Abstract
Study Objectives:
Previous studies with limited follow-up times have suggested that sleep-related traits are associated with an increased risk of incident dementia or cognitive decline. We investigated the association between midlife sleep characteristics and late life cognitive function.
Design:
A follow-up study with a median follow-up time of 22.5 (range 15.8-25.7) years assessing the association between midlife sleep characteristics and later cognitive function.
Setting:
Questionnaire data from 1981 were used in the assessment of sleep characteristics, use of hypnotics, and covariates at baseline. Between 1999 and 2007, participants were assigned a linear cognitive score with a maximum score of 51 based on a telephone interview (mean score 38.3, SD 6.1). Linear regression analyses were controlled for age, sex, education, ApoE genotype, and follow-up time.
Participants:
2,336 members of the Finnish Twin cohort who were at least 65 years of age.
Interventions:
N/A.
Measurements and Results:
Baseline short (< 7 h/day) and long (> 8 h/day) sleepers had lower cognitive scores than participants sleeping 7-8 h/ day (β = -0.84, P = 0.014 and β = -1.66, P < 0.001, respectively). As compared to good sleep quality, poor or rather poor sleep quality was associated with a lower cognitive score (β = -1.00, P = 0.011). Also, the use of hypnotics ≥ 60 days per year was associated with poorer cognitive function (β = -1.92, P = 0.002).
Conclusions:
This is the first study indicating that midlife sleep length, sleep quality, and use of hypnotics are associated with late life cognitive function. Further confirmation is needed, but sleep-related characteristics may emerge as new risk factors for cognitive impairment.
Citation:
Virta JJ; Heikkilä K; Perola M; Koskenvuo M; Räihä I; Rinne JO; Kaprio J. Midlife sleep characteristics associated with late life cognitive function. SLEEP 2013;36(10):1533-1541.
Keywords: Cognition, sleep, hypnotics and sedatives, risk factors, cohort studies
INTRODUCTION
As of today, the medications available for Alzheimer disease (AD) and other dementia disorders are of limited value, offering only symptomatic relief. Therefore, the recognition of possibly modifiable risk factors for dementia and cognitive impairment is of utmost importance. During the last decade, the traditional dementia risk factors, including increasing age, low educational level, and carrying an apolipoprotein E (ApoE) ε4 allele have been supplemented by recognition of the importance of risk factors associated with cardiovascular disease. Still, it seems evident that risk factors recognized thus far only partly explain the variance in dementia risk.1
Cross-sectional studies have suggested that multiple sleep characteristics are associated with poorer cognitive function or are common in dementia patients,2–4 but evidence from prospective studies is more limited. In the Honolulu-Asia Aging study, it was shown that daytime sleepiness was associated with an increased risk of incident dementia and cognitive decline during a 3-year follow-up, whereas insomnia was not.5 The Neurological Diseases in Central Spain study found that 9 or more hours of sleep per day increased the risk of incident dementia 3 years later. In contrast, 5 hours or less of sleep did not increase the risk.6 Likewise, in the HeiDE study, sleeping at least 9 hours per day was associated with impaired verbal memory after a follow-up of 8.5 years as compared to sleeping 7 hours per day.7
Additionally, older women were followed for a mean of 5 years as a part of the Study of Osteoporotic fractures. In that study, a less robust circadian activity rhythm and delayed timing of peak physical activity based on actigraphy was associated with an increased risk of mild cognitive impairment (MCI) or dementia independent of sleep fragmentation and duration.8 In a shorter, one-year follow-up study, poorer sleep quality was associated with an increased risk of incident cognitive impairment in the elderly individuals of the ESA study.9 Also, in the recent Sleep and Cognition study utilizing polysomnography, sleep disordered breathing increased the risk of incident dementia or mild cognitive impairment on average 5 years later, with the risk increase related to hypoxia rather than to sleep fragmentation or duration.10
In contrast to other sleep characteristics, the association between the use of hypnotics—more specifically benzodiaze-pines—and cognitive function has been studied in some detail. In 2005, a meta-analysis found the use of benzodiazepines to be associated with cognitive decline in three of six eligible studies.11
These studies all had follow-up times of at most 10 years, even though the pathological changes of AD are considered to precede symptom onset by at least 10-15 years.12 Therefore, in order to assess potential risk factors for cognitive impairment before the accumulation of such pathological changes, longer follow-up time is needed. In this paper, the results of a 22-year-long follow-up study of the older Finnish Twin cohort are described. The aim of the study was to assess whether midlife sleep characteristics, including the use of hypnotics or tranquilizers, are associated with late life cognitive function. As we had access to data on sleep related traits both in 1975 and 1981, tests for possible exposure time effects were also performed.
METHODS
Subjects
The subjects of the current study were members of the older Finnish Twin Cohort, consisting of same-sex twin pairs born in Finland prior to 1958 with both co-twins alive in 1967.13 The assessment of sleep characteristics was based on postal questionnaires sent to the members of the cohort in 1975 and 1981 with good response rates.14 Between 1999 and 2001, monozygotic (MZ) twin pairs ≥ 65 years old, with both co-twins alive, were asked to participate in a telephone interview assessing their cognitive function (described in detail below). Corresponding dizygotic (DZ) twins and twins of unknown zygosity irrespective of co-twin's vital status were contacted between 2003 and 2007. The twin cohort is unselected and population based; and as adult twins highly resemble the general population,15 it can be seen to be representative of the general elderly population in Finland.
A total of 3,310 subjects were contacted for the telephone interview, but 32 (1.0%) subjects had died since the last registry update for vital status. Of the 3,278 subjects alive at the time of the interview, 2,604 (79.5%) were interviewed successfully. For 2,336 (89.7%) of the successfully interviewed subjects, some questionnaire data (at least information on education) from 1981 were available, and they could be included in the analyses. Therefore, 71.3% of those initially contacted were included in the analyses.
The subjects who had died before the attempted interview would have been older at the time of the interview than those contacted (P < 0.01), but did not differ in educational level or sex distribution. They were not more often obese, heavy or binge drinkers, physically sedentary, or dissatisfied with their lives, but reported having hypertension more often (P = 0.03). They did not differ from the contacted individuals in any of the assessed sleep characteristics. The 942 subjects who could not be included in the analyses either because of an unsuccessful telephone interview or missing questionnaire data were older, less educated, and more often women than those included in the analyses (P < 0.01).
A joint ethics committee of University of Turku and Turku University Hospital approved the study protocol, and informed consent was obtained from the participants or their proxies before the telephone interview.
Assessment of Cognitive Function
The assessment of cognitive function was based on a telephone interview combining 2 validated telephone screening instruments: TELE16 and the Telephone Interview for Cognitive Status (TICS).17 Both TELE and TICS have been shown to correlate strongly with the Mini Mental State Examination score in a group consisting of AD patients and controls (r = 0.87 for TELE and r = 0.86 for TICS, P < 0.0001 for both).18 The assessed cognitive domains include orientation, long-term memory, short-term memory (immediate and delayed recall of a word list), attention, abstraction, calculation, language repetition, and non-verbal praxis. The TELE and TICS share some items, and items included in both screening instruments were asked only once during the interviews. Finally, the interview consisted of 29 items, and the maximum score was 51. This cognitive score was used as a linear variable representing cognitive function, with higher scores indicating better performance.
The assessment of cognitive function with a telephone interview has practical limitations, but careful measures were implemented in order to overcome possible shortcomings, e.g., by asking whether the subject could hear well and whether he/she was feeling rested, and by forbidding the use of pens, pencils, papers, newspapers, and calendars.18
As AD is the most common cause of dementia, we wanted to test whether the factors associated with a lower cognitive score also increased the risk of AD. Therefore, we linked the twin cohort with the records for reimbursed medications of the Social Insurance Institution of Finland, which cover the entire population. The records for reimbursed pharmaceuticals were available until 2004, and individuals who had been prescribed cholinesterase inhibitors (donepezil, rivastigmine or galantamine) or memantine prior to this were considered to have AD. Because the only indication for these pharmaceuticals in Finland is AD, and off-label use of medications is not reimbursed, these subjects are likely to reflect true AD cases. Individuals with both a TELE score > 17.5 and a TICS score > 26.5 were used as controls in these analyses. It has been shown that 96.7% of AD patients have a TELE score < 17, and 90% of AD patients have a TICS score < 26.5.18 Therefore, it is likely that the control group would contain few, if any subjects with AD.
Assessment of Sleep Characteristics
The assessment of midlife sleep characteristics was based on questionnaire data from 1975 and 1981. Data from the 1981 questionnaire were used in the main analyses. Access to data from both 1975 and 1981 allowed us perform exposure time analyses as well. The subjects were asked to report how many hours they usually slept per day, and also how many hours of sleep they needed during the night to be alert the following day. Sleep length was categorized into three classes: short (< 7 h), average (7-8 h), and long (> 8 h).19 Duration of 7-8 hours of sleep/day was chosen as the reference, as it has been shown that people sleeping 7 h/day have the best survival.20 This has also been shown in this cohort.19 A difference of at least one hour between the 2 questions was considered to indicate insufficient sleep.21
Sleep quality was assessed with the question “Do you usually sleep well?” and subjects were divided into 3 sleep quality groups: those who slept well, rather well, and poorly or rather poorly.22 Snoring, a cardiovascular disease risk indicator,23,24 was also assessed: subjects were divided into those who never or seldom snored and those who often or always snored. The use of hypnotics or tranquilizers was assessed with the alternatives: no use, on < 10 days, on 10-59 days, on 60-180 days, and on > 180 days during the last year. The subjects were dichotomized using 60 days per year as the cutoff point. Hypnotics and tranquilizers were grouped together, as both groups of medications were benzodiazepines or benzodiazepine-like agents at time of the baseline assessment. Below, these pharmaceuticals are collectively referred to as hypnotics.
The assessment of the covariates was based on the 1981 questionnaire. Age at the time of the telephone interview was assessed as a linear variable. Follow-up was defined as the time from 1981 to the time of the telephone interview, or to the initiation of AD medication for AD patients in the analyses of AD risk. Educational level was assessed by asking the subjects to classify themselves according to 8 educational categories, and this was then transformed into years of formal schooling and used as a linear variable.25 A 3-level assessment of life satisfaction (satisfied, intermediate, dissatisfied) was based on a self-reported life satisfaction score.26 Other covariates were assessed as binomial variables: obesity (cutoff point 30 kg/m2), hypertension (yes/no), leisure time physical activity (leisure time physical activity at least occasionally/never), alcohol consumption (cutoff point 7 drinks/week for women and 14 drinks/week for men), and binge drinking (at least monthly consumption of ≥ 5 drinks on a single occasion).
ApoE ε4 allele is the most established genetic risk factor for AD.27 Venous blood samples were collected at local health centers, and the samples together with the consent forms were returned to the National Public Health Institute for genotyping. Some subjects had previously provided DNA; they were reconsented for this study by the telephone interviewers, and written consent forms were obtained. The ApoE genotyping was done with an analysis of 2 single-nucleotide polymorphisms (rs429358 and rs7412).28 If only one co-twin of a MZ pair had been genotyped, the same genotype was assumed for the other co-twin as well. Subjects were classified into those with no ε4 alleles, 1-2 ε4 alleles, and unknown genotype; the genotypes were in Hardy-Weinberg equilibrium. The genotype was known for 1,739 (74.4%) of the included subjects.
Statistical Analyses
The statistical analyses were conducted using Stata 11.1 (StataCorp, College Station, TX, USA). As the study population consisted of twins, the subjects cannot be seen as individual observations, and all the analyses were done using robust estimators of variance taking this clustering into account.29 In all analyses, a two-tailed P < 0.05 was considered significant.
The associations between the linear cognitive score and individual sleep characteristics were assessed using linear regression. We report β coefficients, margins of errors at a 95% percent level of confidence (equivalent to 1.96 × standard error), and P values. The associations between the risk of AD and sleep characteristics were assessed using logistic regression analyses, and odds ratios (OR) with 95% confidence intervals (CI) are reported. For categorical variables with ≥ 3 levels, the joint effect of the variable was assessed through a post-estimation test. Seven to eight hours of sleep/day, sufficient sleep, good sleep quality, never or sometimes snoring, and use of hypnotics ≤ 60 days/year were used as the reference categories. In the main analyses, subjects with missing or unknown data for the sleep variables were excluded; hence, the number of subjects varies between individual analyses.
Three different models were tested: an unadjusted model, Model 1, and Model 2. Model 1 included the most established risk factors for cognitive impairment or AD as covariates: age at interview, sex, educational level, ApoE genotype, and follow-up time. Life satisfaction and alcohol consumption can be associated with sleep characteristics and the use of hypnotics, and in our cohort cardiovascular factors (obesity, hypertension, and low physical activity) were associated with the sleep characteristics as well. Hence, Model 2 additionally included life satisfaction, heavy drinking, binge drinking, obesity, hypertension, and leisure time physical activity as covariates. Midlife smoking status (never, past or occasional, current smoker) was not associated with late life cognitive function, and its inclusion as a covariate did not affect the results of Model 2.
To test whether interactions with age, sex, or ApoE geno-type affected the associations between the sleep characteristics and cognitive score, interaction terms were added to Model 1. Subgroup analyses were conducted if the interaction term had a P-value < 0.10.
As the assessed sleep characteristics probably are not independent of each other, additional regression analyses were conducted including all the variables significant in the individual analyses simultaneously in a regression model. Models 1 and 2 were tested in these analyses as well; also, subjects with missing or unknown data on the variables were included. Possible interactions between the significant factors were tested as described above.
RESULTS
Study Population Characteristics
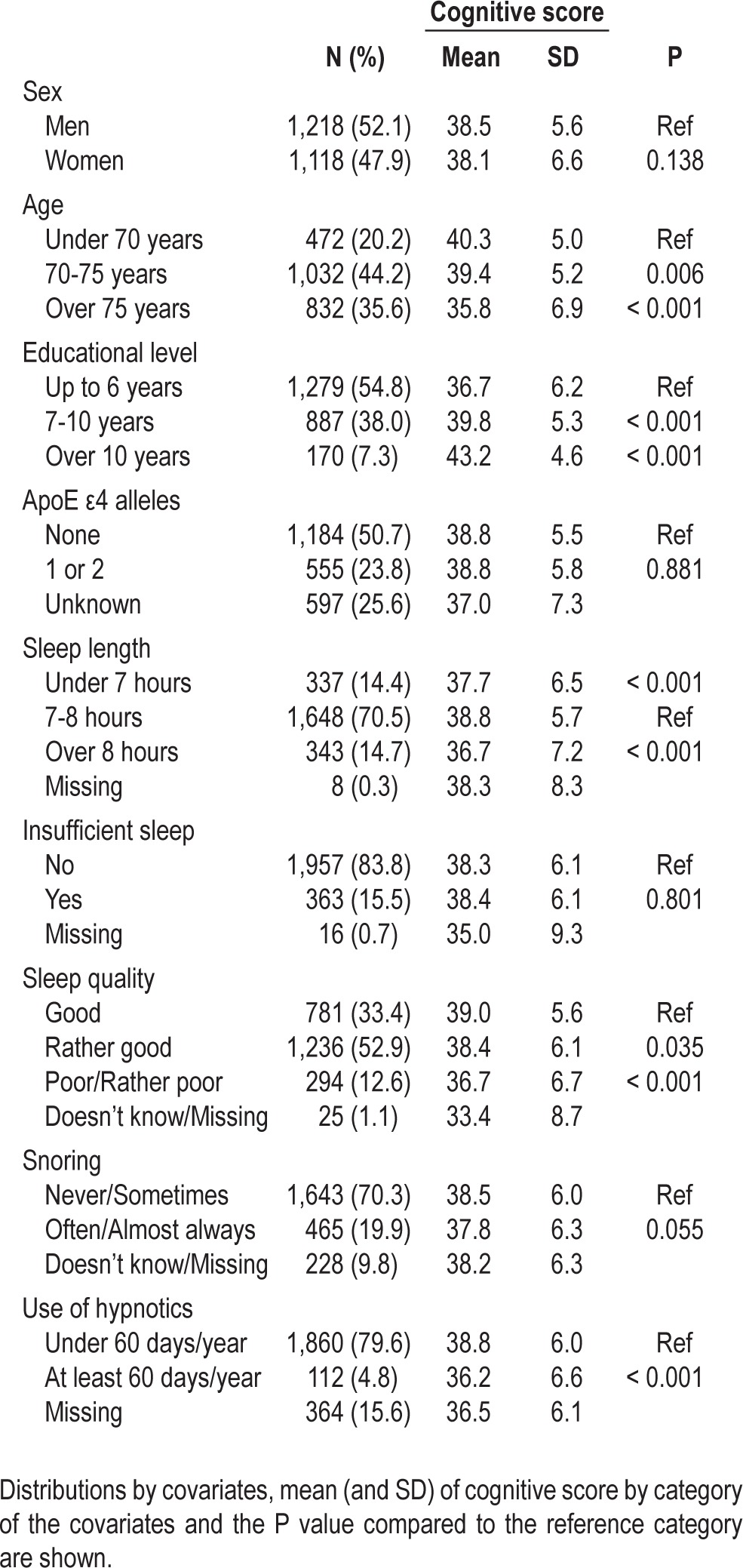
Of 2,336 subjects included in the analyses, 52.1% were men. 28.0% of the subjects were MZ twins, 71.3% DZ twins, and 0.7% twins of unknown zygosity. The subjects were 52.3 (SD 6.2) years old in 1981 and 74.4 (SD 5.2) years at the time of the interview, so the mean follow-up time was 22.1 (SD 2.2) years. The follow-up time of DZ twins was longer than MZ twins (23.0 vs. 19.8 years, P < 0.001). The mean cognitive score of all the included subjects was 38.3 (SD 6.1) years. The cognitive scores based on sex, age, educational level, ApoE genotype, and sleep variables are shown in Table 1. The cognitive scores did not differ between men and women, but higher education and lower age were associated with better cognitive function (P < 0.01). ApoE genotype was not associated with the cognitive score.
Table 1.
Characteristics of the subjects included in the main analyses
Midlife Sleep Characteristics and Later Cognitive Function
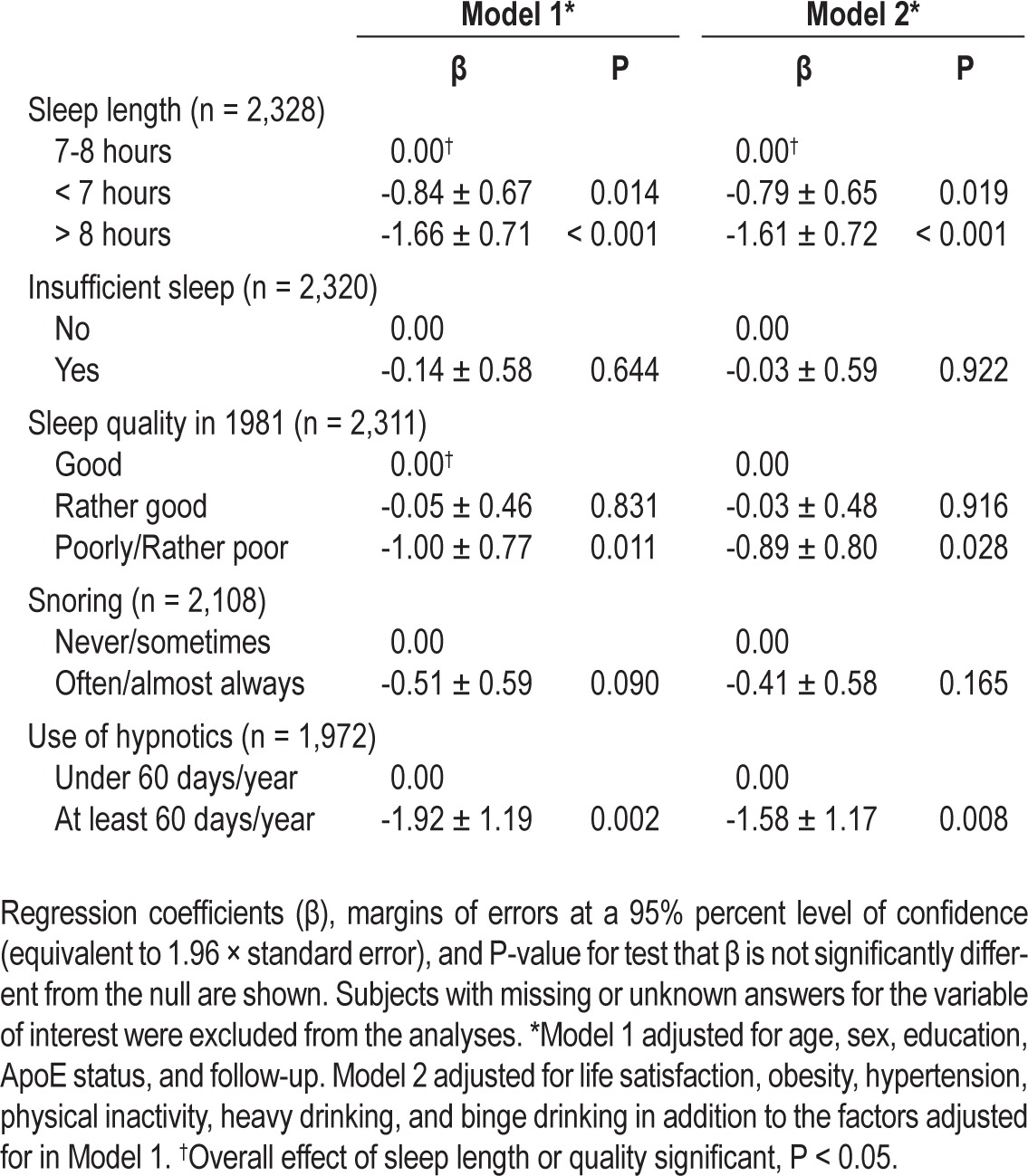
Both short (< 7 h/day) and long sleep (> 8 h/day) at baseline were associated with a lower cognitive score in the unadjusted model when compared to 7-8 h/day. The associations remained significant also in Model 1 and Model 2. Poor or rather poor sleep quality was associated with a lower cognitive score in the unadjusted model, Model 1, and Model 2 when compared to good sleep quality. A linear trend for sleep quality was significant in the unadjusted model and Model 1 (β = -0.99 ± 0.40, P < 0.01 and β = -0.38 ± 0.35, P = 0.03, respectively), but not in Model 2 (β = -0.33 ± 0.36, P = 0.07). Also individuals using hypnotics ≥ 60 days/year had lower cognitive scores in the unadjusted model, Model 1, and Model 2 than those using them < 60 days/year. Insufficient sleep or snoring was not significantly associated with cognitive score. The results of the unadjusted model are shown in Table 1 and the results of Models 1 and 2 in Table 2.
Table 2.
Results of the individual linear regression analyses between the cognitive score and sleep characteristics
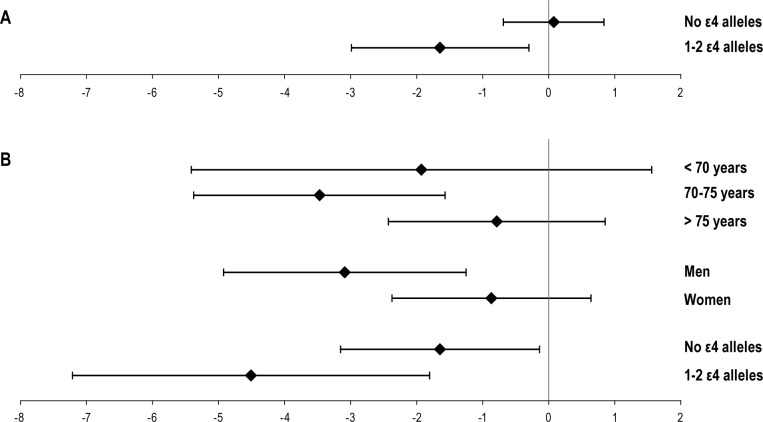
In the interaction analyses, the interaction between insufficient sleep and ApoE genotype was significant (P = 0.03). In subgroup analyses, insufficient sleep was associated with a lower cognitive score in individuals with ≥ 1 ε4 allele (β = -1.65 ± 1.34, P = 0.02), but not in subjects without an ε4 allele (β = 0.07 ± 0.75, P = 0.85). Also the interactions between the use of hypnotics and age (P = 0.04), sex (P = 0.09), and ApoE genotype (P = 0.08) had a P value < 0.10. However, individuals who reported using hypnotics ≥ 60 days/year were older than those who did not (P = 0.02). The association between the cognitive score and use of hypnotics was significant in subjects 70-75 years old at the time of the interview (β = -3.47 ± 1.90, P < 0.01), but not in those younger than 70 (β = -1.93 ± 3.48, P = 0.28) or older than 75 years (β = -0.79 ± 1.64, P = 0.35). The association was significant in men (β = -3.09 ± 1.83, P < 0.01), but not in women (β = -0.87 ± 1.50, P = 0.25). In both ε4 carriers and non-carriers, individuals using hypnotics ≥ 60 days/year had lower cognitive scores, but the effect seemed stronger in the ε4 carriers (β = -4.51 ± 2.70, P < 0.01 and β = -1.65 ± 1.50, P = 0.03). The results of the interaction analyses are also illustrated in Figure 1.
Figure 1.
Illustration of the significant interaction analyses. Diamonds and whiskers indicate β coefficients and 95% confidence intervals, respectively. (A) Results for insufficient sleep (sufficient sleep used as reference and compared to insufficient sleep). (B) Results for the use of hypnotics (< 60 days/year used as reference and compared to ≥ 60 days/year).
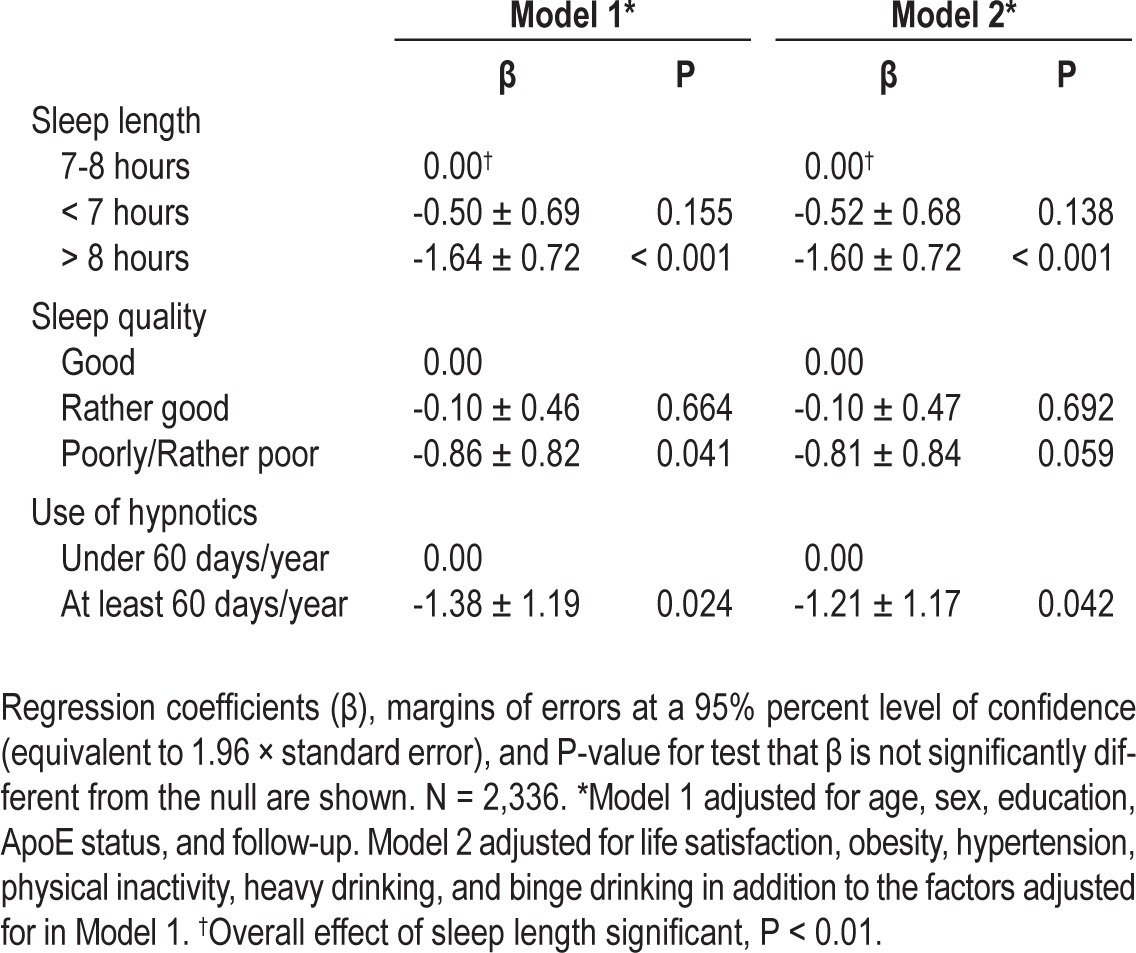
Sleep length, sleep quality, and the use of hypnotics were individually associated with the cognitive score, and because these factors probably are not independent of each other, they were included together into multivariable linear regression models. In these analyses, long sleep was associated with a lower cognitive score than 7-8 h/day. The effect of poor or rather poor sleep quality was significant only in Model 1, but the effect of using hypnotics remained significant in both Model 1 and Model 2. The results of these analyses are shown in Table 3. The analyses were repeated excluding the 390 individuals with unknown sleep duration, sleep quality, or use of hypnotics; in these analyses sleep length and quality were significantly associated with cognition in both models, whereas the use of hypnotics affected cognition only in Model 1 (results not shown). All the two-way interactions between sleep duration, sleep quality, and use of hypnotics were clearly nonsignificant when omitting subjects with missing information on ≥ 1 of the variables (all P > 0.20).
Table 3.
Result of the multivariable linear regression analyses simultaneously including all the sleep characteristics, which were individually associated with the cognitive score.
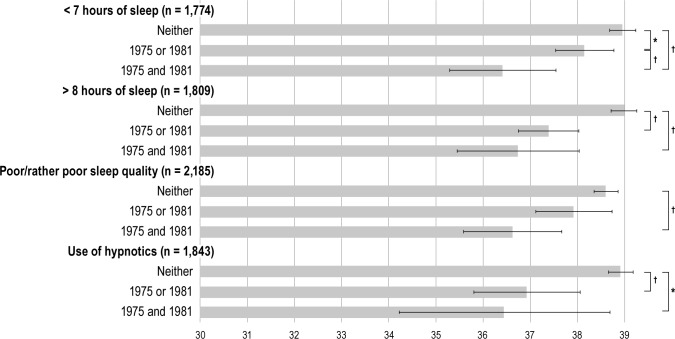
As we had data both from 1981 and 1975, the effects of exposure time could be assessed. These analyses were limited to sleep length, sleep quality, and the use of hypnotics. The analyses included age, sex, educational level, ApoE genotype, and follow-up time as covariates. These analyses included 1,774-2,185 subjects, and the predicted cognitive score means with 95% CIs are shown in Figure 2. Subjects who reported short sleep either only in 1975 or 1981 had significantly lower cognitive scores than subjects who reported 7-8 h of daily sleep in both 1975 and 1981. Subjects reporting short sleep both in 1975 and 1981 had even lower estimated cognitive scores. Similar results were seen for long sleep duration, but the difference between those who reported long sleep only in 1975 or 1981 did not differ from those who reported it in both questionnaires.
Figure 2.
Illustration of the exposure time analyses. Bars and whiskers indicate estimated cognitive score means and 95% confidence intervals, respectively. *Significant difference at P < 0.05. †Significant difference at P < 0.01.
Only subjects who reported poor or rather poor sleep quality in both questionnaires had significantly lower cognitive scores than those who reported good or rather good sleep quality on both instances. Use of hypnotics > 60 days/year in either 1975 or 1981 was associated with a lower cognitive score than use ≤ 60 days/year in both questionnaires. Also, subjects reporting use of hypnotics in both 1975 and 1981 had lower cognitive scores, but the difference from those using them only in either 1975 or 1981 was not statistically significant.
We also conducted more detailed analyses on the effect of changes in sleep length, sleep quality, and use of hypnotics between 1975 and 1981 on cognitive function. The results of these analyses are shown in Table S1 in the supplemental material. Sleep length changed in 29.9%, sleep quality in 31.5%, and use of hypnotics in 5.8% of participants. However, radical changes in sleep characteristics were uncommon, and no clear trends were identified.
Midlife Sleep Characteristics and risk of Alzheimer Disease
A total of 1,326 subjects with adequate questionnaire data were included in the analyses regarding the risk of AD. One hundred seventy-nine individuals (13.5%) had been prescribed AD medications, and the remainder had intact cognitive function by design. Of the 179 individuals with AD medication, 65 had also participated in the telephone interview, and only 6 of them would have been considered cognitively intact in both TELE and TICS. The average age at the end of follow-up in these analyses was 73.6 (SD 5.1) years, and the follow-up time 22.6 (SD 2.3) years. As compared to the controls, AD patients were older (77.2, SD 7.2 years vs. 73.0, SD 4.4 years, P < 0.01), and their follow-up time was shorter (21.2, SD 1.6 years vs. 22.8, SD 2.4 years, P < 0.01). These analyses were not controlled for the number of ε4 alleles, as the genotype was not known for the majority of AD patients.
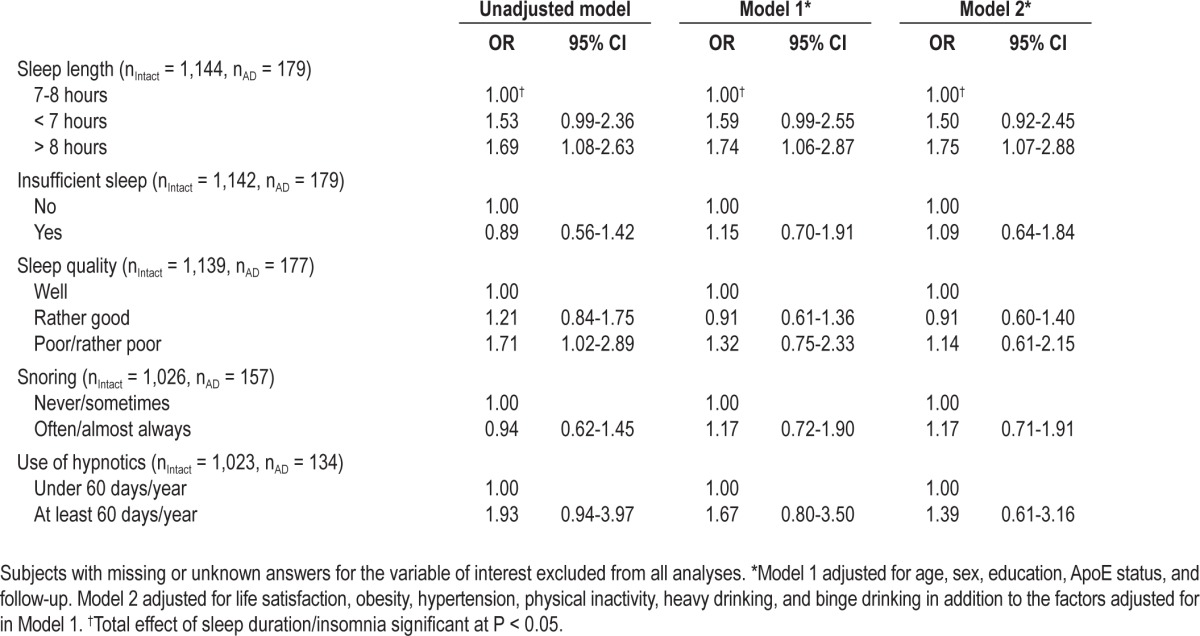
In the analyses of individual risk factors, only long sleep was a significant risk factor for AD. All ORs are shown in Table 4. Long sleep also remained a significant risk factor in a model including sleep duration, sleep quality, and the use of hypnotics, and controlling for age, sex, educational level, and follow-up (OR 1.77, 95% CI 1.08-2.92).
Table 4.
Odds ratios (OR) and 95% CI of Alzheimer disease associated with sleep characteristics
DISCUSSION
In the current study of older Finnish twins, we found that both short and long sleep length in midlife, defined as under 7 and over 8 hours of sleep/day, respectively, were associated with poorer cognitive performance on average 22 years later. Also, poor or rather poor sleep quality and regular use of hypnotics were risk factors for poorer cognitive function. In contrast, snoring or insufficient sleep were not associated with late life cognition. Of the assessed characteristics, long sleep and the use of hypnotics seem most important, as both remained significantly associated with cognitive function in a model including sleep length, sleep quality, and the use of hypnotics. As we had access to questionnaire data both from 1975 and 1981, we were able to assess the exposure time effects of the assessed traits. These analyses suggested that especially longstanding short sleep and poor sleep quality are detrimental for cognitive performance.
Interaction analyses indicated that the association between the use of hypnotics and cognition may be partly dependent on age, sex, or ApoE genotype. In subgroup analyses based on gender, the association between hypnotics and cognition was significant only in men. In similar analyses based on ApoE genotype, the effect was stronger in individuals with at least one ApoE ε4 allele. Also, age seemed to affect the association; only in subjects 70-75 years old at the time of the cognitive assessment did the use of hypnotics affect cognitive performance. These individuals were on average 49.3 (SD 2.9) years old in 1981. However, they also formed the largest age group of our study; hence, the lack of statistically significant findings in other age groups might be attributed to lower statistical power. Finally, a significant association between insufficient sleep and cognition was seen only in ε4 carriers in analyses stratified based on ApoE genotype. However, as insufficient sleep was not associated with cognition in any other analyses, this finding is difficult to interpret.
To assess whether midlife sleep characteristics were also associated with cognitive impairment significant enough to cause clinical AD, we linked the Finnish Twin Cohort with the records for reimbursed medications and identified twins who had been prescribed either a cholinesterase inhibitor or memantine. For a patient to receive reimbursement, the treating physician needed to submit a medical certificate with the diagnosis and supporting clinical evidence. The findings were not completely consistent with cognitive score results, as only long sleepers were found to have a significantly increased risk of AD (OR = 1.75) as compared to individuals sleeping 7-8 hours/day, short sleepers had a 50% increased risk point estimate (P = 0.10) The differences between the two outcomes are understandable, because clinical dementia and a low cognitive score probably reflect at least partly different phenomena. As we only had information on the reimbursed medications until 2004, we probably could not identify a significant number of AD patients. However, as AD is the only indication for cholinesterase inhibitors and memantine in Finland, and the control group consisted of individuals with evidently intact cognitive function according to the telephone interview, the compared groups are probably quite specific.
Our results are consistent with previous studies showing that long sleep duration was associated with an increased risk of incident dementia and cognitive impairment.6,7 In contrast, in the previous studies short sleep was not associated with an increased dementia risk, whereas in our study short sleep was associated with poorer cognitive function. Poor sleep quality has also been associated with incident cognitive impairment in a much shorter follow-up study of elderly subjects.9 Previous literature also supports our finding on the association between the use of hypnotics and cognition, even though previous findings have been inconsistent and have included methodological limitations.11 Sleep disordered breathing has been shown to increase the risk of incident dementia or MCI,10 but we did not find an association between snoring and cognition. Self-reported snoring has been shown to correlate with objective measurements of sleep disordered breathing,30,31 but it is possible that our assessment was not accurate enough to detect a significant association.
Previous prospective studies are consistent with two alternative hypotheses as explanations for the association, as suggested by Lim and Saper.32 In the first case, sleep-related traits, reflecting social, environmental, and biological factors, affect the pathogenetic processes leading to dementia. Alternatively, sleep related traits are signs of existing subclinical neurodegenerative processes or the presence of risk factors for such processes, but do not actually contribute to them. As the pathogenesis of AD is considered to begin 10-15 years prior to clinical symptoms,12 previous studies have had follow-up times that were too short to differentiate between the two hypotheses. To our knowledge, the current study is the first to assess the association between midlife sleep characteristics and late life cognition, and our findings support the first hypothesis. It is possible that the assessed traits are not actual risk factors for cognitive impairment, but mere indicators of other risk factors. However, our findings remained significant even in a model controlling for various known risk factors for dementia, supporting the independent effects of the traits.
We were able to follow a large number of twins from a well-described cohort for over 20 years, and our findings can most likely be generalized to the older Finnish population. The mortality (SMR) of the twin cohort does not differ from the general population up to 2009.33 The analyses were controlled for a number of potential confounders, including ApoE geno-type, the most important genetic risk factor for AD. Additionally, the sleep characteristics were assessed both in 1975 and 1981, allowing exposure time analyses. Educational level is considered to be one of the most important determinants of the risk of dementia and cognitive impairment. In our cohort, the difference in the cognitive score between the highest and lowest educational levels was 6.5 points. In comparison, the effect sizes in the significant findings of our study ranged from 1.1 to 2.6 points, making them less significant but still clinically relevant determinants of cognitive function.
The study design also had its limitations. The assessment of the sleep characteristics was based on self-report. Self-reported short sleep, long sleep, use of hypnotics, and insomnia-related symptoms have all been associated with an increased mortality risk in the twin cohort,19,34 and self-reported snoring is associated with cardiovascular outcomes.23,24 Hence, the assessments can be considered rather robust. Because the sleep characteristics have been associated with mortality and morbidity, the current study most probably was not free of survival bias. However, this survival bias probably shifted the results towards the null hypothesis, i.e., made it less likely to find significant associations. Cognitive function was assessed using a telephone interview consisting of two validated cognitive tests, TELE16 and TICS.17 Both TELE and TICS correlate strongly with the Mini Mental State Examination (MMSE), and in AD patients also with Clinical Dementia Rating (CDR).18 The study protocol did not include cognitive assessment at baseline. Because of the long follow-up time of the study, this is unlikely to have affected the results, as it would be rare to have poor cognitive function at baseline and survive for another 22 years.
An additional limitation of our study design is the lack of follow-up data on sleep characteristics. Considering that poor sleep35 and daytime sleepiness36 are associated with poorer cognitive function in cross-sectional studies, it is possible that our findings are at least partly explained by the persistence of sleep characteristics from midlife to older life.
It is possible that our results are affected by selection bias, as subjects who could not be included in the analyses were older, less educated, and more often women. However, it is unlikely that selection bias significantly affected the findings, as the subjects who had died prior to the interview differed from the interviewed subjects only in age and the prevalence of hypertension.
Our analyses utilizing data on AD medication possibly indicate that sleep characteristics, especially sleeping over 8 hours/ day are specifically a risk factor for AD instead of all or other dementia disorders. However, this could not be reliably tested in the current study, as we did not have data on other dementia diagnoses.
We are not aware of any plausible biological mechanism through which midlife sleep length, sleep quality, or the use of hypnotics could cause declined cognitive performance two decades later, even though it was recently suggested that both hypocretin and melatonin could affect AD pathogenesis.37 To our knowledge, this is the first study to evaluate the link between midlife sleep characteristics and late life cognition, and we think our findings warrant further studies. If the results are confirmed in other cohorts, these sleep characteristics may emerge as new, potentially modifiable early risk factors for cognitive impairment.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The skillful assistance of research nurses Ulla Kulmala-Grïhn, Maarit Mantere, and Kristiina Saanakorpi in interviewing the subjects is gratefully acknowledged. The assistance of Maarit Lappalainen at FIMM with ApoE genotyping is acknowledged. This study was financially supported by the Academy of Finland (project #205954), the Sigrid Juselius Foundation and Clinical grants of Turku University Hospital (EVO). The Finnish Twin Cohort study is part of the Academy of Finland Center of Excellence in Complex Disease Genetics (grant #s 213506, 129680).
SUPPLEMENTAL MATERIAL
Results of the linear regression analyses including changes in sleep variables from 1975 to 1981
REFERENCES
- 1.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735–41. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 2.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 3.Spira AP, Blackwell T, Stone KL, et al. Sleep-disordered breathing and cognition in older women. J Am Geriatr Soc. 2008;56:45–50. doi: 10.1111/j.1532-5415.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 4.Bombois S, Derambure P, Pasquier F, Monaca C. Sleep disorders in aging and dementia. J Nutr Health Aging. 2010;14:212–7. doi: 10.1007/s12603-010-0052-7. [DOI] [PubMed] [Google Scholar]
- 5.Foley D, Monjan A, Masaki K, et al. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc. 2001;49:1628–32. doi: 10.1046/j.1532-5415.2001.t01-1-49271.x. [DOI] [PubMed] [Google Scholar]
- 6.Benito-Leon J, Bermejo-Pareja F, Vega S, Louis ED. Total daily sleep duration and the risk of dementia: a prospective population-based study. Eur J Neurol. 2009;16:990–7. doi: 10.1111/j.1468-1331.2009.02618.x. [DOI] [PubMed] [Google Scholar]
- 7.Loerbroks A, Debling D, Amelang M, Sturmer T. Nocturnal sleep duration and cognitive impairment in a population-based study of older adults. Int J Geriatr Psychiatry. 2010;25:100–9. doi: 10.1002/gps.2305. [DOI] [PubMed] [Google Scholar]
- 8.Tranah GJ, Blackwell T, Stone KL, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70:722–32. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potvin O, Lorrain D, Forget H, et al. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep. 2012;35:491–9. doi: 10.5665/sleep.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–9. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verdoux H, Lagnaoui R, Begaud B. Is benzodiazepine use a risk factor for cognitive decline and dementia? A literature review of epidemiological studies. Psychol Med. 2005;35:307–15. doi: 10.1017/s0033291704003897. [DOI] [PubMed] [Google Scholar]
- 12.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer's disease. Nature. 2009;461:916–22. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaprio J, Sarna S, Koskenvuo M, Rantasalo I. The Finnish Twin Registry: formation and compilation, questionnaire study, zygosity determination procedures, and research program. Prog Clin Biol Res. 1978;24(Pt B):179–84. [PubMed] [Google Scholar]
- 14.Kaprio J, Koskenvuo M. Genetic and environmental factors in complex diseases: the older Finnish Twin Cohort. Twin Res. 2002;5:358–65. doi: 10.1375/136905202320906093. [DOI] [PubMed] [Google Scholar]
- 15.Kaprio J, Silventoinen K. Advanced methods in twin studies. Methods Mol Biol. 2011;713:143–52. doi: 10.1007/978-1-60327-416-6_11. [DOI] [PubMed] [Google Scholar]
- 16.Gatz M, Reynolds C, Nikolic J, Lowe B, Karel M, Pedersen N. An empirical test of telephone screening to identify potential dementia cases. Int Psychogeriatr. 1995;7:429–38. doi: 10.1017/s1041610295002171. [DOI] [PubMed] [Google Scholar]
- 17.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–7. [Google Scholar]
- 18.Järvenpää T, Rinne JO, Räihä I, et al. Characteristics of two telephone screens for cognitive impairment. Dement Geriatr Cogn Disord. 2002;13:149–55. doi: 10.1159/000048646. [DOI] [PubMed] [Google Scholar]
- 19.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007;30:1245–53. doi: 10.1093/sleep/30.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 21.Hublin C, Kaprio J, Partinen M, Koskenvuo M. Insufficient sleep--a population-based study in adults. Sleep. 2001;24:392–400. doi: 10.1093/sleep/24.4.392. [DOI] [PubMed] [Google Scholar]
- 22.Paunio T, Korhonen T, Hublin C, et al. Longitudinal study on poor sleep and life dissatisfaction in a nationwide cohort of twins. Am J Epidemiol. 2009;169:206–13. doi: 10.1093/aje/kwn305. [DOI] [PubMed] [Google Scholar]
- 23.Koskenvuo M, Kaprio J, Partinen M, Langinvainio H, Sarna S, Heikkila K. Snoring as a risk factor for hypertension and angina pectoris. Lancet. 1985;1:893–6. doi: 10.1016/s0140-6736(85)91672-1. [DOI] [PubMed] [Google Scholar]
- 24.Koskenvuo M, Kaprio J, Telakivi T, Partinen M, Heikkila K, Sarna S. Snoring as a risk factor for ischaemic heart disease and stroke in men. BMJ (Clin Res Ed) 1987;294:16–9. doi: 10.1136/bmj.294.6563.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silventoinen K, Kaprio J, Lahelma E. Genetic and environmental contributions to the association between body height and educational attainment: a study of adult Finnish twins. Behav Genet. 2000;30:477–85. doi: 10.1023/a:1010202902159. [DOI] [PubMed] [Google Scholar]
- 26.Allardt E. About dimension of welfare: an explanatory analysis of the Comparative Scandinavian Survey. 1973 Research Report #1. [Google Scholar]
- 27.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–56. [PubMed] [Google Scholar]
- 28.2011. p. 2011. SNPedia. ApoE.
- 29.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–6. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 30.Telakivi T, Partinen M, Koskenvuo M, Salmi T, Kaprio J. Periodic breathing and hypoxia in snorers and controls: validation of snoring history and association with blood pressure and obesity. Acta Neurol Scand. 1987;76:69–75. doi: 10.1111/j.1600-0404.1987.tb03547.x. [DOI] [PubMed] [Google Scholar]
- 31.Telakivi T, Kajaste S, Partinen M, Koskenvuo M, Salmi T, Kaprio J. Cognitive function in middle-aged snorers and controls: role of excessive daytime somnolence and sleep-related hypoxic events. Sleep. 1988;11:454–62. [PubMed] [Google Scholar]
- 32.Lim AS, Saper CB. Sleep, circadian rhythms, and dementia. Ann Neurol. 2011;70:677–9. doi: 10.1002/ana.22637. [DOI] [PubMed] [Google Scholar]
- 33.Kaprio J. The Finnish Twin Cohort Study: an update. Twin Res Hum Genet. 2013:1–6. doi: 10.1017/thg.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Heritability and mortality risk of insomnia-related symptoms: a genetic epidemiologic study in a population-based twin cohort. Sleep. 2011;34:957–64. doi: 10.5665/SLEEP.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 36.Ohayon MM, Vecchierini MF. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162:201–8. doi: 10.1001/archinte.162.2.201. [DOI] [PubMed] [Google Scholar]
- 37.Slats D, Claassen JA, Verbeek MM, Overeem S. Reciprocal interactions between sleep, circadian rhythms and Alzheimer's disease: Focus on the role of hypocretin and melatonin. Ageing Res Rev. 2013;12:188–200. doi: 10.1016/j.arr.2012.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of the linear regression analyses including changes in sleep variables from 1975 to 1981