Abstract
The traditional view of the central nervous system (CNS) as an immune-privileged organ yielded a longstanding perception of such interactions—as seen for example in multiple sclerosis (MS) 1, 2—as intrinsically destructive. This notion is changing with the identification of several homeostatic functions attributable to beneficial T-cell/CNS interaction 3, for example in hippocampal-dependent learning 4 and stress response paradigms 5, and in models of neurodegeneration and CNS injury 6. Here we provide insights into the maintenance, and dynamics of the meningeal T-cell repertoire. We show that meningeal T-cell composition is coupled to the CNS-draining deep cervical lymph nodes (dCLNs), whose surgical removal interrupted the normal flow of meningeal T-cells and resulted in cognitive impairment.
We recently identified the meninges, which envelopes the outer and ventricular surfaces of the brain and spinal cord, as a candidate site for beneficial T-cell interaction with the CNS 3. The meninges are permissive to circulating lymphocytes, harbor MHCII-expressing cell types, and are well situated to broadly affect CNS function 3, 7, 8.
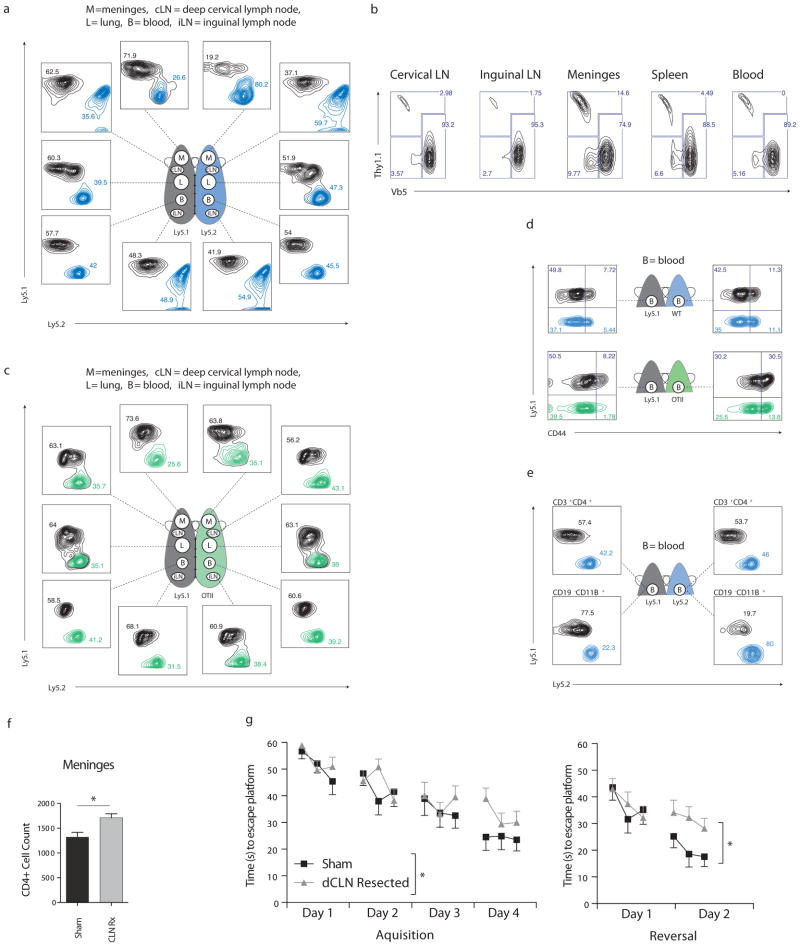
To study the dynamics of CD4+ T-cells at this site, we employed a parabiotic mouse model, enabling us to characterize the relative intra-tissue cell turnover rate in the CD4+ compartment. By day 15 after parabiotic surgery, CD4+ T-cells equilibrate across the blood and lymphoid tissues, however, their turnover in the meninges (and CNS draining dCLN) was severely delayed (Fig. 1a), suggesting that the entry of CD4+ T-cells was gated, passively or actively, by the meninges. We broadly define “passive gating” as an intrinsically CD4-restrictive meningeal environment, and “active gating” as permissive but regulated entry.
Figure 1.
(a) Ly5.1 (Black) and Ly5.2 (Blue) mice were parabiotically joined. After 15 days, each mouse was perfused with PBS and the indicated tissues were analyzed for self and donor CD4+ T-cells by FACS, showing slow relative T-cell turnover in the meninges compared with other tissues and slow turnover in the dCLNs compared with iLNs (representative of n = 4 pairs). (b) wild-type (Thy1.1) splenocytes were adoptively transferred i.v. into OTII recipients, the indicated tissues were analyzed for donor CD4+ T-cells 21 days after transfer to show preferential reconstitution of the meninges and dCLNs. (c) Ly5.1 and OTII mice were parabiotically joined and analyzed after 15 days as in (a) showing preferential reconstitution of wild-type cells in the OTII meninges and dCLNs. (d) CD44 analysis of CD4+ T-cells from blood of wild-type (left) and OTII (right) mice that were parabiotically joined for 15 days (lower panel), and their wild-type Ly5.1 parabiotic counterparts (upper panel) showing incomplete homogenization of CD44+ T-cells between the blood of partner mice. (e) FACS analysis of CD11B+/CD19− populations (lower plots) or CD43+/CD4+ (upper plots) from blood of Ly5.1(left) and ly5.2 (right) mice that were parabiotically joined for 15 days, showing limited monocyte exchange between partners. (f) Total meningeal CD4+ T-cell numbers, (n = 3mice/group *, P < 0.05; Student’s t-test) and (g) MWM acquisition (left) and reversal (right) of deep cervical lymph node-resected or sham operated mice 14 days post surgery, (combined data from 3 independent experiments, sham; n=16mice/group, dCLN resection; n=22mice/group *, P < 0.05; two way repeated measures ANOVA).
To differentiate between active and passive gating, we transferred wild-type Ly5.1 splenocytes into OTII mice. We predicted that given the low CD4+ T-cell turnover in the meninges, seeding by transferred wild-type T-cells (i.e., their “passive gating”) would occur more slowly here than in other tissues. Surprisingly however, the meninges were the most receptive of all the analyzed tissues, with the largest percentage of their total CD4+ T-cells originating from transferred Ly5.1 splenocytes (Fig. 1b). We interpreted this result to mean the meninges is not globally restrictive to CD4+ T-cell entry and residence.
Polyclonal wild-type T-cells are known to expand homeostatically under conditions of lymphopenia or low clonal diversity9. To determine whether homeostatic expansion was driving T-cells from the blood into the meninges, we connected OTII transgenic mice parabiotically with wild-type partners (Fig. 1c). Here, as in the adoptive transfer model, the OTII mouse meninges accepted impressive numbers of donor CD4+ T-cells from the blood. Surprisingly, whereas “naïve” CD44− T-cells equilibrated evenly between parabiotic partners, CD44hi “activated/memory” T-cells did not (Fig. 1d). Not only can this explain the lack of T-cell accumulation in the meninges of the wild-type partner, but it also serves as a cautionary tale concerning a major assumption of the parabiotic model, namely that the unified circulatory system guarantees homogenous leukocyte circulation. In fact, mixing of CD11b+ monocyte populations was also severely diminished (Fig. 1e), suggesting either that some cell types cannot pass through the unified capillary junctions or that they extravasate directly into the site of surgical injury.
Both the uniquely slow T-cell turnover rate in the wild type meninges and the increased seeding of the meninges of OTII mice by wild type T-cells was also apparent in the dCLNs. To explore possible coupling of the meninges and dCLNs, and to find out how interruption of this coupling might affect the T-cell composition of the meninges, we surgically resected dCLNs of wild-type mice. Two weeks after surgery the numbers of CD4+ T-cells in the meninges of operated mice were substantially increased (Fig. 1f). To find out what effect, if any, dCLN resection might have on behavioral outcomes, we subjected resected and sham operated mice to the Morris Water Maze (MWM) behavioral task, and observed significant spatial learning and memory impairment in the dCLN resected group–particularly in the reversal phase of the assay (Fig. 1g).
While the notion that T-cells are required for normal learning and memory is not an intuitive one, every mode of T-cell depletion attempted so far substantiates this necessity (reviewed in 10). This phenomenon is particularly interesting given that the cognitive decline seen in patients undergoing chemotherapy, and in those suffering from age-related senility or HIV-associated dementia, is correlated with a collapse in adaptive immunity that occurs most saliently within the CD4+ T-cell compartment. As we begin to ask what role(s) T-cells are playing in higher brain functions, honing in on the specific localization and migratory behavior of T-cells that operate in the meningeal spaces of the CNS is a likely prerequisite to understanding their ultimate function. Additionally, while the phenotypic readout in this study is learning and memory, a general understanding of T-cell makeup and behavior in the meninges may also be applicable to CNS pathologies such as MS, in which lymphocyte accumulation in the meninges is thought to precede parenchymal infiltration 7.
Methods
Animals
Adult mice were purchased from Jax and housed under standard conditions. All animal procedures were approved by the ACUC at the University of Virginia.
Behavior
Morris Water Maze experiments were conducted using equipment and protocols described by Derecki et al 3 (and in supplementary methods).
Parabiosis and adoptive transfer
Parabiosis was carried out on weight-matched females using a modified surgical protocol as described in supplementary methods.
Cervical lymph node resection and sham surgery
An incision was made midline 5 mm superior to the clavicle, SCM muscle retracted, and dCLN removed with forceps. Sham mice received only the incision and SCM retraction. Detailed protocol and post-op care described in supplementary methods.
Supplementary Material
References
- 1.Steinman L. Multiple approaches to multiple sclerosis. Nat Med. 2000;6(1):15–16. doi: 10.1038/71466. [DOI] [PubMed] [Google Scholar]
- 2.Weiner HL, Selkoe DJ. Inflammation and therapeutic vaccination in CNS diseases. Nature. 2002;420(6917):879–884. doi: 10.1038/nature01325. [DOI] [PubMed] [Google Scholar]
- 3.Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207(5):1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain Behav Immun. 2008;22(6):861–869. doi: 10.1016/j.bbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Cohen H, Ziv Y, Cardon M, Kaplan Z, Matar MA, Gidron Y, et al. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. J Neurobiol. 2006;66(6):552–563. doi: 10.1002/neu.20249. [DOI] [PubMed] [Google Scholar]
- 6.Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T-cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci U S A. 2002;99(24):15620–15625. doi: 10.1073/pnas.232565399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kivisakk P, Imitola J, Rasmussen S, Elyaman W, Zhu B, Ransohoff RM, et al. Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T-cells during experimental autoimmune encephalomyelitis. Ann Neurol. 2009;65(4):457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26(9):485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T-cell survival controlled by clonal abundance. Science. 2006;312(5770):114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 10.Kipnis J, Gadani S, Derecki NC. Pro-cognitive properties of T-cells. Nature reviews Immunology. 2012;12(9):663–669. doi: 10.1038/nri3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.