Abstract
In an effort to further expand the therapeutic range of platinum-based anticancer agents, several new approaches to platinum-based therapy, including nanodelivery, are under active investigation. To better understand the effect of ligand lipophilicity on the encapsulation of Pt(IV) prodrugs within polymer nanoparticles, the series of compounds cis,cis,trans-[Pt(NH3)2Cl2L2] was prepared, where L = acetate, propanoate, butanoate, pentanoate, hexanoate, heptanoate, octanoate, nonanoate, and decanoate. The lipophilicities of these compounds, assessed by reversed-phase HPLC, correlate with the octanol/water partition coefficients of their respective free carboxylic acid ligands, which in turn affect the degree of encapsulation of the Pt(IV) complex within the hydrophobic core of poly(lactic-co-glycolic acid)-block-poly(ethylene glycol) (PLGA-PEG-COOH) nanoparticles. The most lipophilic compound investigated, cis,cis,trans-[Pt(NH3)2Cl2(O2C(CH2)8CH3)2], displayed the best encapsulation. This compound was therefore selected to evaluate the effect of increased platinum concentration on encapsulation. As the platinum concentration was increased, there was an initial increase in encapsulation followed by a decrease due to macroscopic precipitation. Maximal loading occurred when the platinum complex was present at a 40% w/w ratio with respect to polymer during the nanoprecipitation step. Particles formed under these optimal conditions had diameters of approximately 50 nm, as determined by transmission electron microscopy.
Introduction
Following the serendipitous discovery of anticancer activity for cis-[Pt(NH3)2Cl2], or cisplatin1, it has come to be one of the most widely used chemotherapeutic agents for the treatment of testicular, ovarian, bladder, and non-small cell lung cancers (NSCLC), as well as small cell lung cancer (SCLC), melanoma, lymphomas, and myelomas.2 The advantages of platinum-based treatment are perhaps most evident in testicular cancer, where, following the introduction of cisplatin into the arsenal of available treatments, cure rates have risen to > 90%.3 Despite the clinical efficacy demonstrated by platinum-based drugs currently approved by the FDA, intense research is underway to discover variants with enhanced efficacy, lower toxicity, and a more diverse spectrum of activity.4 One approach is to use nano-sized drug delivery vehicles, which offers several potential advantages.5 The controlled release provided by these systems can enhance retention in the bloodstream, their size allows for passive targeting, and their surfaces can be functionalized for active targeting. Our lab has reported the preparation of Pt(IV) constructs designed for delivery by gold nanoparticles,6 single-walled carbon nanotubes,7,8 and polymeric nanoparticles.9–11
Most platinum complexes investigated for their cytotoxic activity are unsuitable for nanoparticle encapsulation. Different approaches have been used to produce analogues that can be integrated into a nanodelivery device. One strategy that we previously described involved the introduction of hydrophobic axial ligands L into the coordination sphere of a cis,cis,trans-[Pt(NH3)2Cl2L2], platinum(IV) complex, allowing it to be encapsulated within the hydrophobic core of a polymeric micelle.9 The micelle used in this prior study was composed of carboxy-terminated amphiphilic poly(lactic-co-glycolic acid)-block-poly(ethylene glycol) (PLGA-PEG-COOH) copolymers, the self-assembly of which has been exploited previously for the construction of other drug delivery vehicles.12 The hexanoate ligands L of the Pt(IV) complex provided lipophilicity sufficient to promote encapsulation within the PLGA nanoparticle core. The remaining ligands in the coordination sphere are such that, following reduction of this Pt(IV) complex, an equivalent of cisplatin would be released as the active Pt(II) cytotoxic payload.
These results raised the question, is the addition of two lipophilic carboxylates to a Pt(II) core complex a readily generalizable strategy for nanodelivery of platinum(IV) anticancer prodrugs? Before exploring different Pt(II) core structures, however, we wanted to understand the precise effect of carboxylate ligand choice on the nanoprecipitation process used to form the polymeric micelles. We therefore undertook the present study of a series of compounds of the form cis,cis,trans-[Pt(NH3)2Cl2(OOCR)2], where R is a straight chain alkyl (Scheme 1). The complexes comprising this series all contain a cis-diamminedichloroplatinum core as the pharmacophore and two axial ligands that vary systematically in length. In previous related work, a series of Pt(IV) carboxylates complexes containing either a cis-1,4-diaminocyclohexanedichloro-platinum13 or a trans-1R,2R-diaminocyclohexanedichloro-platinum pharmacophore14 investigated the influence of axial ligand chain length on their biological properties. The aim of the present work, however, is to exploit the axial ligands for nanoparticle encapsulation with the aim of enhancing anticancer efficacy. The efficiency of nanoparticle encapsulation was investigated and correlated with the physical properties of the carboxylate ligands. The effect of relative platinum complex concentration during the nanoprecipitation procedure on the degree of encapsulation was also studied. Two competing processes, nanoparticle encapsulation and bulk precipitation, were correlated with this parameter, and conditions for maximal loading were determined by finding the optimal balance between these two processes.
Scheme 1.

The synthesis and general structure of the Pt(IV) carboxylate species studied here.
Experimental Section
General Procedures and Chemicals for Synthesis
All chemicals were reagent grade and used as purchased without further purification. Acetic, butanoic, and pentanoic anhydrides were obtained from Sigma-Aldrich. Propanoic, hexanoic, heptanoic, and octanoic anhydrides were purchased from TCI America. Nonanoic and decanoic anhydrides were prepared by the DCC mediated dehydration of the corresponding carboxylic acids,15 which were obtained from TCI America. Solvents were used as received without further purification. Cis,cis,trans-[Pt(NH3)2Cl2(OH)2], compound 1, was prepared as previously described.16 The PLGA-PEG-COOH block co-polymer, prepared as described previously,17 was kindly provided by the Farokhzad Lab (Suresh Gadde, Harvard Medical School). MilliQ water was used for all work involving nanoparticles.
Physical Measurements
1H and 13C{1H} NMR measurements were performed on a Varian Inova-500 spectrometer in the MIT Department of Chemistry Instrumentation Facility with deuterated DMSO as a solvent. NMR chemical shifts (δ) are reported in ppm with respect to tetra-methylsilane and referenced to residual solvent peaks. NMR spectra are presented in Figures S1 to S18. Fourier-transform infrared spectra were recorded on a ThermoNicolet Avatar 360 spectrophotometer using the OMNIC software. All IR samples were prepared as KBr pellets and measurements are reported in cm−1. Electrospray ionization mass spectrometry (ESI-MS) measurements were performed on an Agilent Technologies 1100 series LC/MSD ion trap. Liquid chromatography mass spectrometry (LC-MS) measurements were performed using the same ion source and trap.
Synthesis
Compounds of general formula cis,cis,trans-[Pt(NH3)2Cl2(OOCR)2], where R= methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, octyl, or nonyl, were all prepared in the same manner. Compound 1 (200 mg, 0.60 mmol) was suspended in 8 mL of DMF. To this mixture was added 1.5 mL of the appropriate carboxylic acid anhydride. The reaction mixture was stirred in the dark at 50 °C for 24 h. The resulting yellow to orange solution was filtered through Celite and the volume of the filtrate was reduced to 2 mL in vacuo at 50 °C. This concentrated solution was then added in a dropwise manner to a rapidly stirring volume of diethyl ether (40 mL) forming a pale yellow to white precipitate. The ether suspension was stirred for 30 min, and the solid was collected by filtration and dried under vacuum for 12 h. The detailed characterization of 2 – 10, including spectroscopic and crystallographic data, is collected in the Supporting Information.
Liquid Chromatography-Mass Spectrometry (LC-MS)
Solutions (1:1 methanol/water containing 5% DMF) of 2 through 6 were injected onto an Eclipse XDB-C18 column (3 × 150 mm, Agilent) and eluted with 1:1 methanol/water at a rate of 0.25 mL/min. The elution was isocratic and the temperature of the mobile phase within the column was maintained at 25 °C. The analytes were detected by monitoring the intensity of the negative ion mode base peak, which in all cases was the [M–H]− signal. The dead time of the column was evaluated by monitoring the elution of potassium iodide at 210 nm. This dead time was used in the calculation of the capacity factors of the analyzed Pt(IV) compounds.
Nanoparticle Encapsulation
A 550 μL DMF solution containing 10 mg of PLGA-PEG-COOH and an amount of a platinum complex to give the desired feed, defined as (mg Pt complex/mg polymer) × 100, was prepared. A 500 μL aliquot of this solution was added in a drop-wise manner over the course of 10 min to 5 mL of rapidly stirring MilliQ water. The DMF solutions were added by a mechanical pipette and the nanoprecipitations were carried out in 20 mL glass scintillation vials. The water was stirred magnetically using a 0.5 cm stir bar at approximately 500 rpm. After addition of the DMF solution, the water acquired a milky blue coloration owing to Tyndall scattering of the nanoparticles that formed. At higher loadings, some macroscopic precipitation was also observed. An aqueous solution of poly(vinyl alcohol) (5 mL, 0.1% w/w PVA) was then added along the edge of the vial to bring the final volume to 10.5 mL and the final PVA concentration to approximately 0.05 % w/w. This suspension of nanoparticles was stirred for an additional 20 min and then passed through a 0.45 μm cellulose acetate syringe filter (VWR). If macroscopic precipitation was evident, the suspension was first passed through a plug (0.25 × 1 cm) of Celite in order to avoid excessively clogging of the syringe filter. The filtrate from the 0.45 μm filtration was loaded into an Amicon Centrifugal Filtration Device (100 kDa MWCO regenerated cellulose membrane). The loaded device was centrifuged at 1500 × g for 20 min, concentrating the nanoparticle suspension to approximately 1 mL. This concentrated material was suspended in an additional 10 mL of fresh MilliQ water and centrifuged again under identical conditions. Each sample was washed 3 times in this manner. The final concentrated suspension was diluted to 1.4 mL with MilliQ water for use in further experiments. All nanoprecipitations were carried out in triplicate.
Evaluation of Encapsulation
The metric used to evaluate encapsulation efficacy was the concentration of platinum present in the final 1.4 mL colloidal suspension. This concentration was determined by electrothermal atomic absorption spectroscopy (AAS) using a Perkin-Elmer AAnalyst 600 spectrometer outfitted with a transverse heated graphite atomizer. Platinum absorption was measured at 265.9 nm and a Zeeman background absorption correction was applied. Samples were prepared by diluting nanoparticle suspensions with MilliQ water until the platinum concentration fell within the linear calibration range (50 – 200 μg Pt/L). All AAS measurements were carried out in triplicate and averaged.
Transmission Electron Microscopy
PLGA-PEG-COOH nanoparticles containing compound 10 at a 40% feed, the optimal loading conditions as described below, were prepared and an aliquot was allowed to evaporate on a carbon-coated copper TEM grid. Imaging was conducted by using a JEOL JEM-200CX operated at 120 kV.
Results and Discussion
Synthesis
The nucleophilicity of hydroxo ligands coordinated to Pt(IV) centers provides a convenient synthetic route for modifying Pt(IV) carboxylate compounds via condensation with an appropriate acid anhydride.18 In the present work, this chemistry was carried out in DMF. As the reaction proceeds, the suspension of the dihydroxo compound 1 in DMF is consumed, forming a clear solution of the soluble dicarboxylatoplatinum(IV) product. The differential solubility of the starting material and products both drives the reaction and provides a convenient way to monitor its progress. To purify the compounds, the reaction volume was reduced in vacuo before addition to a large excess of diethyl ether. For many of the compounds, addition in the reverse order yielded only intractable oils. For longer chain carboxylates, the solubility of the products in DMF decreases. For example, 2, with acetate ligands, is readily soluble at concentrations up to 100 mg/mL (0.24 M) at room temperature, but for 10, which has decanoate ligands, a precipitate formed when a warm DMF solution of equivalent molar concentration was cooled to room temperature.
Physical characterization of the compounds confirmed their proposed structures. In the IR spectra of the products, sharp bands corresponding to O–H and Pt–O stretches in the starting material, at 3516 cm−1 and 559 cm−1, respectively, are absent, and new bands in the ranges of 1665 cm−1 to 1620 cm−1 (carbonyl C=O stretch), 1470 cm−1 to 1190 cm−1 (C–H bend) and 2960 cm−1 to 2850 cm−1 (C–H stretch) appear. The remaining notable features of the IR spectra are bands from 1530 cm−1 to 1580 cm−1 (N–H bend) and 3260 cm−1 to 3110 cm−1 (N–H stretch) that are present in both the starting material and the final products.
The 1H NMR spectra of 2 through 10 all contain a resonance at approximately 6.52 ppm, consistent with protons on an ammine ligand bound to a Pt(IV) center. Features present on this signal arise from unresolved coupling of the protons to both 14N and 195Pt nuclei. For 2 – 5, each additional methylene unit in the carboxylate chain produces a well-resolved resonance in the aliphatic region that displays the expected 1H-1H coupling pattern. As additional methylene groups are added from 6 to 10, the overall CH2 signal intensity increases, but individual units were not resolved at 500 MHz. The 13C NMR spectra all display resolved peaks for the expected number of carbon atoms despite nearly overlapping signals (peak-to-peak separations of as little as 0.005 ppm) in some of the longer chains. The mass spectra all show the presence of the desired compound as indicated by [M–H]− peaks in negative ion mode ESI–MS.
Crystallography
The crystal structure of a DMSO solvate of c,c,t-[Pt(NH3)2Cl2(OOCCH3)2], 2·2DMSO, is depicted in Figure 1. Selected crystallographic parameters are presented in Table 1. Full details including bond lengths and angles are collected in Tables S1 and S3. The room temperature structure of a monohydrate of this compound has been previously reported.19 In the previous structure a two-fold rotation axis passed through the platinum, but 2·2DMSO has no such required symmetry. The orientations of the acetate ligands differ in the two structures, results in differing intramolecular hydrogen-bonding interactions, but the bond lengths and angles of the two are comparable. If allowances are made for flexibility of dihedral angles controlling the rotation of the acetate ligands, then the structures have a RMSD of 0.098 Å, as calculated with the program MERCURY.
Figure 1.
Molecular diagrams of 2 (left) and 3 (right). Thermal ellipsoids are drawn at the 50% probability level.
Table 1.
Crystallographic Parameters for 2·2DMSO and 3
| Formula | C8H18Cl2N2O6PtS2 | C6H16Cl2N2O4Pt |
| Formula weight | 568.35 | 446.20 |
| Crystal system | Triclinic | Tetragonal |
| Space group | P1̄ | P43 |
| Color | Colorless | Yellow |
| a, Å | 7.2454(7) | 9.2357(3) |
| b, Å | 10.2263(10) | |
| c, Å | 14.0657(14) | 13.9563(11) |
| α, ° | 72.516(2) | |
| β, ° | 81.044(2) | |
| γ, ° | 69.485(2) | |
| V, Å3 | 929.51(16) | 1190.45(11) |
| Z | 2 | 4 |
| T, K | 100(2) | 100(2) |
| R1a (%) | 3.42 | 1.68 |
| wR2b (%) | 8.79 | 3.71 |
| GOFc | 1.308 | 1.065 |
R1=Σ||Fo| − |Fc||/Σ|Fo|.
wR2= {Σ[w(Fo2−Fc2)2]/Σ[w(Fo2)2]}1/2.
GOF= {Σ[w (Fo2−Fc 2)2]/(n-p)}1/2, where n is the number of data and p is the number of refined parameters.
The crystal structure of 3 was also determined. The apparent Laue symmetry visible in precession camera pseudo-images is 4/mmm, but no successful solution could be obtained in any of the space groups consistent with the 00l, l=4n systematically absent reflections. The abnormally low <E2-1> value of 0.525 and the similarity between Rint(4/mmm) = 0.093 and Rint(4/m) = 0.042 suggested that the crystal may be twinned by merohedry. A Patterson map generated using preliminarily detwinned data revealed the locations of the Pt and Cl atoms. Subsequent refinement using the appropriate twin law afforded a satisfactory crystal structure (Figure 1). Selected crystallographic parameters are presented in Table 1. Full details including bond lengths and angles are collected in Tables S2 and S4.
Evaluation of Lipophilicity
The traditional measure of the lipophilicity of a molecule is the log of the water/octanol partition coefficient (log P).20 Such values are typically obtained by the “shake-flask” method in which the analyte is introduced into a biphasic mixture of n-octanol saturated water and water saturated n-octanol. The compound is allowed to achieve a thermodynamic equilibrium between the two phases and the concentration of analyte in each phase is determined. We find, however, that more reproducible results are obtained by using a reversed-phase high performance liquid chromatography (RP-HPLC) method.21 For many compounds, including platinum complexes, the capacity factor under isocratic elution conditions is linearly proportional to log P values obtained by the shake-flask method.22 Compounds 2 – 6 were analyzed by RP-HPLC and their capacity factors (k) are presented in Table 2. Chromatograms are shown in Figure S20.
Table 2.
Retention Times and Capacity Factors of 2 – 6
| Compound | Retention time (min) | Capacity factor (k) |
|---|---|---|
| 2 | 2.8 | 0.077 |
| 3 | 3.4 | 0.31 |
| 4 | 4.7 | 0.81 |
| 5 | 9.6 | 2.70 |
| 6 | 32.7 | 11.6 |
The lipophilicity of an organic molecule can be accurately modeled as the sum of the contributions from its individual functional groups.23 When the log of the capacity factors of compounds 2 – 6 were plotted against the log P values of acetic through hexanoic acid, a straight line is obtained (Figure 2). This correlation indicates that the effect of adding additional methylene units to the carboxylate chain has approximately the same effect on the platinum complex as it does on the isolated carboxylic acid. Therefore, in subsequent analyses we compare the properties of the platinum complexes with the log P values of the carboxylic acids from which the carboxylate ligands are derived.
Figure 2.

Correlation between the log of the capacity factors for 2 = 6 (abscissa) and the log of the octanol/water partition coefficients of the carboxylic acids from which the carboxylates of 2 – 6 are derived (ordinate).
Nanoparticle Encapsulation
The strategy used here to encapsulate compounds 2 – 10 within a polymeric nanoparticle has been successfully utilized to encapsulate other molecules.12,17,24,25 The process by which the nanoconstruct forms is depicted schematically in Figure 3. An organic solution containing both an amphiphilic block copolymer and a potential guest molecule is added to a large excess of water. Provided that the organic solvent has been properly chosen so as to be readily miscible with water, this process drives self-assembly of the polymer chains into core-shell polymeric micelles. The hydrophobic block is buried within the core and the hydrophilic block forms the solvent-exposed shell. When the polymer chains self-assemble, the additional hydrophobic guest molecule present in the initial organic solution can become trapped within the hydrophobic core of the particle.
Figure 3.

Schematic depiction of the nanoprecipitation process, whereby the amphiphilic block copolymer chains self-assemble into polymeric micelles, trapping hydrophobic guest molecules within.
The encapsulation of complexes for 2 – 10 reflects the lipophilicities of their axial carboxylate ligands. Increasing the chain length increases the overall lipophilicity of the complex, facilitating its partition into the hydrophobic nanoparticle core. The series of platinum complexes reported here was prepared to determine the precise effect of chain length on the encapsulation of platinum complexes within a PLGA-PEG-COOH nanoparticle.
The specific experimental encapsulation procedure used was based upon a method described previously.12 The polymer and platinum complex are dissolved together in DMF and added in a dropwise manner to a tenfold excess volume of water. In previous studies using compound 6,9,10 acetonitrile was employed as the organic solvent, but not all of the compounds prepared here are sufficiently soluble in acetonitrile to allow for a unilateral comparison. When a droplet of the DMF solution comes into contact with the water, the organic solvent rapidly disperses into the aqueous phase forcing the copolymer chains to assemble into micelles. In the process of nanoparticle assembly, lipophilic platinum complexes that were present in the DMF solution are trapped within the hydrophobic core of the polymer nanoparticle.
When attempting to work-up nanoparticle suspensions, particularly during the concentration step in the centrifugal filtration devices, the particles would often aggregate irreversibly. Inclusion of a small amount of a surfactant such as poly(vinyl alcohol) (PVA) during this stage of the preparation eliminated the problem.
When the encapsulation was carried out at a 10% feed – that is, when the platinum complex was present at 10% w/w with respect to polymer in the DMF solution – a consistent trend was observed. As the chain length of the carboxylate ligand increased, the amount of the platinum complex encapsulated increased (Figure 4). Because log P of the carboxylic acid used as the ligand correlates with the lipophilicity of the entire platinum complex (vide supra), the log of the encapsulation efficiency (EE) was plotted against this parameter. The encapsulation efficiency is defined as the % w/w of platinum complex encapsulated with respect to the amount used in the nanoprecipitation. Figure 4 shows that an excellent linear correlation exists between the aforementioned variables.
Figure 4.
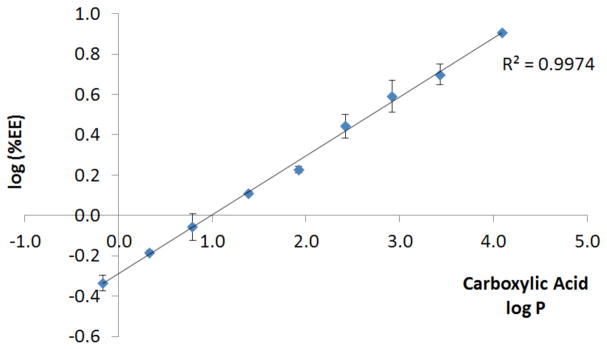
Correlation between log of encapsulation efficiency (%EE) for 2 – 10 (ordinate) and log P of the carboxylic acids from which the carboxylates of 2 – 10 are derived (abscissa).
This correlation is intuitively satisfying, because one would expect that, as the platinum compounds become more lipophilic, they should more readily encapsulate within the hydrophobic core of the nanoparticles. Moreover, the linearity of the correlation attests to the reliability and reproducibility of the procedure.
Because 10 is the most effectively encapsulated at a 10% feed, it was selected to study the effect of feed variation on encapsulation. Initially, as the amount of platinum complex present during the nanoprecipitation was increased, the amount of platinum complex encapsulated increased, as quantified by the loading, the %w/w of platinum compound encapsulated with respect to polymer used in the nanoprecipitation (Figure 5). As the amount of platinum compound increased, however, the amount of macroscopic precipitation that occurred during nanoprecipitation also increased. This precipitation became so appreciable that samples had to be passed through a macroscopic filter aid prior to passage through 0.45 μm cellulose acetate membranes. This macroscopic precipitation also decreased the loading. The competing factors of increased loading and increased macroscopic precipitation resulted in a maximum loading when the feed was 40% (Figure 5).
Figure 5.

Variation in the platinum loaded into PLGA-PEG-COOH nanoparticles as the feed of 10 was varied.
Nanoparticle Characterization
Although a detailed examination of the effect of chain length of the Pt(IV) carboxylate complex on nanoparticle size and morphology was outside the scope of this study, measurements were made to confirm that the nanoparticles displayed characteristics consistent with the formulation provided above. The nanoparticles were imaged by TEM and the results are depicted in Figure 6. The nanoparticles appear as roughly circular black shapes, approximately 50 nm in diameter.
Figure 6.

Transmission electron micrograph of PLGA-PEG-COOH nanoparticles loaded with 10. Scale bar of main image = 500 nm, scale bar of inset = 100 nm.
Conclusion
This work presents a systematic investigation of the effect of ligand lipophilicity on the encapsulation of Pt(IV) prodrugs within PLGA-PEG-COOH nanoparticles. A procedure was optimized for encapsulation of a series of compounds spanning a wide range of lipophilicities. We find that, as the chain length increases, there is a direct and predictable increase in the degree to which the platinum complex is encapsulated. With the most readily encapsulated compound, 10, the effect of platinum feed during nanoprecipitation was investigated. With an increase in concentration of platinum used in the nanoprecipitation, encapsulation also increased but so too did the amount of macroscopic precipitation. At higher feeds, macroscopic precipitation outweighed the increase in encapsulation. An intermediate feed level of 40% allowed for maximum loading of platinum complex 10 into the nanoparticles.
Supplementary Material
Acknowledgments
This work was supported by grant CA034992 from the National Cancer Institute. Spectroscopic instrumentation at the MIT DCIF is maintained with funding from NIH Grant 1S10RR13886-01. T.C.J. received partial funding from the Harvard/MIT CCNE, NIH grant 5-U54-CA151884. We acknowledge Dr. Suresh Gadde (Farokhzad lab, Harvard Medical School) for preparing the PLGA-PEG-COOH used in this study.
Footnotes
Supporting Information. Spectroscopic and crystallographic characterization of 2 – 10, detailed crystallographic parameters for the structure of 2·2DMSO and 3, selected bond lengths and angles of 2·2DMSO and 3, LC-MS chromatograms of 2 – 6. Crystallographic information for 2·2DMSO and 3 are also provided in CIF format. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Rosenberg B, VanCamp L, Trosko JE, Mansour VH. Nature. 1969;222:385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- 2.Wheate NJ, Walker S, Craig GE, Oun R. Dalton Trans. 2010;39:8113–8127. doi: 10.1039/c0dt00292e. [DOI] [PubMed] [Google Scholar]
- 3.O’Dwyer PJ, Stevenson JP, Johnson SW. Clinical Status of Cisplatin, Carboplatin, and Other Platinum-Based Antitumor Drugs. In: Lippert B, editor. Cisplatin - Chemistry and Biochemistry of a Leading Anticancer Drug. Verlag Helvetica Chimica Acta; Zürich, Switzerland: 1999. pp. 31–69. [Google Scholar]
- 4.Kelland L. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 5.Farokhzad OC, Langer R. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 6.Dhar S, Daniel WL, Giljohann DA, Mirkin CA, Lippard SJ. J Am Chem Soc. 2009;131:14652–14653. doi: 10.1021/ja9071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feazell RP, Nakayama-Ratchford N, Dai H, Lippard SJ. J Am Chem Soc. 2007;129:8438–8439. doi: 10.1021/ja073231f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhar S, Liu Z, Thomale J, Dai HJ, Lippard SJ. J Am Chem Soc. 2008;130:11467–11476. doi: 10.1021/ja803036e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Proc Natl Acad Sci USA. 2008;105:17356–17361. doi: 10.1073/pnas.0809154105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC. Proc Natl Acad Sci USA. 2011;108:1850–1855. doi: 10.1073/pnas.1011379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolishetti N, Dhar S, Valencia PM, Lin LQ, Karnik R, Lippard SJ, Langer R, Farokhzad OC. Proc Natl Acad Sci USA. 2010;107:17939–17944. doi: 10.1073/pnas.1011368107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng J, Teply BA, Sherifi I, Sung J, Luther G, Gu FX, Levy-Nissenbaum E, Radovic-Moreno AF, Langer R, Farokhzad OC. Biomaterials. 2007;28:869–876. doi: 10.1016/j.biomaterials.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shamsuddin S, Santillan CC, Stark JL, Whitmire KH, Siddik ZH, Khokhar AR. J Inorg Biochem. 1998;71:29–35. doi: 10.1016/s0162-0134(98)10029-6. [DOI] [PubMed] [Google Scholar]
- 14.Khan SRA, Huang S, Shamsuddin S, Inutsuka S, Whitmire KH, Siddik ZH, Khokhar AR. Bioorgan Med Chem. 2000;8:515–521. doi: 10.1016/s0968-0896(99)00313-2. [DOI] [PubMed] [Google Scholar]
- 15.Chen FMFKK, Benoiton NL. Synthesis. 1978;1978:928–929. [Google Scholar]
- 16.Hall MD, Dillon CT, Zhang M, Beale P, Cai ZH, Lai B, Stampfl APJ, Hambley TW. J Biol Inorg Chem. 2003;8:726–732. doi: 10.1007/s00775-003-0471-6. [DOI] [PubMed] [Google Scholar]
- 17.Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R. Proc Natl Acad Sci USA. 2006;103:6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giandomenico CM, Abrams MJ, Murrer BA, Vollano JF, Rheinheimer MI, Wyer SB, Bossard GE, Higgins JD. Inorg Chem. 1995;34:1015–1021. doi: 10.1021/ic00109a004. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Lee PF, Ranford JD, Vittal JJ, Wong SY. J Chem Soc Dalton. 1999:1209–1212. [Google Scholar]
- 20.Leo A, Hansch C, Elkins D. Chem Rev. 1971;71:525–616. [Google Scholar]
- 21.Harnisch M, Mockel HJ, Schulze G. J Chromatogr. 1983;282:315–332. [Google Scholar]
- 22.Gramatica P, Papa E, Luini M, Monti E, Gariboldi MB, Ravera M, Gabano E, Gaviglio L, Osella D. J Biol Inorg Chem. 2010;15:1157–1169. doi: 10.1007/s00775-010-0676-4. [DOI] [PubMed] [Google Scholar]
- 23.Hansch C, Fujita T. J Am Chem Soc. 1964;86:1616–1626. [Google Scholar]
- 24.Farokhzad OC, Jon SY, Khademhosseini A, Tran TNT, LaVan DA, Langer R. Cancer Res. 2004;64:7668–7672. doi: 10.1158/0008-5472.CAN-04-2550. [DOI] [PubMed] [Google Scholar]
- 25.Fonseca C, Simoes S, Gaspar R. J Control Release. 2002;83:273–286. doi: 10.1016/s0168-3659(02)00212-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



