Abstract
Objective
Scoring systems for predicting mortality after repair of ruptured abdominal aortic aneurysms (RAAAs) have not been developed or tested in a United States population and may not be accurate in the endovascular era. Using prospectively collected data from the Vascular Study Group of New England (VSGNE), we developed a practical risk score for in-hospital mortality after open repair of RAAAs and compared its performance to that of the Glasgow aneurysm score, Hardman index, Vancouver score, and Edinburg ruptured aneurysm score.
Methods
Univariate analysis followed by multivariable analysis of patient, prehospital, anatomic, and procedural characteristics identified significant predictors of in-hospital mortality. Integer points were derived from the odds ratio (OR) for mortality based on each independent predictor in order to generate a VSGNE RAAA risk score, which was internally validated using bootstrapping methodology. Discrimination and calibration of all models were assessed by calculating the area under the receiver-operating characteristic curve (C-statistic) and applying the Hosmer-Lemeshow test.
Results
From 2003 to 2009, 242 patients underwent open repair of RAAAs at 10 centers. In-hospital mortality was 38% (n = 91). Independent predictors of mortality included age >76 years (OR, 5.3; 95% confidence interval [CI], 2.8–10.1), preoperative cardiac arrest (OR, 4.3; 95% CI, 1.6–12), loss of consciousness (OR, 2.6; 95% CI, 1.2–6), and suprarenal aortic clamp (OR, 2.4; 95% CI, 1.3–4.6). Patient stratification according to the VSGNE RAAA risk score (range, 0–6) accurately predicted mortality and identified those at low and high risk for death (8%, 25%, 37%, 60%, 80%, and 87% for scores of 0, 1, 2, 3, 4, and ≥5, respectively). Discrimination (C = .79) and calibration (χ2 = 1.96; P = .85) were excellent in the derivation and bootstrap samples and superior to that of existing scoring systems. The Glasgow aneurysm score, Hardman index, Vancouver score, and Edinburg ruptured aneurysm score correlated with mortality in the VSGNE cohort but failed to identify accurately patients with a risk of mortality >65%.
Conclusions
Existing scoring systems predict mortality after RAAA repair in this cohort but do not identify patients at highest risk. This parsimonious VSGNE RAAA risk score based on four variables readily assessed at the time of presentation allows accurate prediction of in-hospital mortality after open repair of RAAAs, including identification of those patients at highest risk for postoperative mortality.
Ruptured abdominal aortic aneurysm (RAAA) accounts for the preponderance of deaths due to aortic aneurysms and is a major cause of death in the United States (U.S.), United Kingdom, and Europe.1–4 The overall mortality of RAAAs remains at 80% to 90%, with an operative mortality of 40% to 70% after open repair.3–11
Scoring systems have been developed in Canada and the United Kingdom to predict mortality after open repair of RAAAs, including the Glasgow aneurysm score (GAS), the Hardman index, the Vancouver score, and the Edinburg ruptured aneurysm score (ERAS).12–15 However, these scoring systems may be unsuitable for widespread application due to unproven generalizability. First, scoring systems for predicting mortality after open repair of RAAAs have never been developed nor tested in a U.S. population. Second, existing scoring systems have not been validated consistently or robustly.16–19 Third, it is not known if published prediction models are accurate in the current era, which incorporates the increasing and preferential use of endovascular repair (EVAR) of RAAAs when possible.1,3 In modern practice, patients may be selected for open repair because they have difficult anatomy or hemodynamic instability, which makes them unsuitable for EVAR. Thus, patients undergoing open repair of a RAAA in the current era may be at higher risk for mortality than those who underwent open repair in previous studies. In addition, patients currently treated with open repair may be at higher baseline risk than those treated with EVAR, which confounds comparisons of current outcomes.1,19–21 Our objective was to examine the mortality and clinical variables that correlated with mortality after open repair of RAAA in a contemporary U.S. regional cohort, the Vascular Study Group of New England (VSGNE). We sought to develop a practical risk score for prediction of in-hospital mortality after open repair RAAA using prospectively acquired data from the VSGNE and to compare the performance of this risk score to existing scoring systems. We hypothesized that a risk score to predict mortality after open repair of RAAAs based on contemporary outcomes is superior to that of existing risk scores based on non-U.S., noncontemporary outcomes.
METHODS
Database and data collection
The VSGNE is a regional cooperative quality improvement initiative developed in 2002 to study regional outcomes in vascular surgery. Details regarding this registry have been published previously.22 Trained nurses or clinical abstractors entered data prospectively on >100 clinical and demographic variables (www.VSGNE.org). Research analysts were blinded to patient, surgeon, and hospital identity.
Subjects
Our cohort included all patients who underwent open repair of RAAAs from 2003 to 2009 at 10 centers, both community and academic, involved in the registry. All patients in the VSGNE were evaluated for pre-existing demographic variables and medical comorbidities as well as parameters reflective of preoperative severity of illness, including lowest preintubation systolic blood pressure (SBP), mental status, history of preoperative cardiac arrest, hemoglobin, and creatinine. Prehospital characteristics, including transfer status, time from symptoms to incision, and time from admission to incision, also are recorded. The VSGNE also collects detailed anatomic and procedural information, such as AAA size, aortic clamp position, renal/visceral ischemic time, estimated blood loss, procedural time, exposure, anastomotic sites, graft size and configuration, patency of inferior mesenteric artery and hypogastric arteries, use of heparin, mannitol, and renal perfusion, and transfusion of crystalloid and blood products. The VSGNE tracks outcomes including in-hospital mortality and long-term mortality by matching patients with the Social Security Death Index.
Statistical analysis
Descriptive statistics were used to analyze patient, prehospital, anatomic, and procedural characteristics. Univariate analysis of patient comorbidities, pre-hospital factors including time to incision, anatomic parameters, and procedural variables associated with in-hospital mortality were performed with a logistic regression model. The impact of age on mortality was analyzed with univariate and multivariable regression according to multiple strata of age, with the odds ratio (OR) of mortality calculated with the stratum “age <60” used as the reference population. The Youden index ([sensitivity + specificity] − 1) is a frequently used summary measure of the receiver-operating characteristic curve, which enables the selection of an optimal threshold value (cutoff point) for the impact of a continuous variable on an outcome.23 A higher Youden index reflects more accurate predictive ability at a specific cutoff point. In order to identify the optimal cutoff point for determining the impact of age on mortality, a Youden index and the percent of patients classified correctly were determined for each cutoff point of increasing age.
Variables associated with mortality on univariate analysis (P ≤ .2) were initially included in a multivariable regression model, which used stepwise elimination in order to identify variables independently predictive of mortality. We then generated an integer-based VSGNE RAAA risk score based on significant predictors of mortality on multivariable regression. We determined the integer points assigned to each significant predictor by dividing its individual OR for mortality by a common denominator of 2.5 and rounding to the nearest integer. The calibration of the VSGNE RAAA risk score model was tested by applying the model to all individual patients in the dataset and comparing observed and expected mortality across strata of predicted risk. A Hosmer-Lemeshow goodness-of-fit statistic was calculated. The discrimination of the VSGNE RAAA risk score was evaluated via the area under the receiver-operating characteristic curve (AUC). Bootstrapping methodology was used to internally validate the VSGNE RAAA risk score.24 We randomly drew with replacement 1000 random samples of 100%. The AUC was calculated for each sample and compared to the AUC in the original dataset in order to assess the reproducibility of the VSGNE RAAA risk score model.
The GAS, Hardman index, Vancouver score, and ERAS were calculated for each patient in the VSGNE cohort in order to determine the performance of each scoring system in predicting mortality. The ability of each of the existing scoring systems to identify patients of varying mortality risk was determined by calculating patient mortality in the VSGNE cohort for each stratum of increasing risk scores, which represent increasing predicted risk of mortality, in each of the existing scoring systems. The discrimination of each scoring system was assessed in the VSGNE cohort via the AUC, and the calibration of each model was assessed via the Hosmer-Lemeshow test. An α = .05, corresponding to P = .05 and 95% confidence intervals (CIs), was used as a criterion for statistical significance. Statistical computations were performed using SAS software (version 9.1; SAS Institute, Cary, NC).
RESULTS
Patient and prehospital characteristics
Two hundred forty-two patients underwent open repair of an RAAA in 10 academic and community hospitals participating in the VSGNE from 2003 to 2009. The number of repairs over the study period varied according to center (mean [standard deviation] 24.2 [25]; median, 15.5; range, 1–79). Four centers performed six or fewer repairs, two centers performed 15 to 16 repairs, and four centers performed 38 or more repairs. A significant number of patients had symptoms and signs of severe shock, including a preoperative heart rate >100 bpm (20%), preoperative SBP <90 mm Hg (56%), altered mental status (38%), loss of consciousness (20%), and cardiac arrest (14%; Table I).
Table I.
Patient demographics, comorbidities, and prehospital characteristics
| Characteristic | No. (%)a |
|---|---|
| Male | 205 (85.1) |
| Caucasian | 240 (99.6) |
| Age >76 | 105 (43.4) |
| Preoperative heart rate >100 bpm | 47 (19.4) |
| Systolic blood pressure <90 mm Hg | 135 (55.8) |
| Altered mental status | 93 (38.4) |
| Preoperative loss of consciousness | 48 (20.4) |
| Preoperative cardiac arrest | 33 (13.9) |
| Hypertension | 187 (81.1) |
| Diabetes mellitus | 36 (15.6) |
| Coronary artery disease | 74 (33.8) |
| Congestive heart failure | 26 (11.6) |
| Coronary artery bypass graft/percutaneous transluminal coronary angioplasty | 54 (23.9) |
| Smoking | 191 (87.2) |
| Chronic obstructive pulmonary disease | 96 (42.1) |
| Creatinine >2.1 mg/dL (190 μmol/L) | 20 (9.4) |
| Creatine (mg/dL)a | 1.4 ± 0.7 |
| Dialysis | 1 (0.4) |
| Cerebrovascular disease | 14 (6.2) |
| Hemoglobin (g/dL)a | 11.2 ± 2.3 |
| β-blocker | 88 (38.4) |
| Acetylsalicylic acid or Plavix | 110 (45.5) |
| Statin | 74 (33.0) |
| Prior aortic surgery | 10 (4.1) |
| Transferred from outside facility | 132 (54.6) |
| Time from symptom onset to incision, hours, median (IQR) | 6 (10.5) |
| Time from admission to incision, hours, median (IQR) | 1 (2) |
IQR, Interquartile range.
Other units are expressed as mean (standard deviation) except as indicated.
Anatomic and procedural characteristics
The majority of patients were repaired with a transperitoneal approach (95%) and tube graft (67%). The proximal aortic cross-clamp was placed in the infrarenal location in 141 patients (59%) and suprarenal in 98 patients (41%; Table II).
Table II.
Anatomic and procedural characteristics
| Characteristic | No. (%)a |
|---|---|
| Average aneurysm diameter, cm | 7.7 ± 2.1 |
| Aneurysm diameter, cm | |
| <5 | 11 (5.3) |
| 5–7.5 | 85 (40.9) |
| 7.5–10 | 75 (36.1) |
| >10 | 37 (17.8) |
| Transperitoneal exposure | 231 (95.4) |
| Retroperitoneal exposure | 11 (4.6) |
| Infrarenal clamp | 141 (59) |
| Suprarenal clamp | 98 (41) |
| Inferior mesenteric artery | |
| Occluded | 147 (64.8) |
| Ligated | 76 (33.5) |
| Reimplanted | 4 (1.8) |
| Tube graft | 158 (67.2) |
| Aortobi-iliac | 47 (20) |
| Aortobifemoral | 29 (12.3) |
| Heparin administered | 135 (56.7) |
| Mannitol administered | 64 (27.0) |
| Estimated blood loss, L | 4.7 ± 7.2 |
| Units packed red blood cells transfused | 6.7 ± 6.1 |
| Autotransfusion, L | 1.7 ± 1.7 |
| Crystalloid infused, L | 7.9 ± 6.4 |
| Procedural time, minutes | 187.4 ± 85.0 |
| Delayed abdominal closure | 55 (25.5) |
Other units are expressed as mean (standard deviation).
Factors associated with mortality
There were 91 in-hospital deaths (38%). No single risk factor or combination of two factors universally predicted mortality on univariate analysis (Table III). For example, eight of 33 patients who suffered preoperative cardiac arrest survived, including two patients who were older than 76 years. Transfer status, time from symptoms to incision, and time from admission to incision did not impact mortality. Increasing age was generally correlated with increased mortality, although patients in the highest stratum of age (age >85) did not have the highest risk of mortality (Table IV). A cutoff point of 76 years of age yielded the highest Youden index (.38) of any age cutoff point and correctly predicted mortality for 70% of patients, thereby identifying age >76 years as the optimal threshold for analyzing the impact of age on mortality. On multivariable analysis in which age was modeled as a dichotomous variable as either age >76 years or age ≤76 years, independent predictors of mortality included age >76 years, preoperative cardiac arrest, loss of consciousness on presentation, and need for suprarenal aortic clamp (Table V).
Table III.
Univariate predictors of in-hospital mortality
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Age >76 | 4.6 | 2.6–8 | <.0001 |
| Cardiac arrest | 6.8 | 2.9–16 | <.0001 |
| Loss of consciousness | 4.2 | 2.1–8.3 | <.0001 |
| Suprarenal aortic clamp | 2.3 | 1.3–2.8 | .003 |
| Female | 2.1 | 1.0–4.3 | .04 |
| Hypertension | 1.8 | 0.9–3.8 | .12 |
| Creatinine > 2.15 mg/dL | 2.4 | 0.9–6.1 | .064 |
| Lowest preoperative systolic blood pressure < 80 mm Hg | 3.2 | 1.8–5.4 | <.0001 |
| Congestive heart failure | 1.8 | 0.8–4.0 | .18 |
CI, Confidence interval; OR, odds ratio.
Table IV.
Impact of age on in-hospital mortality
| Age strata | n | Univariate analysis
|
Multivariable analysis
|
||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| <60 | 11 | 0.2 | 0.02–1.3 | .08 | Ref | ||
| 60–69 | 65 | 2.3 | 0.3–19.4 | .5 | 1.3 | 0.1–11.6 | .8 |
| 70–79 | 104 | 6.5 | 0.8–52.8 | .08 | 4.3 | 0.5–35.7 | .2 |
| 80–85 | 38 | 19.2 | 2.2–167.1 | .01 | 17.2 | 1.9–15.3 | .01 |
| >85 | 24 | 10.0 | 1.1–90.8 | .04 | 8.6 | 0.9–80.9 | .06 |
CI, Confidence interval; OR, odds ratio.
Table V.
Multivariable predictors of in-hospital mortality
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Age >76 | 5.3 | 2.8–10.1 | <.0001 |
| Cardiac arrest | 4.3 | 1.6–12.0 | .0048 |
| Loss of consciousness | 2.7 | 1.2–6.0 | .018 |
| Suprarenal aortic clamp | 2.4 | 1.3–4.6 | .0057 |
CI, Confidence interval; OR, odds ratio.
Derivation and validation of the VSGNE RAAA risk score
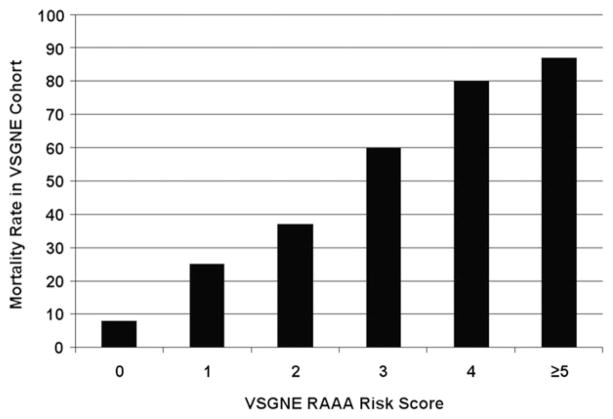
The VSGNE RAAA risk score (range, 0–6) was calculated for each patient by totaling the integer points assigned to each independent predictor of mortality (Table VI). The odds of mortality with each integer increase in risk score increased by a factor of 2.3 (95% CI, 1.79–2.95). Table VII lists the number of patients and the mortality rate in each stratum of VSGNE RAAA risk score. A linear relationship of VSGNE risk score to postoperative mortality was identified (Fig 1). A VSGNE RAAA risk score of 4 identified patients with an 80% risk of mortality, whereas a VSGNE RAAA risk score ≥5 identified patients with an 87% risk of mortality. Discrimination of the VSGNE RAAA risk score in the VSGNE cohort as measured by the AUC was excellent (C = .79; 95% CI, .73–.85). A Hosmer-Lemeshow goodness-of-fit test was not statistically significant, indicating good calibration of the model (χ2 [5 degrees of freedom] = 1.96; P = .85). The VSGNE RAAA risk score model was then applied to 1000 bootstrap samples and showed excellent discrimination (C = .79; 95% CI, .73–.85). In the bootstrap datasets, the odds of mortality with each integer increase in risk score increased by a factor of 2.4 (95% CI, 1.83–3.07). The VSGNE RAAA risk score calculator is available online at www.vsgne.org.
Table VI.
Calculation of VSGNE RAAA risk score
| Variable | OR | Integer points |
|---|---|---|
| Age >76 | 5.3 | 2 |
| Cardiac arrest | 4.3 | 2 |
| Loss of consciousness | 2.7 | 1 |
| Suprarenal clamp VSGNE RAAA risk scorea 0–6 | 2.4 | 1 |
OR, Odds ratio; RAAA, ruptured abdominal aortic aneurysm; VSGNE, Vascular Study Group of New England.
Sample case demonstrating calculation of the VSGNE RAAA risk score in an 80-year-old man who had loss of consciousness but no cardiac arrest and was repaired with suprarenal clamping of the aorta:
Age >76: 2 points
Cardiac arrest: 0 points
Loss of consciousness: 1 point
Suprarenal clamp: 1 point
VSGNE RAAA risk score = 2 + 0 + 1 + 1 = 4
Table VII.
Distribution of patients and mortality according to the VSGNE RAAA risk score
| VSGNE risk score | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| No. of patients (%) | 61 (25.2) | 48 (19.8) | 57 (23.6) | 40 (16.5) | 10 (4.1) | 11 (4.5) | 4 (1.7) |
| No. of deaths | 5 | 12 | 21 | 24 | 8 | 10 | 3 |
RAAA, Ruptured abdominal aortic aneurysm; VSGNE, Vascular Study Group of New England.
Fig 1.
Mortality rate according to the Vascular Study Group of New England (VSGNE) ruptured abdominal aortic aneurysm (RAAA) risk score.
Performance of existing scoring systems in the VSGNE dataset
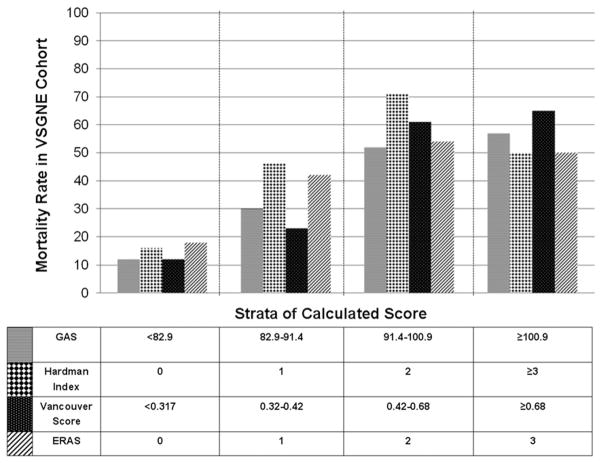
The GAS, Hardman index, Vancouver score, and ERAS were calculated for all patients in the VSGNE cohort according to their respective formulas (Table VIII). The AUC for each of the existing scoring systems ranged from .67 to .74 in the VSGNE dataset, and model calibration was acceptable as evidenced by nonsignificant Hosmer-Lemeshow tests (Table IX). When analyzed according to strata of increasing score (and associated increasing predicted risk of mortality), the ability of each existing scoring system score to predict mortality in the VSGNE dataset was most acceptable in low-risk patients (Fig 2). The GAS, Hardman index, Vancouver score, and ERAS failed to discriminate patients who have >50% mortality risk and failed to identify any patient with >65% risk of mortality (Fig 2).
Table VIII.
Published risk scoring systems for open repair of RAAA
| Risk score | Formula |
|---|---|
| Glasgow aneurysm score | Age + 17 for shock + 7 for myocardial disease + 10 for cerebrovascular disease + 14 for renal disease |
| Hardman index | Score from 1 to 5 depending on number of five risk factors present |
| Risk factors: age >76, electrocardiographic ischemia, creatinine >190 μmol/L, loss of consciousness, hemoglobin (g/dL) <9 | |
| Vancouver score | Ex/(1 + Ex), where x = (−3.44) + [sum of coefficients of significant variables] |
| Variable coefficient | |
| Age .062 × age | |
| Reduced consciousness: Yes 1.14 | |
| Reduced consciousness: No −1.14 | |
| Cardiac arrest: Yes . 6 | |
| Cardiac arrest: No −.6 | |
| Edinburg ruptured aneurysm score | Score from 1 to 3 depending on number of three risk factors present |
| Risk factors: hemoglobin (g/dL) <9, preoperative Glasgow coma scale score <15, preoperative systolic blood pressure <90 mm Hg |
RAAA, Ruptured abdominal aortic aneurysm.
Table IX.
Performance of published risk scoring systems in the VSGNE cohort
| Model | Calibrationa | Discriminationb |
|---|---|---|
| Glasgow aneurysm score | χ2 = 7.2, P = .52 | .74 |
| Hardman index | χ2 = .86, P = .35 | .72 |
| Vancouver score | χ2 = 14.5, P = .07 | .76 |
| Edinburg ruptured aneurysm score | χ2 = 1.55, P = .2 | .67 |
| VSGNE RAAA risk score | χ2 = 1.96, P = .85 | .79 |
RAAA, Ruptured abdominal aortic aneurysm; VSGNE, Vascular Study Group of New England.
Hosmer-Lemeshow test.
C-statistic.
Fig 2.
Mortality rate according to the Glasgow aneurysm score (GAS), Hardman index, Vancouver score, and Edinburg ruptured aneurysm score (ERAS). Patients were grouped and analyzed according to total integer score for the Hardman index and ERAS and according to quartile of increasing risk score for the GAS and Vancouver score. VSGNE, Vascular Study Group of New England.
DISCUSSION
Accurate prediction of mortality after open repair of RAAAs is important for three reasons. First, prognostic tools aid clinical decision making, including the decision to avoid repair in patients with prohibitive risk. Because no existing scoring system has gained widespread acceptance, clinical decisions are often still based primarily on subjective criteria, which may vary widely within and between centers.15 Second, risk adjustment should allow objective evaluation of open RAAA repair in the endovascular era and help control for the selection bias that confounds comparison of open and endovascular RAAA repair.21 Third, repair of RAAAs has been identified by patients, physicians, and third-party payers as a key index procedure by which physicians and institutions can be evaluated.25 As with any outcome measure, accurate risk adjustment is necessary to allow fair and valid comparison between surgeons and institutions that may be treating patients who are very different from each other. Risk-adjusted outcomes also allow appropriate identification of areas for improvement in underperforming institutions. Robust parsimonious models for predicting mortality after repair of RAAAs are clearly needed.
This report represents the first attempt to externally validate existing scoring systems in a U.S. population. When tested in the VSGNE cohort, the discrimination was generally good, with C-statistics ranging from 0.67 to 0.74. Calibration of the existing scoring systems was acceptable in low-risk patients, as evidenced by nonsignificant Hosmer-Lemeshow goodness-of-fit tests. However, all existing scoring systems failed to identify patients at highest risk for mortality. For example, patients with a Hardman index of 2 had 70% mortality. Those with a Hardman index ≥3, who therefore had a higher predicted mortality risk, had an actual mortality of 50%. The highest strata of risk scores according to the GAS (score ≥101), Vancouver score (≥.68), or ERAS (score = 3) were associated with mortality rates of 57%, 65%, and 50% respectively. Based on this analysis, no existing scoring system was able to identify consistently those patients with >65% risk of mortality. This is consistent with previous studies, which demonstrated that the GAS, Hardman index, and Vancouver score failed to identify patients at highest mortality risk when tested in external populations.16,17,26 To our knowledge, this was the first attempt to validate the ERAS in any external cohort.
The VSGNE RAAA risk score, on the other hand, showed superior discrimination (C = .79; 95% CI, .73–.85) than did existing scoring systems and excellent calibration, as it predicted risk well in both low- and high-risk patients, such as those with ≥80% mortality risk. For example, a VSGNE RAAA risk score of 4 was associated with a mortality rate of 80%, whereas a VSGNE RAAA risk score of 5 or 6 was associated with a mortality rate of 87%. In comparison to existing scoring systems, the VSGNE RAAA risk score better identifies patients at highest risk for mortality.
The VSGNE RAAA risk score (range, 0–6) is easily calculated based on four variables readily assessed at the time of presentation (Table VI). The existing scoring systems incorporate variables that require knowledge of the patients’ underlying conditions (GAS: cerebrovascular disease, myocardial disease, renal disease) or additional testing (Harman index: electrocardiographic ischemia, creatinine), which may not be readily available at the time of presentation of a patient with an RAAA.13–15 In addition, the VSGNE RAAA risk score is easier to calculate than the more cumbersome GAS and Vancouver score, without the need for anything but simple addition of integer points based on four dichotomous variables. In addition, based on the fact that all four variables were successfully abstracted in the VSGNE dataset in 231 patients (95%), these variables are readily available for the purpose of risk adjustment at the surgeon, institution, or regional level.
In our multivariable model, age >76 years was most strongly associated with mortality. This was consistent with previous scoring systems, which identified advanced age as a predictor of mortality, including the GAS, Hardman index, and Vancouver score. We analyzed the impact of increasing age in multiple ways. Analysis demonstrated that “age >76” was the optimal cutoff in order to analyze the impact of age as a dichotomous variable. We believed that the ability to accurately model age as a dichotomous variable was valuable because it would allow for creation of a parsimonious risk score that clinicians could quickly calculate. Inclusion of age as a continuous variable, as done in the GAS and Vancouver score, requires more complex calculation that we suspect many find cumbersome. Interestingly, our cutoff of 76 years was identical to that used in the Hardman index and therefore allowed for consistent head-to-head comparison of the two risk scores.
Preoperative cardiac arrest also strongly predicted mortality. Only the Vancouver score included cardiac arrest in the scoring system. The GAS identified “shock,” and the ERAS identified “SBP <90 mm Hg” as similar variables that predicted mortality. We analyzed multiple hemodynamic parameters that identify shock (SBP <80 mm Hg, SBP <90 mm Hg, and HR >100) in our multivariable model, and none was predictive of mortality. “Hypotensive hemostasis,” in which relative hypotension is tolerated without aggressive fluid resuscitation, has been increasingly adopted. In the current era, preoperative shock as measured by traditional parameters does not significantly impact mortality.
“Loss of consciousness” independently predicted mortality. This variable was also identified by the Hardman index, whereas the ERAS identified Glasgow coma scale score <15, and the Vancouver score identified “reduced conscious” as risk factors. When confronted with the need for rapid decision making, clinicians can identify “loss of consciousness” more readily and definitively than determine Glasgow coma scale or “reduced consciousness.”
Finally, our analysis identified the need for a suprarenal clamp as predictive of mortality. Previous studies have also found that placement of a suprarenal aortic clamp has been associated with worse outcomes after repair of both intact and ruptured AAAs.27–30 Based on these data, it is impossible to know if, in fact, a suprarenal clamp was necessitated by the presence of a juxtarenal or pararenal aneurysm or was used at the preference of the surgeon as an alternative to infrarenal dissection and clamping in the setting of extensive inframesocolic hematoma. Nevertheless, these data suggest that the surgeon should avoid suprarenal clamping when at all possible for an infrarenal AAA. In addition, by accounting for the risk associated with the need for a suprarenal clamp, this prediction model potentially allows for comparison of outcomes in cohorts of patients with ruptured AAAs treated with either open or endovascular repair. The use of endovascular repair of RAAAs increased from 5.9% in 2001 to 18.9% in 2006 according to data from Nationwide Inpatient Sample.3 This trend is likely to continue, as mortality of EVAR for RAAAs has generally been reported to be significantly lower than that of open repair, ranging from 18.5% to 31.7%.3,31,32 Endovascular repair is generally used when there is adequate nonaneurysmal infrarenal aortic neck, whereas open repair is often used when there is an adverse anatomy in the neck of the AAA that requires a suprarenal clamp and therefore has increased risk of mortality. Without accounting for the risk associated with suprarenal clamping, valid comparison of the two strategies is difficult, as is comparison of cohorts of patients at different institutions who may be at different risk of mortality.
The need for a suprarenal clamp can often be readily identified based on computed tomographic scanning. In modern algorithms for RAAA management, 78% to 93% of patients undergo preoperative computed tomographic scan.32,33 Thus, the need for a suprarenal clamp can often be readily identified preoperatively by the surgeon for prognostic risk assessment or determined retrospectively for use in risk adjustment for comparative audit. We believe that the validity of the VSGNE RAAA risk score should be tested in a cohort of patients undergoing endovascular repair of ruptured AAAs. When EVAR is applied to patients with an infrarenal AAA, zero points should be assigned for the variable (suprarenal clamp), and the patient’s VSGNE RAAA risk score would then be based on the other three independent predictors represented in the model. If validity in an endovascular cohort is established, the VSGNE RAAA risk score will allow for risk-adjusted comparison of EVAR and open repair of RAAA.34
There are limitations to this study. Although this data-set was generated from a contemporary “real-world” experience of community hospitals and tertiary referral centers, the size of the cohort is limited. There is also heterogeneity between centers with regard to institutional volume, resources, and institutional practices. In addition, the VSGNE RAAA risk score was developed over a period of significant flux in practice patterns, and patients undergoing open repair of ruptured AAAs today are likely at higher risk. Some of the patients who underwent open repair in this cohort may have been treated with endovascular repair at other centers in the United States. Nevertheless, the VSGNE RAAA risk score is the first risk score developed and validated in a prospectively collected U.S. cohort and the first developed in the era of endovascular repair of ruptured AAAs. External, prospective validation in a larger dataset is required before this prediction model can be recommended for risk stratification or comparative audit outside of the VSGNE. There is good evidence that, with appropriate validation, parsimonious prediction models based on a small number of important clinical variables provide risk adjustment of equivalent accuracy to more complicated multivariable models.34
CONCLUSIONS
When tested in the VSGNE cohort, the GAS, Hardman index, Vancouver score, and ERAS demonstrated good overall correlation with mortality but were limited in their ability to identify those patients at highest risk for mortality. The VSGNE RAAA risk score is the first risk score developed and validated in a prospectively collected U.S. cohort and the first developed in the era of endovascular repair of RAAAs. The VSGNE RAAA risk score allows accurate prediction of mortality based on four variables readily assessed in current practice, including identification of those patients at the highest level of risk.
Footnotes
Author conflict of interest: none.
Presented at the 2010 Vascular Annual Meeting of the Society for Vascular Surgery, Boston, Mass, June 10–13, 2010.
AUTHOR CONTRIBUTIONS
Conception and design: WR
Analysis and interpretation: WR, AS, YL, PG, BN, ME, JC, LM
Data collection: YL, WR
Writing the article: WR
Critical revision of the article: AS, YL, PG, BN, ME, JC, LM
Final approval of the article: WR, AS, YL, PG, BN, ME, JC, LM
Statistical analysis: WR, YL
Obtained funding: Not applicable
Overall responsibility: WR
References
- 1.Mureebe L, Egorova N, Giacovelli JK, Gelijns A, Kent KC, McKinsey JF. National trends in the repair of ruptured abdominal aortic aneurysms. J Vasc Surg. 2008;48:1101–7. doi: 10.1016/j.jvs.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 2.Acosta S, Ogren M, Bengtsson H, Bergqvist D, Lindblad B, Zdanowski Z. Increasing incidence of ruptured abdominal aortic aneurysm: a population-based study. J Vasc Surg. 2006;44:237–43. doi: 10.1016/j.jvs.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 3.McPhee J, Eslami MH, Arous EJ, Messina LM, Schanzer A. Endovascular treatment of ruptured abdominal aortic aneurysms in the United States (2001–2006): a significant survival benefit over open repair is independently associated with increased institutional volume. J Vasc Surg. 2009;49:817–26. doi: 10.1016/j.jvs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Giles KA, Pomposelli F, Hamdan A, Wyers M, Jhaveri A, Schermerhorn ML. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg. 2009;49:543–50. doi: 10.1016/j.jvs.2008.09.067. discussion: 550–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dueck AD, Kucey DS, Johnston KW, Alter D, Laupacis A. Survival after ruptured abdominal aortic aneurysm: effect of patient, surgeon, and hospital factors. J Vasc Surg. 2004;39:1253–60. doi: 10.1016/j.jvs.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Bengtsson H, Bergqvist D. Ruptured abdominal aortic aneurysm: a population-based study. J Vasc Surg. 1993;18:74–80. doi: 10.1067/mva.1993.42107. [DOI] [PubMed] [Google Scholar]
- 7.Bown MJ, Sutton AJ, Bell PR, Sayers RD. A meta-analysis of 50 years of ruptured abdominal aortic aneurysm repair. Br J Surg. 2002;89:714–30. doi: 10.1046/j.1365-2168.2002.02122.x. [DOI] [PubMed] [Google Scholar]
- 8.Dardik A, Burleyson GP, Bowman H, Gordon TA, Williams GM, Webb TH, et al. Surgical repair of ruptured abdominal aortic aneurysms in the state of Maryland: factors influencing outcome among 527 recent cases. J Vasc Surg. 1998;28:413–20. doi: 10.1016/s0741-5214(98)70126-0. discussion: 420–1. [DOI] [PubMed] [Google Scholar]
- 9.Johansen K, Kohler TR, Nicholls SC, Zierler RE, Clowes AW, Kazmers A. Ruptured abdominal aortic aneurysm: the Harborview experience. J Vasc Surg. 1991;13:240–5. discussion: 245–7. [PubMed] [Google Scholar]
- 10.Lawrence PF, Gazak C, Bhirangi L, Jones B, Bhirangi K, Oderich G, et al. The epidemiology of surgically repaired aneurysms in the United States. J Vasc Surg. 1999;30:632–40. doi: 10.1016/s0741-5214(99)70102-3. [DOI] [PubMed] [Google Scholar]
- 11.Wakefield TW, Whitehouse WM, Jr, Wu SC, Zelenock GB, Cronenwett JL, Erlandson EE, et al. Abdominal aortic aneurysm rupture: statistical analysis of factors affecting outcome of surgical treatment. Surgery. 1982;91:586–96. [PubMed] [Google Scholar]
- 12.Chen JC, Hildebrand HD, Salvian AJ, Taylor DC, Strandberg S, Myckatyn TM, et al. Predictors of death in nonruptured and ruptured abdominal aortic aneurysms. J Vasc Surg. 1996;24:614–20. doi: 10.1016/s0741-5214(96)70077-0. discussion: 621–3. [DOI] [PubMed] [Google Scholar]
- 13.Hardman DT, Fisher CM, Patel MI, Neale M, Chambers J, Lane R, et al. Ruptured abdominal aortic aneurysms: who should be offered surgery? J Vasc Surg. 1996;23:123–9. doi: 10.1016/s0741-5214(05)80042-4. [DOI] [PubMed] [Google Scholar]
- 14.Samy AK, Murray G, MacBain G. Glasgow aneurysm score. Cardiovasc Surg. 1994;2:41–4. [PubMed] [Google Scholar]
- 15.Tambyraja A, Murie J, Chalmers R. Predictors of outcome after abdominal aortic aneurysm rupture: Edinburgh Ruptured Aneurysm Score. World J Surg. 2007;31:2243–7. doi: 10.1007/s00268-007-9181-5. [DOI] [PubMed] [Google Scholar]
- 16.Tambyraja AL, Fraser SC, Murie JA, Chalmers RT. Validity of the Glasgow Aneurysm Score and the Hardman Index in predicting outcome after ruptured abdominal aortic aneurysm repair. Br J Surg. 2005;92:570–3. doi: 10.1002/bjs.4907. [DOI] [PubMed] [Google Scholar]
- 17.Tambyraja AL, Murie JA, Chalmers RT. Prediction of outcome after abdominal aortic aneurysm rupture. J Vasc Surg. 2008;47:222–30. doi: 10.1016/j.jvs.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 18.Tambyraja AL, Lee AJ, Murie JA, Chalmers RT. Prognostic scoring in ruptured abdominal aortic aneurysm: a prospective evaluation. J Vasc Surg. 2008;47:282–6. doi: 10.1016/j.jvs.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 19.Patterson BO, Karthikesalingam A, Hinchliffe RJ, Loftus IM, Thompson MM, Holt PJ. The Glasgow Aneurysm Score does not predict mortality after open abdominal aortic aneurysm in the era of endovascular aneurysm repair. J Vasc Surg. 2011;54:353–7. doi: 10.1016/j.jvs.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Bohm N, Wales L, Dunckley M, Morgan R, Loftus I, Thompson M. Objective risk-scoring systems for repair of abdominal aortic aneurysms: applicability in endovascular repair? Eur J Vasc Endovasc Surg. 2008;36:172–7. doi: 10.1016/j.ejvs.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Hinchliffe RJ, Powell JT, Cheshire NJ, Thompson MM. Endovascular repair of ruptured abdominal aortic aneurysm: a strategy in need of definitive evidence. J Vasc Surg. 2009;49:1077–80. doi: 10.1016/j.jvs.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE) J Vasc Surg. 2007;46:1093–101. doi: 10.1016/j.jvs.2007.08.012. discussion: 1101–2. [DOI] [PubMed] [Google Scholar]
- 23.Akobeng AK. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007;96:644–7. doi: 10.1111/j.1651-2227.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 24.Efron B, Tibshirani R. Bootstrap methods of standard errors, confidence intervals, and other methods of statistical accuracy. Stat Sci. 1986;1:54–77. [Google Scholar]
- 25.Agency for Healthcare Research and Quality. Inpatient quality indicators overview. Available at: http://www.qualityindicators.ahrq.gov/Modules/iqi_overview.aspx.
- 26.Leo E, Biancari F, Nesi F, Pogany G, Bartolucci R, De Pasquale F, et al. Risk-scoring methods in predicting the immediate outcome after emergency open repair of ruptured abdominal aortic aneurysm. Am J Surg. 2006;192:19–23. doi: 10.1016/j.amjsurg.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 27.Bauer EP, Redaelli C, von Segesser LK, Turina MI. Ruptured abdominal aortic aneurysms: predictors for early complications and death. Surgery. 1993;114:31–5. [PubMed] [Google Scholar]
- 28.Beck AW, Goodney PP, Nolan BW, Likosky DS, Eldrup-Jorgensen J, Cronenwett JL. Predicting 1-year mortality after elective abdominal aortic aneurysm repair. J Vasc Surg. 2009;49:838–43. doi: 10.1016/j.jvs.2008.10.067. discussion: 843–4. [DOI] [PubMed] [Google Scholar]
- 29.Green RM, Ricotta JJ, Ouriel K, DeWeese JA. Results of supraceliac aortic clamping in the difficult elective resection of infrarenal abdominal aortic aneurysm. J Vasc Surg. 1989;9:124–34. [PubMed] [Google Scholar]
- 30.Johnston KW. Ruptured abdominal aortic aneurysm: six-year follow-up results of a multicenter prospective study. Canadian Society for Vascular Surgery Aneurysm Study Group. J Vasc Surg. 1994;19:888–900. doi: 10.1016/s0741-5214(94)70015-x. [DOI] [PubMed] [Google Scholar]
- 31.Mastracci TM, Garrido-Olivares L, Cina CS, Clase CM. Endovascular repair of ruptured abdominal aortic aneurysms: a systematic review and meta-analysis. J Vasc Surg. 2008;47:214–21. doi: 10.1016/j.jvs.2007.07.052. [DOI] [PubMed] [Google Scholar]
- 32.Starnes BW, Quiroga E, Hutter C, Tran NT, Hatsukami T, Meissner M, et al. Management of ruptured abdominal aortic aneurysm in the endovascular era. J Vasc Surg. 2010;51:9–17. doi: 10.1016/j.jvs.2009.08.038. discussion: 17–8. [DOI] [PubMed] [Google Scholar]
- 33.Sharif MA, Lee B, Makar RR, Loan W, Soong CV. Role of the Hardman index in predicting mortality for open and endovascular repair of ruptured abdominal aortic aneurysm. J Endovasc Ther. 2007;14:528–35. doi: 10.1177/152660280701400414. [DOI] [PubMed] [Google Scholar]
- 34.Osborne NH, Ko CY, Upchurch GR, Jr, Dimick JB. Evaluating parsimonious risk-adjustment models for comparing hospital outcomes with vascular surgery. [accepted Aug 28, 2012];J Vasc Surg. 2010 52:400–5. doi: 10.1016/j.jvs.2010.02.293. Submitted Jul 5, 2012. [DOI] [PubMed] [Google Scholar]