi: 10.1016/j.tripleo.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 28.Friedman M, Hamilton C, Samuelson CG, et al. Compliance and efficacy of titratable thermoplastic versus custom mandibular advancement devices. Otolaryngol Head and Neck Surg. 2012;147:379–86. doi: 10.1177/0194599812439683. [DOI] [PubMed] [Google Scholar]
- 29.Almeida FR, Parker JA, Hodges JS, et al. Effect of a titration polysomnogram on treatment success with a mandibular repositioning appliance. J Clin Sleep Med. 2009;5:198–204. [PMC free article] [PubMed] [Google Scholar]
- 30.Levendowski DJ, Morgan T, Westbrook P. Initial evaluation of a titration appliance for temporary treatment of obstructive sleep apnea. J Sleep Disord Ther. 2011;1:1. doi: 10.4172/2167-0277.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The body mass index (kg/m2) and baseline apnea-hypopnea index (events/h) values for each participant by outcome with oral appliance therapy (●: therapeutic success; ○: therapeutic failure).
Comparison of MATRx test receiver operator characteristic (ROC) curve to ROC curve for age and ANC pre-test predictors. AUC: area under curve; CI: confidence interval; ANC: adjusted neck circumference.
The effective target protrusive position (ETPP) plotted against baseline apnea-hypopnea index (AHI) (top) and body mass index (bottom) for participants in the predicted success group.
The number of participants in the predicted success (PS) group plotted as a function of the percent of protrusive position at which successful treatment was achieved. Full protrusion is the distance from resting position to maximum protrusion.