Abstract
Background/Objectives
Controversy exists regarding statins’ effects on cognitive decline in the healthy elderly. Prior longitudinal studies show mixed results; most have not evaluated normal and MCI subjects separately.
Design, Setting, and Participants
Participants were enrolled in the National Institute of Aging network of Alzheimer’s Disease Centers. We conducted a longitudinal study, comparing baseline cognition and rate of decline in statin users (n=1244) and non-users (n=2363) among research volunteers with normal cognition at baseline, evaluated an average 4.1 times over 3.4 years. Comparable analyses were conducted for 763 users and 917 non-users with mild cognitive impairment (MCI) at baseline (3.9 visits during 2.8 years). We conducted repeated measures analyses adjusted for age, gender, education, comorbidities, and family history of dementia.
Measurements
Cognitive performance was assessed via ten neuropsychological indices and the Clinical Dementia Rating Sum of Boxes (CDR-SOB).
Results
Among those with normal cognition at baseline, statin users performed significantly better across all visits in attention (Trails A). They also showed significantly slower annual worsening in CDR-SOB scores (p=0.006), and borderline significantly slower worsening in MMSE scores, compared with non-users (adjusting for multiple comparisons). For MCI subjects, statin users performed significantly better across all visits on attention measures (Trails A), verbal skills (Category Fluency) and executive functioning (Trails B, Digit Symbol, and Digits Backward). However, there were no differences in cognitive decline between users and non-users.
Conclusion
This study indicates elderly subjects with normal cognition at baseline who use statins have a slower rate of annual worsening in CDR-SOB than non-users.
Keywords: cognitive decline, statins
INTRODUCTION
There is controversy about the effects of statins on cognition and cognitive decline in aging. Epidemiologic findings have been mixed regarding statin use and cognition.1–3 Prospective observational studies have mostly found a significantly lower risk of dementia or incident Alzheimer’s disease (AD) in statin users4–13, although negative results have been obtained as well.14–15 Both Li et al.9 and Rockwood et al.8 analyzed their data by age and found a strong beneficial effect for subjects less than age 80 at baseline, but not in those older than 80.
Two other studies found less cognitive decline among AD patients taking statins. 16–17 The potential for a neuroprotective effect of statins has resulted in two recent multicenter clinical trials for treatment of AD, both of which showed no benefit over time.18–19 However, disease-modifying therapies may be unlikely to be successful if initiated in mild to moderate disease, since there is already extensive AD pathology and irreversible degeneration at those stages.
In support of a possible early benefit of statins, there have been four positive (i.e., protective) observational studies of statins and cognition in non-demented older adults.4,13,20–21 Of these, Betterman et al.4 recently conducted a secondary analysis of clinical trial of the effect ginkobiloba within 3069 elderly patients (age 75+), in which dementia and cognition were the primary endpoints. A significantly decreased rate of decline was found for the Modified Mini-Mental State Examination (3MSE) and the AD Assessment Scale (ADAS-Cog), for current statin users vs. non-users who were normal at baseline. However, no effect on rate of decline was found among those who had mild cognitive impairment (MCI) at baseline.
Against this positive background, however, are other longitudinal studies of cognition (both randomized trials and observational studies) among non-demented subjects that have yielded negative results.14,22–25 Shepherd et al.22 (see also Trompet et al.25) and Collins et al.23 conducted clinical trials of statins (pravastatin and simvastatin, respectively) in which cognitive was assessed as a secondary endpoint, among a population with cardiovascular disease or strong risk factors for cardiovascular disease. Collins et al.23 only had an evaluation of cognition at follow-up, not at baseline. Furthermore, about a third of the subjects were non-compliant with statin use or non-use, which was not taken into account in the intention-to-treat analysis. Among the observational studies, Ancelin et al.14 studied cognitive decline among 6830 community residents followed for 7 years, 16% of whom were taking statins. These investigators found no significant protective effects on visual memory (Benton Visual Retention Test), attention (Trails A), and set shifting speed (Trails B). A limitation of this study is that trends in cognition over time were not evaluated using continuous scores, but instead were dichotomized into ‘decliners’ and ‘non-decliners’. Benito-Leon et al.24 followed 548 community residents age 65+ in Spain for a median of two years. No differences in cognition were observed between statin-users and non-users at baseline, as measured by a comprehensive battery of cognitive tests at the end of a 2-year follow-up. This study is limited by relatively small numbers of subjects and short follow-up.
Last year the FDA required alerts of rare instances of memory loss to be listed on statin medications (NY Times, Feb 28, 2012). These alerts, along with informal communications on the web, have alarmed some patients and families about the adverse risks of statin use among those concerned with memory loss and cognitive decline. Given the mixed results of studies and public messaging, additional studies of statins and cognition are warranted.
In the current investigation, we further explored the effects of statin use on cognitive functioning and change over time in a sample of over 5,000 research participants in the NIH-NIA supported Alzheimer’s Disease Centers. We investigated separately whether statins affect cognitive decline in subjects with normal cognition at baseline and subjects with MCI at baseline. These research volunteer shad repeated evaluations of cognitive performance and information about statin use at each annual follow-up as part of the Uniform Data Set (UDS), a standardized assessment and data protocol maintained by the National Alzheimer’s Coordinating Center. Repeated observations enabled us to characterize a population taking statins consistently throughout follow-up, an advantage over a number of prior studies that queried statin use only at baseline. We also had available a standardized battery of neuropsychological measures that examined a wide range of cognitive areas, as opposed to using an index of overall cognitive status such as the 3MSE. This rich data set allowed us to determine whether certain areas such as executive functioning are more vulnerable to the effects of statins. The minimum requirement of at least three annual visits, resulting in an average follow-up of 3 years, provided a strong test of whether statins are associated with longitudinal cognitive changes.
METHODS
Data Collection
Variables were collected as part of the UDS, with 31 participating NIH-NIA Alzheimer’s Disease Centers nationwide. The UDS consists of longitudinal data obtained by annual comprehensive evaluations of thousands of research volunteers.26,27 We ascertained the effects of statins on cognitive performance over time among those classified as either normal or MCI at baseline.
Participants
We used information from the UDS as of June 2011. Recruitment strategies vary across the ADCs, and as such, participants may come from clinics and/or the community.26 Inclusion criteria for the current study required that participants had a diagnosis of normal cognition or MCI from the clinicians at each center (n=6600). The diagnosis of MCI follows guidelines set forth by an Expert Panel28, including clinical judgment that a person is not cognitively normal and does not meet diagnostic criteria for dementia, has preserved or only minimally impaired functional abilities, and has evidence of cognitive impairment or decline based on self or informant report as well as objective cognitive tests. A diagnosis of normal cognition is made when a person does not meet criteria for either MCI or dementia. All participants signed consent forms approved by the Institutional Review Boards at their study sites.
Measures
The outcome measures used in the analyses are listed below.
Clinical Dementia Rating Sum of Boxes (CDR-SOB)
The CDR was administered using a structured interview format with the patient and their informant to assess the patient’s current cognitive and functional status.29 Areas involving memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care are each rated for their level of impairment. The CDR-SOB provides a composite of the overall level of impairment.
Neuropsychological Measures
Cognitive test scores were based on the core battery of measures collected by the ADCs.27 The MMSE was used to assess overall cognitive status30. Attention was assessed by the maximum number of correct trials for digits forward31 and the number of seconds needed to sequence numbers using a pencil (Trails A).32 Language was examined via the 30-item version of the Boston Naming Test.33 The evaluation of memory included verbal episodic memory (immediate and delayed story recall)31 and semantic memory (timed generation of animal names in 60 seconds)(category fluency).34 Finally, executive functioning was measured via set shifting tasks involving mental manipulation of digits31 and rapid alternation of numbers/letters and symbols (Trails B and Digit Symbol).31,32
Statin use
Data on self-reported medication use at each visit were available from the UDS database. We restricted the analyses to those subjects with consistent reporting of statin use across all visits; i.e., subjects always using statins (n=2029) across all visits or subjects never using statins at any visit (n=3309). These two groups represented 81% of the total population of 6600; the remainder intermittently reported use of statins. For clarity of presentation, we simply refer to the two groups as statin users and non-users. We believe this dichotomization provides a cleaner comparison of long-term vs. never statin use than including sporadic users and considering statin use as a time-dependent variable.
Statistical Analysis
We conducted longitudinal linear regression analyses to determine if there was a difference in cognitive change over time for statin users vs. non-users (PROC MIXED, SAS). All participants had at least three observations, spaced approximately a year apart. Subjects could have varying number of visits. We used a repeated measures analysis with a compound symmetry correlation matrix, equivalent to an analysis in which subjects are included as a random effect.
We first ran separate models to evaluate the main effects of time (a continuous variable coded 1, 2, ….6, corresponding to visit number which are approximately a year apart) and statins on cognitive tests (use of time as a continuous variable as time from first visit yielded virtually identical results). These models assessed whether statin users performed on the average better on cognitive tests over the course of follow-up compared to non-users. We then explored whether cognition among patients using statins worsened over time more rapidly than those not using statins. To test this, we added to the main effects model an interaction term, between time as a continuous variable and statin use as a dichotomous variable (always use vs. never use during follow-up). Graphical representation of the different slopes for change over time between statin and non-statin users was based on adding the coefficient of the interaction term to the coefficient for the time variable for non-statin users in the interaction model; the starting point for the cognitive test in the figures was the value of the cognitive outcome at the initial visit 1.
There were 11 dependent variables including the CDR-SOB of Boxes, the MMSE(total score), number of seconds to complete Trails A and Trails B, maximum number of correct trials for digits forward and digits backwards, number of story units recalled immediately after hearing a story and after a delay, number of completed pairings within 90 seconds on the Digit Symbol subtest, number of correct responses on the Boston Naming Test, and number of animal names generated in 60 seconds. For Trails A and B we conducted analyses of both the original variable and the log transformed variable, as the latter satisfied the normality assumption and the former did not. Results were concordant, and we report the untransformed results for ease of interpretation.
The CDR-SOB also did not fulfill normality assumptions, as this variable is not continuous (0, .5, 1….up to a maximum of 18 in our data), with most data in the lower part of the distribution. We therefore modeled CDR-SOB as a multinomial (ordinal) variable using a proportional odds logistic regression model (SAS PROC GLIMMIX, assuming a multinomial distribution, and a variance component correlation matrix for correlated multiple observations per subject). To compare the fit for this model versus a linear regression model, we calculated the mean square error, with error defined as the different between observed and predicted values. We defined the predicted value as the ordinal CDR-SOB category with the highest probability from the model for each subject, and subtracted this predicted value from the observed, and then squared the result. We summed this value across all subjects and took the square root. For an analogous procedure in PROC MIXED, we defined ‘predicted’ as the ordinal CDR value closest to that predicted by the model, and then proceeded as described above for the GLIMMIX multinomial model. When graphing the predicted CDR-SOB of boxes for statin and non-statin users, we used the predicted CDR-SOB for each subject with the highest probability from the logistic regression, and then averaged these predicted CDR-SOB scores across all subjects in each group (statin vs. non-statin).
Variables that could confound the relationship between statin use and cognitive function were entered a priori in all the statistical models. These covariates included age (continuous), race (white, non-white), gender, education (no high school degree, high school degree, > high school), and presence versus absence of a self-reported history of baseline diabetes, hypertension, heart disease (e.g., myocardial infarction, atrial fibrillation, or congestive heart failure), stroke/TIA, or depression within the last two years. We also included a variable for hypertension, which was divided into three levels; no history of hypertension (referent), history of hypertension but not current high blood pressure (>80 diastolic BP or >120 systolic), current high blood pressure.
As we conducted 11 tests for those with normal cognition at baseline, and 11 tests for subjects with MCI at baseline, we adjusted the threshold p-value for declaring significance using a false discovery rate approximation, such that the threshold p-value for determining statistical significance was (α(m+1)/2m, where α is the original conventional threshold of 0.05, and m is the number of tests. Thus, we used a threshold p-value of .05[(22+1)/(2*22)], or 0.026.35
Results
Table 1 shows the demographic and clinical features of the participants stratified by their cognitive status at baseline (normal, MCI) and by statin use (non-user, user). As expected, statin users were significantly more likely to report a history of heart disease, diabetes, hypertension, and stroke. Among those cognitively normal at baseline, statin users had a significantly higher (i.e., worse) level of CDR-SOB.
Table 1.
Comparison of Baseline Characteristics by Statin Use*
| Non-Statin users | Statin Users | p value | |
|---|---|---|---|
| Normal at baseline | N=2363 | N=1224 | |
| Number of visits | 4.2 ± 1.0 | 4.0 ± 1.0 | <.0001 |
| Follow-up time, years | 3.4 ± 1.1 | 3.3 ± 1.1 | 0.0036 |
| Baseline values | |||
| Age, years | 72.6 ± 10.8 | 72.8 ± 8.17 | 0.64 |
| Education, years | 15.5 ± 3.0 | 15.6 ± 3.03 | 0.33 |
| Male Gender, n (%) | 658 (27.8) | 547 (44.7) | <0.0001 |
| White, n (%) | 2001 (84.9) | 1053 (86.0) | 0.35 |
| Heart disease, n (%) | 397 (16.9) | 430 (35.4) | <0.0001 |
| Diabetes, n (%) | 104 (4.41) | 212 (17.4) | <0.0001 |
| Depression, n (%) | 377 (16.0) | 243 (19.9) | 0.0035 |
| Hypertension, n (%) | |||
| No history of hypertension, no current hypertension | 1401 (61.6) | 445 (37.6) | <0.0001 |
| History of hypertension but now controlled | 458 (20.1) | 425 (35.9) | |
| With or without history, but now uncontrolled | 415 (18.2) | 314 (26.5) | |
| First degree relative with dementia, n (%) | 1126 (55.6) | 602 (58.7) | 0.10 |
| Stroke/TIA, n (%) | 106 (4.50) | 88 (7.22) | 0.0006 |
| MMSE | 29.0 ± 1.3 | 28.9 ± 1.3 | 0.06 |
| % subjects with CDR sum of boxes>0 | 10.1 | 15.0 | <.0001 |
| MCI at baseline | N=917 | N=763 | |
| Number of visits | 3.9 ± 0.9 | 3.9 ± 0.9 | 0.90 |
| Follow-up time, years | 2.9 ± 1.2 | 2.8 ± 1.1 | 0.15 |
| Baseline values | |||
| Age, years | 74.3 ± 9.9 | 73.7 ± 8.3 | 0.15 |
| Education, years | 15.0 ± 3.3 | 15.1 ± 3.3 | 0.52 |
| Male Gender, n (%) | 400 (43.6) | 417 (54.7) | <0.0001 |
| White, n (%) | 769 (84.0) | 614 (80.5) | 0.06 |
| Heart disease, n (%) | 177 (19.5) | 303 (40.0) | <0.0001 |
| Diabetes, n (%) | 58 (6.3) | 135 (17.7) | <0.0001 |
| Depression, n (%) | 322 (35.3) | 261 (34.3) | 0.67 |
| Hypertension, n (%) | <0.0001 | ||
| No history of hypertension, no current hypertension | 526 (59.7) | 261 (35.7) | |
| History of hypertension but now Controlled | 193 (21.9) | 255 (34.9) | |
| History of hypertension but now Uncontrolled | 162 (18.4) | 215 (29.4) | |
| First degree relative with dementia, n (%) | 426 (55.4) | 357 (57.2) | 0.50 |
| Stroke/TIA, n (%) | 62 (6.8) | 96 (12.7) | <0.0001 |
| MMSE | 27.2 ± 2.4 | 27.4 ± 2.2 | 0.11 |
| % subjects with CDR sum of boxes>0 | 86.9 | 87.7 | 0.64 |
unadjusted for other covariates
Six different statins were used by participants: simvastatin (41%), atorvastatin (37%), lovastatin (10%), pravastatin (6%), rosuvastatin (5%), and fluvastatin (1%).
Table 2 shows the coefficients for the main effects for statin use and time (from the main effects model), and their corresponding p-values, as well as the coefficient for the interaction between statin use and time (after adding an interaction term to the main effects model), and its corresponding p-value. The main effects term for those normal at baseline show that, across all visits, statin users had significantly better scores on Trails A, and borderline significantly better scores on Digit Symbol. For those with MCI at baseline, statin users had significantly better scores across all visits for Trails A, Trails B, Digit Symbol, Category Fluency, and Digits Backward. The main effects term for time indicate the change over time for each test (statin users and non-users combined). Most tests, as expected, showed significant deterioration over time. Exceptions were the Boston Naming among normals, and notably the Logical Memory tests for immediate and delayed recall among normals, which showed significant improvement over time, which probably reflects learning how to do the test (this same phenomenon was not seen for MCI subjects).
Table 2.
Longitudinal linear regression analyses of cognitive tests regressed on time and statin users vs. non-users*
| Diagnostic group/test | Regression coefficient** for statin users vs. non- users (p value) | Regression coefficient** for change in outcome over time (p value) | Regression coefficient Interaction term between time and statin use (p-value) |
|---|---|---|---|
| Normal at baseline | |||
| Trails A (secs) | −1.4 (0.009) | 0.53 (<0.0001) | −0.12 (0.41) |
| Trails B (secs) | −2.6 (0.12) | 3.05 (<0.0001) | −0.27 (0.53) |
| Boston Naming (# of correct) | 0.02 (0.85) | 0.02 (0.17) | 0.00 (0.95) |
| MMSE (total score) | 0.08 (0.10) | −0.09 (<0.0001) | 0.04 (0.05) |
| CDR sum boxes*** | −0.03 (0.29) | 0.08 (<0.0001) | −0.03 (0.006) |
| Logical Memory immediate recall (# of units) | −0.08 (0.57) | 0.24 (<0.0001) | −0.04 (0.25) |
| WAIS Digit Symbol (# of correct) | 0.79 (0.07) | −0.34 (0.03) | −0.03 (0.67) |
| Category Fluency ( # of animals) | −0.18 (0.35) | −0.19 (<0.0001) | −0.01 (0.82) |
| Logical Memory delayed recall (# of units) | −0.14 (0.36) | 0.33 (<0.0001) | −0.03 (0.50) |
| Digits Span Forward (# of points) | −0.02 (0.76) | −0.05 (<0.0001) | 0.00 (0.92) |
| Digits Span Backward (# of points) | 0.02 (0.76) | −0.04 (<0.0001) | 0.01 (0.80) |
| MCI at baseline | |||
| Trails A (secs) | −3.4 (0.008) | 2.73 (<0.0001) | −0.23 (0.55) |
| Trails B (secs) | −13 (0.0008) | 9.65 (<0.0001) | −0.75 (0.50) |
| Boston Naming (# of correct) | 0.24 (0.34) | −0.43 (<0.0001) | 0.05 (0.43) |
| MMSE (total score) | 0.20 (0.22) | −0.62 (<0.0001) | −0.05 (0.73) |
| CDR sum boxes*** | −0.09 (0.44) | 0.54 (<0.0001) | −0.05 (0.19) |
| Logical Memory immediate recall (# of units) | 0.14 (0.55) | −0.26 (<0.0001) | −0.03 (0.60) |
| WAIS Digit Symbol (# of correct) | 1.66 (0.01) | −1.3 (<0.0001) | 0.14 (0.32) |
| Category Fluency (# of animals) | 0.53 (0.04) | −0.53 (<0.0001) | 0.05 (0.48) |
| Logical Memory delayed recall (# of units) | −0.07 (0.80) | −0.13 (<0.0001) | −0.04 (0.55) |
| Digit Span Forward (# of points) | 0.12 (0.23) | −0.14 (<0.0001) | 0.02 (0.47) |
| Digit Span Backward (# of points) | 0.26 (0.01) | −0.15 (<0.0001) | 0.02 (0.41) |
adjusted for age (continuous), gender, race, education (<high school, high school, high school+), family history (first degree relative) with dementia, depression in last 2 years (yes/no), and history of heart disease at baseline (yes/no), diabetes at baseline (yes/no), uncontrolled/controlled hypertension at baseline (yes/no), stroke/TIA (yes/no) at baseline. Coefficients for columns 2–3 come from a main effects model; coefficient in column 4 is from a separate model with an interaction term.
Higher scores are better for Boston Naming, MMSE, Logical Memory, WAIS Digit Symbol, Category Fluency, and Digit Span tests. Higher scores are worse for Trails A and B and CDR sum of boxes.
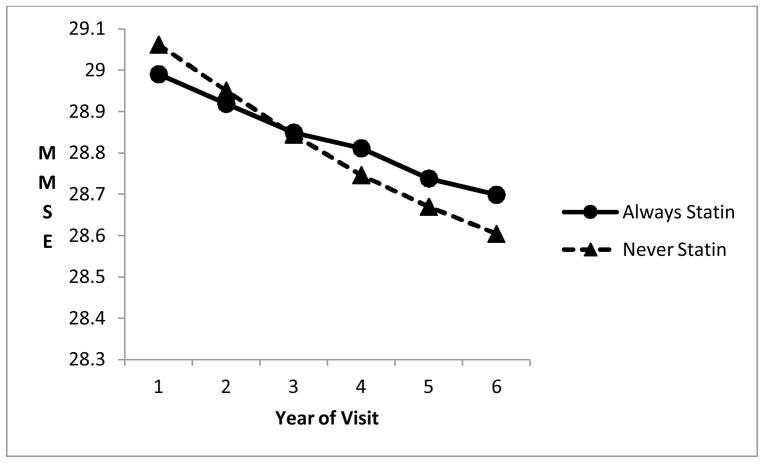
Statin users normal at baseline had significantly less deterioration over time in CDR sum of boxes (higher scores worse) than non-users, adjusted for multiple comparisons (significance judged with p<0.026). They also showed borderline significantly less decline in MMSE scores. Use of a ordinal logistic regression model as an alternative to linear regression for CDR sum of boxes resulted in a stronger interaction term, with a p-value of 0.0002.
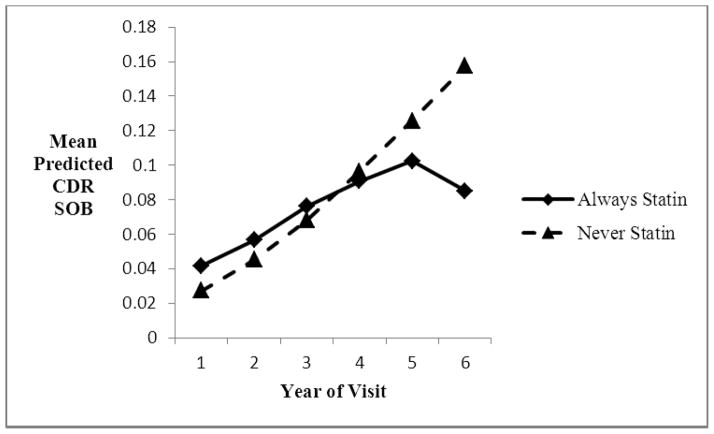
Of particular interest is the interaction term between time (or visit number) and statin use, which indicates whether change over time differed in statin users vs. non-users. Among those cognitively normal at baseline, there was significantly less deterioration over visits (i.e., over time) for the statin users for the CDR-SOB, and borderline significant less decline for the MMSE (adjusting for multiple comparisons), although the differences in the slopes were not marked (Figures 1 and 2). There were no significant differences in change over time for those diagnosed with MCI at baseline.
Figure 1.
Decline in MMSE over annual visits for those normal at baseline, always statin-users vs. non-users, from linear regression model.
Figure 2.
Increase in mean predicted CDR sum of boxes over annual visits for those normal at baseline, always statin-users vs. non-users, using predicted CDR category from the logistic regression model..
Using an ordinal logistic regression model instead of a linear regression model for the CDR sum of boxes resulted again in a highly significant interaction term between time and statin use (p=0.0002 compared to p=0.006 for the linear regression model), for those with normal cognition at baseline. This model fit the data better (root mean squared error 0.67) than the linear regression model (root mean squared error 0.82). For those with MCI at baseline, the ordinal logistic model resulted in a non-significant interaction term between time and statin use (p= 0.92).
Supplemental stratified analyses considered the approximately 60% of the population with APOE data. We stratified the data by APOE4 status (variant present or absent), and then evaluated the interaction term between statins and time as before. We found that a suggestion of a protective effect of statins against MMSE decline among those cognitively normal at baseline was seen only in those without the APOE4 variant, although this protective effect was not significant (p=0.08 for interaction term between statin use and visit); no suggestion of a protective effect was found for those with the variant (p= 0.95) Among MCI patients, a significant protective effect for MMSE was seen for those APOE4 negative(p=0.03 for the interaction term); for APOE4 positive MCI patients, the interaction term was in the wrong direction and not significant (p=0.10). For the CDR-SOB, among normals a protective effect of statins against decline was seen for both APOE4 positive(p=0.01 for the interaction term between statin use and visit) and APOE4 negative subjects (p=0.01 for the interaction term), using the ordinal logistic regression model. Among MCI patients, statins did not affect decline over time regardless of APOE genotype. In these targeted analyses no multiple comparison adjustments were used.
DISCUSSION
Our data provide evidence that statin users exhibited better function during follow-up than non-users on cognitive tests evaluating attention (Trails A), and executive functioning (Trails B, Digit Symbol), after adjustment for other covariates. Furthermore, among those with normal cognition at baseline, overall cognitive deterioration over time (measured by the MMSE and the CDR-SOB) was significantly less pronounced among statin users vs non-users. No such protective effect was seen among those who were diagnosed MCI at baseline, perhaps reflecting that statins exert an effect only before significant deterioration is observed.
A neuroprotective effect in those with normal cognition was confined to two measures and must therefore be interpreted cautiously. However, our findings concur with those of Bettermann et al.,4 who also found a protective effect against cognitive decline only among subjects with normal cognition at baseline, but not among those with MCI at baseline, using the 3MSE and the ADAS-Cog. Other statin studies have not separated subjects between those normal or mildly impaired at baseline, which may account for differences in findings.
There are a number of hypotheses regarding the way that statins may affect cognitive function. Several statins cross the blood-brain barrier, and animal and cell studies have shown that statins can decrease A β production, thereby being neuroprotective.36,37 Reduction of plasma cholesterol by statins lowers risk of cardiovascular disease and may lead to less cognitive decline, although this remains an issue of debate. However, statins could mediate neuroprotection via a wide variety of other mechanisms including anti-inflammatory, anti-oxidant, and anti-thrombotic actions, in addition to pleiotropic cell biological effects on regulation of gene expression, cytoskeletal function, and membrane traffic38.
Strengths of our study include a large sample size, standardized cognitive testing, reasonably long follow-up, and clinical diagnoses enabling us to differentiate between those diagnosed with normal cognition and those with MCI at baseline.
Limitations to our study include the use multiple outcomes (with the potential of false positives). To this end we report our data with and without adjustment for multiple comparisons. A second limitation is our use of observational data rather than randomized assignment of statin use, as in clinical trials. Observational data are subject to confounding, unlike clinical trials. On the other hand, we have controlled in the analyses for the major likely confounders. Furthermore, clinical trial data thus far on cognitive decline have their own limitations, given that they been restricted to patients with cardiovascular disease or risk factors for cardiovascular disease, or persons with AD. In contrast, our observational data included those with and without a history of cardiovascular disease, and those who had normal cognition at baseline. In this sense our results may be more generalizable. Furthermore, the available clinical trial data from cardiovascular cohorts consider cognition as a secondary end-point and use only screening instruments to measure cognition, rather than a comprehensive evaluation of domains such as attention, memory, language and executive functioning as in the present study.
Our data are also subject to possible selection biases. One type of selection bias is ‘indication’ bias, which has been discussed in the present context by other authors.8 Indication bias could occur if those who were less susceptible to cognitive decline were more likely to be prescribed statins, which might occur for example if statin users were perhaps both more health-conscious and healthier than other subjects. However, in our data we have information on health status, and were able to control for differential health status among those taking and not taking statins. Furthermore, in our data (Table 1), those taking statins at baseline were in fact in worse health at baseline, with significantly more prevalence of hypertension, diabetes, and heart disease. Thus, even if we had not been able to adjust for differential health status, such worse health would be expected to cause statin uses to show more, not less, cognitive decline.
Another related type of selection bias could occur if different types of subjects were more likely to volunteer for research. For example, those with high levels of comorbidities, and possibly worse cognition, might be less likely to serve as research volunteers and more likely to take statins. However the reverse situation is also possible, in that subjects with comorbidities, more statins, and worse cognition might be more likely to volunteer as research participants. In either case, while such possible biases might affect comparison of cognition between statin users and non-users across a longitudinal series of tests, they would not be likely to affect a comparison of the longitudinal rate of decline over time between the two groups.
In sum, our study confirms and extends the results others4 have observed, demonstrating a modest but positive effect of statin use for those with normal cognition, but not for those with MCI. To confirm these results directly with clinical trials, the study designs would necessarily randomize statin and placebo treatment in cognitively normal individuals and monitor cognitive decline as the primary outcome. Such studies have not been performed, but warrant further consideration given the urgent need for treatments to reduce the prevalence of MCI and dementia. Moreover, our additional evidence suggesting that statins may protect from cognitive decline should mitigate concerns about widespread use of statins in the elderly because of possible cognitive side effects of statins that have been reported in rare cases.
Acknowledgments
We thank the Alzheimer’s Disease Centers’ participants for their willingness to devote their time to research, and the staff members who work tirelessly to make the research possible.
Funding: Supported by the Emory Alzheimer’s Disease Research Center (NIH-NIA 5 P50 AG025688) (AIL). This project was accomplished through the auspices of the National Alzheimer’s Coordinating Center (NACC), a NIH-NIA funded Center that facilitates collaborative research.
Sponsor’s Role: NACC provided the data used in this study under cooperative agreement number U01 AG016976.
Footnotes
Conflict of Interest: No author has any conflict of interest, be that financial, personal, or potential.
Authors’ contributions:
Dr. Steenland - study concept and design, data analysis, writing, interpretation, study supervision
Ms. Zhao – data analysis
Dr. Goldstein –writing, interpretation
Dr. Levey – writing, interpretation
Contributor Information
Liping Zhao, Email: lzhao2@emory.edu.
Felicia C. Goldstein, Email: fgoldst@emory.edu.
Allan I. Levey, Email: alevey@emory.edu.
References
- 1.Rojas-Fernandez CH, Cameron JC. Is statin-associated cognitive impairment clinically relevant? A narrative review and clinical recommendations. Ann Pharmacother. 2012;46:549–557. doi: 10.1345/aph.1Q620. [DOI] [PubMed] [Google Scholar]
- 2.Sabbagh MN, Sparks DL. Statins to treat Alzheimer’s disease: An incomplete story. Expert Rev Neurother. 2012;12:27–30. doi: 10.1586/ern.11.171. [DOI] [PubMed] [Google Scholar]
- 3.van Vliet P. Cholesterol and late-life cognitive decline. J Alzheimers Dis. 2012;30:S147–162. doi: 10.3233/JAD-2011-111028. [DOI] [PubMed] [Google Scholar]
- 4.Bettermann K, Arnold AM, Williamson J, et al. Statins, risk of dementia, and cognitive function: secondary analysis of the Ginkgo Evaluation of Memory Study. J Stroke Cerebrovasc Dis. 2011 Jan 12; doi: 10.1016/j.jstrokecerebrovasdis.2010.11.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beydoun MA, Beason-Held LL, Kitner-Triolo MH, et al. Statins and serum cholesterol’s associations with incident dementia and mild cognitive impairment. J Epidemiol Community Health. 2011;65:949–957. doi: 10.1136/jech.2009.100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haag MD, Hofman A, Koudstaal PJ, et al. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2009;80:13–17. doi: 10.1136/jnnp.2008.150433. [DOI] [PubMed] [Google Scholar]
- 7.Cramer C, Haan MN, Galea S, et al. Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology. 2008;71:344–350. doi: 10.1212/01.wnl.0000319647.15752.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockwood K, Kirkland S, Hogan DB, et al. Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch Neurol. 2002;59:223–227. doi: 10.1001/archneur.59.2.223. [DOI] [PubMed] [Google Scholar]
- 9.Li G, Shofer JB, Rhew IC, Kukull WA, et al. Age-varying association between statin use and incident Alzheimer’s disease. J Am Geriatr Soc. 2010;58:1311–1317. doi: 10.1111/j.1532-5415.2010.02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sparks DL, Kryscio RJ, Sabbagh MN, et al. Reduced risk of incident AD with elective statin use in a clinical trial cohort. Curr Alzheimer Res. 2008;5:416–421. doi: 10.2174/156720508785132316. [DOI] [PubMed] [Google Scholar]
- 11.Wolozin B, Wang SW, Li NC, et al. Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med. 2007;5:20. doi: 10.1186/1741-7015-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solomon A, Sippola R, Soininen H, et al. Lipid-lowering treatment is related to decreased risk of dementia: A population-based study (FINRISK) Neurodegener Dis. 2010;7:180–182. doi: 10.1159/000295659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szwast SJ, Hendrie HC, Lane KA, et al. Association of statin use with cognitive decline in elderly African Americans. Neurology. 2007;69:1873–1880. doi: 10.1212/01.wnl.0000279333.77404.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ancelin ML, Carrière I, Barberger-Gateau P, et al. Lipid lowering agents, cognitive decline, and dementia: The three-city study. J Alzheimers Dis. 2012;30:629–637. doi: 10.3233/JAD-2012-120064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zandi PP, Sparks DL, Khachaturian AS, et al. Cache County Study investigators. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry. 2005;62:217–224. doi: 10.1001/archpsyc.62.2.217. [DOI] [PubMed] [Google Scholar]
- 16.Masse I, Bordet R, Deplanque D, et al. Lipid lowering agents are associated with a slower cognitive decline in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2005;76:1624–1629. doi: 10.1136/jnnp.2005.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg PB, Mielke MM, Tschanz J, et al. Effects of cardiovascular medications on rate of functional decline in Alzheimer disease. Am J Geriatr Psychiatry. 2008;16:883–892. doi: 10.1097/JGP.0b013e318181276a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sano M, Bell KL, Galasko D, et al. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology. 2011;77:556–563. doi: 10.1212/WNL.0b013e318228bf11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman HH, Doody RS, Kivipelto M, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010;23;74:956–964. doi: 10.1212/WNL.0b013e3181d6476a. [DOI] [PubMed] [Google Scholar]
- 20.Bernick C, Katz R, Smith NL, et al. Cardiovascular Health Study Collaborative Research Group. Statins and cognitive function in the elderly: The Cardiovascular Health Study. Neurology. 2005;65:1388–1394. doi: 10.1212/01.wnl.0000182897.18229.ec. [DOI] [PubMed] [Google Scholar]
- 21.Yaffe K, Barrett-Connor E, Lin F, et al. Serum lipoprotein levels, statin use, and cognitive function in older women. Arch Neurol. 2002;59:378–384. doi: 10.1001/archneur.59.3.378. [DOI] [PubMed] [Google Scholar]
- 22.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment - beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J Internal Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd J, Blauw GJ, Murphy MB, et al. PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 24.Collins R, Armitage J, Parish S, et al. Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363:757–767. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- 25.Benito-León J, Louis ED, Vega S, et al. Statins and cognitive functioning in the elderly: A population-based study. J Alzheimers Dis. 2010;21:95–102. doi: 10.3233/JAD-2010-100180. [DOI] [PubMed] [Google Scholar]
- 26.Trompet S, van Vliet P, de Craen AJ, et al. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J Neurol. 2010;257:85–90. doi: 10.1007/s00415-009-5271-7. [DOI] [PubMed] [Google Scholar]
- 27.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer’s Coordinating Center (NACC) database: The Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 28.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Wechsler D. The Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: The Psychological Corporation; 1981. [Google Scholar]
- 33.Army US. Army Individual Test Battery. Washington, D.C: War Department, Adjutant General’s Office; 1944. [Google Scholar]
- 34.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 35.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 36.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Statist Soc B (Methodological) 1995;57:289–300. [Google Scholar]
- 37.McGuinness B, O’Hare J, Craig D, Bullock R, et al. Cochrane review on ‘Statins for the treatment of dementia’. Int J Geriatr Psychiatry. 2012 Apr 2; doi: 10.1002/gps.3797. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Pac-Soo C, Lloyd DG, Vizcaychipi MP, et al. Statins: the role in the treatment and prevention of Alzheimer’s neurodegeneration. J Alzheimers Dis. 2011;27:1–10. doi: 10.3233/JAD-2011-110524. [DOI] [PubMed] [Google Scholar]
- 39.Jeger R, Dieterle T. Statins: Have we found the Holy Grail? Swiss Med Wkly. 2012;142:w13515. doi: 10.4414/smw.2012.13515. [DOI] [PubMed] [Google Scholar]