Abstract
BACKGROUND
Aldosterone (ALDO), a critical regulator of sodium homeostasis, mediates its effects via activation of the mineralocorticoid receptor (MR) through mechanisms that are not entirely clear. Striatin, a membrane associated protein, interacts with estrogen receptors in endothelial cells.
METHODS
We studied the effects of MR activation in vitro and in vivo on striatin levels in vascular tissue.
RESULTS
We observed that dietary sodium restriction was associated with increased striatin levels in mouse heart and aorta and that striatin and MR are present in the human endothelial cell line, (EA.hy926), and in mouse aortic endothelial cells (MAEC). Further, we show that MR co-precipitates with striatin in vascular tissue. Incubation of EA.hy926 cells with ALDO (10−8 mol/l for 5–24 h) increases striatin protein and mRNA expression, an effect that was inhibited by canrenoic acid, an MR antagonist. Consistent with these observations, incubation of MAEC with ALDO increased striatin levels that were likewise blocked by canrenoic acid. To test the in vivo relevance of these findings, we studied two previously described mouse models of increased ALDO levels. Intraperitoneal ALDO administration augmented the abundance of striatin protein in mouse heart. We also observed that in a murine model of chronic ALDO-mediated cardiovascular damage following treatment with NG-nitro-L-arginine methyl ester plus angiotensin II an increased abundance of striatin protein in heart and kidney tissue.
CONCLUSION
Our results provide evidence that increased striatin levels is a component of MR activation in the vasculature and suggest that regulation of striatin by ALDO may modulate estrogen’s nongenomic effects.
Keywords: aldosterone, angiotensin, animal physiology, antagonists, blood pressure, endothelial cells, heart tissue, hypertension, inflammation, L-NAME, mineralocorticoid receptor, RAAS
Aldosterone (ALDO) is regulated by sodium intake and has direct adverse effects on the vasculature.1 Administration of mineralocorticoids leads to heart lesions.2 Impaired left ventricular diastolic function and prolonged PQ intervals are observed in patients with primary hyperaldosteronism.3,4 In addition, circulating ALDO levels correlate with cardiac damage5 and clinical studies with mineralocorticoid receptor (MR) antagonists show decreased morbidity and mortality in patients with heart failure.6 Consistent with these observations, animal studies demonstrated that mineralocorticoid excess may lead to myocardial inflammation and fibrosis.7,8 In vitro studies demonstrate direct effects of ALDO on plasminogen activator inhibitor-1 expression in cardiac cells9 and cardiac fibrosis and heart failure have been observed in a transgenic mouse model overexpressing 11β-hydroxysteroid dehydrogenase type 2 in cardiomyocytes.10 Moreover, MR interacts specifically with the cardiac myosin binding protein, suggesting a role of MR in cardiac remodeling.11
Increased ALDO levels lead to numerous signaling events.12 ALDO’s classical epithelial effect is to increase the transport of sodium via the effects of MR as a ligand-dependent transcription factor. These effects of ALDO are mediated in part by vesicular trafficking to the plasma membrane of proteins such as vacuolar H+-ATPase, Na+/H+ exchange isoform 3 and the epithelial sodium channel.13,14 There is evidence that ALDO mediates epithelial sodium channel trafficking to lipid rafts in the plasma membrane of A6 cells. However the mechanisms by which ALDO alters vascular tissue are not clear.
Striatin is abundant in neurons where it is proposed to function as a scaffolding protein that interacts with mediators of vesicular trafficking.15–17 Striatin contains several protein association domains: a caveolin (cav)-binding motif, a coiled-coil structure, a Ca2+-calmodulin-binding site and a large WD-repeat domain.15,18 There is evidence that striatin’s WD-repeat domain interacts with GαI protein and Protein Phosphatase 2A (PP2A) allowing for rapid activation of several transduction molecules (e.g., endothelial nitric oxide synthase) and mitogen-activated protein kinase 19
More recently, striatin has been shown to be a key intermediary of the effects of estrogen receptor-α (ERα) activation.20 Lu et al. provided evidence that striatin’s N-terminal segment interacts with the DNA binding domain of ERα in the immortalized human endothelial cell line (EA.hy926) cells. This interaction organizes ERα-endothelial nitric oxide synthase membrane signaling leading to rapid nongenomic activation of endothelial nitric oxide synthase in EA.hy926 cells. Thus, the association between ERα and striatin raise the possibility that striatin may also interact with the MR.
We determined the effects of salt restriction on striatin levels in vascular tissue. We studied the in vivo effects of dietary sodium restriction in mice and observed marked increases in the levels of striatin in the heart. Our results also show that vascular tissue express striatin and that striatin co-precipitates with MR. We then studied the direct effects of ALDO on striatin levels in human and mouse endothelial cells and tested the in vivo relevance of our findings in two mouse models of MR activation. Our results suggest that MR interacts with striatin and are consistent with a direct effect of ALDO on striatin levels in vivo.
METHODS
Mouse aortic endothelial cells
Aortic endothelial cells were isolated as previously described.21 Briefly, thoracic aortas were perfused with PBS containing 1,000 U/ml heparin and dissected out and placed in 20% Fetal bovine serum-Dulbecco’s Modified Eagle Medium with 1,000 U/ml heparin. Aortas were filled with 2 mg/ml collagenase II (Sigma-Aldrich, St Louis, MO). Endothelial cells were cultured in type I collagen-coated T25 flasks in 20% Fetal bovine serum-Dulbecco’s Modified Eagle Medium containing: 100 U/ml penicillin, 100 μg streptomycin, 2 mmol/l L-glutamine, 1% MEM amino acids, 1% sodium pyruvate, 100 μg/ml heparin (Invitrogen, Carlsbad, CA), 100 μg/ml endothelial cell growth supplements (Sigma-Aldrich) and incubated in 5% CO2 at 37 °C in a humidified atmosphere. The cells were used at passages 2–3. The purity of the cultures was confirmed by testing specific monoclonal antibodies against vWF and PECAM-1.
Electrophoresis, immunoblot, and immunoprecipitation
Analyses were performed as previously described.22 Tissues were homogenized in radioimmunoprecipitation assay buffer (Santa Cruz Biotechnology, Santa Cruz, CA). Proteins were analyzed using enhanced chemiluminescence (Perkin-Elmer Life Sciences, Boston, MA). Quantitative data are presented as fold change relative to controls. Primary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA): rabbit anti-MR (cat. no. sc11412, 1:1000), BD Transduction Laboratories (San Diego, CA): mouse monoclonal anti-striatin (cat. no. 610838, 1:1000), Sigma-Aldrich: mouse monoclonal anti-β-actin (cat. no. A5316, 1:1000) and AbCam (Cambridge, MA): rabbit anti-β-tubulin (cat. no. ab6046, 1:1000). For immunoprecipitation experiments, protein extracts (500 μg) were mixed with 1 μg of corresponding primary antibody and 50 μl μMACS protein A or G MicroBeads (Miltenyi Biotec, Auburn, CA) and then incubated at 4 °C for 1–2 h. The mixture was then loaded on top of Miltenyi MACS separation columns and eluted according to manufacturer’s protocol. The immunoprecipitates were then analyzed as described.22
Real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction was performed as previously described.23 Briefly, total mRNA was extracted using the RNeasy mini kit (Qiagen, CA, USA). The ABI PRISM 7000 Sequence Detection System real-time quantitative PCR (Applied Biosystems, Foster City, CA) was used to perform the real-time PCR using TaqMan Gene Expression Assays for both mouse or human striatin (Applied Biosystems). Reactions were analyzed with the ABI software using the ΔΔCT method. Target gene expression was normalized to 18S rRNA levels.
siRNA knockdown of striatin
EA.hy926 cells were transfected with ON-TARGETplus siRNA predesigned duplex specific for striatin obtained from Dharmacon RNAi Technologies, Thermo Scientific (Chicago, IL). Control/blank siRNA was transfected in parallel with striatin siRNA following the manufacturer’s protocols and using the Dharmafect 1 siRNA Transfection Reagent from Dharmacon RNAi Technologies. The cells were harvested for western blot analyses 48 h post-transfection.
EA.hy926
EA.hy926 cells were grown as originally described in 10% Fetal bovine serum-Dulbecco’s Modified Eagle Medium and split 1:16 at confluence.24
Animals
Our studies followed guidelines approved by the Institutional Animal Care and Use Committee at Harvard Medical School that conforms with the Guide for the Care and Use of Laboratory Animals (US National Institutes of Health Publication No. 85–23). We studied male mice (C57Bl/6J) from Jackson Laboratory (Bar Harbor, ME). Animals were housed in a room lighted 12 h/day at an ambient temperature of 22 ± 1 °C and were allowed 1–3 weeks to recover after arrival with free access to Purina Lab Chow 5001 (Ralston Purina, St Louis, MO) and tap water unless stated otherwise.
Dietary sodium restriction model
Twelve-week-old male mice were randomized to either high-salt (HS) (4% NaCl) or low-salt (LS) (0.08% NaCl) diets (Purina, St Louis, MO) for 11 days to achieve sodium balance as previously described.23 Animals were euthanized under isoflurane anesthesia, the thoracic and abdominal cavities were opened, and the aortas and hearts were rapidly excised. Tissues were placed in liquid nitrogen after collection.22,23
In vivo ALDO administration model
Male C57BL/6 mice (Charles River, Wilmington, MA) were uninephrectomized at 11 week of age and allowed 7 day recovery time. Starting at 12 week of age, all animals were fed 3% NaCl diet (Purina Test Diet Salt Series based on Basal Diet 5755, Pharmaserv, Framingham, MA) for 1 week. Mice were administered via intraperitoneal injection of 125 μl mouse serum as control or 250 ng (~10 μg/kg) ALDO (Acros Organics, Geel, Belgium) diluted in 250 nl ethanol as described previously.25 Five animals in each control and experimental groups were euthanized as described above, at the following intervals after the injection: 0 h (no injection), 0.5, 1, 2, and 3 h.
AngII/L-NAME mouse model
Animals received placebo or a combination treatment of NG-nitro-L-arginine methyl ester (L-NAME) (0.2 mg m/l drinking fluid) and angiotensin II (AngII) (2.8 mg/kg/day), unless otherwise stated. L-NAME (Sigma-Aldrich) was administered in drinking water from days 1 to 11. Vehicle or Ang II (American Peptide, Sunnyvale, CA) was administered on days 8 through 11 via Alzet osmotic subcutaneous minipumps (Model 1007D, DURECT, Cupertino, CA). All mice consumed a high-sodium diet, 4% NaCl-supplemented rodent chow. Animals were euthanized on day 11 as described above.
RESULTS
EA.hy926 cells and early cultures of mouse aortic endothelial cells express striatin and the MR
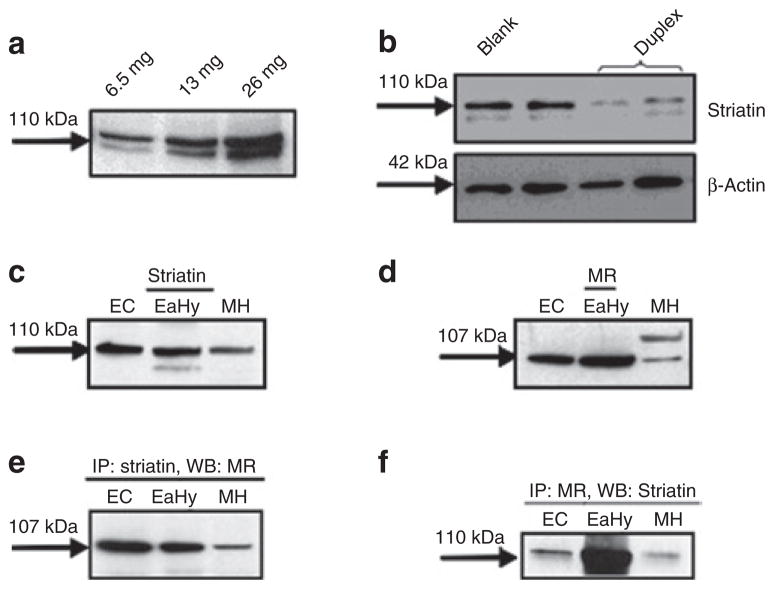
We performed western blot analyses and observed the presence of striatin in EA.hy926 cells (Figure 1a).24 We examined the specificity of our striatin antibody by reducing striatin levels in EA.hy926 cells with striatin-specific siRNA. As shown in Figure 1b, the striatin band intensity at 110 kDa was reduced following striatin siRNA in these cells. These results provide evidence for the specificity of this antibody to recognize striatin. To determine whether the presence of striatin was unique to EA.hy926 cells, we prepared early cultures of mouse aortic endothelial cells (MAEC) following established protocols.21 We confirmed that EA.hy926 cells and MAEC were endothelial in origin by positive immunohistochemical staining for vWF and PECAM-1 (CD31), two endothelial cell markers, but lacked staining for α-smooth muscle actin, a smooth muscle cell marker (data not shown). MAEC and mouse heart likewise expressed striatin (Figure 1c) and MR (Figure 1d).
Figure 1.
Striatin and the mineralocorticoid receptor are expressed and co-immunoprecipitate in early cultures of mouse aortic endothelial cells, EA.hy926 cells, and mouse heart tissue. (a) EA.hy926 cells were lysed and protein levels quantified as described in the Methods. Cell lysates (6.5–26.0 μg) were analyzed by western blotting for striatin levels. (b) Prior to cell lysis and western blotting for striatin, EA.hy926 cells were either untransfected (blank, lane 1) or transfected with scrambled siRNA (lane 2) or anti-striatin duplex (lanes 3–4), as described under Methods. Early cultures of mouse aortic endothelial cells (MAEC), EA.hy926 cells and mouse heart tissue were processed for western blotting for striatin (c) or (d) mineralocorticoid receptor (MR). (e) Immunoprecipitation with striatin flowed by western blot for MR. (f) Immunoprecipitation with MR followed by western blot for striatin as described under Methods. EA.hy926, human endothelial cell line.
In EA.hy926 cells, striatin co-precipitates with the ERα subunit.20 We studied whether a similar relationship would occur with MR. Our results show that immunoprecipitation of EA.hy926 cells, mouse heart, and MAEC with anti-striatin antibody allows detection of MR (Figure 1e,f).
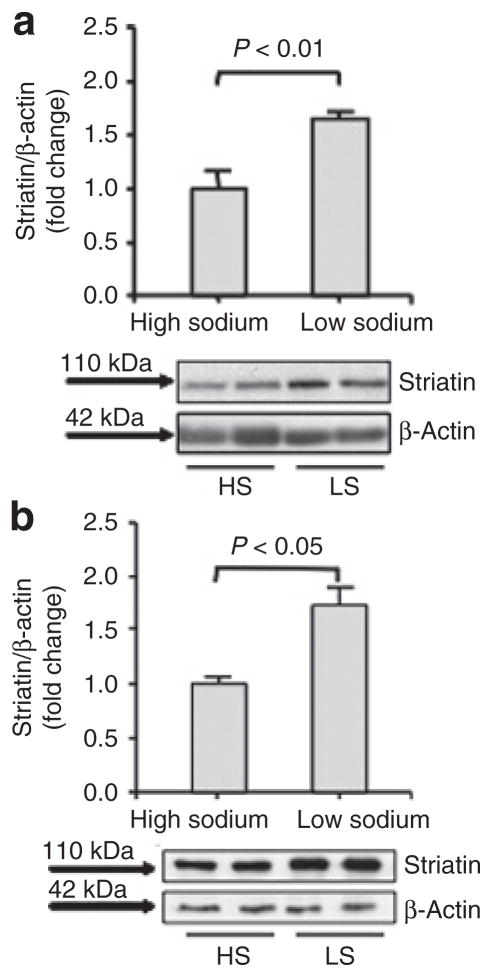
Dietary sodium restriction increases striatin levels in mouse hearts and aorta
We studied a sodium restriction mouse model that we described leads to increased ALDO levels.26 As shown previously, mice given a LS diet for 11 days had significantly higher plasma ALDO levels (64.18+/−14.30 ng/dl, (n = 6)) than those on a HS diet (34.26+/−4.99 ng/dl (n = 7)) for 11 days. We isolated heart tissue from these mice and observed that striatin levels were significantly higher in mice on LS when compared to mice on a HS diet as were their serum ALDO levels (Figure 2a). Consistent with these results, we also observed that striatin levels were significantly higher in aortas from LS mice when compared to HS mice (Figure 2b). We also measured blood pressure in these mice by tail cuff.23 As shown previously under these conditions, LS was associated with lower blood pressure than HS (108.8 ± 2.8 mm Hg vs 114.7 ± 3.1 mm Hg, LS vs. HS respectively, mean ± standard deviation, P < 0.03).23
Figure 2.
Dietary sodium restriction increases striatin levels in mouse hearts. (a) Mouse heart tissues were obtained from mice maintained on a high-sodium (HS) (n = 7) or low-sodium (LS) diet (n = 6) for 11 days. (b) Mouse aorta were obtained from mice maintained on a high- (n = 3) or low-sodium diet (n = 4) for 11 days. Tissue homogenates were analyzed for striatin and β-actin protein levels as described in Methods.
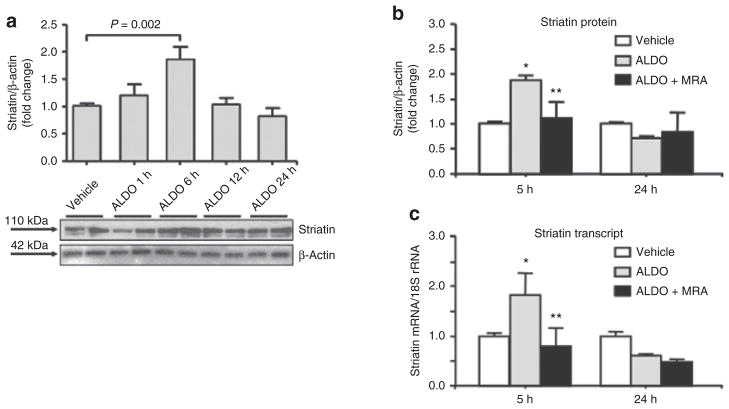
ALDO increases the expression of striatin in endothelial cells via activation of the MR
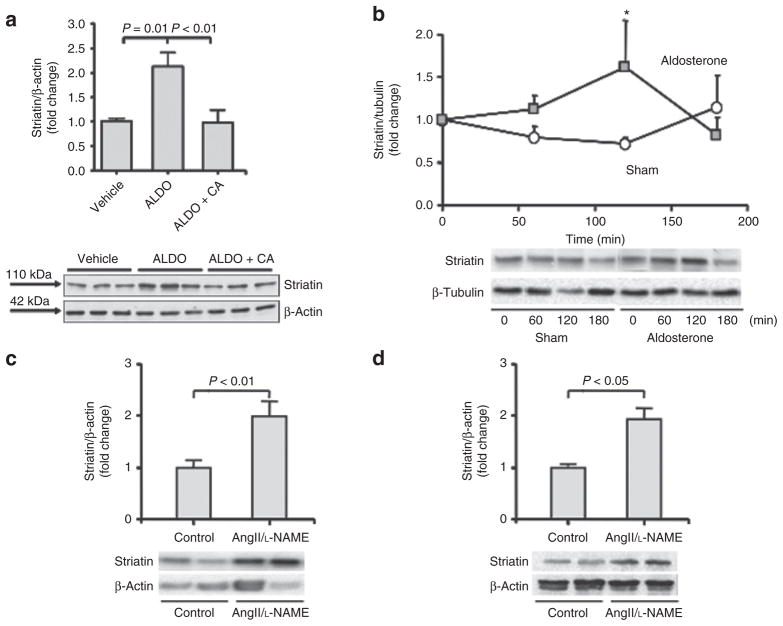
We characterize the direct in vitro effects of ALDO on striatin levels in EA.hy926 cells incubated with 10 nmol/l ALDO for 1–24 h at 37 °C. Our results show that incubation of cells with ALDO increased striatin levels by 5 h that returned to baseline by 12 h (Figure 3a). We then studied the effect of cells incubated with 10 nmol/l ALDO in the presence or absence of canrenoic acid, a competitive ALDO antagonist to MR, on striatin levels. We confirmed that ALDO increased striatin protein levels and that preincubation with 1 μmol/l canrenoic acid prevented this increase (Figure 3b). The effect of ALDO on striatin mRNA expression levels using quantitative real-time polymerase chain reaction with specific Taqman Assays for striatin was analyzed. We observed that 10 nmol/l ALDO for 5 h at 37 °C led to increased expression of striatin mRNA in these cells (Figure 3c). These results suggest a genomic effect of ALDO that is specific for striatin expression. Consistent with this proposal, we have identified several potential MR response elements in the 5′ region of the striatin gene (data not shown). Furthermore, treatment for 5 h with 100 nmol/l dexamethasone had no significant effect on striatin mRNA levels in EA.hy926 cells, suggesting specificity of ALDO-mediated MR activation (data not shown). We also studied the effect of ALDO on striatin levels in MAEC, and observed that ALDO stimulated an increase in striatin protein levels that was likewise sensitive to canrenoic acid (Figure 4a).
Figure 3.
The aldosterone-induced increases in striatin expression are mediated via activation of the mineralocorticoid receptor (MR) in EA.hy926 cells. (a) EA.hy926 cells were incubated with 10 nmol/l ALDO for 1, 5, 12, 24 h. Cell lysates were analyzed for striatin protein levels as described in Methods. Data were normalized to β-actin. (b) EA.hy926 cells were incubated with vehicle (white bars) or 10 nmol/l ALDO (gray bars) for 5 h and 24 h in the absence or presence (black bars) of the MR antagonist, canrenoic acid (MRA) and analyzed for striatin protein normalized to β-actin (b) and mRNA normalized to (c) 18S rRNA. *P < 0.05 vs. vehicle-treated cells. **P < 0.05 vs. ALDO-treated cells. ALDO, aldosterone; EA.hy926, human endothelial cell line.
Figure 4.
The in vivo and in vitro effects of increased aldosterone on striatin levels in mice. (a) Aldosterone (ALDO) increases striatin expression via activation of the mineralocorticoid receptor (MR) in early cultures of mouse aortic endothelial cells. Mouse aortic endothelial cells (MAEC) were incubated with 10 nmol/l ALDO for 5 h, in the presence or absence of the MR antagonist canrenoic acid. Cell lysates were analyzed for striatin and β-actin protein levels as described in Methods. (b) Acute in vivo administration of aldosterone increases striatin levels in mouse heart. Heart tissues were obtained from mice at 0, 1, 2, or 3 h after acute intraperitoneal administration of ALDO (10 μg/kg, gray squares) or vehicle (Sham, white circles). Tissue homogenates were analyzed for striatin and β-tubulin protein levels as described in Methods. *P < 0.05 vs. Sham. (c,d) Treatment of mice with Ang II/L-NAME increases striatin levels in mouse (c) heart and (d) kidney. Heart and kidney tissues were obtained from mice treated with placebo (control) or L-NAME/AngII for 11 days. Tissue homogenates were analyzed for striatin and β-actin protein levels as described in Methods. EA.hy926, human endothelial cell line. AngII, angiotensin II; L-NAME, NG-nitro-L-arginine methyl ester.
Acute in vivo administration of ALDO increases striatin levels in mouse hearts
To characterize the in vivo relevance of these findings, we studied the effects of ALDO on striatin levels in mouse heart by using additional mouse models of MR activation. We analyzed the effects of an acute in vivo ALDO administration on striatin levels in the heart. We included at each time point a response to vehicle injection, thus allowing us to distinguish the effects of experimental method from those of ALDO administration. Male C57BL/6 mice on a 3% NaCl diet were injected IP with either 10 μg/kg of ALDO or vehicle and sacrificed at the following time points after the injection: 0 h (no injection), 1, 2, and 3 h (five mice in each group at each time point). We observed that in isolated heart tissue from ALDO-treated mice, striatin levels increased when compared to vehicle injected mice (Figure 4b). Plasma ALDO concentrations were determined in each animal as previously reported.25 Plasma ALDO levels increased only in the ALDO injected group where the highest levels reached 280 ng/dl at 30 min.
Treatment of AngII/L-NAME increases striatin levels in mouse hearts and kidneys
We previously studied an animal model of acute, generalized, multiple organ injury that is secondary to vascular inflammation.27,28 In this model, we treat mice with the nitric oxide synthase inhibitor, L-NAME, in combination with AngII. This treatment induces myocardial damage initiated at the vascular level that can be prevented by MR blockade or by adrenalectomy.27,29 Thus we used this AngII/L-NAME model as a “stressor” to assess the interaction of MR activation on striatin. Our results show that AngII/L-NAME treatment is associated with increased striatin levels in heart (Figure 4c) and kidney tissues (Figure 4d) when compared to vehicle treated control. In these studies, AngII/L-NAME treatment increased circulating ALDO levels (122.1+/−34.1 ng/dl (n = 6)) when compared to vehicle treatment (34.26+/−4.99 ng/dl (n = 7); P = 0.008). In addition, we measured blood pressure in these mice. As shown previously, AngII/L-NAME was associated with increased blood pressure vs. vehicle treated mice (120 ± 3.8 mm Hg vs 161 ± 23 mm Hg, vehicle vs. AngII/L-NAME respectively, mean ± standard deviation, P < 0.04).22
DISCUSSION
The studies presented herein characterized the effects of MR activation on striatin levels, a protein that has been shown to be associated with steroid receptors. We provide evidence that MR activation leads to increased striatin levels in two cell lines and heart tissue from three in vivo mouse models of MR activation. Furthermore, our studies in both human and mouse endothelial cells demonstrate that ALDO increases striatin protein and mRNA levels via MR activation in vitro and suggest the existence of a striatin/MR complex.
Striatin, abundant in the brain, is detected in fibroblasts and cardiac tissue.15,30,31 We now report its endogenous expression in both mouse and human endothelial cells. Striatin and its family members are characterized by caveolin binding sites30,32,33 and are reported to function as molecular anchors and scaffolds that interact with cav-133 in caveolae mediating the downstream, nongenomic transduction effects of estrogen. 20 Striatin has also been shown to interact with PP2A to regulate vesicular trafficking.17,31 Few studies have reported on the physiological functions of striatin. In one study, down-regulation of striatin in vivo using antisense oligonucleotides against striatin resulted in decreased locomotor activity and reduced dendritic growth in vitro.34
The cav-1 rich caveolae are docking sites for several steroid receptors, such as ERα and the androgen receptor.35,36 Recent evidence shows MR present in vascular tissue caveolae and that cav-1 is critical for the cellular effects of ALDO.22,37,38 Furthermore, ALDO mediates epithelial sodium channel and cav trafficking to lipid rafts in A6 cells.39 Our results using co-immunoprecipitation studies expand on these observations by documenting that striatin is associated with another steroid receptor, MR. The large number of receptors and proteins, such as striatin, that anchor at the caveolae strongly suggest that alterations in components of the caveolae may have major effects on signaling pathways. Thus, it is tempting to speculate that MR forms a complex with striatin and cav-1 in vascular tissue and that modulation of striatin levels may alter vascular function via modulation nitric oxide signaling and PP2A signaling. Indeed, ALDO has been reported to regulate endothelial nitric oxide synthase by PP2A activation in endothelial cells.40
We examined three animal models of MR activation to study the chronic and acute effects of ALDO on striatin levels in the heart. In one model, we take advantage of the fact that the renin-angiotensin-ALDO system is modified during changes in dietary sodium intake where chronic LS diet is associated with increased ALDO.26 We have also used an animal model of generalized multiple organ injury that is secondary to vascular inflammation where we treat mice with the nitric oxide synthase inhibitor L-NAME and AngII to induce myocardial damage.1,27,29 We reasoned that if striatin is ALDO sensitive, then changing dietary sodium intake or administrating L-NAME followed by AngII should likewise modulate striatin levels. We observed that in both chronic models of MR activation, the levels of striatin were increased in heart tissue. Consistent with these effects, in our acute model of MR activation by intraperitoneal ALDO injection when compared to placebo injection, similar results were observed. It is important to note that this latter experimental design allows us to separate the effects of the experimental method from those of ALDO. Indeed, we have previously reported that intraperitoneal injections with either ALDO or placebo were associated with increases in circulating corticosterone levels in this murine model.25 However, only in mice that were injected with ALDO did circulating ALDO increase. These in vivo observations further support the contention that ALDO regulates striatin and that the MR/striatin complex may mediate some of the membrane- related effects of ALDO. However, the cellular effects of increasing striatin levels and how this may in turn modulate steroid receptor function (e.g., MR and ERα activation) are at the present unknown. Further studies are needed to determine the potential mechanisms involved and should include the in vivo use of an MR antagonist, to determine whether striatin plays a role in end-organ damage.
There are some limitations to our studies. Sodium restriction and AngII/L-NAME also lead to changes in AngII, renin levels, and blood pressure. However a potential role for AngII and renin on striatin is unlikely since (i) the in vitro studies confirm that ALDO has a direct effect on striatin and (ii) acute in vivo administration of ALDO leads to suppression of AngII and renin. Therefore, in all three in vivo studies both ALDO and striatin are increased while opposite effects on AngII levels are seen during acute ALDO administration when compared to the salt restriction. Regarding blood pressure, dietary sodium restriction was associated with lower blood pressure when compared to high salt while AngII/L-NAME showed opposite effects on blood pressure. Thus it seems that striatin increases in these in vivo mouse models of MR activation are independent of blood pressure changes.
Our findings are consistent with the hypothesis that MR activation is a key factor in regulating striatin expression in heart and endothelial cells. We posit that some of the chronic and acute effects of MR activation in vivo may be secondary to the effects of increased ALDO on striatin expression. Thus, our results together with those of others implicate striatin as a critical component of a protein complex that mediates the effects of steroid receptor activation.
Acknowledgments
EA.hy926 cells were a kind gift from Dr Cora J Edgell and were obtained from the Tissue Culture Facility at the University of North Carolina Lineberger Comprehensive Cancer Center. The anti-MR antibody used in immunoprecipitation experiments (clone 2D6) was the kind gift of Dr. Celso Gomez-Sanchez (University of Mississippi Medical Center, Jackson, MS). This work was supported by grants from the National Institutes of Health: [R01HL096518, 3R01HL096518-01S1 and R21ES014462] to JRR and [R01HL090632] to AR. LHP was supported by a career development award form the American Heart Association #0735609T and a KL2 Medical Research Investigator Training (MeRIT) [KL2RR025757] award from Harvard Catalyst/The Harvard Clinical and Translational Science Center (NIH Award #KL2 RR 025757). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Stier CT, Jr, Chander PN, Rocha R. Aldosterone as a mediator in cardiovascular injury. Cardiol Rev. 2002;10:97–107. doi: 10.1097/00045415-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Young MJ, Funder JW. Mineralocorticoids, salt, hypertension: effects on the heart. Steroids. 1996;61:233–235. doi: 10.1016/0039-128x(96)00020-7. [DOI] [PubMed] [Google Scholar]
- 3.Rossi GP, Di Bello V, Ganzaroli C, Sacchetto A, Cesari M, Bertini A, Giorgi D, Scognamiglio R, Mariani M, Pessina AC. Excess aldosterone is associated with alterations of myocardial texture in primary aldosteronism. Hypertension. 2002;40:23–27. doi: 10.1161/01.hyp.0000023182.68420.eb. [DOI] [PubMed] [Google Scholar]
- 4.Rossi GP, Sacchetto A, Visentin P, Canali C, Graniero GR, Palatini P, Pessina AC. Changes in left ventricular anatomy and function in hypertension and primary aldosteronism. Hypertension. 1996;27:1039–1045. doi: 10.1161/01.hyp.27.5.1039. [DOI] [PubMed] [Google Scholar]
- 5.Arora RB. Role of aldosterone in myocardial infarction. Ann N Y Acad Sci. 1965;118:539–554. doi: 10.1111/j.1749-6632.1965.tb33976.x. [DOI] [PubMed] [Google Scholar]
- 6.Pitt B, Reichek N, Willenbrock R, Zannad F, Phillips RA, Roniker B, Kleiman J, Krause S, Burns D, Williams GH. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003;108:1831–1838. doi: 10.1161/01.CIR.0000091405.00772.6E. [DOI] [PubMed] [Google Scholar]
- 7.Brilla CG, Weber KT. Reactive and reparative myocardial fibrosis in arterial hypertension in the rat. Cardiovasc Res. 1992;26:671–677. doi: 10.1093/cvr/26.7.671. [DOI] [PubMed] [Google Scholar]
- 8.Young M, Head G, Funder J. Determinants of cardiac fibrosis in experimental hypermineralocorticoid states. Am J Physiol. 1995;269:E657–E662. doi: 10.1152/ajpendo.1995.269.4.E657. [DOI] [PubMed] [Google Scholar]
- 9.Chun TY, Pratt JH. Aldosterone increases plasminogen activator inhibitor-1 synthesis in rat cardiomyocytes. Mol Cell Endocrinol. 2005;239:55–61. doi: 10.1016/j.mce.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Qin W, Rudolph AE, Bond BR, Rocha R, Blomme EA, Goellner JJ, Funder JW, McMahon EG. Transgenic model of aldosterone-driven cardiac hypertrophy and heart failure. Circ Res. 2003;93:69–76. doi: 10.1161/01.RES.0000080521.15238.E5. [DOI] [PubMed] [Google Scholar]
- 11.Bratton MR, Gomez-Sanchez EP, Gomez-Sanchez CE, Subauste JS. The myosin binding protein is a novel mineralocorticoid receptor binding partner. Mol Cell Endocrinol. 2004;217:221–227. doi: 10.1016/j.mce.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Adler GK, Chen R, Menachery AI, Braley LM, Williams GH. Sodium restriction increases aldosterone biosynthesis by increasing late pathway, but not early pathway, messenger ribonucleic acid levels and enzyme activity in normotensive rats. Endocrinology. 1993;133:2235–2240. doi: 10.1210/endo.133.5.8404675. [DOI] [PubMed] [Google Scholar]
- 13.Moe OW. Acute regulation of proximal tubule apical membrane Na/H exchanger NHE-3: role of phosphorylation, protein trafficking, and regulatory factors. J Am Soc Nephrol. 1999;10:2412–2425. doi: 10.1681/ASN.V10112412. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen J, Kwon TH, Frøkiaer J, Knepper MA, Nielsen S. Maintained ENaC trafficking in aldosterone-infused rats during mineralocorticoid and glucocorticoid receptor blockade. Am J Physiol Renal Physiol. 2007;292:F382–F394. doi: 10.1152/ajprenal.00212.2005. [DOI] [PubMed] [Google Scholar]
- 15.Castets F, Bartoli M, Barnier JV, Baillat G, Salin P, Moqrich A, Bourgeois JP, Denizot F, Rougon G, Calothy G, Monneron A. A novel calmodulin-binding protein, belonging to the WD-repeat family, is localized in dendrites of a subset of CNS neurons. J Cell Biol. 1996;134:1051–1062. doi: 10.1083/jcb.134.4.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moqrich A, Mattei MG, Bartoli M, Rakitina T, Baillat G, Monneron A, Castets F. Cloning of human striatin cDNA (STRN), gene mapping to 2p22-p21, and preferential expression in brain. Genomics. 1998;51:136–139. doi: 10.1006/geno.1998.5342. [DOI] [PubMed] [Google Scholar]
- 17.Baillat G, Moqrich A, Castets F, Baude A, Bailly Y, Benmerah A, Monneron A. Molecular cloning and characterization of phocein, a protein found from the Golgi complex to dendritic spines. Mol Biol Cell. 2001;12:663–673. doi: 10.1091/mbc.12.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartoli M, Monneron A, Ladant D. Interaction of calmodulin with striatin, a WD-repeat protein present in neuronal dendritic spines. J Biol Chem. 1998;273:22248–22253. doi: 10.1074/jbc.273.35.22248. [DOI] [PubMed] [Google Scholar]
- 19.Tan B, Long X, Nakshatri H, Nephew KP, Bigsby RM. Striatin-3 gamma inhibits estrogen receptor activity by recruiting a protein phosphatase. J Mol Endocrinol. 2008;40:199–210. doi: 10.1677/JME-07-0132. [DOI] [PubMed] [Google Scholar]
- 20.Lu Q, Pallas DC, Surks HK, Baur WE, Mendelsohn ME, Karas RH. Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor alpha. Proc Natl Acad Sci USA. 2004;101:17126–17131. doi: 10.1073/pnas.0407492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi M, Inoue K, Warabi E, Minami T, Kodama T. A simple method of isolating mouse aortic endothelial cells. J Atheroscler Thromb. 2005;12:138–142. doi: 10.5551/jat.12.138. [DOI] [PubMed] [Google Scholar]
- 22.Pojoga LH, Romero JR, Yao TM, Loutraris P, Ricchiuti V, Coutinho P, Guo C, Lapointe N, Stone JR, Adler GK, Williams GH. Caveolin-1 ablation reduces the adverse cardiovascular effects of N-omega-nitro-L-arginine methyl ester and angiotensin II. Endocrinology. 2010;151:1236–1246. doi: 10.1210/en.2009-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pojoga LH, Yao TM, Sinha S, Ross RL, Lin JC, Raffetto JD, Adler GK, Williams GH, Khalil RA. Effect of dietary sodium on vasoconstriction and eNOS-mediated vascular relaxation in caveolin-1-deficient mice. Am J Physiol Heart Circ Physiol. 2008;294:H1258–H1265. doi: 10.1152/ajpheart.01014.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turchin A, Guo CZ, Adler GK, Ricchiuti V, Kohane IS, Williams GH. Effect of acute aldosterone administration on gene expression profile in the heart. Endocrinology. 2006;147:3183–3189. doi: 10.1210/en.2005-1674. [DOI] [PubMed] [Google Scholar]
- 26.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 27.Rocha R, Stier CT, Jr, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, Adler GK. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141:3871–3878. doi: 10.1210/endo.141.10.7711. [DOI] [PubMed] [Google Scholar]
- 28.Oestreicher EM, Martinez-Vasquez D, Stone JR, Jonasson L, Roubsanthisuk W, Mukasa K, Adler GK. Aldosterone and not plasminogen activator inhibitor-1 is a critical mediator of early angiotensin II/NG-nitro-L-arginine methyl ester-induced myocardial injury. Circulation. 2003;108:2517–2523. doi: 10.1161/01.CIR.0000097000.51723.6F. [DOI] [PubMed] [Google Scholar]
- 29.Martinez DV, Rocha R, Matsumura M, Oestreicher E, Ochoa-Maya M, Roubsanthisuk W, Williams GH, Adler GK. Cardiac damage prevention by eplerenone: comparison with low sodium diet or potassium loading. Hypertension. 2002;39:614–618. [PubMed] [Google Scholar]
- 30.Castets F, Rakitina T, Gaillard S, Moqrich A, Mattei MG, Monneron A. Zinedin, SG2NA, and striatin are calmodulin-binding, WD repeat proteins principally expressed in the brain. J Biol Chem. 2000;275:19970–19977. doi: 10.1074/jbc.M909782199. [DOI] [PubMed] [Google Scholar]
- 31.Moreno CS, Park S, Nelson K, Ashby D, Hubalek F, Lane WS, Pallas DC. WD40 repeat proteins striatin and S/G(2) nuclear autoantigen are members of a novel family of calmodulin-binding proteins that associate with protein phosphatase 2A. J Biol Chem. 2000;275:5257–5263. doi: 10.1074/jbc.275.8.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benoist M, Gaillard S, Castets F. The striatin family: a new signaling platform in dendritic spines. J Physiol Paris. 2006;99:146–153. doi: 10.1016/j.jphysparis.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Gaillard S, Bartoli M, Castets F, Monneron A. Striatin, a calmodulin-dependent scaffolding protein, directly binds caveolin-1. FEBS Lett. 2001;508:49–52. doi: 10.1016/s0014-5793(01)03020-4. [DOI] [PubMed] [Google Scholar]
- 34.Moreno CS, Lane WS, Pallas DC. A mammalian homolog of yeast MOB1 is both a member and a putative substrate of striatin family-protein phosphatase 2A complexes. J Biol Chem. 2001;276:24253–24260. doi: 10.1074/jbc.M102398200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlegel A, Wang C, Pestell RG, Lisanti MP. Ligand-independent activation of oestrogen receptor alpha by caveolin-1. Biochem J. 2001;359:203–210. doi: 10.1042/0264-6021:3590203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu ML, Schneider MC, Zheng Y, Zhang X, Richie JP. Caveolin-1 interacts with androgen receptor. A positive modulator of androgen receptor mediated transactivation. J Biol Chem. 2001;276:13442–13451. doi: 10.1074/jbc.M006598200. [DOI] [PubMed] [Google Scholar]
- 37.Callera GE, Montezano AC, Yogi A, Tostes RC, Touyz RM. Vascular signaling through cholesterol-rich domains: implications in hypertension. Curr Opin Nephrol Hypertens. 2007;16:90–104. doi: 10.1097/MNH.0b013e328040bfbd. [DOI] [PubMed] [Google Scholar]
- 38.Kifor I, Kifor O, Yao TM, Ricchiuti V, Martinez D, Oestreicher E, Roubsanthisuk W, Williams GH. Angiotensin II, aldosterone, and the caveolae. I. Autocrine signaling via the caveolae. J Hypertens. 2002;20:S175. [Google Scholar]
- 39.Hill WG, An B, Johnson JP. Endogenously expressed epithelial sodium channel is present in lipid rafts in A6 cells. J Biol Chem. 2002;277:33541–33544. doi: 10.1074/jbc.C200309200. [DOI] [PubMed] [Google Scholar]
- 40.Nagata D, Takahashi M, Sawai K, Tagami T, Usui T, Shimatsu A, Hirata Y, Naruse M. Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO synthase activity. Hypertension. 2006;48:165–171. doi: 10.1161/01.HYP.0000226054.53527.bb. [DOI] [PubMed] [Google Scholar]