Summary
Mutations in the C. elegans gene ced-3 prevent almost all programmed cell deaths, so that in a ced-3 mutant there are many extra cells. We show that the pharyngeal neuron M4 is essential for feeding in wild-type worms, but in a ced-3 mutant, one of the extra cells, probably MSpaaaaap (the sister of M4), can sometimes take over M4’s function. The function of MSpaaaaap, unlike that of M4, is variable and subnormal. One possible explanation is that its fate, being hidden by death and not subject to selection, has drifted randomly during evolution. We suggest that such cells may play roles in the evolution of cell lineage analogous to those played by pseudogenes in the evolution of genomes.
Introduction
During multicellular development many cells are born only to die later, without serving any obvious function. This phenomenon, referred to as programmed cell death, occurs in organisms as diverse as bacteria (Kaiser et al., 1979), nematodes (Sulston and Horvitz, 1977; Sulston et al., 1983) insects (Taylor and Truman, 1974) and vertebrates (Hamburger and Oppenheim, 1982). Cell death is especially common in the development of nervous systems In the hermaphrodite of the nematode Caenorhabditis elegans, for instance, 82% (107/131) of the programmed cell deaths that occur are in lineages that lead only to neurons and glia, although only 37% (358/959) of the nuclei in a mature hermaphrodite are neurons or glia (Sulston and Horvitz, 1977; Sulston et al., 1983).
One possible explanation for the ubiquity of programmed cell death is that the cells that die are evolutionary relics. These cells may have once served some function that later became unnecessary or even deleterious. Cell death would thus eliminate unnecessary cells. It might also cause adaptive changes in behavior or physiology. There is evidence that specific cell deaths can have profound biological effects. For instance, the gross difference between the one-armed gonad of Panagrellus redivivus (a nematode related to C. elegans) and the two-armed gonad of C. elegans can be explained by the death of the posterior distal tip cell in P redivivus (Sternberg and Horvitz, 1981). A single cell death can thus account for a major change in anatomy. Single mutations that cause new cell deaths can also lead to changes in behavior. For instance, mutations in the C. elegans gene egl-7 cause the two HSN motoneurons to die, resulting in animals that cannot lay eggs normally (Trent et al., 1983).
The evolutionary relic theory suggests that cells that die during wild-type development might have hidden functions that could be restored by preventing cell death. How might one prevent cell death, in order to test this prediction? Ellis and Horvitz (1986) identified a C. elegans gene, ced-3, that is necessary for almost all programmed cell deaths, so that in a ced-3 mutant, cells that would normally die instead survive. The phenotype of a ced-3;egC-1 double mutant is evidence that ced-3 is a good tool for revealing functions hidden by programmed cell death. As described above, egl-1 worms are egg-laying-defective because of the death of the HSN neurons. In a ced-3;egl-1 double mutant, the programmed death of the HSN neurons is prevented by ced-3, so the HSNs survive. In fact, the HSNs not only survive, but function, as demonstrated by the double mutant’s ability to lay eggs normally (Ellis and Horvitz, 1986). However, this observation does not clarify the possible evolutionary significance of cell death. We wanted to test whether a cell that dies during wild-type development, and which therefore cannot have been under selective pressure to function, might function in a ced-3 mutant,
In the course of studying the function of the pharyngeal nervous system, we discovered that the neuron M4 is necessary for the proper operation of the pharynx in young worms. Since M4 has a sister that dies during wild-type development, and since in ced-3 worms cell death survivors sometimes acquire some characteristics of their sisters (Ellis and Horvitz, 1986) we hoped that MSpaaaaap, the sister of M4, might have a hidden M4 function. Using a laser microbeam to kill cells, we found that one of the extra cells in ced-3 worms, tentatively identified as MSpaaaaap, could become a functioning M4 neuron about half of the time, although it rarely or never fully replaced M4.
Results
M4 Is Necessary for Normal Feeding in Young Worms
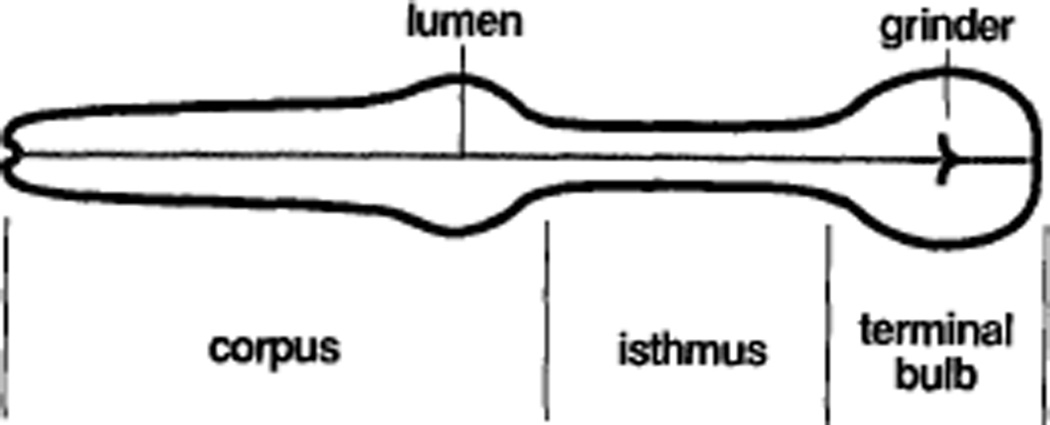
The pharynx of C. elegans is a muscular pump that sucks in bacteria, grinds them up, and passes the debris back to the intestine. The pharynx is a nearly self-contained organ, surrounded by a basement membrane, and containing a nervous system and muscles of its own. It has three parts: the corpus, which is responsible for accumulating bacteria, the terminal bulb, which is responsible for grinding the bacteria up, and the isthmus, which connects the corpus to the terminal bulb (Figure 1) (Albertson and Thomson, 1976). The isthmus regulates the passage of bacteria from the corpus to the terminal bulb by propagating peristaltic waves.
Figure 1. Anatomy of the Pharynx.
Anterior is to the left.
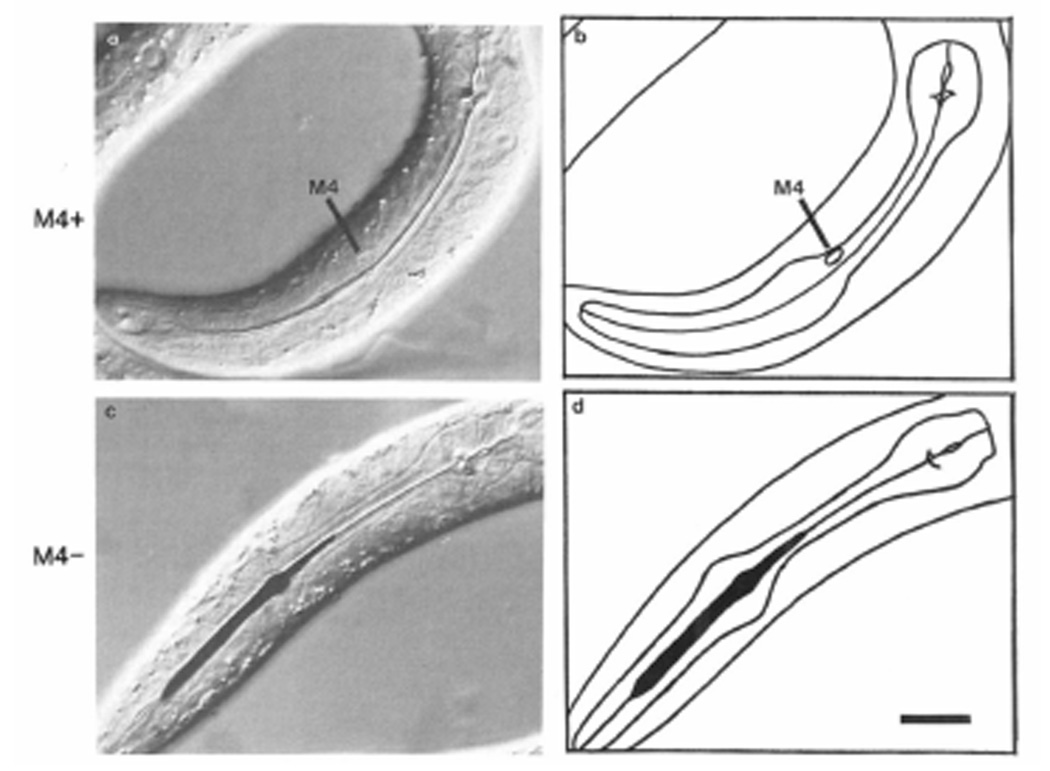
As part of a general survey of the function of the pharyngeal nervous system, we killed the neuron M4 in newly hatched worms. M4 innervates the posterior isthmus muscles (Albertson and Thomson, 1976). When M4 is killed, the posterior isthmus remains closed. The corpus and terminal bulb muscles still contract in synchrony, but since the isthmus is closed, the corpus quickly becomes stuffed with bacteria, while the terminal bulb grinds nothing. In Figure 2 we show an intact worm (Figures 2a and 2b) and a worm lacking M4 (Figures 2c and 2d), both of which were fed ink. The lumen of the pharynx is closed in the intact worm, but the corpus and anterior isthmus are stuffed with large amounts of ink in the worm that has no M4.
Figure 2. Comparison of Intact Pharynx with Pharynx Lacking M4.
(a) Nomarski differential interference contrast micrograph of the pharynx of an intact wild-type worm. (b) Tracing of (a). Anterior is left, dorsal up. Wild-type eggs were placed on an NGM plate that had been spread with ink. After 4–5 hr, the animals were mounted in sodium azide, which anesthetizes them, and photographed. The line down the center of the pharynx is the lumen, which is closed in normal anesthetized worms. The ink eaten by intact worms like this one is passed back to the intestine (not shown). (c) Nomarski photomicrograph of the pharynx of a wild-type worm in which M4 had been killed. (d) Tracing of(c). The worm was placed on an ink plate immediately after M4 was killed, and photographed 4–5 hr later under anesthesia. The lumen of the front half of the pharynx, including the entire corpus and the front half of the isthmus, is stuffed with ink. Scale bar = 10 µm.
Because food does not get to the intestine, these M4-deficient worms starve. They stop growing, and never get bigger than a well-fed larva would be about 12 hr after hatching. Many of them wander out of the bacterial lawn and off the agar, never to be seen again. The first row of Table 1 shows that of ten wild-type animals in which M4 was killed shortly after hatching, six were lost within 2 days, while the other four arrested as larvae. The animals that do not get lost can continue to be active for up to 2 weeks.
Table 1.
Growth Arrest in Worms Lacking M4
| Developmental Arrest Stage | |||||||
|---|---|---|---|---|---|---|---|
| Row | Genotype | Cell(s) Killed | lost | early | stunted | normal | |
| 1 | + | M4 | 6 | 4 | 0 | 0 | |
| 2 | unc-29 | M4 | 2 | 49 | la | 0 | |
| 3 | unc-54 | M4 | 4 | 10 | 0 | 0 | |
| 4 | ced-3 | none | 2 | 0 | 1 | 18 | |
| 5 | unc-29;ced-3 | none | 1 | 2 | 0 | 10 | |
| 6 | ced-3 | M4(a) | 7 | 12 | 12 | 1 | |
| 7 | unc-29;ced-3 | M4(a) | 0 | 10 | 7 | 1 | |
| 8 | ced-3 | M4(p) | 1 | 2 | 0 | 7 | |
| 9 | unc-29;ced-3 | M4(p) | 0 | 2 | 2 | 10 | |
| 10 | ced-3 | M4(a) + M4(p) | 4 | 19 | 0 | 0 | |
| 11 | unc-29;ced-3 | M4(a) +M4(p) | 0 | 17 | 0 | 0 | |
This worm produced one egg after 23 days.
Animals of the indicated genotype were operated on within 4 hr of hatching. All operated worms were checked 1 day later to make sure the operation was successful. For ced-3 and unc-29;ced-3 worms, M4(a) is the anterior of the two cells at the M4 position, and M4(p) the posterior. lost means that the worm was lost within 3 days after hatching. early means that the worm never produced progeny. stunted means that the worm produced progeny, but grew slowly, was smaller than normal, or had a starved appearance. normal means that the worm was indistinguishable from an intact wild-type worm.
The defect in pharyngeal function described above is a specific result of killing M4. When any or all of the remaining 19 pharyngeal neurons are killed, the corpus is never stuffed, and the worm grows to adulthood (138/138 cases) and produces abundant progeny (unpublished data). Furthermore, developmental arrest or retardation occurs only rarely after operations that do not affect pharyngeal function: 115/117 worms were normal after such operations, and the remaining 2 were stunted (i.e., they became small, starved adults, as described in Experimental Procedures; also unpublished data).
To find out whether the worms that do not get lost are a representative sample of the worms operated on, we also performed M4 kills in unc-54 and unc-29 mutants. These mutants move poorly, due to, respectively, absence of the major body muscle myosin heavy chain (Epstein et al., 1974), and absence of a particular nicotinic acetylcholine receptor, the levamisole receptor (Lewis et al., 1980b). unc-29 mutations have no visible effect on the pharynx, and unc-29 worms become healthy adults in the same time as wild-type. unc-54 does not affect the pharynx, since the pharyngeal muscles and body muscles contain distinct myosin heavy chains (Mackenzie et al., 1978). When M4 was killed, all but one of the worms of both genotypes arrested before they could produce progeny (Table 1, rows 2–3). (Most if not all intact unc-29 or unc-54 worms grow up to become normal adults.) Therefore, we believe that the worms that got lost when M4 was killed in wild-type arrested early, just like those that were not lost. The only difference among the different genotypes was that, whereas the corpuses of wild-type and unc-54 worms were always stuffed when M4 was killed, the corpuses of unc-29 worms were often only partly full of bacteria, and sometimes contained no bacteria at all. (One possible explanation is that the levamisole receptor has some function in the pharynx.)
TO learn when M4 function is required, we killed M4 in wild-type worms of various ages, then looked to see how far they developed (Table 2). Of 25 worms in which M4 was killed before the first larval molt at 13 hr, none reached adulthood. Worms that were 13 hr old or older at the time of the operation were sometimes able to become stunted adults and produce progeny, and worms that were more than 21 hr old (the time of the second larval molt) were sometimes completely normal.
Table 2.
Requirement for M4 as a Function of Age
| Age (hr) |
Developmental Arrest Stage |
|||
|---|---|---|---|---|
| lost | early | stunted | normal | |
| 1 | 1 | 3 | 0 | 0 |
| 2 | 4 | 3 | 0 | 0 |
| 3 | 0 | 6 | 0 | 0 |
| 5 | 4 | 3 | 0 | 0 |
| 7 | 6 | 1 | 0 | 0 |
| 8 | 3 | 4 | 0 | 0 |
| 9 | 5 | 2 | 0 | 0 |
| 12 | 4 | 3 | 0 | 0 |
| 13 | 4 | 0 | 3 | 0 |
| 15 | 1 | 0 | 5 | 0 |
| 16 | 2 | 1 | 3 | 0 |
| 18 | 4 | 0 | 3 | 0 |
| 19 | 1 | 0 | 3 | 0 |
| 21 | 0 | 1 | 6 | 0 |
| 23 | 2 | 0 | 4 | 1 |
| 24 | 1 | 0 | 2 | 3 |
M4 was killed in wild-type worms at the indicated time after hatching. (The worms were maintained at 20°C.) The first larval molt occurred at 13 hr, and the second at 21 hr. The developmental stage the worms eventually reached was then determined. The categories lost, early, stunted, and normal are the same as in Table 1. In this experiment, unlike those of Table 1, not all operated worms were checked for successful operation. The underlined entries each include one worm that was checked.
In this experiment, unlike all the others, we did not verify the results of the operation in every worm. Because we wanted to set upper bounds on the extent of growth after killing M4, we verified the kill in the largest, healthiest worms one day after the operation. Of 17 operated worms checked, 2 were unsatisfactory. Our success rate in the more difficult operations done in ced-3 animals was at least 75% (probably much higher, see Experimental Procedures). We therefore believe that most of the worms in Table 2 were successfully operated. The 15 verified worms are underlined in Table 2. Their distribution parallels the distribution of unverified worms.
A Cell Death Survivor Can Partially Replace M4 in a ced3 Mutant
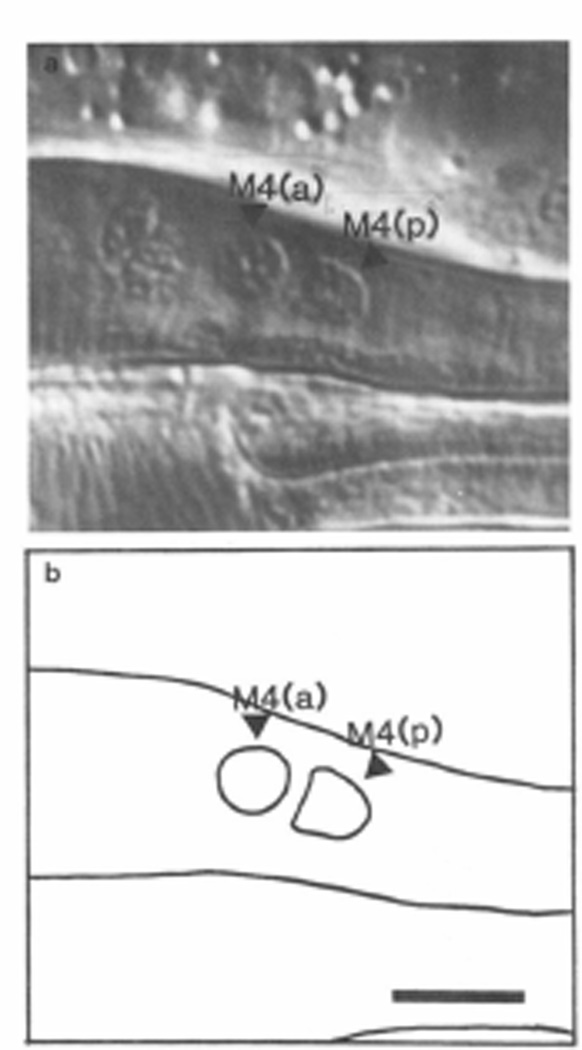
MSpaaaaa, the mother of M4, divides to produce two daughters. M4 is the anterior daughter, and MSpaaaaap the posterior. MSpaaaaap dies shortly after this division in wild-type worms. Mutations in the gene ced-3 prevent most programmed cell deaths (Ellis and Horvitz, 1986). In about half of ced-3 worms, there are two similar nuclei visible where M4 is normally, one, M4(a), anterior to the other, M4(p) (Figure 3). (On the basis of counts of nuclei in ced-3 pharynxes, R. Ellis has suggested that in the animals where this extra nucleus is not near M4, it is in the anterior dorsal terminal bulb (personal communication.) This is not unexpected, since at the beginning of pharyngeal morphogenesis, the precursors of the anterior terminal bulb are very close to those of the posterior corpus (Sulston et al., 1983).) It seemed likely that of M4(a) and M4(p), one was M4 and the other MSpaaaaap. When we killed both M4(a) and M4(p) in ced-3 or unc-29;ced-3 worms, all of the animals arrested early (Table 1, rows 10 and 11) just as when M4 was killed in wild-type. This result proves that no other cell in ced-3 worms has M4 function.
Figure 3. Two Nuclei at the M4 Position in ced-3.
(a) Nomarski photograph of the posterior metacarpus and anterior isthmus of a ced-3 worm, showing two nuclei where wild-type has only M4. (b) Tracing of (a). Anterior left, dorsal up. Scale bar = 5 µm.
To find out which of the two was M4, we killed either M4(a) or M4(p) in ced-3 or unc-29;ced-3 worms (Table 1, rows 6–9). When M4(p) was killed and M4(a) left intact, 17/23 worms were completely normal (pooling results from both genotypes, and ignoring lost worms). But when M4(a) was killed, only 2/43 worms were normal. All the subnormal ced-3 worms and most of the subnormal unc- 29;ced-3 worms had stuffed corpuses (like unc-29 M4- deficient worms), which indicates that they were lacking some M4 function. Therefore it seemed clear that M4(a) was M4 in most of the worms. (This conclusion assumes that M4 function is not impaired in ced-3 worms.)
The same experiment demonstrated that the extra cell sometimes had partial M4 function. Although only two of the worms in which M4(a) was killed became normal adults in three days, 19/43 did eventually become stunted adults and produce some progeny. M4(a) was killed within 4 hr of hatching in these animals; Table 2 and rows l–3 of Table 1 show that when M4 is killed this early in ced- 3(+) worms, they almost never become adults. In comparing these results with the results of killing both M4(a) and M4(p) (Table 1, rows 10 and ll), one sees that this partial function is due entirely to M4(p).
Two of the 43 worms in which M4(a) had been killed seemed completely normal. Does this mean that MSpaaaaap can infrequently fully replace M4? An alternative explanation is that the placement of M4 and MSpaaaaap is not constant: that in these two animals, M4(a) was MSpaaaaap and M4(p) was M4, and that therefore MSpaaaaap was killed, leaving M4. If M4(p) is sometimes M4, then one should sometimes see a partial or complete loss of M4 function when M4(p) is killed. In fact, there were 6/23 subnormal worms when M4(p) was killed (Table 1, rows 8 and 9). Although there is some background arrest even in intact ced-3 worms (Table 1, rows 4 and 5), one of the two subnormal M4(p)-deficient ced-3 worms had a stuffed corpus, and three of the four subnormal M4(p)-deficient unc-29;ced-3 worms had partially stuffed corpuses, strongly suggesting a deficit of M4 function in these four. (We very rarely see stuffed corpuses in intact wild-type or ced-3 worms [data not shown], and none of the mock-operated animals in rows 4 and 5 of Table 1 had stuffed corpuses.) Therefore, although M4 is usually anterior to MSpaaaaap, it seems likely that it can occasionally be posterior, and our data are consistent with the hypothesis that MSpaaaaap can never fully replace M4.
Discussion
M4 Is Necessary for Normal Pharyngeal Function in Young Worms
We have shown that when the pharyngeal neuron M4 is killed in a young worm, the posterior half of the isthmus fails to open. As a result, bacteria are not passed from the corpus to the terminal bulb, and the worms starve. The posterior half of the isthmus is precisely the area innervated by M4 (Albertson and Thomson, 1976). Therefore the effect of killing M4 makes sense in terms of its anatomy. The requirement for M4 function in young worms is quite strict: combining data from rows l–3 of Table 1 with data from Table 2, we find only l/95 Ll worms lacking M4 managed to grow to adulthood and produce progeny. (The exceptional worm produced a single egg after 23 days. Normal worms produce about 300 eggs in 6 days.)
When M4 was killed in slightly older worms, the worms survived, and the isthmus was capable of some function. We do not know why older worms survive the loss of M4. One possibility is that M4 continues to function long after its nucleus has been destroyed. If so, M4 function must continue much longer in older animals than in young, as a severe deficit in M4 function is obvious a few hours after the operation in young worms. For instance, the photograph of Figure 2c was taken 4– 5 hr after M4 was killed. Yet when M4 was killed in 24 hr old animals, they continued to eat for days. We watched these worms eat, and the isthmus worked normally. It may be that once the worm reaches the L2 stage, M4 can function without its nucleus. Alternatively, the isthmus muscles might acquire the ability to contract without M4 after about 24 hr of development.
In a ced-3 Mutant, a Cell Death Survivor Can Partially Replace the Neuron M4
There are two arguments that lead to the conclusion that in some ced-3 worms both M4(a) and M4(p) are capable of some level of M4 function. The first argument is statistical. When M4(a) was killed, 49% (21143) of the worms that did not get lost became adults. When M4(p) was killed, 83% (19/23) became adults. Since 83% + 49% = 132%, both M4(a) and M4(p) must be capable of functioning in at least 32% of the worms. These numbers are inconsistent with the hypothesis that only one of M4(a) and M4(p) can function in a given worm (see Statistical Methods, Experimental Procedures).
A more convincing argument is provided by the partial M4 function in many of the cases in which M4(a) was killed. This partial function is clearly distinct from the full function of intact wild-type and most ced-3 worms, and from the lack of function in wild-type worms when M4 has been killed. Since the partial function vanishes when both M4(a) and M4(p) are killed, M4(p) has M4function in these animals. It is also clear that M4(a) would have had M4 function in these worms had it not been killed, since intact ced-3 worms always have full M4 function (28/31 cases in Table 1 grew to become normal adults, and none of these 31 nor many casually observed ced-3 worms had stuffed corpuses).
Therefore, in some ced-3 worms, both M4(a) and M4(p) have some level of M4 function. Since there is only one cell at this position in wild-type worms, one of these two presumably dies during wild-type development. We there-fore conclude that some cell that dies in wild-type worms survives and can partially replace M4 in ced-3 worms. Our conclusion strengthens and extends that of Ellis and Horvitz (1986) that in ced-3 worms survivors of cells that die during wild-type development can differentiate. At that time, the only available criterion of differentiation for such cells was synthesis of serotonin or dopamine, and elaboration of neurotransmitter-containing processes with the correct morphology. We have shown that a cell that dies during wild-type development is capable of much more than synthesis of a neurotransmitter: it can, when its death is suppressed, express all the characteristics necessary for the function of a neuron.
Since ced-3 has not been observed to affect cells that do not undergo programmed cell death, we assume that one of the two cells M4(a) and M4(p) is actually M4. When M4(a) is killed, there is usually (41/43 worms) a deficit in M4 function, whereas when M4(p) is killed, M4 function is usually normal (17/23 worms). Therefore it seems reasonable to conclude that M4 is usually the anterior of the two. The identity of the other cell is much less firm. We suspect that this cell is MSpaaaaap, the sister of M4, because of the evidence that cell death survivors in ced-3 tend to end up adjacent to their sisters (Ellis and Horvitz, 1986). We attempted to verify this identification by direct observation of developing embryos, but were unable to follow MSpaaaaap beyond 430 min when, as described by Sulston et al. (1983) lineages become very difficult to observe because of the movement of the embryo. The variable positions of the many extra cells in ced-3 embryos make it impossible to learn to recognize reproducible patterns in the moving embryos, as Sulston et al. were able to do in the wild type. Nevertheless, there is one piece of direct evidence indicating that the extra cell is MSPaaaaap. J. Yuan (personal communication) has made genetic mosaic animals in which some cells are mutant for ced-3, while others carry a wild-type ced-3 gene. She finds two nuclei at the M4 position only when M4 and cells related to M4 (i.e., cells derived from the embryonic mesoblast MS) are mutant for ced-3.
The precise identity of M4(p) is not crucial to our main conclusion as long as M4(p) is a cell death survivor. It seems unlikely that M4(p) is a cell normally present in wild-type worms, since ced-3 does not seem to affect cells present in the wild type. Several workers have compared ced-3 and wild-type worms, and for every nucleus examined in wild-type worms, there is a nucleus with similar appearance in the same place in ced-3 (Ellis and Horvitz, 1986; Ft. Ellis and J. Y. Yuan, personal communication; our unpublished observations). Furthermore, all embryonic and postembryonic cell lineages that have been followed in ced-3 worms are identical to those in wild-type except for the absence of programmed cell death (Ellis and Horvitz, 1986; our unpublished data).
Cell Death Survivors Have Variable, Subnormal Fates
Although MSpaaaaap could partially replace M4, it did so in only about half of ced-3 worms, and it rarely or never (<6%) fully replaced M4. Other workers using fluorescent stains that detect neurotransmitters (Ellis and Horvitz, 1986, S. Mclntire, personal communication) have also observed that the fates of cell death survivors in ced-3 worms are variable. In contrast, cells that do not die during wild-type development have strikingly reproducible fates. Why are cell death survivors different?
One possible explanation is that ced-3 mutations do not completely suppress cell death, so that these cells are sick, and unable to reliably express their hidden fates. There are observations that are difficult to reconcile with this explanation. ced-3 fully and reliably restores HSN function in egl-1 mutant worms (as discussed above; Ellis and Horvitz, 1986). Since we do not know how egl-1 causes HSN death, one might discount this observation by proposing that HSN death in egl-1 mutants is different from the programmed cell deaths that occur in wild-type worms. However, ced-3 also fully and reliably suppresses HSN death in egl-41 mutants (C. Desai, personal communication). egl-41 has been shown to be involved in sex determination (Hodgkin et al., 1985), and the egl-47 mutation transforms hermaphrodites toward males. The HSNs die during wild-type male embryogenesis, so HSN death in egl-41 is probably due to activation of the normal male developmental pathway. These observations strongly suggest that ced-3 mutations can fully rescue a cell that would otherwise die.
A more plausible explanation is that there is competition of some kind. For instance, perhaps in a ced-3 mutant M4 and MSpaaaaap are born equivalent in potential, but compete with each other for target sites, and the one that ends up anterior to the other usually wins. Variability could result because the winner is inefficient at blocking the loser, or because the loser recovers variably after the winner is removed. This hypothesis could be tested by killing M4 shortly after its birth. Unfortunately, this experiment is very difficult for the same reasons that hindered our attempts to determine the embryonic lineage of M4(p).
A third explanation derives from thinking about other developmental mutants of C. elegans. The variability of the fates of cell death survivors is a special case of a common observation in C. elegans: developmental mutants are usually more variable in development than the wild type (Horvitz and Sulston, 1980). Sternberg and Horvitz (1981) have suggested that the constancy of wild-type C. elegans development is a result of evolutionary fine-tuning. This idea, that constancy in development is a result of selection, fits perfectly with the idea of variability in the hidden fates of cells that normally die. Because these cells die shortly after they are born, there is no selective pressure to prevent random drift of their hidden fates. Thus we expect these hidden fates to be variable. It also makes sense that the function of the rescued HSN neurons in ced-3;egl-1 and ced-3;egl-41 double mutants is not variable. The HSNs have an important function in wild worms, and therefore have been under selective pressure.
The evolutionary explanation is not inconsistent with the competition explanation mentioned above. For instance, the position of a cell might be important in determining its fate, and the variable fate of MSpaaaaap might be a consequence of its variable position, which in turn is a consequence of the lack of selection for its function. In fact, cell death survivors in ced-3 are more variable in position than cells that survive in wild-type.
Chalfie et al. (1981) have suggested that “cell duplications” may play a role in the evolution of cell lineages analogous to that of gene duplications in the evolution of genomes. Perhaps this analogy between the evolution of lineages and genomes can be extended even further. A cell that always dies during wild-type development might be considered a pseudocell, by analogy to pseudogenes (Vanin, 1985), which, although present in the genome, have no function and are subject to random evolutionary drift. One can imagine that pseudocells might play roles in the evolution of cell lineage similar to those proposed for pseudogenes in the evolution of genomes.
Aside from any role it might play in evolution, programmed cell death is useful to us, because it allows US to recognize that a cell has a hidden fate, one not subject to selection. Using ced-3, we can look at those fates. We find that they are variable. This variability suggests that in the absence of selection, cell fates can drift. Such drift might occur in any cell not subject to selection, whether or not the cell dies during wild-type development. In this way, new functions might evolve by drift of cell fates.
Experimental Procedures
General Methods and Strains
C. elegans was cultured as described by Brenner (1974). Worms were kept at 20%. The wild-type strain was N2 (Brenner, 1974), and the mutant strains used were CB190: unc-54 (e/90) I (Brenner, 1974), MT1522: ced-3(n7I7)IV(Ellisand Horvitz, 1986), PR1152: cha-1 (p1752)IV (Rand and Russell, 1984), CB1072: unc-29(e/072) I (Lewis et al., 1980a), and MT3299: unc-29(e/072) /; ced-3 (n717) IV.
Laser Killing of Cells
Nuclei were destroyed by the laser microbeam method of Sulston and White (1980), using an optical system like that of Sternberg and Horvitz (1981). We used a VSL-DYE (mirror) dye laser excited by a VSL-337 nitrogen laser, both made by Laser Science, Inc. (Cambridge, MA). The dye solution was 5 mM coumarin 120 (7-amino-Cmethylcoumarin) in absolute ethanol. This dye produces light with a wavelength of about 440 nm. Beam intensity is controlled by neutral density filters in the vertical illuminator.
We obtained newly hatched worms by picking eggs, placing them at 20°C; for less than 4 hr, and then picking any larvae that had hatched. unc-54 eggs were prepared by hypochlorite treatment of adults (Sulston and Brenner, 1974), since unc-54 worms do not lay eggs. Staged worms (Table 2) were obtained by picking eggs, waiting 1–1.5 hr. picking larvae that had hatched during this time to a new plate, and waiting until the larvae had reached the desired age.
The worms were anesthetized with sodium azide. We picked about a dozen worms into a 1 µl drop of S Basal at the corner of a pad of 5% agar, 10 mM sodium azide in M9 salts. (M9 salts and S Basal are described by Sulston and Brenner [1974].) Using a hair attached to the end of a Pasteur pipette, we drew the worms to the center of the pad with a small volume of liquid, then dropped a coverslip on them. Worms can only bend dorsal-ventral (Croll, 1970), so if the coverslip was put on before they had stopped moving, most of them were on their sides.
The intensity of the laser beam was adjusted with neutral density filters so that it was just possible to make a hole in the coverslip. Usually the total optical density of the filters was 0.7–0.8. an attenuation of 5 to d-fold. To destroy a nucleus, we focused the laser spot in the center of the nucleus and fired 2–3 set bursts at a repetition rate of about 10 shots per second. Each shot raises a pock mark, so that at 10 per second the place being shot at looks like a tiny boiling spot. A few bursts are enough to kill a neuron. Within a minute the nucleus becomes refractile. Unusable worms (e.g., worms whose orientation made the operation difficult) were blown up by firing at the underside of the coverslip over them. After all the worms on the slide had been either successfully operated on or blown up, the coverslip was slid off and the unexploded worms were picked onto a plate. They began to move in a few minutes and were fully recovered in an hour. For mock kills (Table 1, rows 4 and 5), we mounted worms for laser surgery, used the laser to destroy unusable ones, and then recovered the rest.
Unless otherwise noted, we examined each worm the day after the operation lo check for undesired structural damage and to verify that the desired cells and only the desired cells had been killed. We also noted whether the corpus (see Figure 1) was stuffed with bacteria. We placed the worm either in a drop of 10 mM sodium azide on a pad of 5% agar and 10 mM sodium azide in M9 salts, or in a drop of 30 mM sodium azide on a pad of 5% agar in distilled water, and then looked at the worm with Nomarski optics. Of 245 kills done to generate the data of Table 1. 183 (75%) were verified. The other 62 worms failed verification for the following reasons: 23 (9%) were lost or died before verification; in 23 (9%), nuclei were poorly visible; 8 (3%) were intact (probably these worms had simply been overlooked when doing the kills); 8 (3%) had damage beyond that intended. It is useful to compare these results with the verifications of the mock kills: 33/43 (77%) verified (i.e., the pharynx showed no signs of damage); 3 (7%) were lost or died before verification; in 1 (2%) nuclei were poorly visible; 6 (14%) were damaged. (The damage in these six was similar to the most common kinds of damage seen after real kills.) These data show that the 25% that failed to pass verification failed for the most part for reasons that had nothing to do with the laser, and suggest that our real success rate was probably much higher than the nominal 75%. The only exceptions are the worms in which nuclei were poorly visible; these animals were more frequent after real kills than mock kills. Most of them were M4, M4(a). or M4(a) + M4(p) kills, which disrupt pharyngeal function. One day after operation these worms were usually small larvae with stuffed corpuses, and it was difficult to see nuclei in the pharynx. We followed the development of these worms; the results were the same as for the verified kills of the same class.
Developmental Arrest Stages
We examined animals every day with a dissecting microscope and noted their approximate size, whether they were active, the color of the intestine (in healthy, well-fed worms the intestine is brown, while in starved worms it is clear), whether they had a vulva (an adult cuticular structure), and whether they produced progeny. If a worm could not be found on two successive days, it was marked as lost. Normally worms stay in the bacterial lawn in the center of a petri plate. Worms that cannot sense bacteria (nonchemotactic mutants, for instance) tend to leave the lawn, crawl up on the plastic at the edge of the plate, and dry up. Worms lacking M4 also tend to do this, perhaps because of starvation, or because sensory nerves in the pharynx are confused by the continual presence of bacteria in the corpus and their continual absence in the terminal bulb.
We assigned each worm to one of four developmental arrest categories: lost, early, srunted, or normal. lost includes those worms that were lost within three days after hatching, so that we could not determine their stage of arrest. early includes those worms that never produced progeny. The vast majority of these never produced a vulva, indicating that they never became adults. stunted includes those worms that produced a vulva and progeny, but were detectably more starved than normal, based on small size, decreased intestinal pigment, and retarded development. The normal category includes worms that became large, active adults with brown intestines and produced abundant progeny in three days.
Feeding Ink to Worms
We fed ink to worms to make a photograph that would clearly contrast the stuffing of the corpus in worms lacking M4 with the closed lumen in intact worms. Black Koh-i-noor rapidraw ink (Koh-i-noor, Blooms-bury, NJ) was diluted 100-fold into distilled water, and the suspension was spread on NGM plates one day before the experiment, Several N2 eggs were put on one such plate. On another plate, we put about 20 N2 worms whose M4 neurons had just been killed. Photographs were taken 4–5 hr later. Intact worms often had one or two particles of ink in the pharynx, especially in the terminal bulb. The worms lacking M4 always had considerably more ink, although only a third were as stuffed as the one shown in Figure 2. Stuffing with ink is less reliable than with bacteria, presumably because the larger ink particles block the lumen of the pharynx.
Statistical Methods
We used a G test of independence (Sokal and Rohlf, 1981) to test the hypothesis that in every ced-3 worm, exactly one of M4(a) and M4(p) has some M4 function. The rationale for this test is as follows. When M4(p) was killed, 19123 worms produced progeny (Table 1, rows 8 and 9, (stunted + normal)/(early + stunted + normal)). Therefore M4(a) had some M4 function in 19/23 worms. Similarly, by killing M4(a) (Table 1, rows 6 and 7) we measured the frequency with which M4(p) has some M4 function as 21/43. By the above hypothesis, only those 22/43 worms in which M4(p) did not have any M4 function had any M4 function in M4(a). Therefore 19/23 and 22/43 must be independent estimates of the same ratio. The G test gives G = 6.65 with 1 degree of freedom, corresponding to a probability of about 0.010. Therefore we reject the hypothesis that only one of M4(a) and M4(p) has some M4 function.
This test is conservatively biased because we have neglected the effect of background death in ced-3 mutant worms (i.e., even when no cells are killed, some ced-3 worms do not become adults, as shown by rows 4 and 5 of Table 1). A more sophisticated test assumes that with probability p M4(a) has some M4 function and M4(p) has none, with probability (1-p) M4(p) has some M4 function and M4(a) has none, and that a ced-3 worm that has some M4 function will reach adulthood with probability q. p and q were estimated by maximum likelihood using the data from rows 4–9 of Table 1, and a G test performed to determine whether the predicted frequencies matched the actual (Sokal and Rohlf, 1981). This test gave G = 8.64 with 1 degree of freedom, corresponding to a probability of 0.003.
The same test can be used to test the hypothesis that exactly one of M4(a) and M4(p) has full M4 function, by using the ratio (normal)/(early + stunted + normal). (The alternative hypothesis is that a cell death survivor sometimes has full M4 function.) For this model the fit is good: G = 1.14, probability about 0.29. Therefore we have no evidence that a cell death survivor ever has full M4 function. As a bonus, we get estimates of p and q. M4(a) has full M4 function in 93 ± 4% (SEM) of ced-3 worms, and ced-3 worms with full M4 function grow to normal adults 87 ± 5% (SEM) of the time.
Acknowledgments
We thank Junking Yuan for suggesting we check whether M4- worms could be rescued by a ced-3 mutation, and we thank Chand Desai, Ron Ellis, Steve Mclntire, and Junying Yuan for sharing unpublished results. L. A. was supported by a postdoctoral fellowship from the Damon Runyon-Walter Winchell Cancer Fund. This work was supported by research grants GM24663 and GM24943 from the U.S. Public Health Service.
References
- 1.Albertson DG, Thomson JN. The pharynx of Caenorhabditis elegans. Phil. Trans. Roy. Sot. London Ser. B. 1976;275:299–325. doi: 10.1098/rstb.1976.0085. [DOI] [PubMed] [Google Scholar]
- 2.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalfie M, Horvitz HR, Sulston JE. Mutations that lead to reiterations in the cell lineages of C. elegans. Cell. 1981;24:59–69. doi: 10.1016/0092-8674(81)90501-8. [DOI] [PubMed] [Google Scholar]
- 4.Croll NA. The Behavior of Nematodes. London: Edward Arnold; 1970. [Google Scholar]
- 5.Ellis HM, Horvitz HR. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986;44:817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- 6.Epstein HF, Waterston RH, Brenner S. A mutant affecting the heavy chain of myosin in Caenorhabdiris elegans. J. Mol. Biol. 1974;90:291–300. doi: 10.1016/0022-2836(74)90374-x. [DOI] [PubMed] [Google Scholar]
- 7.Hamburger V, Oppenheim RW. Naturally occurring neuronal death in vertebrates. Neurosci. Commentaries. 1962;7:39–55. [Google Scholar]
- 8.Hodgkin J, Doniach T, Shen M. The sex determination pathway in the nematode Caenorhabditis elegans: variations on a theme. Cold Spring Harbor Symp. Quant. Biol. 1985;50:585–593. doi: 10.1101/sqb.1985.050.01.071. [DOI] [PubMed] [Google Scholar]
- 9.Horvitz HR, Sulston JE. Isolation and characterization of cell-lineage mutants of the nematode C. elegans. Genetics. 1980;96:435–454. doi: 10.1093/genetics/96.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaiser D, Manoil C, Dworkin M. Myxobacteria: cell interactions, genetics, and development. Ann. Rev. Microbial. 1979;33:595–639. doi: 10.1146/annurev.mi.33.100179.003115. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JA, Wu CH, Berg H, Levine JH. The genetics of levamisole resistance in the nematode C. elegans. Genetics. 1980a;95:905–928. doi: 10.1093/genetics/95.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis JA, Wu CH, Levine JH, Berg H. Levamisole-resistant mutants of the nematode C. elegans lack pharmacological acetylcholine receptors. Neuroscience. 1980b;5:967–989. doi: 10.1016/0306-4522(80)90180-3. [DOI] [PubMed] [Google Scholar]
- 13.Mackenzie JM, Schachat F, Epstein HF. Immunocyto- chemical localization of two myosins within the same muscle cells in Caenorhabditis elegans. Cell. 1978;14:413–419. doi: 10.1016/0092-8674(78)90010-7. [DOI] [PubMed] [Google Scholar]
- 14.Rand JB, Russell RL. Choline acetyltransferase-deficient mutants of the nematode C. elegans. Genetics. 1984;106:227–248. doi: 10.1093/genetics/106.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sokal RR, Rohlf FJ. Biometry. Second Edition. San Francisco: W. H. Freeman; 1981. [Google Scholar]
- 16.Sternberg PW, Horvitz HR. Gonadal cell lineages of the nematode Panagrellus mdivivus and implications for evolution by the modification of cell lineage. Dev. Biol. 1981;88:147–166. doi: 10.1016/0012-1606(81)90226-8. [DOI] [PubMed] [Google Scholar]
- 17.Sulston JE, Brenner S. The DNA of C. elegans. Genetics. 1974;77:95–104. doi: 10.1093/genetics/77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode Caenorhabditis elegans. Dev. Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- 19.Sulston JE, White JG. Regulation and cell autonomy during post-embryonic development of Caenorhabditis elegans. Dev. Biol. 1980;78:577–597. doi: 10.1016/0012-1606(80)90353-x. [DOI] [PubMed] [Google Scholar]
- 20.Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 21.Taylor HM, Truman JW. Metamorphosis of the abdominal ganglia of the tobacco hornworm, Manduca sexta: changes in populations of identified motoneurons. J. Comp. Physiol. 1974;90:367–388. [Google Scholar]
- 22.Trent C, Tsung N, Horvitz HR. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics. 1983;104:619–647. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanin EF. Processed pseudogenes: characteristics and evolution. Ann. Rev. Genet. 1985;19:253–272. doi: 10.1146/annurev.ge.19.120185.001345. [DOI] [PubMed] [Google Scholar]