Abstract
A major goal of HIV research is to develop vaccines reproducibly eliciting broadly neutralizing antibodies (bNAbs). This has proved to be challenging, however. One suggested explanation for this difficulty is that epitopes seen by bNAbs mimic self, leading to immune tolerance. We generated “knock-in” mice expressing bNAb 4E10, which recognizes the membrane proximal external region of gp41. Unlike b12 knock-in mice, described in the accompanying study, 4E10HL mice were found to undergo profound negative selection of B cells, indicating that 4E10 is, to a physiologically significant extent, autoreactive. Negative selection occurred by various mechanisms including receptor editing, clonal deletion and receptor downregulation. Despite significant deletion, small amounts of IgM and IgG anti-gp41 were found in the sera of 4E10HL mice. On a Rag1−/− background 4E10HL mice had virtually no serum immunoglobulins of any kind. These results are consistent with a model in which B cells with 4E10 specificity are counterselected, raising the question of how 4E10 was generated in the patient from whom it was isolated. This represents the second example of an MPER-directed bNAb that is apparently autoreactive in a physiological setting. The relative conservation in HIV of the 4E10 epitope might reflect the fact that it is under less intense immunological selection as a result of B cell self-tolerance. The safety and desirability of targeting this epitope by a vaccine is discussed in light of the newly-described bNAb 10E8.
INTRODUCTION
Although no HIV vaccine exists, passive transfer of a number of broadly neutralizing antibodies, bNAbs, can protect in animal models of disease (1-10). Hence protection from HIV by vaccination is theoretically possible, but our lack of understanding of how to elicit bNAbs by immunization is a significant stumbling block. Here we focus on bNAbs 4E10, and, in the accompanying article, b12, which were until recently two of the most potent and broadly neutralizing HIV antibodies known (11). Generation of mouse models expressing B cells of these specificities could aid in optimizing antigens capable of triggering such desirable B cells.
bNAb 4E10 was isolated by Katinger and colleagues. It neutralizes isolates from multiple clades with modest potency. Isolated from an HIV infected patient as a hybridoma by fusion of peripheral blood cells with a heterohybridoma cell line (12), 4E10 antibody genes were recombinantly expressed, and the secreted IgG tested for cross neutralization (13). 4E10 recognizes a linear stretch of amino acids in gp41, in the membrane proximal external region (MPER), centered on amino acids NWF(D/N)IT (14). In the co-crystal structure, the epitope is in helical conformation, forming a somewhat amphipathic structure with a hydrophobic face on one side, with W in the epitope involved in 36% of the contacts with 4E10 (15). The 4E10 combining site is also unusually hydrophobic in parts. Five of six CDRs are involved in epitope binding. But much of the long and hydrophobic H-chain CDR3 does not directly contact the gp41 peptide. Cardoso et al speculated that 4E10’s H-chain CDR3 might contribute to viral binding by contacting the surface of the viral membrane through the tip of CDRH3, which is not involved in peptide binding, but is predicted to be near the viral surface. Support for this notion was provided by enhanced binding of 4E10 seen in the presence of membranes (16) and in studies showing that viral neutralization, but not MPER peptide binding, was dependent upon CDRH3 residues (17, 18).
Surprisingly, in addition to their ability to bind to HIV Env, both 4E10 and b12 have been suggested to be autoantibodies (19). This conclusion was based mainly on antibody binding studies and was also extended to the antibody 2F5, which recognizes an epitope adjacent to that of 4E10 (19, 20). 2F5 has autoreactive properties when introduced as knock-in transgenes in mice (21). Recently, 4E10 was found to bind weakly to many human proteins present on protein microarrays, and to bind under “stringent” ELISA conditions to splicing factor 3B subunit 3 (20). These findings have been interpreted to suggest that Env might have evolved to protect against the elicitation of neutralizing antibody by mimicking autoantigen. Among the assays in which 4E10 scored positive was in binding to HEp-2 cells, a clinical assay for autoantibodies, and in ELISA involving self constituents immobilized on microtiter plates, with 4E10 in solution. 4E10 bound to cardiolipin, phosphatidlyserine, phosphatidylcholine, phosphatidylethanolamine, and the lupus autoantigen Ro (SSA). In addition, 4E10 had anticoagulant activity, a hallmark of anti-phospholipid syndrome, though this activity was weak (Scherer et al, 2007). Haynes et al. suggested that tolerance to self explains the difficulty in generating antibodies to the 4E10 determinant and the relative ineffectiveness of immunogens based on the MPER. 4E10, but not 2F5, reacted weakly in anti-phospholipid assays and modestly prolonged activated partial thromboplastin time in vivo (22, 23). In the same study, b12 was found to bind to ribonucleoprotein, double stranded DNA, centromere protein, histones, and HEp-2 cells (19). The implication is that 4E10 and b12 cells are normally suppressed by immune tolerance, but might respond under extraordinary circumstances. Patient responders might be resistant to AIDS, but with a propensity to lupus-like autoimmunity. If these speculations were correct, they would have enormous implications for HIV vaccine design.
In this study we have generated knock-in mice in which the variable portions of 4E10 was introduced to the physiological mouse immunoglobulin (Ig) H and L loci by gene targeting. The mice were then analyzed for B cell development and function. The results suggest that expression in mice can be a useful tool to establish the physiological significance of putative antibody autoreactivity and in so doing help us prioritize vaccine epitopes.
MATERIALS AND METHODS
Gene targeting
Targeting was carried out as essentially described in the accompanying article but using 4E10 variable region coding sequences in place of b12 sequences.
Mice
All experiments were performed in accordance with relevant institutional and national guidelines and were approved by the TRSI Institutional Animal Care and Use Committee. C57Bl/6J, Rag1−/−, EIIa-cre, and IgHa mice on the B6 background (B6.Cg-Igha Thy1a Gpi1a/J) were from Jackson Laboratories. hCκ mice (24) were bred for >10 generations to C57Bl/6J and then interbred to generate B6.IgHa/a.Cκh/h mice for interbreeding with transgenic strains.
Flow cytometry and serum ELISA analyses
Analyses for surface markers and serum antibodies were performed as described in the accompanying article.
HIV antigens
Peptide C1 sequence: QIQQEKNMYELLALDKWASLWNWFDITKWLWYIKYGVYIVK-biotin. Design and preparation of MPER scaffold T93 has been described (25).
ELISPOT
PVDF Enzyme-linked immunosorbent spot (ELISPOT) plates (Millipore, Billerica, MA, USA) were coated with 10 μg/mL of the MPER scaffold T93 in PBS and incubated overnight at 4°C. The rest of the assay was performed as previously described (26).
Statistical analysis
Statistical analyses were performed as described in the accompanying article.
RESULTS
Generation of 4E10 H and L knock-in mice
As in the accompanying article, C57BL/6 ES cells were modified by gene targeting to introduce HIV antibody H- and L-chain variable exons, replacing the respective J clusters. Targetings were verified by PCR assay and Southern blotting (Suppl. Figs. 1,2).
Analysis of 4E10 knock-in mice reveals evidence of physiological autoreactivity
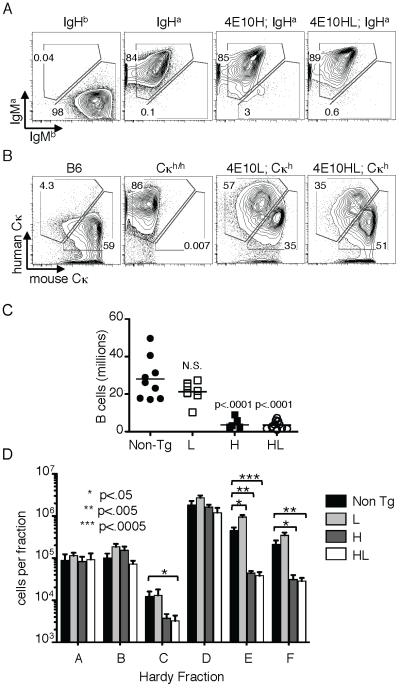
Usage of targeted H-chains was analyzed in B cells of 4E10H and HL mice carrying an IgHa wild type allele (the targeted allele was IgHb). Unlike b12 mice (see accompanying paper), 4E10HL mice and 4E10H mice failed to demonstrate efficient feedback suppression of endogenous Ig genes, as indicated by extensive expression on splenic B cells of IgM derived from the wild type allele (IgMa) (Fig. 1A). Analysis of κ allele usage in 4E10L and HL mice similarly showed frequent usage of the endogenous allele (marked using a human Cκ targeted replacement allele, Cκh (24)), however about half of the cells appeared to express only the targeted 4E10L chain (Fig. 1B). Furthermore, 4E10H and HL mice had significantly reduced splenic B cell numbers (Fig. 1C). Analysis of BM B cell fractions revealed a block in B cell development in 4E10H and HL mice, primarily at the preB to B cell transition (Hardy fraction D to E (27)), however, there was also a reduction in the late proB cell fraction C (Fig. 1D).
Figure 1.
B cell development and allelic inclusion in 4E10 mice.
A,B, Knock-in mice carrying serologically distinguishable endogenous H and L chain constant regions were generated by breeding homozygous knock-in mice with IgHa/a; Cκh/h mice. Spleen B cells of the indicated genotypes were assessed by flow cytometry using antibodies against IgMb and mouse Cκ, to detect the transgene H and L alleles, respectively, and antibodies against IgMa and human Cκ to identify chains derived from endogenous genes. Data is representative of results obtained with 4-10 mice/group. C, Reduced splenic B cell numbers in 4E10 knock-in mice. B220+CD19+ splenic B cells were enumerated from mice of the indicated genotypes. Each data point represents the value obtained in one mouse. Data were from at least three independent experiments. D, Analysis of BM B cell developmental fractions according to the staining scheme of Hardy (27). Fractions represent developmental stages as follows: A-C, proB; D, small preB; E, immature B; F, recirculating B. Number of mice analyzed was 6 for 4E10HL and 4 for the other genotypes, carried out in one experiment.
Weak, but reproducible, spontaneous anti-gp41 secretion in 4E10HL mice
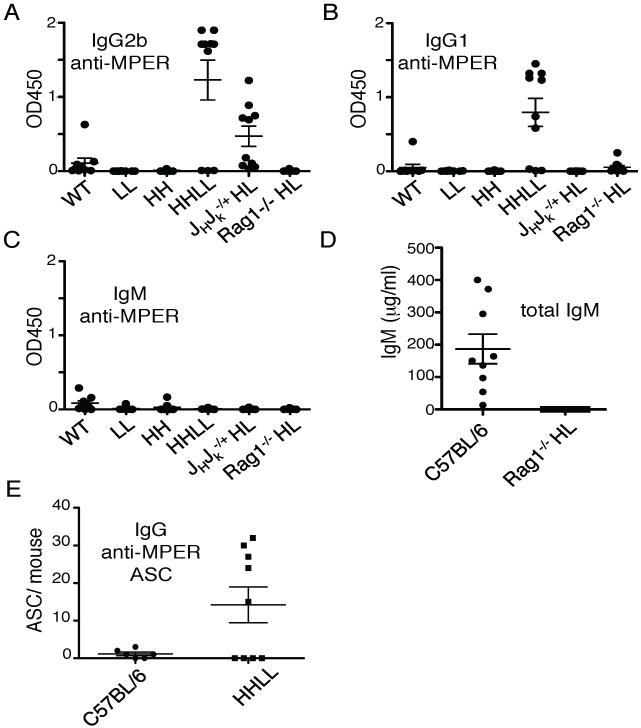
Despite the poor retention of 4E10 BCR expression on most B cells, analysis of serum antibodies revealed that 4E10HL mice, but not mice carrying only the H or L transgene alone, expressed detectable serum IgG anti-gp41 activity (Fig. 2A). We presume that this indicates that there was some secretion of 4E10HL-like antibody. Serum IgM anti-gp41 activity was also seen, though this correlated less well with coexpression of 4E10 H and L (Fig. 2B). Moreover, a subset of 4E10HL mice produced IgG reactive with cardiolipin (Fig. 2C). Consistent with the notion that 4E10 is an autoantibody, the 4E10HL sera expressing the highest levels of IgG anti-gp41 activity also contained IgG reactive with cardiolipin (r=0.9, Fig. 2D), whereas sera from 4E10H and 4E10L mice lacked both activities (Fig. 2A,C). Total Ig levels in 4E10HL mice were slightly subnormal, though this was only statistically significant for IgG1 (Fig. 3). We therefore conclude that in 4E10HL mice some 4E10-like antibody was produced despite the obvious negative regulation of B cells carrying this specificity.
Figure 2.
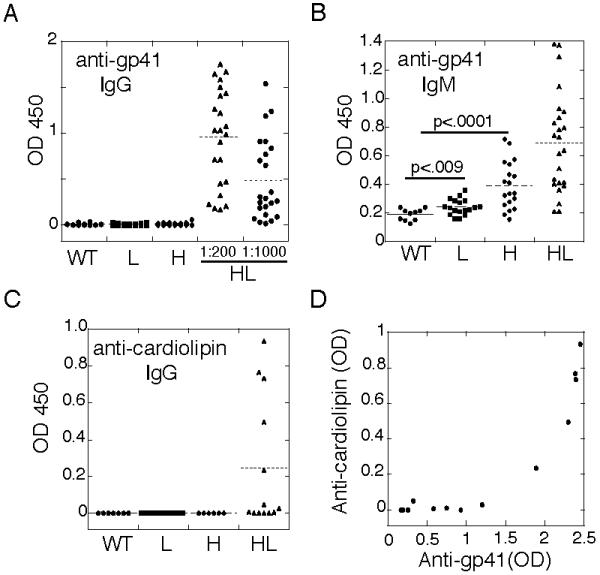
Analysis of spontaneous anti-gp41 activity in the sera of 4E10 HL mice. Sera from mice of the indicated genotypes were tested for reactivity to gp41 and were from mice 6-10 weeks old. A,B, Total IgG and IgM anti-gp41 activity of normal sera from individual adult mice of the indicated genotypes. Readings were from ELISA using serum at 1:200 dilution unless otherwise indicated. C, IgG anti-cardiolipin assay. D, Correlation between anti-gp41 and anti-cardiolipin IgG activity.
Figure 3.
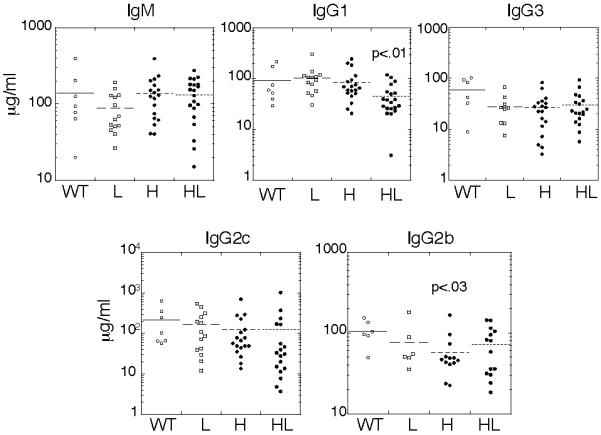
Normal serum Ig levels of mice of the indicated genotypes carrying one or no copies of the knock-in H and L alleles. Each point represents the levels measured in an individual mouse.
Enforced expression of 4E10 leads to B cell deletion
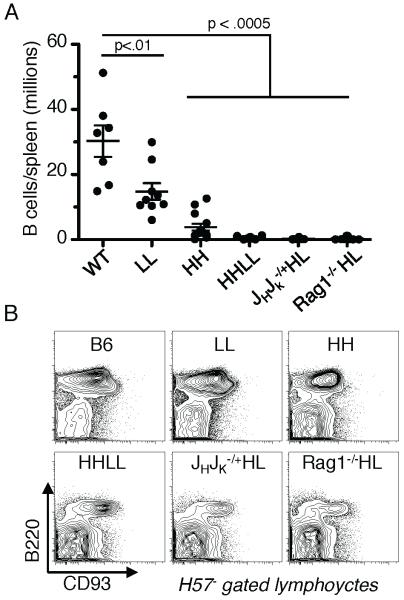
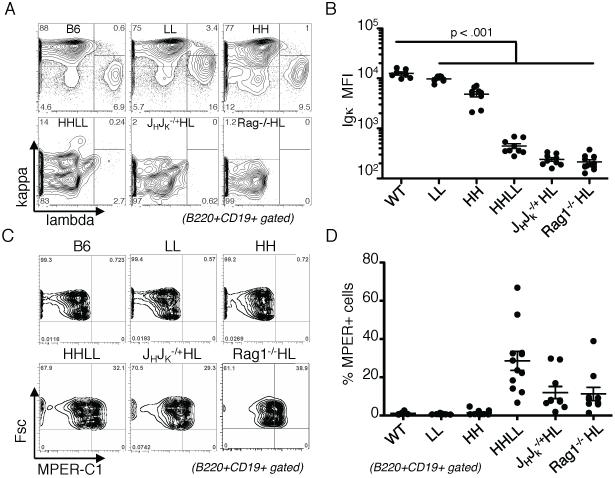
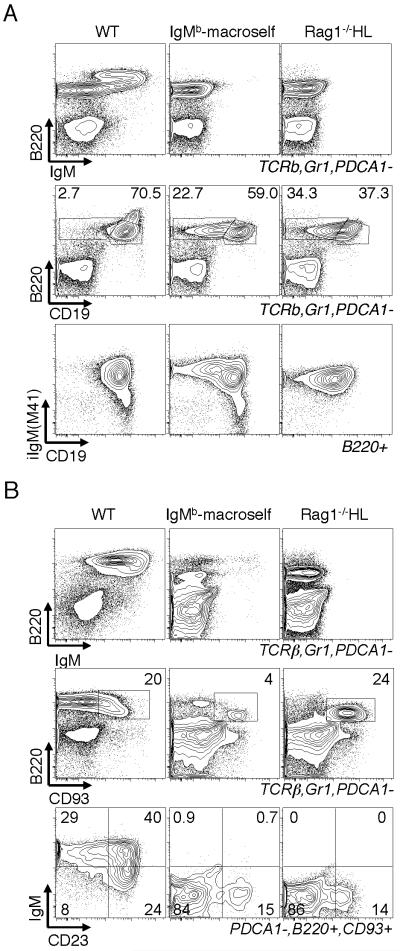
In an effort to enforce 4E10 BCR expression, we carried out genetic strategies to increase the dosage of transgenic receptors or to reduce editing by competing endogenous receptors. We generated mice homozygous for the 4E10 H chain knock-in (HH mice), L chain knock-in (LL mice) or compound homozygotes (HHLL mice). In addition, we bred 4E10HL mice to the Rag1−/− background or to mice with deletions of JH and JCκ clusters, yielding Rag1−/−;HL and JH +/− Jκ+/−; HL mice, respectively. If the 4E10 BCR were innocuous and reduced BCR expression was the cause of the subnormal B cell development, it should be to some extent rescued in HHLL mice. In contrast, if 4E10 were autoreactive, the increased barriers to editing and increased BCR expression in the 4E10 BCR in HHLL mice would lead to stronger negative selection and a further decrease in B cells. B cell numbers in the periphery were indeed further reduced, rather than rescued, by increasing knock-in gene copy number or suppressing endogenous Ig gene rearrangements (Fig. 4A). Total B cell numbers in mice with enforced 4E10 BCR expression amounted to <106/spleen. In addition, those B cells that populated the spleen were made up of a much higher proportion of CD93+ cells (Fig. 4B). These few remaining cells had reduced BCR density (Fig. 5A,B), yet some carried low levels of 4E10HL receptors, as indicated by their ability to bind to MPER peptide tetramerized on streptavidin (Fig. 5C,D). HH or LL mice, which carry the same transgenes without their partner, had higher BCR surface densities than HHLL mice, indicating that each individual chain was well expressed, and suggesting that B cells expressing both chains had downregulated their BCRs. Consistent with this interpretation, BM B cells in Rag1−/−;HL mice included a major subset with downregulated IgM and CD19 levels (Fig. 6A right column of panels). The B220+CD19low population (left box in central panel) is diagnostic of cells undergoing prolonged attempts at receptor editing (28). As a positive control in this experiment, we used BM cells from IgMb-macroself transgenic mice, which defined this phenotype (28)(middle column of panels). The rare splenic B cells of Rag1−/−;HL mice were CD93+, showed downregulated IgM, and had relatively low levels of CD23 (Fig. 6B), placing the cells in the T3′ category of highly autoreactive B cells. Overall, we conclude that the 4E10 BCR is, to a physiologically significant extent, autoreactive and is counterselected by various mechanisms including receptor editing, clonal deletion and receptor downregulation.
Figure 4.
Effect of enforcing 4E10 expression on B cell numbers and maturation. Spleen cells of mice homozygous for 4E10 H or L targeted loci, or double homozygotes (respectively LL, HH and HHLL), were compared to 4E10HL hemizygous mice deficient in the second allele (JHJk−/+HL) or lacking Rag1 (Rag1−/−HL). A, Total B cell numbers (CD19+B220+) in spleens. B, Analysis of CD93 and B220 coexpression in splenic lymphocytes gated to exclude TCRβ+ cells using MAb H57.
Figure 5.
Analysis of BCR surface density and MPER binding among splenic B cells from mice with enforced 4E10 expression. A,B, Analysis of sIgL density on splenic B cells of mice of the indicated genotypes. C,D, Analysis of the frequency of MPER binding splenic B cells using the biotinylated C1 peptide. Values from individual mice obtained over at least 2 experiments are indicated by dots.
Figure 6.
BCR internalization and developmental block of B cells in Rag1−/−HL mice. A, Analysis of BM B cells for surface markers B220, IgM, CD19 and intracellular IgM (iIgM). In the top two rows of panels BM cells were gated to exclude most myeloid and T cells. For iIgM analysis (lower row) cells were B220+ gated. IgMb-macroself Tg mice express a superantigen reactive to IgMb (28). B, Analysis of spleen B cells from mice of the indicated genotype. Second row of panels shows the gating used for the analysis on the lower row.
Anti-gp41 secretion in 4E10 mice with enforced transgene expression
These data raised the question of how the 4E10 B cell arose in the patient from whom it was isolated. As mentioned above, 4E10HL mice produced anti-gp41 serum antibodies. We therefore asked if MPER-reactive antibodies were still made in the mice with enforced 4E10 expression. Interestingly, low, but reproducible levels of IgG2a and IgG1 anti-MPER activity were found in most HHLL and JH +/− Jκ+/−; 4E10HL sera, but little to none was detected in Rag1−/−;4E10HL sera(Fig. 7A,B). IgM anti-MPER levels were uniformly low (Fig. 7C). Consistent with these findings, Rag1−/−;4E10HL mice lacked any detectable serum IgM. As Rag1−/−;4E10HL mice are unable to express any endogenous Ig chains and lack T cells, we infer that anti-MPER production might require T cells, B cells expressing a mixture of both endogenous and 4E10 Ig molecules, or both. In any case, 4E10-like antibody levels were low and maintained by a surprisingly small number of plasma cells, as determined by ELISPOT analysis of HHLL mice (Fig. 7E). Most anti-MPER plasma cells were found in the spleen and mesenteric LN, but combined analysis of several lymphoid organs enumerated fewer than 100 antibody-secreting cells (ASC) combined per mouse (Fig. 7E). Overall, though tolerance is evidently not complete, these data establish that 4E10HL B cells are negatively selected even when B cell competition is lacking and editing is inhibited.
Figure 7.
Analysis of spontaneous anti-MPER activity in the sera of in mice with limited ability to edit because of 4E10 gene homozygosity or gene knockout. A,B, Analysis of IgG2b and IgG1 anti-MPER titers in mice of the indicated genotypes. C, IgM anti-MPER levels in the same samples shown in A,B. D, Total IgM levels in Rag1−/−;HL mice compared to WT controls. E, Assessment of IgG anti-MPER antibody forming cell (AFC) numbers in HHLL mice. BM, SP and selected LN were assessed for anti-gp41 activity using an ELISpot assay. Serological assays shown were carried out in a single experiment for optimal comparison, but were similar to results in assays done with the same sera at different dilutions or on different days. Each point represents the mean value from an independent mouse.
DISCUSSION
B cells in knock-in mice expressing 4E10 are negatively regulated by immune tolerance processes. In contrast to b12 mice (discussed in the companion paper), 4E10HL mice clearly have suppressed B cell production, mainly by central tolerance and clonal deletion, leading to B cell lymphopenia. The remaining B cells that appear in the periphery of these mice have lost 4E10H chain expression and a substantial fraction also failed to express 4E10L. However, analysis of normal sera indicated that at least some B cells in this model make 4E10 antibody. It is likely that the phenotype of 4E10 knock-in mice is a consequence of 4E10’s autoreactivity, as might have been predicted by Haynes et al and subsequent studies (20). (However, the claim that b12 is autoreactive (19) was not supported by our studies presented in the companion manuscript.) Our findings, along with the study by Verkoczy et al on 2F5 knock-in mice (21), underscore the value of biological measures of autoreactivity. In this regard, it is of interest that B cell line transfectants expressing 4E10HL appeared to be difficult to propagate and appeared to have desensitized receptors, while cells carrying b12HL were propagated more easily and could signal Ca++ mobilization upon stimulation with soluble trimers (29).
Splenic B cells of 4E10 HHLL mice had downregulated BCRs compared to 4E10 HH or LL mice. As 4E10 HH and LL splenic B cells individually had surface BCR expression >10-fold higher than B cells coexpressing 4E10H and L chains together, the 4E10 transgenes appear to be well expressed. Similar transgenic expression is predicted for b12 (see accompanying paper) and 4E10 mice because we used identical targeting vectors, including promoter elements. We infer that the low B cell numbers and expression of endogenous H chains among escaped B cells in 4E10HL mice were the result of inefficient H chain editing followed by the preferential survival of cells that eliminated the 4E10 specificity through expression of endogenous Ig chains. 4E10H mice also had reduced B cell numbers. The 4E10 H-chain CDR3 is quite hydrophobic, and might yield an autoreactive antibody when combined with a multitude of endogenous L-chains. The 4E10 H-chain locus lacks unrearranged JH elements, but might be silenced by recombinase-mediated destruction by D-to-VDJ rearrangements at cryptic heptamer sites (30) or be replaced by VH-to-VDJ rearrangements, which can be functional (31). When endogenous Ig rearrangements were blocked in Rag1−/−;4E10HL mice, B cell development was aborted at the preB/B cell transition with the few remaining B cells exhibiting BCR down-modulation.
The autoreactivity and negative selection of 4E10 B cells established in this study, along with similar data in the 2F5 system (21), and an independently generated 4E10 mouse (Laurent Verkoczy, Duke, published while this paper was in revision (32)), raise the questions of how 4E10 was initially produced and whether it would be effective or desirable to target the MPER for human vaccination. A larger epitope overlapping with those of 2F5 and 4E10 is seen by the broadly neutralizing antibody 10E8 (33). 10E8 is both more potent than 4E10 and 2F5, and unlike 4E10, appears not to be polyreactive. It would be of interest to confirm this in a 10E8 knock-in model and to assess in both knock-in models the immunization conditions that promote or prevent antibody formation to defined MPER epitopes. Short of barring a B cell response, a tolerance barrier could slow the evolution of B cell maturation in the germinal center toward desirable epitopes, particularly if the autoantigen in question is present in the germinal center environment (34, 35). One possible explanation for the autoreactivity of both 2F5 and 4E10 is that both recognize relatively short, linear peptide determinants, which by chance might have many cross-reactive homologues in the body. A high affinity autoantigen recognized by 2F5 has been identified (20), but even low affinity interactions can efficiently promote B cell tolerance when the autoantigen is sufficiently accessible (36, 37). It is tempting to speculate, but premature to conclude, that conformational epitopes might be more specific and hence safer vaccine targets than linear ones.
Supplementary Material
Acknowledgements
The authors thank the TSRI Mouse Genetics facility for excellent technical assistance, Roberta Pelanda (NJH) for the pVKR2neo targeting vector, and Dennis Huszar, GenPharm Intl, Marilia Cascalho, U Michigan, and Michel Nussenzweig, Rockefeller Univ. for Jκ−/−, JH−/− and hCκ mice, respectively.
Abbreviations used
- BM
bone marrow
- bNAb
broadly neutralizing antibody
- Env
envelope
- LN
lymph nodes
- MPER
membrane proximal external region
- SP
spleen
REFERENCES
- 1.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 2.Conley AJ, Kessler JA, 2nd, Boots LJ, McKenna PM, Schleif WA, Emini EA, Mark GE, 3rd, Katinger H, Cobb EK, Lunceford SM, Rouse SR, Murthy KK. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J. Virol. 1996;70:6751–6758. doi: 10.1128/jvi.70.10.6751-6758.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gauduin MC, Parren PW, Weir R, Barbas CF, Burton DR, Koup RA. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat. Med. 1997;3:1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- 4.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 5.Mascola JR, Lewis MG, Stiegler G, Harris D, VanCott TC, Hayes D, Louder MK, Brown CR, Sapan CV, Frankel SS, Lu Y, Robb ML, Katinger H, Birx DL. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parren PW, Ditzel HJ, Gulizia RJ, Binley JM, Barbas CF, 3rd, Burton DR, Mosier DE. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a neutralizing human monoclonal antibody against the gp120 CD4-binding site. AIDS. 1995;9:F1–6. doi: 10.1097/00002030-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 2009;15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 10.Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton DR, Stanfield RL, Wilson IA. Antibody vs. HIV in a clash of evolutionary titans. Proc.Natl.Acad.Sci.U.S.A. 2005;102:14943–14948. doi: 10.1073/pnas.0505126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauer A. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res.Hum.Retroviruses. 1994;10:359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 13.Kunert R, Wolbank S, Stiegler G, Weik R, Katinger H. Characterization of molecular features, antigen-binding, and in vitro properties of IgG and IgM variants of 4E10, an anti-HIV type 1 neutralizing monoclonal antibody. AIDS Res.Hum.Retroviruses. 2004;20:755–762. doi: 10.1089/0889222041524571. [DOI] [PubMed] [Google Scholar]
- 14.Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, Moore JP, Stiegler G, Katinger H, Burton DR, Parren PW. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 2001;75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardoso RM, Zwick MB, Stanfield RL, Kunert R, Binley JM, Katinger H, Burton DR, Wilson IA. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity. 2005;22:163–173. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Ofek G, Tang M, Sambor A, Katinger H, Mascola JR, Wyatt R, Kwong PD. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J. Virol. 2004;78:10724–10737. doi: 10.1128/JVI.78.19.10724-10737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam SM, Morelli M, Dennison SM, Liao HX, Zhang R, Xia SM, Rits-Volloch S, Sun L, Harrison SC, Haynes BF, Chen B. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20234–20239. doi: 10.1073/pnas.0908713106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherer EM, Leaman DP, Zwick MB, McMichael AJ, Burton DR. Aromatic residues at the edge of the antibody combining site facilitate viral glycoprotein recognition through membrane interactions. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1529–1534. doi: 10.1073/pnas.0909680107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 20.Yang G, Holl TM, Liu Y, Li Y, Lu X, Nicely NI, Kepler TB, Alam SM, Liao HX, Cain DW, Spicer L, Vandeberg JL, Haynes BF, Kelsoe G. Identification of autoantigens recognized by the 2F5 and 4E10 broadly neutralizing HIV-1 antibodies. J. Exp. Med. 2013 doi: 10.1084/jem.20121977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verkoczy L, Chen Y, Bouton-Verville H, Zhang J, Diaz M, Hutchinson J, Ouyang YB, Alam SM, Holl TM, Hwang KK, Kelsoe G, Haynes BF. Rescue of HIV-1 broad neutralizing antibody-expressing B cells in 2F5 VH × VL knockin mice reveals multiple tolerance controls. J. Immunol. 2011;187:3785–3797. doi: 10.4049/jimmunol.1101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vcelar B, Stiegler G, Wolf HM, Muntean W, Leschnik B, Mehandru S, Markowitz M, Armbruster C, Kunert R, Eibl MM, Katinger H. Reassessment of autoreactivity of the broadly neutralizing HIV antibodies 4E10 and 2F5 and retrospective analysis of clinical safety data. AIDS. 2007;21:2161–2170. doi: 10.1097/QAD.0b013e328285da15. [DOI] [PubMed] [Google Scholar]
- 23.Scherer EM, Zwick MB, Teyton L, Burton DR. Difficulties in eliciting broadly neutralizing anti-HIV antibodies are not explained by cardiolipin autoreactivity. AIDS. 2007;21:2131–2139. doi: 10.1097/QAD.0b013e3282a4a632. [DOI] [PubMed] [Google Scholar]
- 24.Casellas R, Shih TA, Kleinewietfeld M, Rakonjac J, Nemazee D, Rajewsky K, Nussenzweig MC. Contribution of receptor editing to the antibody repertoire. Science. 2001;291:1541–1544. doi: 10.1126/science.1056600. [DOI] [PubMed] [Google Scholar]
- 25.Correia BE, Ban YE, Holmes MA, Xu H, Ellingson K, Kraft Z, Carrico C, Boni E, Sather DN, Zenobia C, Burke KY, Bradley-Hewitt T, Bruhn-Johannsen JF, Kalyuzhniy O, Baker D, Strong RK, Stamatatos L, Schief WR. Computational design of epitope-scaffolds allows induction of antibodies specific for a poorly immunogenic HIV vaccine epitope. Structure. 2010;18:1116–1126. doi: 10.1016/j.str.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Avery DT, Kalled SL, Ellyard JI, Ambrose C, Bixler SA, Thien M, Brink R, Mackay F, Hodgkin PD, Tangye SG. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J. Clin. Invest. 2003;112:286–297. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duong BH, Ota T, Aoki-Ota M, Cooper AB, Ait-Azzouzene D, Vela JL, Gavin AL, Nemazee D. Negative selection by IgM superantigen defines a B cell central tolerance compartment and reveals mutations allowing escape. J. Immunol. 2011;187:5596–5605. doi: 10.4049/jimmunol.1102479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ota T, Doyle-Cooper C, Cooper AB, Huber M, Falkowska E, Doores KJ, Hangartner L, Le K, Sok D, Jardine J, Lifson J, Wu X, Mascola JR, Poignard P, Binley JM, Chakrabarti BK, Schief WR, Wyatt RT, Burton DR, Nemazee D. Anti-HIV B Cell Lines as Candidate Vaccine Biosensors. J. Immunol. 2012;189:4816–4824. doi: 10.4049/jimmunol.1202165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taki S, Schwenk F, Rajewsky K. Rearrangement of upstream DH and VH genes to a rearranged immunoglobulin variable region gene inserted into the DQ52-JH region of the immunoglobulin heavy chain locus. Eur. J. Immunol. 1995;25:1888–1896. doi: 10.1002/eji.1830250715. [DOI] [PubMed] [Google Scholar]
- 31.Chen C, Nagy Z, Prak EL, Weigert M. Immunoglobulin heavy chain gene replacement: a mechanism of receptor editing. Immunity. 1995;3:747–755. doi: 10.1016/1074-7613(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Zhang J, Hwang KK, Bouton-Verville H, Xia SM, Newman A, Ouyang YB, Haynes BF, Verkoczy L. Common Tolerance Mechanisms, but Distinct Cross-Reactivities Associated with gp41 and Lipids, Limit Production of HIV-1 Broad Neutralizing Antibodies 2F5 and 4E10. J. Immunol. 2013 doi: 10.4049/jimmunol.1300770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aït-Azzouzene D, Kono DH, Gonzalez-Quintial R, McHeyzer-Williams LJ, Lim M, Wickramarachchi D, Gerdes T, Gavin AL, Skog P, McHeyzer-Williams MG, Nemazee D, Theofilopoulos AN. Deletion of IgG-switched autoreactive B cells and defects in Fas(lpr) lupus mice. J. Immunol. 2010;185:1015–1027. doi: 10.4049/jimmunol.1000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan TD, Wood K, Hermes JR, Butt D, Jolly CJ, Basten A, Brink R. Elimination of germinal-center-derived self-reactive B cells is governed by the location and concentration of self-antigen. Immunity. 2012;37:893–904. doi: 10.1016/j.immuni.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 36.Lang J, Jackson M, Teyton L, Brunmark A, Kane K, Nemazee D. B cells are exquisitely sensitive to central tolerance and receptor editing induced by ultralow affinity, membrane-bound antigen. J. Exp. Med. 1996;184:1685–1697. doi: 10.1084/jem.184.5.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell DM, Dembic Z, Morahan G, Miller JF, Burki K, Nemazee D. Peripheral deletion of self-reactive B cells. Nature. 1991;354:308–311. doi: 10.1038/354308a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.