Abstract
Background
Fine needle aspiration (FNA) is the standard to evaluate thyroid nodules for malignancy. The aim of this study was to determine the influence of patient age and gender on the rate of thyroid nodule malignancy by FNA.
Methods
A database of 3,981 consecutive patients who underwent thyroid FNA between 2002 and 2009 was reviewed. The percentages of benign, indeterminate, and malignant biopsies based on patient age and gender were determined. Statistical analysis was performed using SPSS.
Results
Our patient population included 2,766 women (mean age ± SD, 52 ± 15.2) and 964 men (mean age ± SD, 59 ± 13.8). Of the 3,722 (93.5%) patients with diagnostic FNAs, 196 (5.3%) had malignant FNA cytology. Malignant FNAs were twice as frequent in patients age ≤45 vs. those >45 (8.1% vs. 4.0%, p<0.001). Overall, men had more indeterminate (10.2% vs. 6.3%, p<0.001) and malignant (6.7% vs. 4.8%, p=0.034) FNAs than women. Malignant FNAs in men were greatest in patients over age 45 (6.0% vs. 3.2%, p=0.001). The incidence of malignant FNAs for women peaked in their 30s (10.4%) whereas the incidence of malignant FNAs for men peaked 10 years later in their 40s (12.1%). Both men and women had the lowest incidence of malignant FNAs in their 70s (2.3% and 1.9% respectively).
Conclusion
The typical 5% risk of thyroid nodule malignancy on FNA varies depending on patient age and gender. A patient’s age and gender should therefore be considered when counseling someone of their risk of thyroid cancer by FNA.
Keywords: thyroid cancer, thyroid nodule, fine needle aspiration, gender, age, indeterminate biopsy
INTRODUCTION
Thyroid nodules are a common finding in the United States with 19–67% of the general population having one or more thyroid nodules detectable by ultrasound (1). However, studies show that only 1 in 20 of these nodules is cancerous (2). With the high incidence of thyroid nodules and low rate of cancer among nodules, it is important to differentiate between cancerous and non-cancerous nodules before surgical intervention is sought.
Fine needle aspiration (FNA) is currently the standard diagnostic procedure used to evaluate thyroid nodules prior to surgery (3). As a diagnostic tool, FNA results are about 95% accurate when compared with final surgical pathology (4). Because FNA is accurate and cost-effective, the American Thyroid Association (ATA) recommends FNA of all thyroid nodules > 1 cm (3). Overall, about 5–10% of thyroid FNAs will have malignant cytology, 10–25% will be indeterminate or suspicious for cancer, and 60–70% will be benign (5, 6). Patients with nodules that are malignant or suspicious for cancer by FNA usually undergo thyroid surgery. The routine use of FNA prior to surgery has significantly decreased the number of patients with benign nodules undergoing thyroid surgery (4).
Previous studies suggest that patient characteristics, such as age or gender, may impact the risk of a thyroid nodule being malignant when evaluated by FNA (6–10). While these studies show correlations between rates of malignancy of thyroid nodules and factors, like age or gender, they often only look at a subset of FNA diagnoses (such as follicular neoplasms) and usually only examine patients that went on to surgical excision, which creates an inherent bias, especially in regards to the risk of malignancy. Furthermore, the number of thyroid nodules incidentally discovered in patients has grown in recent years due to the increased use of imaging in medicine (11, 12). This rise in technology has increased the number of FNA biopsies, and has changed the patient population undergoing FNA at our institution. As a result, the overall number of patients undergoing FNA at our institution has risen significantly (13).
Previous studies, combined with a changing population undergoing thyroid nodule FNA, indicated a need to reexamine the incidence of thyroid nodule malignancy on FNA based on different groups of age and gender. The aim of this study was to determine the influence of age and gender on the rate of thyroid nodule malignancy by FNA in order to assess which patient groups may have a higher risk of cancer prior to FNA.
METHODS
A database of 3,981 consecutive patients who underwent thyroid FNA between February 2002 and December 2009 at the University of Wisconsin was reviewed. All FNAs performed during this time period were included in this study. Data recorded in the database included patient gender, age at the time of FNA, whether the FNA was diagnostic or non-diagnostic, and the FNA result.
Diagnostic FNAs were first classified into groupings based on a modification of the Bethesda System for Reporting Thyroid Cytopathology (13, 14). These groupings were then more broadly classified as benign, indeterminate, or malignant. Benign FNA cytology included benign biopsies, benign colloid nodules, and thyroiditis. Indeterminate FNA cytology included diagnoses of follicular/Hurthle cell neoplasm, and suspicious for papillary thyroid carcinoma. Malignant FNA cytology included the following diagnosis categories: papillary thyroid carcinoma, medullary thyroid carcinoma, anaplastic thyroid carcinoma, lymphoma, and other carcinoma.
Patients were then grouped into categories of age and gender. First, patients were divided into two age groups: ages 45 and younger and older than age 45. Age 45 was chosen as a cutoff since it is significant for thyroid cancer staging (3). In addition, patients were grouped into age by decade. The mean age ± standard deviation, the rate of diagnostic FNAs, as well as the incidences of benign, indeterminate, and malignant biopsies out of the diagnostic FNAs in each age-gender group were determined. Comparison between groups was performed using Chi-square and independent t-tests with p-values <0.05 being considered statistically significant. Multivariate logistic regression was performed to assess the independence of the age and gender variables with respect to FNA result. Statistical analysis was performed using SPSS software (version 19, Chicago, IL).
RESULTS
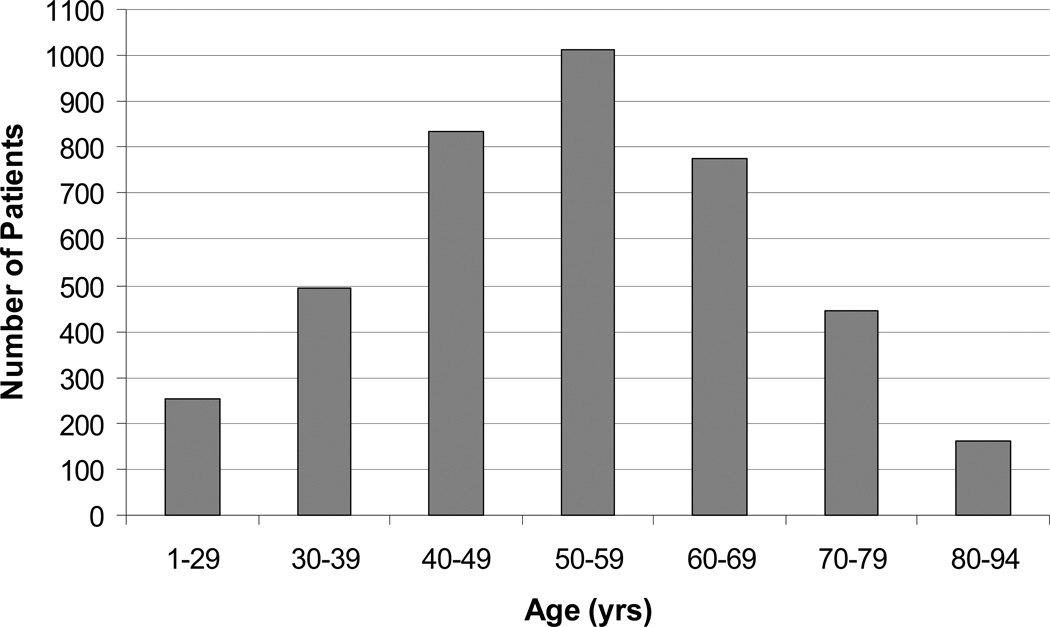
Of the 3,981 FNAs, the majority of patients were women (76%) and the average age was 53 years (range 1–94). The distribution of patients by age was normal with 25% (1013/3981) of patients undergoing FNA in their 50s (Figure 1). Six and one-half percent (259/3981) of patients had a non-diagnostic FNA result, and were excluded from the remainder of our analysis. Of the 93.5% (3722/3981) of patients with diagnostic FNAs, 5.3% (196/3722) had malignant FNA diagnoses, 7.2% (269/3722) had indeterminate FNA results, and 87.5% (3257/3722) had benign FNA diagnoses.
Figure 1.
Distribution of ages within the patient population (N=3,981; mean age ± SD of 53 ± 15.2).
The differences in FNA results observed between patients 45 and younger and patients older than 45 is shown in Table 1. Patients under age 45 were more likely to be female (86% vs. 71%, p<0.0001). The percentages of diagnostic and indeterminate FNAs were similar between age groups. However, benign FNAs were more common in patients older than age 45 (88.4% vs. 85.5%, p=0.01) and malignant FNAs were twice as frequent in patients younger than age 45 (8.1% vs. 4.0%, p<0.001).
Table 1.
FNA diagnosis by age
| FNA diagnosis | Age ≤45 (N=1,215) |
Age >45 (N=2,766) |
P-value |
|---|---|---|---|
| Diagnostic | 94.2% (1,145) | 93.2% (2,577) | NS |
| Benign | 85.5% | 88.4% | 0.01 |
| Indeterminate | 6.4% | 7.6% | NS |
| Malignant | 8.1% | 4.0% | <0.001 |
| Non-Diagnostic | 5.8% | 6.8% | NS |
% malignant, indeterminate, benign out of diagnostic FNAs
There were also significant gender differences in our FNA findings. Men were on average older than women undergoing FNA (59±13.8 yrs vs. 52±15.2 yrs, p<0.001). Women had more diagnostic and benign FNAs than men, while men had more indeterminate and malignant FNAs than women (Table 2). This gender-based difference for malignant FNAs was especially evident in patients over age 45 with 6.0% of men and 3.2% of women having malignant FNAs in this age category (p=0.001).
Table 2.
FNA diagnosis by gender
| FNA diagnosis | Male (N=964) |
Female (N=3,017) |
P-value |
|---|---|---|---|
| Diagnostic | 92.0% (887) | 94.0% (2,835) | 0.03 |
| Benign | 83.2% | 88.9% | <0.001 |
| Indeterminate | 10.2% | 6.3% | <0.001 |
| Malignant | 6.7% | 4.8% | 0.03 |
| Non-Diagnostic | 8.0% | 6.0% | 0.03 |
% malignant, indeterminate, benign out of diagnostic FNAs
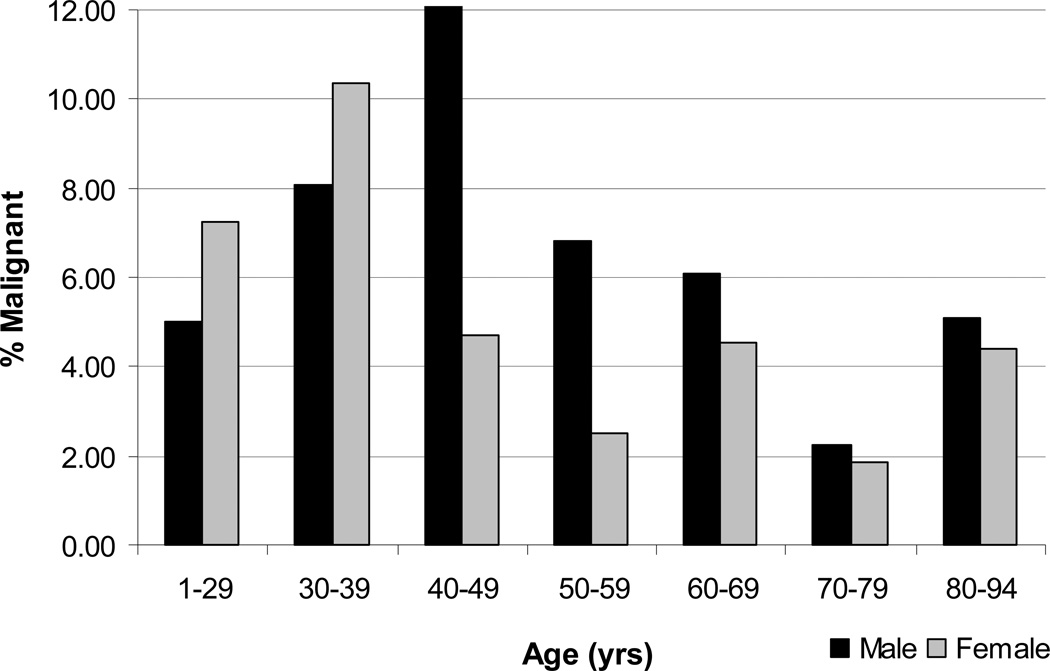
The rate of malignant FNA diagnoses based on gender and age varied by decade and is shown in Figure 2. The incidence of malignant FNAs for women was elevated from ages 1 to 29 (7.3%), peaked in their 30s (10.4%), and was lowest in their 70s (1.9%). In addition, the incidence of malignant FNAs in women was less than 5% in all age groups 40 years and older. The incidence of malignant FNAs for men was slightly elevated in their 30s (8.1%), peaked 10 years later than women in their 40s (12.1%), and was also lowest in their 70s (2.3%). The incidence of malignant FNAs in men was less than 7% in all age groups 50 years and older. Interestingly, both men and women older than 70 years of age had a low incidence of malignancy on FNA. Lastly, multivariate logistic regression to assess the independence of the age by decade and gender variables with respect to FNA result revealed that older patients were less likely to have a malignant FNA result when controlled for gender (OR=0.87; 95% CI: 0.81–0.94; p=0.001).
Figure 2.
Percentage of malignant FNAs by age and gender comparing males to females within age ranges (N=3,722).
DISCUSSION
Knowing a patient’s pre-FNA risk of thyroid nodule malignancy is important in patient counseling, and for optimizing the use and interpretation of FNA results. One way of knowing a patient’s risk of cancer prior to biopsy, is by examining the relationship between patient characteristics and the rate of thyroid cancer on FNA in a large population of patients. For this study, our objective was to determine the influence of two patient characteristics, age and gender, on the rate of thyroid nodule malignancy diagnosed by FNA. We found that the risk of thyroid nodule malignancy on FNA varies considerably depending on patient age and gender. Specifically, the rates of thyroid cancer diagnosed by FNA were higher in men and in younger patients (age ≤45 yrs). The peak incidences of malignancy were between ages 30–39 in women and ages 40–49 in men.
Overall in our study, 5.3% of patients with diagnostic FNAs had a malignant FNA diagnosis which is similar to the average 4% malignancy rate reported in the literature (15). The rate of benign FNAs in our population (87.5%) is higher than the 60–70% rate cited in the literature (4, 15). However, as shown in a previous study at our institution (13), this can probably be explained by the increase in the number of benign nodules meeting ATA criteria for biopsy in recent years due to increased detection of nodules through imaging and increases in biopsies of multiple nodules in a multi-nodular goiter (12, 14). Our rate of non-diagnostic FNA results (6.5%) was lower than that reported in some series and reviews which report rates of 15–20% of non-diagnostic FNAs (10, 15, 16). However, one study found that ultrasound guidance lowered the rate of non-diagnostic FNA results to 3.5% (17). Therefore, our low rate of non-diagnostic FNA results is expected due to a transition to ultrasound guidance on all FNAs performed at our institution within the time of this study (13). The distribution of FNA diagnoses in our experience is therefore reasonable compared to other populations and changes reflect the increased use of technology in medicine.
It is well established that women are far more likely than men to have thyroid nodules (11, 15, 18, 19). Our population was consistent with this knowledge, with the majority (76%) of our patients being women. Though thyroid nodules were diagnosed three times as frequently in women, our study showed that men were more likely than women to have an indeterminate (10.15% vs. 6.31%, p<0.001) or malignant (6.65% vs. 4.83%, p=0.034) FNA result. This finding is consistent previous literature looking at the relationship between gender and other patient features and the risk of malignancy by FNA in patients with follicular neoplasms (6), and cold thyroid nodules (7), and one study which examined patient characteristics and the rate of papillary thyroid cancer (PTC) on FNA (10). Our study not only confirmed that men are more likely to have a malignant FNA result, but also showed that this is true when looking at all patients undergoing FNA in our population and not just a subset of FNA biopsies or diagnoses.
Age affected the risk of thyroid cancer on FNA in the overall population and separately for both men and women. Though previous studies have looked at correlations between FNA diagnosis and age, they often only look at a subset of FNA diagnoses or only examine age as a continuous variable, which makes clinical interpretation of the results less meaningful. We looked at age categorically both in broad categories and then roughly by decade for all FNA biopsies performed within the study period at our institution. In our overall population, we found that malignant FNAs were twice as frequent in patients ages 45 years and younger when compared to older patients (8.1% vs. 4.0%, p<0.001). This finding is in agreement with Rago et al (10), which when examining age as a continuous variable found that patients diagnosed with PTC were younger in a large, mildly iodine deficient population in Italy. Our data shows a similar finding in a US population.
More specifically, our study found peak incidences of malignancy at ages 30–39 in women (10.4%) and ages 40–49 in men (12.1%) with the lowest incidence of malignancy for both gender occurring at ages 70–79 (< 3% in each gender). This contrasts with previous literature. In a 1992 study on FNA of cold thyroid nodules, Belfiore et al (7) found the highest rates of malignancy in older patients (ages 71–80) and in patients younger than 20 years, with their lowest rate of malignancy between ages 31–40 years. There are a couple of potential explanations for this discrepancy. Firstly, this study was carried out between 1980 and 1990. Guidelines for FNA biopsy have changed over the years, and this has changed the population of patients undergoing FNA (13). Secondly, this study looked only at patients with cold thyroid nodules. Since radioactive iodine uptake scans are no longer routinely obtained at our institution, the diagnosis distribution trends by FNA seen for cold thyroid nodules is different than for all types of thyroid nodules combined.
Since our population was largely female, we wanted to see if the trends observed for malignant FNA and age by decade would be changed by controlling for gender in a multivariate analysis. This analysis reinforced our observations discussed above. It showed that older patients were less likely to have a malignant FNA result while controlling for gender. (OR=0.87; 95% CI: 0.81–0.94; p=0.001). Therefore, based on our study, age and gender impact FNA result independent of one another.
Patient characteristics other than age and gender may also influence the rate of thyroid nodule malignancy on FNA. One limitation of this study is that other information, such as multiple vs. single nodularity, nodule size, patient irradiation status, or final surgical pathology, were not able to be analyzed as this data was not available as part of this dataset. However, our results are clinically meaningful and are easily applicable in most clinical situations since age and gender is known for virtually every patient, whereas other variables may or may not be known prior to imaging or FNA. Also, by including all FNAs performed at our institution, and not limiting it to only those that went on to surgical excision, these results may be applied to a more general population undergoing thyroid nodule FNA, instead of only a select subgroup of patients or only patients who elected to undergo surgery. While we were unable to assess the accuracy of FNA in this overall population due to the lack of pathologic follow-up in the majority of patients, we have previously examined the accuracy of FNA from our institution using our surgical database, and FNA at our institution has been shown to be 96% accurate (20). In addition, our dataset does not include the specific reason an FNA was done on each patient in the study. Though this is a limitation, we do have the knowledge that FNA is performed at our institution largely based on guidelines outlined by the American Thyroid Association (3), which is considered standard of care at most US institutions.
In conclusion, the risk of thyroid nodule malignancy on FNA is not the same in every patient. In our population, the risk of thyroid cancer by FNA varied from as low as 1.9% for women in their 70s to as high as 12.1% for men in their 40s. This range varies widely from the overall 5% population risk of thyroid nodule malignancy. Therefore, age and gender should be considered when counseling a patient regarding their risk of a malignancy prior to FNA. This is especially important for patients in groups of both elevated cancer risk (greater than 10% risk) and lessened cancer risk (less than 3% risk) to ensure optimal FNA result interpretation and appropriate thyroid nodule management in these patients. Patient age and gender are therefore important in determining a patient’s risk of thyroid cancer prior to FNA and may be used by the physician to help patients with thyroid nodules make an informed decision regarding the appropriate course of care.
References
- 1.Tan GH, Gharib H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med. 1997;126:226–231. doi: 10.7326/0003-4819-126-3-199702010-00009. [DOI] [PubMed] [Google Scholar]
- 2.Hegedüs L. Clinical practice. The thyroid nodule. N Engl J Med. 2004;351:1764–1771. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 3.Cooper DS, Doherty GM, Haugen BR, Hauger BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM, Cancer ATAAGToTNaDT. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 4.Gharib H, Goellner JR. Fine-needle aspiration biopsy of the thyroid: an appraisal. Ann Intern Med. 1993;118:282–289. doi: 10.7326/0003-4819-118-4-199302150-00007. [DOI] [PubMed] [Google Scholar]
- 5.Gharib H, Goellner JR, Johnson DA. Fine-needle aspiration cytology of the thyroid. A 12-year experience with 11,000 biopsies. Clin Lab Med. 1993;13:699–709. [PubMed] [Google Scholar]
- 6.Tuttle RM, Lemar H, Burch HB. Clinical features associated with an increased risk of thyroid malignancy in patients with follicular neoplasia by fine-needle aspiration. Thyroid. 1998;8:377–383. doi: 10.1089/thy.1998.8.377. [DOI] [PubMed] [Google Scholar]
- 7.Belfiore A, La Rosa GL, La Porta GA, Giuffrida D, Milazzo G, Lupo L, Regalbuto C, Vigneri R. Cancer risk in patients with cold thyroid nodules: relevance of iodine intake, sex age, and multinodularity. Am J Med. 1992;93:363–369. doi: 10.1016/0002-9343(92)90164-7. [DOI] [PubMed] [Google Scholar]
- 8.Tyler DS, Winchester DJ, Caraway NP, Hickey RC, Evans DB. Indeterminate fine-needle aspiration biopsy of the thyroid: identification of subgroups at high risk for invasive carcinoma. Surgery. 1994;116:1054–1060. [PubMed] [Google Scholar]
- 9.Banks ND, Kowalski J, Tsai HL, Somervell H, Tufano R, Dackiw AP, Marohn MR, Clark DP, Umbricht CB, Zeiger MA. A diagnostic predictor model for indeterminate or suspicious thyroid FNA samples. Thyroid. 2008;18:933–941. doi: 10.1089/thy.2008.0108. [DOI] [PubMed] [Google Scholar]
- 10.Rago T, Fiore E, Scutari M, Santini F, Di Coscio G, Romani R, Piaggi P, Ugolini C, Basolo F, Miccoli P, Pinchera A, Vitti P. Male sex, single nodularity, and young age are associated with the risk of finding a papillary thyroid cancer on fine-needle aspiration cytology in a large series of patients with nodular thyroid disease. Eur J Endocrinol. 2010;162:763–770. doi: 10.1530/EJE-09-0895. [DOI] [PubMed] [Google Scholar]
- 11.Ezzat S, Sarti DA, Cain DR, Braunstein GD. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994;154:1838–1840. doi: 10.1001/archinte.154.16.1838. [DOI] [PubMed] [Google Scholar]
- 12.Kang HW, No JH, Chung JH, Min YK, Lee MS, Lee MK, Yang JH, Kim KW. Prevalence, clinical and ultrasonographic characteristics of thyroid incidentalomas. Thyroid. 2004;14:29–33. doi: 10.1089/105072504322783812. [DOI] [PubMed] [Google Scholar]
- 13.Coorough N, Hudak K, Buehler D, Selvaggi S, Sippel R, Chen H. Fine Needle Aspiration of the Thyroid: A Contemporary Experience of 3981 Cases. J Surg Res. 2011 doi: 10.1016/j.jss.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 14.Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009;19:1159–1165. doi: 10.1089/thy.2009.0274. [DOI] [PubMed] [Google Scholar]
- 15.Burch HB. Evaluation and management of the solid thyroid nodule. Endocrinol Metab Clin North Am. 1995;24:663–710. [PubMed] [Google Scholar]
- 16.Castro MR, Gharib H. Thyroid fine-needle aspiration biopsy: progress, practice, and pitfalls. Endocr Pract. 2003;9:128–136. doi: 10.4158/EP.9.2.128. [DOI] [PubMed] [Google Scholar]
- 17.Danese D, Sciacchitano S, Farsetti A, Andreoli M, Pontecorvi A. Diagnostic accuracy of conventional versus sonography-guided fine-needle aspiration biopsy of thyroid nodules. Thyroid. 1998;8:15–21. doi: 10.1089/thy.1998.8.15. [DOI] [PubMed] [Google Scholar]
- 18.Vander JB, Gaston EA, Dawber TR. The significance of nontoxic thyroid nodules. Final report of a 15-year study of the incidence of thyroid malignancy. Ann Intern Med. 1968;69:537–540. doi: 10.7326/0003-4819-69-3-537. [DOI] [PubMed] [Google Scholar]
- 19.Rojeski MT, Gharib H. Nodular thyroid disease. Evaluation and management. N Engl J Med. 1985;313:428–436. doi: 10.1056/NEJM198508153130707. [DOI] [PubMed] [Google Scholar]
- 20.Yu XM, Patel PN, Chen H, Sippel RS. False-negative fine-needle aspiration of thyroid nodules cannot be attributed to sampling error alone. Am. J. Surg. 2012;203:331–334. doi: 10.1016/j.amjsurg.2011.09.016. discussion 334. [DOI] [PubMed] [Google Scholar]