Summary
The cysteinyl leukotrienes (cys-LTs) are three structurally similar, but functionally distinct lipid mediators of inflammation. The parent cys-LT, LTC4, is synthesized by and released from mast cells, eosinophils, basophils, and macrophages, and is converted to the potent constrictor LTD4 and the stable metabolite, LTE4. While only two cys-LT-selective receptors (CysLTRs) have been identified, cloned, and characterized, studies dating back three decades predicted the existence of at least three functional CysLTRs, each with a characteristic physiological function in airways and other tissues. The recent demonstration that mice lacking both known CysLTRs exhibit full (and in some instances, augmented) physiological responses to cys-LTs verifies the existence of unidentified CysLTRs. Moreover, the ability to manipulate receptor expression in both whole animal and cellular systems reveals that the functions of CysLTRs are controlled at multiple levels, including receptor-receptor interactions. Finally, studies in transgenic mice have uncovered a potentially major role for cys-LTs in controlling the induction of Th2 responses to common allergens. This review focuses on these recent findings and their potential clinical implications.
Introduction
The cysteinyl leukotrienes (cys-LTs) are highly potent peptide-conjugated arachidonic acid-derived mediators closely linked to the pathobiology of allergic inflammation [1, 2]. Cys-LTs are also now thought to play roles in the pathophysiology of cardiovascular disease [3], cancer [4], fibrosis [5], and immune host defence[6]. Thus, a comprehensive understanding of the cellular targets and mechanisms of action of cys-LTs is potentially important to all of these processes. Cys-LTs were the first specific mediators successfully targeted for drug development in asthma [7–9], and cys-LT targeted drugs are now widely prescribed. While efficacious and useful, clinical responses to existing antagonists of the type 1 cysteinyl leukotriene receptor (CysLT1 receptor) are heterogeneous [10]. Furthermore, increasingly precise molecular tools and transgenic animal studies have revealed that cys-LTs and their receptors comprise of a much more complex network than originally thought. This complexity is likely due to cross-regulation [11, 12] and physical complexes among the receptors [13, 14], close interactions with other receptors, and novel receptors [15, 16]. Each of these factors carries substantial implications for the modulation of the cys-LT system for therapy. The purpose of this review is to contextualize this complexity in terms of its potential therapeutic implications.
Production of cysteinyl leukotrienes
Cysteinyl leukotrienes are generated by eosinophils, basophils, mast cells, macrophages, and myeloid dendritic cells in response to activation [17]. In each cell type, arachidonic acid is oxidized by 5-lipoxygenase (5-LO) to generate the unstable precursor leukotriene A4 (LTA4) [18], and subsequently leukotriene C4 synthase (LTC4S) converts LTA4 to the parent cys-LT, LTC4 [19]. After energy-dependent export from the cell [20], LTC4 is sequentially converted to LTD4 [21], and then to the final and most stable cys-LT, LTE4 [22]. Thus, three different ligands (LTC4, LTD4, and LTE4) arise from a single intracellular synthetic event by successive enzymatic conversions. In addition to this intracellular pathway, there is also a transcellular mechanism for cys-LT generation that can be carried out by cells that express LTC4S but not the proximal enzyme 5-LO in the pathway (e.g. platelets, endothelial cells). In the latter mechanism, the LTC4S-expressing cells can convert extracellular LTA4 (released by neutrophils or other cells with an active 5-LO enzyme) [23], and may serve as an additional source of cys-LTs in certain inflammatory states. Indeed, a recent study demonstrated that adherent platelets accounted for ~60% of the LTC4S activity and cys-LT generation by granulocytes from individuals with aspirin exacerbated respiratory disease (AERD), a disorder consistently associated with high levels of cys-LT production (see below) [24].
The relevance of cysteinyl leukotrienes to asthma
The bioactivities of the cys-LTs in the pre-clinical setting, particularly their potency as smooth muscle constrictors, spurred interest in these mediators as potential therapeutic targets in asthma. The ability to monitor urinary levels of LTE4 as a reflection of systemic cys-LT generation in vivo allowed for the proof that cys-LTs are generated by subjects with acute asthma exacerbations [2]. Individuals with AERD, a variant of asthma characterized by nasal polyposis, chronic eosinophilic sinusitis, and idiosyncratic respiratory reactions induced upon ingestion of aspirin or other drugs that block COX-1, have especially high baseline levels of urinary LTE4 (3–4-fold higher than aspirin-tolerant asthmatic controls), and a marked (approximately tenfold) further increment in these levels in response to oral challenge with aspirin [25]. The role of cys-LTs in asthma has been well validated by clinical trials using the available drugs. The 5-LO inhibitor zileuton, which reduces urinary cys-LT excretion by ~50%, and selective antagonists of CysLT1 receptor both improve lung function, reduce the frequency of asthma exacerbations [7, 26], and reduce the severity of reactions to aspirin challenge in individuals with AERD [27]. Collectively, these findings establish a role for the cys-LTs as pertinent mediators of asthma, particularly in AERD, due to the high levels of cys-LTs generated in this disease variant.
Physiological and pharmacological evidence for multiple cysteinyl leukotriene receptors
While the three cys-LTs share certain functions in vivo, including smooth muscle contraction and vascular leak [28–30], there were important differences identified in early studies that suggested additional and distinct functions for each. Pharmacological profiling of guinea-pig lung demonstrated that LTC4 and LTD4 were equipotent as constrictors, whereas LTE4 was inactive. Remarkably, however, LTE4 was 10-times more potent for inducing guinea-pig tracheal ring contractions in vitro than was LTC4 or LTD4 [31, 32]. Intravenously administered LTC4 and LTD4 elicited large changes in dynamic lung compliance without major effects on resistance (indicative of primarily peripheral airway effects), whereas LTE4 elicited a robust increase in resistance (indicative of effects on the central airways), and changes in compliance [31]. Furthermore, LTE4, but not LTC4 or LTD4, enhanced contractile responses of guinea-pig tracheal smooth muscle to histamine. This priming effect of LTE4 for histamine responsiveness was prevented by pre-treatment of the tracheal tissue with indomethacin, indicating a key collaborative role for a cyclooxygenase (COX) product [32], putatively identified as thromboxane A2 [33]. Together, these in vitro and in vivo functional findings predicted the existence of at least three receptors for cys-LTs: a high affinity receptor for LTD4, a lower affinity receptor for LTC4, and a separate receptor for LTE4, with the latter potentially capable of eliciting the secondary production of a prostanoid.
Studies on human subjects also provided compelling evidence for the existence of at least three receptors for cys-LTs. In both non-asthmatic and asthmatic subjects, inhalation of LTC4 and LTD4 elicited airflow obstruction at doses several 1000-fold lower than histamine [30, 34, 35]. Although, LTE4 was only ~40-fold more potent than histamine in reducing expiratory flow rates in non-asthmatic subjects [36], asthmatic subjects were much more sensitive to LTE4, as they developed airflow obstruction after inhalation of LTE4 at doses 10-fold lower than the doses required to elicit this response in non-asthmatic controls. However, asthmatic and non-asthmatic subjects had equivalent dose-responses to LTC4 and LTD4 [29]. Moreover, subjects with AERD demonstrated even greater (16-fold) sensitivity to LTE4-induced bronchoconstriction than did aspirin-tolerant asthmatic controls [37], but their responses to LTC4 and histamine were similar to those in aspirin-tolerant controls. In another study, inhalation of LTE4, but not of LTD4, provoked the accumulation of eosinophils and basophils into the bronchial mucosa and sputum of asthmatic subjects when the two cys-LTs were administered at doses titrated to produce an equivalent degree of bronchoconstriction [38]. Inhalation of LTE4 also enhanced the sensitivity of asthmatic subjects to histamine-induced bronchoconstriction, an effect that was blocked by pre-treatment with oral indomethacin [39]. These studies support the concept that LTE4 acts through a receptor(s) that is distinct from those responsible for the actions of LTC4 and LTD4, and suggest that expression and/or function of the LTE4 receptor may be selectively up-regulated in asthma, specifically enhancing pulmonary responsiveness to it. Lastly, as was the case for guinea-pig trachea, LTE4 can potentiate hyperresponsiveness to histamine by the induction of or collaboration with COX products.
Molecular pharmacology of the CysLTRs and cross-regulation of the CysLT1 receptor
The CysLT1 and CysLT2 receptors are both G-protein coupled receptors (GPCRs) and were cloned and characterized several years after the original descriptive pharmacology predicted their properties. These two GPCRs arise from different chromosomes and share only 38% sequence homology [40, 41]. Human and mouse CysLT1 receptors are 87% identical [42] and the CysLT2 receptors are 74% identical [43], suggesting a high level of functional conservation through evolution. Both receptors are structural homologues of the purinergic (P2Y) receptors that are specialized to recognize extracellular nucleotides [44], with 25–34% identity at the amino acid level. CysLT1 receptor binds LTD4 with high affinity (10−9 M) and LTC4 with lesser affinity (10−8 M), whereas CysLT2 receptor binds both LTC4 and LTD4 with equal affinity (10−8 M). Neither receptor exhibits substantial affinity for LTE4 in radioligand binding assays nor does LTE4 elicit strong signalling responses in cells expressing CysLT1 receptor or CysLT2 receptor in isolation [16, 40, 41]. It is therefore unlikely that the pharmacology of LTE4 in vivo is attributable to CysLT1 receptor and CysLT2 receptor alone.
The CysLT1 and CysLT2 receptors are broadly expressed by structural and hematopoietic cells. Some cell types (vascular smooth muscle) express mostly Cys-LT1 receptors [40], whereas others (endothelial cells) dominantly express CysLT2 receptors [43]. Both receptors are expressed by cells of the innate (macrophages, monocytes, eosinophils, basophils, mast cells, dendritic cells) and adaptive (T cells, B cells) immune system, implying potentially cooperative functions in immunity and inflammation [17]. Studies of human mast cells ex vivo showed cys-LTs promoted proliferation of the cells through transactivation of the c-kit tyrosine kinase [45] and downstream phosphorylation of extracellular signal regulated kinase (ERK), and also induced cytokine generation [46]. LTD4-induced mast cell proliferation and cytokine production were completely eliminated by knockdown of CysLT1 receptors [12, 13] or by blockade of CysLT1 receptors with MK571 [45, 46], an early prototype of the clinically available CysLT1 receptor-selective antagonists. These studies suggested that the functions of cys-LTs included a direct role as agonists for activation of immune effector cells.
As the CysLT1 and CysLT2 receptors both mediate calcium flux and activate signalling cascades when expressed in isolation [47], it was surprising that blockade or knockdown of CysLT1 receptors in mast cells eliminated most LTD4-mediated signalling [13, 44, 48] despite the presence of CysLT2 receptors on these cells. In fact, the knockdown of CysLT2 receptors substantially potentiated LTD4-induced signalling and cytokine generation [13]. The absence of CysLT2 receptors resulted in approximately twofold higher levels of CysLT1 receptor protein expressed on the cell surface, without changing the total cellular content of CysLT1 receptors. CysLT1 receptors and CysLT2 receptors were found to heterodimerize in primary mast cells, a relatively common feature of GPCRs that recognizes similar ligands [49]. Thus CysLT2 receptors, by interacting with CysLT1 receptors, limit the surface expression levels and signalling ability of the latter receptors, at least on mast cells. This cross-regulation is likely to be important in vivo, since mice lacking CysLT2 receptors (cysltr2−/− mice) show exaggerated ear swelling responses to direct intracutaneous challenges to LTD4 relative to wild-type controls [15]. The absence of CysLT2 receptors may also facilitate the formation of CysLT1 receptor homodimers [41] that are strong signalling units for LTD4 (Fig. 1).
Fig. 1.
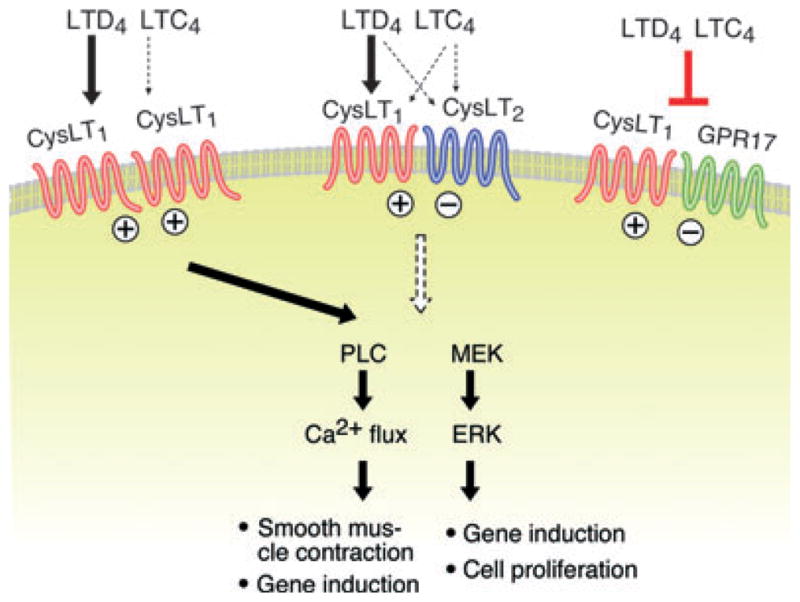
Homotypic and heterotypic interactions of the CysLT1 receptor. CysLT1 receptors can form homodimers that bind LTD4 with high affinity and LTC4 with lesser affinity, causing both calcium flux and extracellular signal regulated kinase activation that lead to smooth muscle contraction, cytokine gene induction, and cell proliferation. Both CysLT2 receptors and GPR17 can heterodimerize with CysLT1 receptors, attenuating signalling through the latter receptor and, in the case of GPR17, markedly attenuating ligand binding. (+) signs indicate positive signalling, (−) signs denote inhibitory signalling, and ⊥ indicates blockade of ligand binding,
The CysLT2 receptor is not the only GPCR likely to cross-regulate the functions of CysLT1 receptors in vivo. A P2Y-like GPCR termed GPR17 shows sequence homology to the CysLTRs, and was reported to be a dual-responsive receptor for both cys-LTs and nucleotides when over-expressed in an astrocytoma cell line [50]. GPR17 heterodimerizes with CysLT1 receptors in transfectants and in primary macrophages [14], but does not exhibit specific binding to LTD4 in these contexts. Instead, co-transfection of CysLT1 receptors with GPR17 markedly reduced the binding affinity of CysLT1 receptors for LTD4 (Fig. 1) Importantly, mice lacking GPR17 [51] show exaggerated Th2 responses and eosinophilic pulmonary inflammation in the house dust mite-induced model of pulmonary disease, whereas mice lacking CysLT1 receptors (cysltr1−/− mice) [14] or LTC4 synthase (ltc4s−/− mice) [52] show dramatically attenuated responses in this model. In addition, in vitro studies indicate that CysLT1 receptors can be heterologously desensitized by nucleotides that signal through its homologues, the P2Y1, P2Y2, P2Y4, and P2Y6 receptors, through protein kinase C-induced phosphorylation [11]. Collectively, these studies argue that multiple mechanisms exist to limit the magnitude and duration of signalling events occurring through CysLT1 receptors, reflecting a need to maintain homeostasis of the powerful biological effects induced in vivo by LTD4.
Novel receptors for cysteinyl leukotrienes
As noted previously, both animal and human studies suggested that biological responses to LTE4 were likely mediated by receptors that were different from those responsible for the effects of LTC4 and LTD4. As LTE4, but not LTD4, elicits eosinophil and basophil recruitment to the bronchial mucosa of asthmatic subjects [38], a mouse model was employed to determine the mechanism(s) responsible. Cys-LTs alone failed to cause eosinophil recruitment to the lungs of naïve BALB/c mice. However, when administered intranasally to mice that were sensitized and challenged with ovalbumin (OVA), LTE4, but not LTD4, significantly increased the numbers of eosinophils recovered from the BAL fluid, and also increased the extent of bronchovascular leucocyte infiltration and goblet cell metaplasia [53]. Remarkably, these effects of LTE4 were completely intact in double-knockout mice lacking both CysLT1 and CysLT2 receptors (cysltr1/cysltr2−/− mice), providing further evidence for the presence of a distinct LTE4 receptor [53].
A computer modelling study had predicted that the ADP-binding P2Y12 receptor could potentially recognize LTE4 as a surrogate ligand [54]. Thus, sensitized BALB/c mice were treated with clopidogrel, a thienopyridine drug that is an antagonist of P2Y12 receptors [55]. Clopidogrel completely eliminated the increments in eosinophil recruitment, inflammation, and goblet cell metaplasia induced by LTE4. The actions of LTE4 in this model were also absent in mice lacking P2Y12 receptors. Depletion of platelets, the major cell type expressing P2Y12 receptors, from the sensitized mice before their challenges with OVA and LTE4 also eliminated the ability of LTE4 to amplify pulmonary eosinophilia [53]. This study established that the ability of LTE4 to amplify bronchial eosinophilia depends not on classical CysLTRs, but rather on ADP-reactive P2Y12 receptors and platelets, implying the existence of an effector pathway that is likely to resist blockade by conventional CysLT1 receptor antagonists. As naïve mouse airways fail to contract directly to cys-LTs [56], it was not possible to assess whether P2Y12 receptors mediate bronchoconstriction in response to LTE4, or whether the involvement of platelets explains the indomethacin-sensitive aspects of airway responses to LTE4 observed in previous studies of human and guinea-pig [32, 39].
Three lines of evidence supported the concept that P2Y12 receptors were bona fide receptors for LTE4. First, computer modelling predicted the ability of P2Y12 receptors to bind LTE4 [54]. Second, heterologous expression by transfection of human P2Y12 receptors in Chinese hamster ovary (CHO) cells permitted both calcium flux [54] (when the cells were co-transfected with the Gα16 G protein subunit) and phosphorylation of ERK [53] in response to stimulation with LTE4. Third, targeted knockdown of P2Y12 receptor expression in the human mast cell line LAD2 markedly reduced the ability of the cells to generate chemokines and cytokines when stimulated with LTE4, whereas reactivity to LTD4 was unaffected; conversely, knockdown of CysLT1 receptors eliminated the cellular responses to LTD4 without altering responses to LTE4 [16, 53]. Although these findings indicate that P2Y12 receptors mediate certain functional and signalling responses to LTE4, heterologously expressed P2Y12 receptors failed to exhibit specific binding of radiolabelled LTE4 [53]. Curiously, however, LTE4 competitively inhibited the binding of ADP (the natural ligand for P2Y12 receptors) to the membranes of LAD2 cells, and this competition was eliminated by knockdown of P2Y12 receptors from the parent cell line [53]. Collectively, these observations imply that P2Y12 may function as a component of a complex with another GPCR that specifically recognizes LTE4 (Fig. 2, top) This would be consistent with the previously recognized complexes formed by CysLT1 receptors with both CysLT2 receptors and GPR17 [13, 14] (Fig. 1).
Fig. 2.
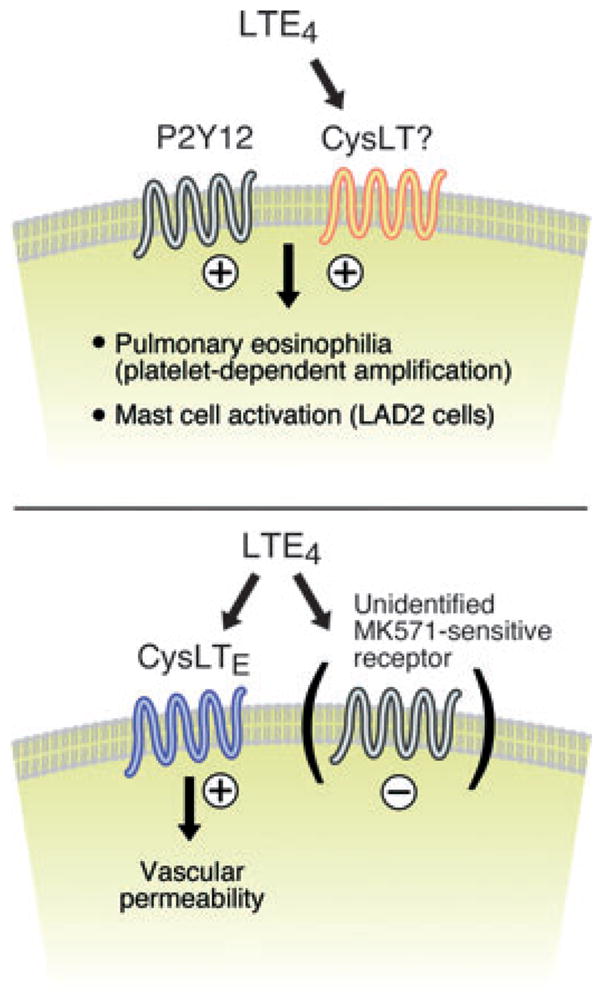
Hypothetical receptors for LTE4 based on functional studies in mice lacking both CysLT1 receptors and CysLT2 receptors. An LTE4-binding receptor combines functionally with P2Y12 receptors (Top) to facilitate the recruitment of eosinophils to the bronchial mucosa in a platelet-dependent manner, while inducing chemokine generation by mast cells. In the cutaneous microvasculature, a putative G-protein coupled receptors (CysLTE receptor) directly mediate vascular leakage in response to LTE4 that is potentiated by the presence of MK571 (and likely other CysLT1 receptor antagonists), suggesting the presence of a lukast-sensitive negative regulator. (+) signs indicate positive signalling, (−) signs denote inhibitory signalling.
Additional studies using cysltr1/cysltr2−/− mice led to the recognition of another potential LTE4-reactive receptor in the cutaneous microvasculature. Cys-LTs caused swelling of the ear skin when injected intradermally into wild-type BALB/c mice. The dose-dependent ear oedema elicited by injection of LTD4 and LTC4 in the cysltr1/cysltr2−/− strain was equivalent to that in the wild-type controls [15], indicating that vascular responses to the cys-LTs, surprisingly, did not require the presence of either classical receptor. Remarkably, and in sharp contrast to the pharmacology of the cloned receptors, LTE4 was the most potent agonist, eliciting the same extent of ear swelling in cysltr1/cysltr2−/− mice at a dose of 0.008 nmol as the response of the WT mice to 0.5 nmol (a 64-fold increase in sensitivity to LTE4). The LTE4-mediated vascular leak in the cysltr1/cysltr2−/− strain was inhibited by pre-treatment of the mice with pertussis toxin or a Rho kinase inhibitor, supporting that the mechanism involved a GPCR linked to Gαi proteins and Rho kinase [15], and was blocked by ~30% by treatment of the mice with indomethacin, reminiscent of the indomethacin sensitivity of the LTE4 response of guinea-pig tracheal rings [32] and human airway smooth muscle [39]. However, the ear swelling response to LTE4 was resistant to clopidogrel, indicating that it did not depend on P2Y12 receptors. Curiously, treatment of the cysltr1/cysltr2−/− mice with MK571 dramatically increased the extent of their swelling response to LTE4, implying the ability of the drug to block targets other than CysLT1 receptors when the latter receptor is deleted, and that the LTE4 receptor(s) responsible for the effects of LTE4 in the cutaneous vasculature are insensitive to CysLT1 receptor antagonists. This receptor has been designated a ‘CysLTE receptor’, pending molecular identification (Fig. 2, bottom). Given the potentiation of the putative CysLTE receptor function by MK571, it seems likely that a lukast-susceptible GPCR restrains the former receptor, analogous to the arrangement for GPR17 and CysLT2 receptors in the case of the CysLT1 receptor (Fig. 1).
Summary and potential clinical implications
Since the cloning of the two classical CysLTRs, the incremental availability of antibody reagents and the application of molecular technology and genetics to the cys-LT system have not only provided mechanistic explanations for observations made in classical pharmacological and physiological studies, but have revealed much greater complexity and functional diversification of the cys-LT system than could possibly have been anticipated. While a comprehensive list of potential clinical implications is beyond the scope of this article, we present three areas of cys-LT biology that could greatly impact the field of asthma and allergy in the near future.
Cross-regulation of CysLT1 receptors
Given that at least two receptors (CysLT2 receptor and GPR17) substantially dampen CysLT1 receptor function in vivo, it is possible that functional variation in either receptor could influence clinical reactivity to CysLT1 receptor antagonists, susceptibility to LTD4-induced bronchoconstriction, or both. In support of this concept, polymorphic sequence variants of the human CysLT2 receptor have been reported that predict response to CysLT1 receptor antagonists. Other sequence variants of the CysLT2 receptor predict the magnitude of bronchoconstriction induced in response to oral aspirin challenge in individuals with AERD [57], a feature of the reaction that is sensitive to CysLT1 receptor antagonists. To date, no studies have addressed whether variants in GPR17 influence sensitivity to LTD4 or clinical responses to CysLT1 receptor antagonists. Interestingly, the P2Y receptors capable of inducing heterologous desensitization of CysLT1 receptor (P2Y1, P2Y2, P2Y4, and P2Y6) [11] are all sufficiently structurally similar to the CysLTRs that they are susceptible to blockade by clinically active CysLT1 receptor antagonists in vitro [58], and the same is true of GPR17 [50]. It is thus possible that the ability of CysLT1 receptor antagonists to block these putative negative regulators could ‘cancel out’ the benefits of these drugs and attenuate the potential therapeutic benefit for some individuals. Perhaps this could account for some of the heterogeneity of response to these agents [59].
Receptors for LTE4
As LTE4 is abundant due to its long half-life, the existence of distinct receptors that are not blocked by currently available antagonists is potentially of great importance. This may be particularly true of AERD, in which high systemic levels of LTE4 are accompanied by selectively increased sensitivity to bronchoconstriction in response to inhaled LTE4 [37]. The dependence of LTE4-mediated increases in eosinophil recruitment to the respiratory mucosa on P2Y12 and platelets [53] suggest a potential application for thienopyridine drugs in the treatment of asthma and AERD. The molecular identification of CysLTE receptor could open new avenues towards the development of antagonists with a more complete blockade of cys-LT-dependent pathobiological effects, including antagonists of LTC4S to prevent the formation of cys-LTs. Finally, the dependence of certain LTE4-induced pulmonary effects on the presence of COX products [39] may explain why desensitization to aspirin produces a rapid loss of LTE4-mediated bronchoconstriction in individuals with AERD [60], and why sequence variants in the gene encoding the receptor for thromboxane (TPR) are risk alleles for AERD [61].
The role of cysteinyl leukotrienes in sensitization to common allergens
Myeloid dendritic cells release LTC4 via an innate, Dectin 2-dependent mechanism that can be activated by dust mite and Aspergillus fumigatus allergens [62]. The fact that dendritic cells from ltc4s−/− and cysltr1−/− mice fail to induce sensitization to these allergens in adoptive transfer experiments, and that mice lacking either CysLT1 receptor or LTC4S are resistant to the induction of Th2 responses by dust mite allergens in vivo imply that cys-LTs play a critical role in the sensitization phase of this immune response [52]. Although there is no direct proof at this stage that this is the case in humans, variants of the genes encoding the CysLT1 receptor [63], the CysLT2 receptor [64], and LTC4S [65] that associate with atopy in general or sensitization to dust mites in particular have all been reported in various human populations. As the CysLT1 receptor is the key receptor in this process, early life intervention with the available antagonists could potentially alter or delay sensitization to Dectin 2-activating allergens in infants at high risk.
In summary, the full complexity of the cys-LT receptor system is still being determined. The development of mice lacking the classical CysLTRs, alone and in combination has proven invaluable. Studies using these mice verified older studies suggesting the existence of distinct receptors for LTE4, demonstrated that the function of the CysLT1 receptor is regulated by other GPCRs, and identified previously unanticipated functions for cys-LTs in fibrosis [5] and Th2 immunity [52]. Each of these carries potential implications for the clinical setting.
Footnotes
Conflict of Interest Statement: JAB has received honoraria and grant support from Merck Pharmaceuticals, and has consulted for Altana and LPath.
References
- 1.Drazen JM. Inhalation challenge with sulfidopeptide leukotrienes in human subjects. Chest. 1986;89:414–9. doi: 10.1378/chest.89.3.414. [DOI] [PubMed] [Google Scholar]
- 2.Drazen JM, O’Brien J, Sparrow D, et al. Recovery of leukotriene E4 from the urine of patients with airway obstruction. Am Rev Respir Dis. 1992;146:104–8. doi: 10.1164/ajrccm/146.1.104. [DOI] [PubMed] [Google Scholar]
- 3.Riccioni G, Back M, Capra V. Leukotrienes and atherosclerosis. Curr Drug Targets. 2010;11:882–7. doi: 10.2174/138945010791320881. [DOI] [PubMed] [Google Scholar]
- 4.Magnusson C, Mezhybovska M, Lorinc E, Fernebro E, Nilbert M, Sjolander A. Low expression of CysLT1R and high expression of CysLT2R mediate good prognosis in colorectal cancer. Eur J Cancer. 2010;46:826–35. doi: 10.1016/j.ejca.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Beller TC, Friend DS, Maekawa A, Lam BK, Austen KF, Kanaoka Y. Cysteinyl leukotriene 1 receptor controls the severity of chronic pulmonary inflammation and fibrosis. Proc Natl Acad Sci U S A. 2004;101:3047–52. doi: 10.1073/pnas.0400235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machado ER, Ueta MT, Lourenco EV, et al. Leukotrienes play a role in the control of parasite burden in murine strongyloidiasis. J Immunol. 2005;175:3892–9. doi: 10.4049/jimmunol.175.6.3892. [DOI] [PubMed] [Google Scholar]
- 7.Israel E, Cohn J, Dube L, Drazen JM. Effect of treatment with zileuton, a 5-lipoxygenase inhibitor, in patients with asthma. A randomized controlled trial. Zileuton Clinical Trial Group. JAMA. 1996;275:931–6. [PubMed] [Google Scholar]
- 8.Knorr B, Matz J, Bernstein JA, et al. Montelukast for chronic asthma in 6- to 14-year-old children: a randomized, double-blind trial. Pediatric Montelukast Study Group. JAMA. 1998;279:1181–6. doi: 10.1001/jama.279.15.1181. [DOI] [PubMed] [Google Scholar]
- 9.Israel E, Chervinsky PS, Friedman B, et al. Effects of montelukast and beclomethasone on airway function and asthma control. J Allergy Clin Immunol. 2002;110:847–54. doi: 10.1067/mai.2002.129413. [DOI] [PubMed] [Google Scholar]
- 10.Drazen JM, Silverman EK, Lee TH. Heterogeneity of therapeutic responses in asthma. Br Med Bull. 2000;56:1054–70. doi: 10.1258/0007142001903535. [DOI] [PubMed] [Google Scholar]
- 11.Capra V, Ravasi S, Accomazzo MR, et al. CysLT1 receptor is a target for extracellular nucleotide-induced heterologous desensitization: a possible feedback mechanism in inflammation. J Cell Sci. 2005;118:5625–36. doi: 10.1242/jcs.02668. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y, Borrelli L, Bacskai BJ, Kanaoka Y, Boyce JA. P2Y6 receptors require an intact cysteinyl leukotriene synthetic and signaling system to induce survival and activation of mast cells. J Immunol. 2009;182:1129–37. doi: 10.4049/jimmunol.182.2.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang Y, Borrelli LA, Kanaoka Y, Bacskai BJ, Boyce JA. CysLT2 receptors interact with CysLT1 receptors and down-modulate cysteinyl leukotriene dependent mitogenic responses of mast cells. Blood. 2007;110:3263–70. doi: 10.1182/blood-2007-07-100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maekawa A, Balestrieri B, Austen KF, Kanaoka Y. GPR17 is a negative regulator of the cysteinyl leukotriene 1 receptor response to leukotriene D4. Proc Natl Acad Sci U S A. 2009;106:11685–90. doi: 10.1073/pnas.0905364106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maekawa A, Kanaoka Y, Xing W, Austen KF. Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. Proc Natl Acad Sci U S A. 2008;105:16695–700. doi: 10.1073/pnas.0808993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paruchuri S, Jiang Y, Feng C, Francis SA, Plutzky J, Boyce JA. Leukotriene E4 activates peroxisome proliferator-activated receptor gamma and induces prostaglandin D2 generation by human mast cells. J Biol Chem. 2008;283:16477–87. doi: 10.1074/jbc.M705822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol. 2004;173:1503–10. doi: 10.4049/jimmunol.173.3.1503. [DOI] [PubMed] [Google Scholar]
- 18.Malaviya R, Malaviya R, Jakschik BA. Reversible translocation of 5-lipoxygenase in mast cells upon IgE/antigen stimulation. J Biol Chem. 1993;268:4939–44. [PubMed] [Google Scholar]
- 19.Lam BK, Penrose JF, Freeman GJ, Austen KF. Expression cloning of a cDNA for human leukotriene C4 synthase, an integral membrane protein conjugating reduced glutathione to leukotriene A4. Proc Natl Acad Sci U S A. 1994;91:7663–7. doi: 10.1073/pnas.91.16.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam BK, Owen WF, Jr, Austen KF, Soberman RJ. The identification of a distinct export step following the biosynthesis of leukotriene C4 by human eosinophils. J Biol Chem. 1989;264:12885–9. [PubMed] [Google Scholar]
- 21.Carter BZ, Shi ZZ, Barrios R, Lieberman MW. gamma-glutamyl leukotrienase, a gamma-glutamyl transpeptidase gene family member, is expressed primarily in spleen. J Biol Chem. 1998;273:28277–85. doi: 10.1074/jbc.273.43.28277. [DOI] [PubMed] [Google Scholar]
- 22.Lee CW, Lewis RA, Corey EJ, Austen KF. Conversion of leukotriene D4 to leukotriene E4 by a dipeptidase released from the specific granule of human polymorphonuclear leucocytes. Immunology. 1983;48:27–35. [PMC free article] [PubMed] [Google Scholar]
- 23.Maclouf J, Antoine C, Henson PM, Murphy RC. Leukotriene C4 formation by transcellular biosynthesis. Ann N Y Acad Sci. 1994;714:143–50. doi: 10.1111/j.1749-6632.1994.tb12038.x. [DOI] [PubMed] [Google Scholar]
- 24.Laidlaw TM, Kidder MS, Bhattacharyya N, et al. Cysteinyl leukotriene overproduction in aspirin exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood. 2012;119:3790–8. doi: 10.1182/blood-2011-10-384826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christie PE, Tagari P, Ford-Hutchinson AW, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143:1025–9. doi: 10.1164/ajrccm/143.5_Pt_1.1025. [DOI] [PubMed] [Google Scholar]
- 26.Liu MC, Dube LM, Lancaster J. Acute and chronic effects of a 5-lipoxygenase inhibitor in asthma: a 6-month randomized multicenter trial. Zileuton Study Group. J Allergy Clin Immunol. 1996;98:859–71. doi: 10.1016/s0091-6749(96)80002-9. [DOI] [PubMed] [Google Scholar]
- 27.Berges-Gimeno MP, Simon RA, Stevenson DD. The effect of leukotriene-modifier drugs on aspirin-induced asthma and rhinitis reactions. Clin Exp Allergy. 2002;32:1491–6. doi: 10.1046/j.1365-2745.2002.01501.x. [DOI] [PubMed] [Google Scholar]
- 28.Soter NA, Lewis RA, Corey EJ, Austen KF. Local effects of synthetic leukotrienes (LTC4, LTD4, LTE4, and LTB4) in human skin. J Invest Dermatol. 1983;80:115–9. doi: 10.1111/1523-1747.ep12531738. [DOI] [PubMed] [Google Scholar]
- 29.Griffin M, Weiss JW, Leitch AG, et al. Effects of leukotriene D on the airways in asthma. N Engl J Med. 1983;308:436–9. doi: 10.1056/NEJM198302243080807. [DOI] [PubMed] [Google Scholar]
- 30.Weiss JW, Drazen JM, Coles N, et al. Bronchoconstrictor effects of leukotriene C in humans. Science. 1982;216:196–8. doi: 10.1126/science.7063880. [DOI] [PubMed] [Google Scholar]
- 31.Drazen JM, Venugopalan CS, Austen KF, Brion F, Corey EJ. Effects of leukotriene E on pulmonary mechanics in the guinea pig. Am Rev Respir Dis. 1982;125:290–4. doi: 10.1164/arrd.1982.125.3.290. [DOI] [PubMed] [Google Scholar]
- 32.Lee TH, Austen KF, Corey EJ, Drazen JM. Leukotriene E4-induced airway hyperresponsiveness of guinea pig tracheal smooth muscle to histamine and evidence for three separate sulfidopeptide leukotriene receptors. Proc Natl Acad Sci U S A. 1984;81:4922–5. doi: 10.1073/pnas.81.15.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacques CA, Spur BW, Johnson M, Lee TH. The mechanism of LTE4-induced histamine hyperresponsiveness in guinea-pig tracheal and human bronchial smooth muscle, in vitro. Br J Pharmacol. 1991;104:859–66. doi: 10.1111/j.1476-5381.1991.tb12518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss JW, Drazen JM, McFaddenx ER, Jr, et al. Comparative bronchoconstrictor effects of histamine, leukotriene C, and leukotriene D in normal human volunteers. Trans Assoc Am Physicians. 1982;95:30–5. [PubMed] [Google Scholar]
- 35.Weiss JW, Drazen JM, McFadden ER, Jr, et al. Airway constriction in normal humans produced by inhalation of leukotriene D Potency, time course, and effect of aspirin therapy. JAMA. 1983;249:2814–7. [PubMed] [Google Scholar]
- 36.Davidson AB, Lee TH, Scanlon PD, et al. Bronchoconstrictor effects of leukotriene E4 in normal and asthmatic subjects. Am Rev Respir Dis. 1987;135:333–7. doi: 10.1164/arrd.1987.135.2.333. [DOI] [PubMed] [Google Scholar]
- 37.Christie PE, Schmitz-Schumann M, Spur BW, Lee TH. Airway responsiveness to leukotriene C4 (LTC4), leukotriene E4 (LTE4) and histamine in aspirin-sensitive asthmatic subjects. Eur Respir J. 1993;6:1468–73. [PubMed] [Google Scholar]
- 38.Gauvreau GM, Parameswaran KN, Watson RM, O’Byrne PM. Inhaled leukotriene E(4), but not leukotriene D(4), increased airway inflammatory cells in subjects with atopic asthma. Am J Respir Crit Care Med. 2001;164:1495–500. doi: 10.1164/ajrccm.164.8.2102033. [DOI] [PubMed] [Google Scholar]
- 39.Christie PE, Hawksworth R, Spur BW, Lee TH. Effect of indomethacin on leukotriene4-induced histamine hyperresponsiveness in asthmatic subjects. Am Rev Respir Dis. 1992;146:1506–10. doi: 10.1164/ajrccm/146.6.1506. [DOI] [PubMed] [Google Scholar]
- 40.Heise CE, O’Dowd BF, Figueroa DJ, et al. Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem. 2000;275:30531–6. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- 41.Lynch KR, O’Neill GP, Liu Q, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–93. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 42.Mollerup J, Jorgensen ST, Hougaard C, Hoffmann EK. Identification of a murine cysteinyl leukotriene receptor by expression in Xenopus laevis oocytes. Biochim Biophys Acta. 2001;1517:455–9. doi: 10.1016/s0167-4781(00)00271-2. [DOI] [PubMed] [Google Scholar]
- 43.Hui Y, Yang G, Galczenski H, et al. The murine cysteinyl leukotriene 2 (Cys-LT2) receptor. cDNA and genomic cloning, alternative splicing, and in vitro characterization. J Biol Chem. 2001;276:47489–95. doi: 10.1074/jbc.M107556200. [DOI] [PubMed] [Google Scholar]
- 44.Mellor EA, Maekawa A, Austen KF, Boyce JA. Cysteinyl leukotriene receptor 1 is also a pyrimidinergic receptor and is expressed by human mast cells. Proc Natl Acad Sci U S A. 2001;98:7964–9. doi: 10.1073/pnas.141221498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang Y, Kanaoka Y, Feng C, Nocka K, Rao S, Boyce JA. Cutting edge: Interleukin 4-dependent mast cell proliferation requires autocrine/intracrine cysteinyl leukotriene-induced signaling. J Immunol. 2006;177:2755–9. doi: 10.4049/jimmunol.177.5.2755. [DOI] [PubMed] [Google Scholar]
- 46.Mellor EA, Austen KF, Boyce JA. Cysteinyl leukotrienes and uridine diphosphate induce cytokine generation by human mast cells through an interleukin 4-regulated pathway that is inhibited by leukotriene receptor antagonists. J Exp Med. 2002;195:583–92. doi: 10.1084/jem.20020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans JF. Cysteinyl leukotriene receptors. Prostaglandins Other Lipid Mediat. 2002;69:587–97. doi: 10.1016/s0090-6980(02)00057-6. [DOI] [PubMed] [Google Scholar]
- 48.Mellor EA, Frank N, Soler D, et al. Expression of the type 2 receptor for cysteinyl leukotrienes (CysLT2R) by human mast cells: functional distinction from CysLT1R. Proc Natl Acad Sci U S A. 2003;100:11589–93. doi: 10.1073/pnas.2034927100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franco R, Casado V, Cortes A, et al. Basic concepts in G-protein-coupled receptor homo- and heterodimerization. ScientificWorldJournal. 2007;7:48–57. doi: 10.1100/tsw.2007.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ciana P, Fumagalli M, Trincavelli ML, et al. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25:4615–27. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maekawa A, Xing W, Austen KF, Kanaoka Y. GPR17 regulates immune pulmonary inflammation induced by house dust mites. J Immunol. 2010;185:1846–54. doi: 10.4049/jimmunol.1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barrett NA, Rahman OM, Fernandez JM, et al. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J Exp Med. 2011;208:593–604. doi: 10.1084/jem.20100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paruchuri S, Tashimo H, Feng C, et al. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med. 2009;206:2543–55. doi: 10.1084/jem.20091240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nonaka Y, Hiramoto T, Fujita N. Identification of endogenous surrogate ligands for human P2Y12 receptors by in silico and in vitro methods. Biochem Biophys Res Commun. 2005;337:281–8. doi: 10.1016/j.bbrc.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 55.Savi P, Zachayus JL, Delesque-Touchard N, et al. The active metabolite of Clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proc Natl Acad Sci U S A. 2006;103:11069–74. doi: 10.1073/pnas.0510446103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin TR, Gerard NP, Galli SJ, Drazen JM. Pulmonary responses to bronchoconstrictor agonists in the mouse. J Appl Physiol. 1988;64:2318–23. doi: 10.1152/jappl.1988.64.6.2318. [DOI] [PubMed] [Google Scholar]
- 57.Park JS, Chang HS, Park CS, et al. Association analysis of cysteinyl-leukotriene receptor 2 (CYSLTR2) polymorphisms with aspirin intolerance in asthmatics. Pharmacogenet Genomics. 2005;15:483–92. doi: 10.1097/01.fpc.0000166456.84905.a0. [DOI] [PubMed] [Google Scholar]
- 58.Mamedova L, Capra V, Accomazzo MR, et al. CysLT1 leukotriene receptor antagonists inhibit the effects of nucleotides acting at P2Y receptors. Biochem Pharmacol. 2005;71:115–25. doi: 10.1016/j.bcp.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malmstrom K, Rodriguez-Gomez G, Guerra J, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann Intern Med. 1999;130:487–95. doi: 10.7326/0003-4819-130-6-199903160-00005. [DOI] [PubMed] [Google Scholar]
- 60.Arm JP, O’Hickey SP, Spur BW, Lee TH. Airway responsiveness to histamine and leukotriene E4 in subjects with aspirin-induced asthma. Am Rev Respir Dis. 1989;140:148–53. doi: 10.1164/ajrccm/140.1.148. [DOI] [PubMed] [Google Scholar]
- 61.Kim SH, Choi JH, Park HS, et al. Association of thromboxane A2 receptor gene polymorphism with the phenotype of acetyl salicylic acid-intolerant asthma. Clin Exp Allergy. 2005;35:585–90. doi: 10.1111/j.1365-2222.2005.02220.x. [DOI] [PubMed] [Google Scholar]
- 62.Barrett NA, Maekawa A, Rahman OM, Austen KF, Kanaoka Y. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J Immunol. 2009;182:1119–28. doi: 10.4049/jimmunol.182.2.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thompson MD, Capra V, Takasaki J, et al. A functional G300S variant of the cysteinyl leukotriene 1 receptor is associated with atopy in a Tristan da Cunha isolate. Pharmacogenet Genomics. 2007;17:539–49. doi: 10.1097/FPC.0b013e328012d0bf. [DOI] [PubMed] [Google Scholar]
- 64.Thompson MD, Storm van’s GK, Galczenski H, et al. A cysteinyl leukotriene 2 receptor variant is associated with atopy in the population of Tristan da Cunha. Pharmacogenetics. 2003;13:641–9. doi: 10.1097/00008571-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 65.Acevedo N, Vergara C, Mercado D, Jimenez S, Caraballo L. The A-444C polymorphism of leukotriene C4 synthase gene is associated with IgE antibodies to Dermatophagoides pteronyssinus in a Colombian population. J Allergy Clin Immunol. 2007;119:505–7. doi: 10.1016/j.jaci.2006.10.002. [DOI] [PubMed] [Google Scholar]


