Abstract
Recent insight into the critical role of pro-inflammatory cytokines, particularly tumor necrosis factor-α (TNF-α), in bone regeneration has heralded a new direction in the design of bone tissue engineering constructs. Previous studies have demonstrated that continuous delivery of 50 ng/ml TNF-α to mesenchymal stem cells (MSCs) cultured on three-dimensional (3D) biodegradable electrospun poly(ε-caprolactone) (PCL) microfiber meshes stimulates mineralized matrix deposition, a marker of osteogenic differentiation. Since TNF-α exhibits a biphasic pattern of expression following bone fracture in vivo, the goal of this study was to investigate the effects of temporal patterns of TNF-α delivery on in vitro osteogenic differentiation of MSCs cultured on 3D electrospun PCL scaffolds. MSCs were cultured for 16 days and exposed to continuous, early, intermediate, or late TNF-α delivery. To further elucidate the effects of TNF-α on osteogenic differentiation, the study design included MSCs pre-cultured both in the presence and absence of the typically required osteogenic supplement dexamethasone. Mineralized matrix deposition was not observed in constructs with dexamethasone-naïve MSCs, suggesting that TNF-α is not sufficient to trigger in vitro osteogenic differentiation of MSCs. For MSCs precultured with dexamethasone, TNF-α suppressed ALP activity, an early marker of osteogenic differentiation, and stimulated mineralized matrix deposition, a late stage marker of MSC osteogenic differentiation. By elucidating the impact of temporal variations in TNF-α delivery on MSC osteogenic differentiation, our results offer insight into the regenerative mechanism of TNF-α and provide the necessary design parameters for a novel tissue engineering strategy that rationally controls TNF-α signaling to stimulate bone regeneration.
Keywords: mesenchymal stem cells, bone tissue engineering, inflammation, scaffold, tumor necrosis factor-alpha
1. Introduction
Inflammation is an immediate, highly regulated biological response to bone injuries, including those due to fracture, surgical intervention, and massive trauma, and it plays a vital role in priming bone regeneration (1-5). Although bone has the ability to heal without scarring, impaired or completely absent osseous union occurs in large bone defects due to inadequate distribution of critical molecular and cellular signals (2). Informed by bone biology research and motivated by the limitations of surgical reconstruction techniques, tissue engineering efforts to date have focused on stimulating bone regeneration via delivery of various pro-regenerative bioactive molecules. Recent insight into the critical role of pro-inflammatory cytokines, particularly tumor necrosis factor-α (TNF-α), in bone healing has heralded a new direction in the rational design of bone tissue engineering constructs (1).
A complex balance exists between bone tissue and the immune system, which generates the inflammatory response, and this must be taken into account in the development of biomaterials-based tissue engineering strategies to rationally control inflammation. For instance, precise spatial and temporal control is necessary when delivering TNF-α because prolonged inflammatory signalling, as seen in chronic inflammatory disease and severe foreign body reactions to materials, stimulates bone destruction (1, 6). In contrast, during the normal response to bone injury, expression of TNF-α rises immediately, returns to baseline within a few days, and then is elevated again in later stages of bone remodeling (2, 7, 8). Obliterating this TNF-α signaling has been shown to significantly impair in vivo bone fracture healing by delaying endochondral ossification (9, 10). At the same time, repeated local injections of TNF-α during the first two days after bone injury have been shown to significantly increase fracture callus mineralization at four weeks (4).
Motivated by this significant impact on in vivo bone regeneration, ongoing in vitro studies have focused on elucidating the pro-regenerative mechanism of TNF-α, particularly with regards to its impact on mesenchymal stem cell (MSC) osteogenic differentiation (4, 11-21). However, these studies have exposed several inadequacies in the typical methods used for in vitro mesenchymal stem cell osteogenic differentiation. For instance, two osteogenic supplements typically used to stimulate in vitro MSC osteogenic differentiation, ascorbic acid and dexamethasone, antagonize TNF-α signaling (16, 19, 20), which has necessitated the development of alternate cell culture protocols to avoid concurrent delivery. The use of dexamethasone supplementation as an in vitro technique to simulate in vivo MSC osteogenic differentiation has also been called into question because corticosteroids like dexamethasone are absent in the in vivo bone fracture environment. In fact, their presence has been shown to negatively impact bone homeostasis and healing (1, 8).
We have previously reported one strategy to overcome this limitation, by preculturing MSCs in dexamethasone-replete (+dex) osteogenic medium, and then using medium without dexamethasone (-dex) to evaluate the effects of TNF-α (16). Using this strategy, we studied the effects of several different concentrations of TNF-α on MSCs cultured on three-dimensional (3D) biodegradable electrospun poly(ε-caprolactone) (PCL) microfiber meshes, and demonstrated that continuous delivery of 50 ng/ml TNF-α to MSCs stimulates mineralized matrix deposition, a marker of osteogenic differentiation (16).
Electrospun PCL microfiber scaffolds represent an emerging design in tissue engineering, where the majority of studies have focused on nanofibrous scaffolds (22, 23). Although nanoscale fibers more closely resemble the natural components of the extracellular matrix, a major challenge with nanofibrous electrospun scaffolds has been the nanoscale pore sizes that are typically achieved, which impede nutrient diffusion as well as cell infiltration and migration (22, 23). We have previously demonstrated that these limitations can be addressed by modulating electrospinning parameters to achieve PCL microfiber meshes, which support attachment and infiltration of MSCs undergoing in vitro osteogenic differentiation (16, 21, 23).
The objective of this work was to address another gap in our understanding of TNF-α, by examining the effects of temporal variations in TNF-α delivery on MSC osteogenic differentiation. We hypothesized that a temporal pattern of TNF-α delivery similar to that seen in vivo would further enhance MSC osteogenic differentiation. MSCs were cultured in 3D biodegradable electrospun PCL scaffolds for 4-16 days and exposed to continuous, early, intermediate, or late TNF-α delivery. The goal of this study was to investigate the effects of early, intermediate, late, and continuous temporal patterns of TNF-α delivery on MSC osteogenic differentiation.
2. Materials and Methods
2.1. Experimental Design
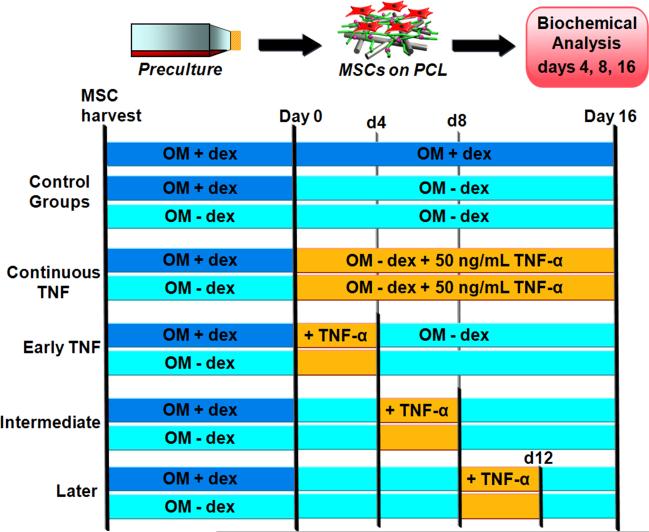
This study had a three-factor design with 2 preculture conditions (“+dex” vs. “-dex”), 4 TNF-α delivery periods (“Continuous,” “Early,” “Intermediate,” and “Later”), and 3 time points, as depicted in Figure 1. All experimental groups received the same concentration (50 ng/ml) of TNF-α during the various delivery periods. Negative control groups consisted of MSCs (either +dex or -dex precultured) on PCL scaffolds cultured in -dex media without TNF-α. The positive control consisted of +dex precultured MSCs that continued to receive +dex media following seeding onto PCL scaffolds. Due to the large number of groups in this study, the entire design was repeated twice; the MSCs for each trial were obtained from independent isolations, and (n = 4) scaffolds per group per timepoint were included in each trial.
Figure 1.
Schematic depiction of the three-factor design used, incorporating 2 preculture conditions (“+dex” vs. “-dex”), 4 TNF-α delivery periods (“Continuous,” “Early,” “Intermediate,” and “Later”), and 3 time points (days 4, 8, 16). OM represents osteogenic media (α-MEM, 10% v/v fetal bovine serum, 50 μg gentamicin/ml, 1.25 μg amphotericin-B/ml, 10 mM β-glycerophosphate, 50 μg/ml ascorbic acid) either with or without 10-8 M dexamethasone (“+dex” vs. “-dex”).
An additional study, presented in the Appendix, was included in the design to control for any change in MSC differentiation as well as response to TNF-α due to serum lot. Although the fetal bovine serum used in this study was from the same manufacturer (Gemini Bio-Products, West Sacramento, CA) as that used in a previous study using TNF-α and osteogenically differentiating MSCs (16), the serum used for the studies described herein was derived from a different serum lot. Thus, the previously reported TNF-α dosage study (16), which examined the impact of continuous delivery of 0.1, 5, and 50 ng/ml of TNF-α on MSCs cultured within 3D electrospun PCL meshes for 16 days, was repeated with the new serum to establish a baseline for comparison of the results of the temporal patterning study. The results are reported in the Appendix.
2.2. Electrospun Scaffold Generation
Electrospun PCL meshes were prepared according to an established protocol (16). The PCL (Lactel, Pelham, AL) had a number-average molecular weight (Mn) of 72,100 ± 1400 Da and polydispersity index (Mw/Mn) of 1.60 ± 0.02, determined via gel permeation chromatography (Phenogel Linear Column with 5 μm particles, Phenomenex, Torrance, CA; Differential Refractometer 410, Waters, Milford, MA) using a calibration curve generated with polystyrene standards (Fluka, Switzerland). An 18 w/w% solution of PCL in 5:1 (by vol) chloroform:methanol was loaded into a 10 ml syringe and positioned in a previously described electrospinning apparatus (16, 23). A 16 gauge blunt-tip needle (Brico Medical Supplies, Inc., Metuchen, NJ) was attached to the syringe such that the needle tip was 33 cm from a glass collecting plate placed in front of the system's grounded copper plate. A voltage difference of 33 kV was applied to the needle tip and the PCL solution was pumped out of the needle tip at 40 ml/h using a syringe pump.
Scaffolds with a diameter of 8 mm were cut from the resulting electrospun PCL meshes using an arch punch (C.S. Osborne & Co., Harrison, NJ). The thickness of each scaffold was measured using digital micro-calipers (Mitutoyo, Aurora, IL) and, for consistency with previous work (16, 21), scaffolds with thickness 0.90-1.10 mm were used for the study. For each MSC isolation, (n = 2-3) electrospun PCL meshes were required to obtain a sufficient number of scaffolds for the study. Each batch of meshes was prepared on the same day using the same lot of PCL and the same electrospinning parameters. Scaffolds were stored under an inert nitrogen atmosphere at -20°C in a non-defrosting freezer to prevent degradation prior to use.
2.3. Scaffold Characterization
For each electrospun mesh, (n = 3) scaffolds were mounted onto aluminum stubs, sputter-coated with 30 nm of gold, and imaged using scanning electron microscopy (SEM) (FEI Quanta 400 Environmental, Hillsboro, OR) at a 7.31 kV accelerating voltage and 3.0 spot size. Predefined coordinates on each scaffold were imaged and the diameters of (n = 45 fibers) from the top and (n = 45 fibers) from the bottom of each mesh were measured according to an established protocol (16, 21, 23). The porosity of n = 50 scaffolds from each mesh was determined using gravimetric analysis as previously described (16, 21, 23).
2.4 Mesenchymal Stem Cell Isolation
All MSC isolations were approved by the Rice University Institutional Animal Care and Use Committee and adhered to the National Institutes of Health guidelines. For each of the two independent trials of the temporal variation study described in the Experimental Design section above and depicted in Figure 1, MSCs were isolated from the pooled femoral and tibial bone marrow of 8 male syngeneic Fischer 344 rats weighing 150-175 g, according to established methods (24, 25). For the dosage study shown in the Appendix, MSCs were isolated in the same manner from the pooled marrow of 5 male Fischer 344 rats. For each trial of the temporal study shown in Figure 1, bone marrow suspensions from each of the 8 rats were split into two portions and were cultured in either +dex or -dex media for 7 days. Both types of media consisted of α-MEM (Sigma, St. Louis, MO) with 10% v/v fetal bovine serum (Gemini), 50 μg/ml gentamicin, 1.25 μg/ml amphotericin-B, 10 mM β-glycerophosphate, and 50 μg/ml ascorbic acid (all from Sigma). The +dex media also contained 10-8 M dexamethasone (Sigma). For the dosage study, all MSCs were cultured in +dex media. In all cases, culture media was exchanged on days 1, 3, and 5 to remove non-adherent cells. As in previous studies, the adherent cells were designated as “mesenchymal stem cells,” based on this cell population's established osteogenic potential under relevant in vitro conditions (16, 26, 27).
2.5. TNF-α Reconstitution and Dilution
For each trial of the temporal study shown in Figure 1, lyophilized recombinant rat TNF-α (R&D Systems, Minneapolis, MN) was reconstituted at 50 ng/μl on the day of scaffold seeding using sterile phosphate-buffered saline (PBS) containing 0.1 w/w% bovine serum albumin (Sigma), according to the manufacturer's instructions. For the dosage study described in the Experimental Design, recombinant rat TNF-α was reconstituted at 100 ng/μl on the day of scaffold seeding, as described above. This stock solution was then further diluted to create stock solutions of 0.1, 5, and 50 ng/μl. For all experiments, 4 μl of the relevant TNF-α stock solution were added to 4 ml of media used for construct culture, resulting in the desired 0.1, 5, or 50 ng/ml TNF-α.
2.6. Scaffold Seeding and Culture
Prior to MSC seeding, electrospun PCL scaffolds were sterilized via exposure to ethylene oxide gas for 14h and then prewet using a decreasing ethanol gradient (from 100% to 70%), according to established protocols (16, 23). The ethanol was exchanged with sterile ultrapure (Type I) water (Super-Q Water Purification System, EMD Millipore, Billerica, MA), and then with -dex media. The scaffolds in -dex media were incubated at 37°C overnight, and then press-fit into seeding cassettes that were placed in ultra-low attachment 6-well plates (1 cassette/well) (Corning Incorporated Life Sciences, Lowell, MA) as previously described (16).
For seeding, nearly confluent MSCs were lifted from the cell culture flasks with 0.25% trypsin/EDTA (Gibco, Carlsbad, CA), counted with a hemocytometer, centrifuged, and resuspended at 1.25 million cells/ml in -dex osteogenic media. 0.2 ml of the resuspended MSCs were added drop-wise to each scaffold, which had already been press-fit into a seeding cassette. After an attachment period of 4h, each well was filled with 8 ml of -dex media and the relevant groups were supplemented with either 10-8 M dexamethasone or 50 ng/ml TNF-α (according to the design shown in Figure 1). For the dosage study, groups were supplemented with either dexamethasone or various TNF-α concentrations, as described in the Experimental Design above. In all cases, constructs were removed from the seeding cassettes after 24h and transferred to new ultra-low attachment 6-well plates for the duration of the study. The wells were filled with 4 ml of -dex media, supplemented with dexamethasone or TNF-α as appropriate, and media were changed every two days thereafter.
2.7. Cellularity, Alkaline Phosphatase Activity, and Calcium Content Assays
For each of the two independent trials of the TNF-α temporal study, and separately for the dosage study, on days 4, 8, and 16, (n = 4) scaffolds from each group were removed, rinsed twice with 4 ml of PBS, and flash-frozen in 1 ml of sterile ultrapure (Type I) water (EMD Millipore) using liquid nitrogen. The cells were lysed using three consecutive freeze/thaw/sonication cycles (10 min at -80°C, 10 min at 37°C, 10 min sonication). Biochemical assays were performed according to protocols detailed elsewhere (16, 24). Briefly, the double-stranded DNA content of each resulting cell lysate solution was quantified using the fluorometric PicoGreen assay (Molecular Probes, Eugene, OR) and then converted to the number of cells per construct. The alkaline phosphatase (ALP) activity of the cell lysates was quantified using an established colorimeteric assay based on the rate of conversion of the colorless substrate p-nitrophenyl phosphate disodium salt hexahydrate (Sigma), to a yellow product, p-nitrophenol. Next, each construct was removed from the aqueous cell lysate, immersed in 1 ml of 1 N acetic acid, and left on a shaker table at 200 rpm overnight to dissolve matrix-bound calcium. Calcium content was quantified using an established colorimetric assay that is based on a calcium chelating agent (Arsenazo III; Genzyme Diagnostics PEI, Charlottetown, Prince Edward Island, Canada) that changes color upon binding to free calcium in an acid solution (16, 24). Samples and standards were run in triplicate for all assays, and samples were diluted as necessary to fall within the range of the standards for each assay.
2.8. Histological Sample Preparation and Analysis
At the final timepoint of each trial of the temporal study (depicted schematically in Figure 1), n = 1 construct from each of the three control groups and from each of the two “continuous” TNF-α groups was rinsed twice with PBS and fixed overnight in 5 ml of 10% buffered formalin phosphate. Constructs were then passed through an ascending (70% to 100%) ethanol gradient and stored in 100% ethanol at 4°C. Prior to sectioning, each construct was cut in half (so that cross-sectional images could be obtained) and embedded in HistoPrep (Fisher Scientific, Pittsburgh, PA). Frozen 5 μm thick sections (CM1850 cryotome, Leica Microsystems, Bannockburn, IL) were mounted on Superfrost Plus slides (Fisher Scientific) and incubated at 45°C on a slide warmer for 4-5 days according to an established protocol for optimal section adhesion (16).
Sections were rehydrated and then stained with von Kossa (5% w/w silver nitrate in milliQ water) under UV for 30 min. The slides were rinsed with water followed by 95% ethanol and then alcoholic eosin Y (Sigma) was applied as a counter-stain for 3 min. Excess eosin was removed with 95% ethanol. The slides were allowed to air dry, and then imaged with a light microscope (Eclipse E600, Nikon, Melville, NY) equipped with a camera (3CCD Color Video Camera DXC-950P, Sony, Park Ridge, NJ) and attached to a computer. Light microscope images were calibrated using the standard two-image method to correct for differences in background lighting and in dark-current effects in the detector (28).
2.9. Statistical Analysis
Fiber diameters and porosities are reported as mean ± standard deviation for n = 45 fibers and n = 50 scaffolds, respectively. The average fiber diameter of the top and bottom of each PCL microfiber mesh, along with the average porosity, were compared at 95% confidence using one-way analysis of variance. For the temporal variation study, the biochemical assay results are reported as mean ± standard deviation for n = 8 constructs (n = 4 constructs from each of two independent trials). The cellularity, ALP activity, and calcium content data from each of the two trials were combined and analyzed as a randomized complete block design at 95% confidence using two-way analysis of variance. Each trial was set as a block. Multiple pair-wise comparisons were made using the Bonferroni post-hoc analysis method at 95% confidence. For the TNF-α dosage study, the cellularity, ALP activity, and calcium content values represent the mean ± standard deviation for n = 4 constructs from each group at each timepoint. The data were compared using one-way analysis of variance with 95% confidence. Multiple pair-wise comparisons were made using the Bonferroni post-hoc analysis method at 95% confidence.
3. Results
3.1. Scaffold Morphology
The morphology of representative scaffolds from each study is shown in Figure 2, imaged via SEM at varying magnifications. Table 1 lists the average fiber diameter and porosity of the PCL microfiber meshes used in this work. The tops and bottoms of the scaffolds used in the two repetitions of the temporal study (“Temporal trial 1” and “Temporal trial 2” in Table 1), as well as in the dosage study described in the Appendix (“Dosage Study” in Table 1), generally had statistically equivalent average fiber diameters (p > 0.05), with the exception of one of the meshes used for Temporal trial 2, which had both top and bottom average fiber diameters that were significantly larger than the other values (p < 0.05). Nearly every mesh had a statistically unique porosity (p < 0.05). However, the magnitude of the differences was small, with the values ranging from 78.8 ± 1.4% to 84.9 ± 0.9% (Table 1).
Figure 2.
SEM images of representative electrospun PCL meshes generated for this study, at 300× magnification. The left (a) and center (b) panels correspond to the meshes used for the first and second repetitions of the temporal study, respectively. The right panel (c) depicts a representative scaffold from the batch used for the TNF-α dosage study described in the Appendix. Scale bars shown in (a) is 100 μm and applies to all panels.
Table 1.
Comparison of fiber diameters and porosities of electrospun PCL meshes
| Parameter | Temporal trial 1 | Temporal trial 2 | Dosage study | |||||
|---|---|---|---|---|---|---|---|---|
| Mesh 1 | 2 | 3 | Mesh 4 | 5 | Mesh 6 | 7 | 8 | |
| Fiber diameter, top (μm) | 10.0 ± 1.0 | 9.6 ± 1.5 | 9.9 ± 1.5 | 11.1 ± 1.1* | 9.0 ± 1.3** | 8.9 ± 1.9** | 8.6 ± 1.9** | 8.3 ± 1.8** |
| Fiber diameter, bottom (μm) | 10.7 ± 1.2 | 10.0 ± 1.9 | 11.2 ± 2.0 | 15.9 ± 1.9* | 12.5 ± 1.4** | 11.0 ± 1.6 | 10.4 ± 1.2 | 12.0 ± 1.7** |
| Porosity (%) | 80.1 ± 1.6 | 84.4 ± 1.0* | 83.9 ± 1.4* | 78.8 ± 1.4* | 81.7 ± 0.9* | 80.4 ± 2.6 | 83.3 ± 1.0* | 84.9 ± 0.9* |
Values represent mean ± standard deviation for n = 45 for fiber diameters from the top and bottom of each mesh, and n = 50 scaffolds for porosity. For each parameter (row), * indicates values that significantly differ from all other values (p < 0.05), while values marked with ** differ from all other values (p < 0.05), but not from each other.
3.2. Effect of Varying TNF-α Concentration
To establish a baseline for comparison of the results of this work to a previous study examining the effect of varying TNF-α dose on MSC osteogenic differentiation (16), the prior study was repeated using media supplemented with the same batch of fetal bovine serum used for the temporal studies described in this work. For this dosage study, +dex precultured MSCs were seeded onto PCL scaffolds and continuously exposed to 0.1, 5, or 50 ng/ml of TNF-α over 16 days. Figure A1 depicts the cellularity, ALP activity, and calcium content of constructs, and the results are fully discussed in the Appendix. Briefly, as in the prior study (16), TNF-α stimulated construct mineralization in a dose-dependent fashion. Continuous delivery of 50 ng/ml TNF-α group resulted in significantly elevated calcium content compared to the other experimental groups and also compared to the negative control (+dex/-dex/-TNF; Figure A1d). This concentration of TNF-α was thus selected for use in the temporal variation studies described below and depicted schematically in Figure 1.
Figure A1.
Construct cellularity (a), ALP activity on a per-cell basis (b), and calcium content (c) with varying TNF-α concentration. The three control groups consisted of: acellular PCL scaffolds incubated in media (Acellular), as well as scaffolds seeded with +dex precultured MSCs that were then cultured in either +dex or -dex media (+dex/+dex/-TNF and +dex/-dex/-TNF, respectively). All three experimental groups consisted of +dex precultured MSCs seeded on PCL scaffolds and cultured in -dex media that contained varying amounts of TNF-α (0.1, 5, and 50 ng/ml, respectively). The inset in (b) depicts the full height of the day 16 bar for the positive control (+dex/+dex/-TNF). Note that (c) is plotted on a logarithmic scale to facilitate comparison with Figure 5. The legend in (a) applies to all panels and insets. Bars represent average ± standard deviation of (n = 4) scaffolds per group at each timepoint. Within a timepoint, groups with * differ from all other groups (p < 0.05), while ** differ from all other groups but not each other (p < 0.05). On day 16, + indicates that d4 and d16 values of that group differ (p < 0.05), while # indicates that values at all timepoints differ (p < 0.05).
3.3. Construct Cellularity
Figure 3 depicts construct cellularity at each timepoint. The cellularity of the positive control group (+dex/+dex/-TNF) followed a unique pattern with a peak on day 8 and a dramatic drop in cell number on day 16. This group had significantly fewer cells than any other group on day 16 (p < 0.05). The cellularity of the +dex-precultured negative control group (+dex/-dex/-TNF) followed a pattern similar to that of the positive control, with a peak at day 8, but this trend was not statistically significant. In contrast, the dexamethasone-naïve control (-dex/-dex/-TNF) showed an increase from day 4 to day 8 (p < 0.05), with a plateau in cell count through day 16.
Figure 3.
Scaffold cellularity with various TNF-α temporal patterns. MSCs precultured with osteogenic supplements (preculture “+dex”) and dexamethasone-naïve MSCs (preculture “-dex”) were seeded on PCL scaffolds and cultured for 16 days in dexamethasone-free media (culture “-dex”; the only exception is the positive control). Experimental groups were exposed to continuous (days 0-16), early (days 0-4), intermediate (days 4-8), or later (days 8-12) delivery of 50 ng/ml TNF-α. Each bar represents the average ± standard deviation of (n = 8) scaffolds. For each timepoint, values marked with the same letter (A-D) do not differ (p > 0.05). Values marked with different letters differ significantly, and those marked with * differ from all other groups at that timepoint (p < 0.05). For each group, # indicates that all three timepoint values for this group differ (p < 0.05), while + indicates that the day 16 value differs from the day 4 value (p < 0.05).
All groups exposed to TNF-α showed a significant (p < 0.05) increase in cellularity from days 4 to 16 (Figure 3). For both +dex and -dex precultured MSCs, early exposure to TNF-α significantly reduced day 4 cellularity compared to the analogous control groups (p < 0.05). The continuous TNF-α groups (+dex/-dex/d0-16 TNF and -dex/-dex/d0-16 TNF) also had significantly lower day 8 cell counts compared to all other groups (p < 0.05), except for the positive control (Figure 3).
3.4. Construct ALP Activity
Figure 4 depicts the ALP activity of each group on a per-cell basis, which is an early marker of MSC osteogenic differentiation. All three control groups (+dex/+dex/-TNF, +dex/-dex/-TNF, and -dex/-dex/-TNF) showed increasing ALP activity over time, such that the day 16 value was significantly higher than the day 4 value for each group (p < 0.05). The +dex/+dex/-TNF positive control had much greater ALP activity at days 8 and 16 (p < 0.05) compared to any of the other groups cultured in -dex media (inset of Figure 4). In contrast to the increasing trend over time seen for the controls, all experimental groups showed a decrease in ALP activity following TNF-α exposure. The most dramatic effect was seen in the continuous TNF-α delivery groups (+dex/-dex/d0-16 TNF and -dex/-dex/d0-16 TNF), which showed a monotonic decrease over time resulting in significantly lower ALP activity compared to any other group on day 16 (p < 0.05; Figure 4).
Figure 4.
Alkaline phosphatase (ALP) activity with various TNF-α temporal patterns. Absolute ALP activity was normalized to the corresponding cellularity shown in Figure 3. The inset depicts the full height of the day 8 and day 16 bars for the positive control (+dex/+dex/-TNF). Each bar represents the average ± standard deviation of (n = 8) scaffolds per group at each timepoint. For each timepoint, groups not marked with the same letter (A-J) differ significantly (p < 0.05). Values marked with * differ from all other groups at that timepoint (p < 0.05). For each group, # indicates that values at each timepoint for this group differ (p < 0.05), while + indicates that the day 16 value differs from the day 4 value (p < 0.05).
This suppression of ALP activity was directly related to the pattern of TNF-α delivery. For the +dex-precultured “early” TNF-α group (+dex/-dex/d0-4 TNF), ALP activity on day 4 was significantly higher than that of the corresponding control group (+dex/-dex/-TNF), then dropped significantly by day 8, and rose again on day 16 (p < 0.05 for all differences). The dexamethasone-naïve “early” TNF-α group (-dex/-dex/d0-4 TNF) had significantly reduced ALP activity on both days 4 and 8 compared to its respective TNF-α-free control (-dex/-dex/-TNF), but also showed a significant increase in ALP activity on day 16 (p < 0.05). Similarly, “intermediate” (days 4-8), and “late” (days 8-12) TNF-α delivery induced a significant drop (p < 0.05) in ALP activity between days 4 and 8, and days 8 to 12, respectively (Figure 4); this pattern was seen in both the +dex and -dex precultured versions of the groups.
3.5. Construct Mineralization
Mineralized matrix deposition, a late marker of MSC osteogenic differentiation, is shown in Figure 5. The positive control (+dex/+dex/-TNF) had the highest calcium content of any group at each timepoint (p < 0.05), with a day 16 value of 520 ± 96 μg per scaffold. The dexamethasone-naïve control (-dex/-dex/-TNF), showed a slight, yet significant (p < 0.05), increase in calcium content from day 4 to day 16, from 4.9 ± 0.8 to 5.8 ± 0.8 μg/scaffold. However, none of the corresponding dexamethasone-naïve experimental groups showed any change in calcium content over time.
Figure 5.
Construct mineralization with varying TNF-α temporal patterns. Insoluble calcium was extracted from each construct using acetic acid and the measured calcium content is shown, plotted on a logarithmic scale. Groups correspond to those shown in Figures 3-4. Each bar represents the average ± standard deviation of (n = 8) scaffolds per group at each timepoint. For each timepoint, groups marked with * differ from all other groups at that timepoint (p < 0.05), while values marked with “A” differ from all other groups (p < 0.05), but not from each other. For each group, # indicates that values at each timepoint for this group differ (p < 0.05), while + indicates that the day 16 value differs from the day 4 value (p < 0.05).
In contrast, although the +dex-precultured control group (+dex/-dex/-TNF) showed a slight increase in calcium content from day 4 to day 16, from 5.7 ± 1.7 to 10.8 ± 3.3 μg/scaffold, three of the corresponding experimental groups (continuous, early, and intermediate TNF-α delivery) also showed a significant increase in calcium content from day 4 to day 16 (p < 0.05; Figure 5). Continuous delivery of TNF-α (+dex/-dex/d0-16 TNF) resulted in significant levels of mineralized matrix deposition on days 8 and 16, with a day 16 value of 69.7 ± 31.2 μg/scaffold. Early TNF-α delivery (+dex/-dex/d0-4 TNF) also stimulated significant (p < 0.05) mineralized matrix deposition, although the day 8 and day 16 values for this group were lower than those of the continuous (+dex/-dex/d0-16 TNF) group. In contrast, intermediate and late TNF-α delivery (+dex/-dex/d4-8 and +dex/-dex/d8-12) resulted in only slight increases in calcium content from days 4 to 16 that did not differ significantly from the trend observed in the corresponding (+dex/-dex/-TNF) control group (p > 0.05)
3.6. Cell and Mineral Distribution by Histology
Cellularity and calcium content were qualitatively confirmed via histological analysis of day 16. Figure 6 depicts representative sections stained with von Kossa, which dyes mineralized matrix black, and eosin, which stains cells and non-mineralized matrix reddish-pink. Higher densities of calcium deposits (blue arrows) and cells (yellow arrows) were observed in the top half of each construct, with a 5-10 μm thick pink-stained layer of cells and non-mineralized matrix visible at the top of each section, except for the acellular scaffold included for reference. Cells infiltrated 200-500 μm into each microfiber scaffold. In some cases, particularly in the positive (+dex/+dex/-TNF) control, the pink color of the cells was obscured by the dark black color of the calcium deposits. Consistent with the calcium data in Figure 5, the positive control group (+dex/+dex/-TNF) stained much darker and contained more calcium deposits (blue arrows) than any other group (Figure 6).
Figure 6.
Representative cross-sections of day 16 constructs stained with von Kossa, which stains mineralized matrix black (blue arrows), and eosin, which stains cells and non-mineralized matrix reddish-pink (yellow arrows). Prior to scaffold seeding, MSCs were precultured in complete osteogenic media (+dex) or in dexamethasone-free media (-dex). After MSCs were seeded onto the 3D scaffolds, all groups were cultured in -dex media, except for the positive control (+dex/+dex/-TNF), which continued to receive dexamethasone. The two experimental groups shown were continuously supplemented with 50 ng/mL TNF-α from day 0 to 16. An image of an acellular scaffold is included for reference. Images were captured at 10× original magnification. Scale bar in upper left corner represents 200 μm and applies to all images.
4. Discussion
This study evaluated several temporal patterns of delivery of the pro-inflammatory cytokine TNF-α and demonstrated that continuous 16-day delivery supported osteogenic differentiation of MSCs cultured in 3D electrospun PCL microfiber meshes to the greatest extent. MSCs were cultured in 3D biodegradable electrospun PCL scaffolds for 4-16 days and exposed to continuous, early, intermediate, or late TNF-α delivery (Figure 1). To our knowledge, this is the first study to examine the temporal effects of TNF-α on MSC osteogenic differentiation within 3D polymeric scaffolds. Recent studies have reported the in vitro effects of TNF-α on various osteoprogenitors (4, 11-21, 29-31), with most studies exposing cells to continuous delivery of TNF-α for several days to weeks. The early and late patterns of TNF-α delivery used herein were chosen to mimic the biphasic pattern of TNF-α signaling observed in vivo, where TNF-α levels rise immediately after bone fracture, return to baseline within several days, and then rise again during the later stages of bone remodeling (8).
PCL was selected for the generation of electrospun microfiber mesh scaffolds because it is biocompatible and biodegradeable, with a slow degradation rate that would be beneficial in future in vivo implantation studies by providing structural support of a bone defect during tissue ingrowth, then degrading to allow for bone remodelling (16, 23, 32, 33). As in previous studies of PCL microfiber meshes (16, 21, 23), successful cell attachment and infiltration to a depth of 200-500 μm was observed (Figure 6). Our choice of material and scaffold architecture was additionally motivated by a previous study reporting that 3D PCL microfiber mesh scaffolds supported in vitro osteogenic differentiation of MSCs in the absence of the typically required osteogenic supplement dexamethasone (16). However, this prior finding may be attributable to the batch of MSCs used, as it was not reproduced in any of the studies described in the present work, where the negative controls had minimal changes in calcium content over time (+dex/-dex/-TNF and -dex/-dex/-TNF, Figure 5).
Although providing supplemental dexamethasone is an established technique to trigger in vitro osteogenic differentiation of MSCs and thus create a model of bone healing (27, 34, 35), we adopted a modified cell culture protocol in this work so as to avoid concurrent delivery of dexamethasone and TNF-α. This is because dexamethasone is an anti-inflammatory agent that is known to antagonize TNF-α signalling (1, 16, 21). In this work, we addressed this issue with two different strategies: (1) by preculturing MSCs in +dex media and then delivering TNF-α in the absence of dexamethasone; and (2) by delivering TNF-α to dexamethasone-naïve MSCs. The first strategy has been used in a previously published study (16), and consistent with the findings reported in that work, the preliminary dosage study reported herein indicated that TNF-α exposure stimulated continued mineralized matrix deposition by +dex precultured MSCs in the absence of dexamethasone (see Appendix, Figure A1c).
Motivated by this finding, the temporal studies included both +dex precultured and dexamethasone-naïve MSCs treated with TNF-α in the absence of dexamethasone (Figure 1). Prior studies of undifferentiated human MSCs have shown that TNF-α significantly affects in vitro protein expression, proliferation, and migration (11, 12, 29). In this work, dexamethasone-naïve MSCs did not exhibit any substantial mineralized matrix deposition (Figure 5), suggesting that TNF-α is not sufficient to trigger in vitro osteogenic differentiation of MSCs, but instead stimulates and supports progression of +dex precultured cells toward terminal differentiation.
This finding is nevertheless exciting because it represents an important step toward a more clinically realistic in vitro culture model. Continuous delivery of dexamethasone is typically required to maintain in vitro osteogenic differentiation of +dex precultured MSCs (27, 35), as is apparent when comparing the positive and negative controls in this work. The positive control (+dex/+dex/-TNF) showed significant mineralized matrix deposition during the study, while essentially none was seen for the +dex precultured negative control (+dex/-dex/-TNF; Figure 5). However, the use of dexamethasone to trigger MSC osteogenic differentiation does not replicate the actual in vivo biology. In fact, administration of corticosteroids suppresses in vivo bone healing and raises the risk for bone necrosis (1, 8). Thus, although the positive (+dex/+dex/-TNF) control had the highest calcium content of any group in Figure 5, the significant values noted for the continuous and early TNF-α groups (+dex/-dex/d0-16 TNF and +dex/-dex/d0-4 TNF) represent an important step toward the development of a more realistic in vitro model of bone regeneration.
For MSCs precultured with osteogenic supplements, TNF-α suppressed ALP activity, an early marker of osteogenic differentiation, and stimulated mineralized matrix deposition, a late stage marker of MSC osteogenic differentiation. TNF-α delivery suppressed ALP activity in a dose- and time-dependent manner (Figures A1b and 4). This is in stark contrast to the positive control group (+dex/+dex/-TNF), which showed a monotonic increase in ALP activity over time resulting in the highest day 16 level of any group (inset of Figure 4). The dose-dependent suppression of ALP by TNF-α is consistent with a previous study of MSCs cultured within 3D PCL scaffolds (16), but differs from several other studies of 2D-cultured osteoprogenitors, where delivery of TNF-α in -dex media stimulated both ALP activity and calcium deposition (4, 13-15). The results for the +dex-precultured cells also differ considerably from those of prior studies that concurrently delivered TNF-α and dexamethasone, which found that continuous TNF-α delivery suppressed both early and late markers of MSC osteogenic differentiation (ALP and calcium content, respectively) in both 2D and 3D culture (17, 18).
The time-dependent effects of TNF-α on MSC differentiation reported herein represent the first such study in the literature. The results for the early temporal delivery period suggest that the TNF-α-induced suppression of ALP activity is reversible once exposure to TNF-α ceases (Figure 4), and that TNF-α stimulates some mineralized matrix deposition when delivery is commenced during the first 4 days of MSC culture (Figure 5). This effect of early TNF-α delivery is consistent with a recent report from an in vivo fracture healing model, where daily local injections of TNF-α during the first two days after injury resulted in significantly increased fracture callus mineralization four weeks later (4). In contrast to in vivo findings, where prolonged TNF-α exposure is detrimental to bone healing (1, 8), we found that +dex precultured MSCs that were continuously exposed to TNF-α for 16 days (+dex/-dex/d0-16 TNF) had the second-highest amount of mineralized matrix deposition. The calcium content of these constructs was significantly greater than that of both negative controls and was exceeded only by the positive control group (Figure 5). This effect of continuous TNF-α is consistent with numerous other reports of in vitro MSC culture in -dex media (4, 13, 14, 16, 29, 30). This likely stems from the limited variation in vitro compared to in vivo, where fracture healing involves changes in thousands of genes (36, 37). TNF-α is known to have significant interplay with several other signaling pathways, e.g., that of the bone morphogenetic proteins (29, 38), a factor that will be incorporated in future studies.
The cellularity of many of the groups in Figure 3 followed a similar trend over time. A notable exception is the positive control group, whose cellularity peaked at day 8 and then significantly dropped by day. This trend is consistent with previous results (16, 24), and may reflect the reduced self-renewal of the MSCs as they progress down the osteogenic lineage. In contrast, TNF-α exposure resulted in increased cell counts, with the most dramatic effect in the continuous (days 0-16) TNF-α groups (Figure 3).
Comparison of the preliminary dosage study described in the Appendix to the previously published work with the same design (16) indicates that the change in the batch of serum affected the attachment, proliferation, and/or survival of the MSCs on the PCL scaffolds, as well as the magnitude of ALP activity and mineralized matrix deposition, without altering the general trends amongst the groups. Since serum is derived from blood by removing the cellular components and clotting factors, its composition can vary in terms of the concentration of hormones, growth factors, and cytokines. For human MSCs, serum selection often involves an extensive screening process (27), but serum for MSCs from other species is not typically subjected to such rigorous screening. These procedures may need to be revised as more complicated in vitro cell culture models are developed. However, some of the differences between the results in the Appendix and those in prior work (16) may also be accounted for by the batch-to-batch variations in the MSCs, as can be seen by comparing the positive control group's ALP activity (Figure 4 vs. A1b) and calcium content (Figure 5 vs. A1c) in both the temporal and dosage studies described herein.
Thus, the results of this study are not only important for understanding the role of TNF-α in bone regeneration, but are also useful steps toward the development of a more clinically realistic in vitro model of the fracture healing microenvironment. A limitation of this work is that only a single cell type was involved, in contrast to the mixture of cell types present during in vivo fracture healing. A recent in vitro study indicated that TNF-α has a significant effect on osteogenic differentiation of MSCs co-cultured with osteoblasts (31). Another recent study reported that continuous exposure to TNF-α had a negative effect on in vitro osteogenic differentiation of MSCs cultured on PCL scaffolds coated with bone-like extracellular matrix (21), a result that is more consistent with in vivo findings, where short-term TNF-α exposure promotes bone healing, while long-term exposure is detrimental. By elucidating the impact of temporal variations in TNF-α delivery, as well as the interplay between TNF-α and the anti-inflammatory osteogenic supplement dexamethasone, our results greatly improve our understanding of the impact of TNF-α on in vitro MSC osteogenic differentiation.
5. Conclusions
This work elucidated the impact of temporal variations in TNF-α delivery, as well as the interplay between TNF-α and the anti-inflammatory osteogenic supplement dexamethasone, on in vitro MSC osteogenic differentiation in 3D biodegradable electrospun PCL scaffolds. TNF-α delivery initiated during the early (days 0-4) portion of MSC culture induced cell proliferation, suppressed ALP activity, and stimulated mineralized matrix deposition, findings indicating MSC osteogenic differentiation. This effect of TNF-α was observed only for +dex precultured MSCs, and not in dexamethasone-naïve MSCs, suggesting that TNF-α is not sufficient to trigger differentiation, but can support mineralized matrix deposition that would otherwise require the presence of dexamethasone. Precise spatial and temporal control is critical for the development of tissue engineering strategies to modulate inflammation because both insufficient as well as excessive amounts of pro-inflammatory signals have detrimental effects on bone. The findings presented herein will facilitate the design of future in vitro and in vivo experiments to probe the interplay of TNF-α with other growth factors and cytokines typically present at the site of bone injury and regeneration, which will ultimately enable the design of new strategies to rationally control TNF-α signaling and stimulate bone regeneration.
Acknowledgements
This research was supported by the National Institutes of Health (NIH R01 AR057083) (AGM). PMM was supported by training fellowships from the Keck Center Nanobiology Training Program of the Gulf Coast Consortia (NIH Grant No. 5 T90 DK070121-04) and the NIH Biotechnology Training Program (NIH Grant No. 5 T32 GM008362-19) for portions of this work.
Appendix
To establish a baseline for comparison, a previously study (16) was repeated using the same batch of fetal bovine serum used for the temporal studies described in this work. Figure A1 depicts the cellularity, ALP activity, and calcium content of constructs consisting of +dex precultured MSCs seeded onto PCL scaffolds and continuously exposed to 0.1, 5, or 50 ng/ml of TNF-α over 16 days.
Most of the groups had similar cell numbers at each timepoint (Figure A1a). The acellular constructs (“Acellular”) had nearly zero cells at all timepoints, as expected. The positive (+dex/+dex/-TNF) and negative (+dex/-dex/-TNF) control groups showed a peak in cellularity at day 8 followed by a drop on day 16. The negative control had significantly higher cell count than any other group on day 8, while the positive control had significantly lower cellularity than any other group on day 16, except for the acellular constructs (p < 0.05 in both cases). In contrast to the control groups, all three experimental groups showed a steady increase in cellularity over time, such that the day 4 and 16 values differed significantly in all three cases (p < 0.05). The 5 ng/ml and 50 ng/ml TNF-α groups had a particularly dramatic increase in cellularity after day 8, such that the cellularity of these groups greatly exceeded that of any other group on day 16 (p < 0.05; Figure A1a).
Figure A1b shows the ALP activity on a per-cell basis in response to continuous delivery of various TNF-α concentrations. The acellular group was not included in this analysis because of the near-zero cellularity of these constructs at each timepoint (Figure A1a). The two control groups (+dex/+dex/-TNF and +dex/-dex/-TNF), as well as group receiving the lowest dose of TNF-α (0.1 ng/ml), all showed a monotonic increase in ALP activity over time. The negative control and the 0.1 ng/ml TNF-α group had similar values at all three timepoints, while the positive control had at least a six-fold higher day 16 ALP activity than any other group (p < 0.05; inset of Figure A1b). In contrast, the two experimental groups that received higher doses of TNF-α (5 and 50 ng/ml) showed decreasing ALP activity over time, with day 16 values that were 20-fold lower than the 0.1 ng/ml TNF-α group (p < 0.05).
Insoluble calcium content, a late stage marker of osteogenic differentiation, is shown in Figure A1d. As expected, the acellular control showed no mineralization over time and the positive control (+dex/+dex/-TNF) had significantly higher (p < 0.05) calcium content than all other groups on day 16. Exposure to all concentrations of TNF-α induced significant mineralized matrix deposition. At day 4, all three experimental groups (0.1, 5, and 50 ng/ml TNF-α) had significantly higher calcium content than the corresponding negative control (+dex/-dex/-TNF) (p < 0.05). This difference was again present on days 8 and 16, though only for the two higher doses of TNF-α (5 and 50 ng/ml). The day 16 calcium content of the experimental groups showed a dose-response trend, with 50 ng/ml TNF-α resulting in significantly higher mineralized matrix deposition (p < 0.05) compared to all other groups except for the positive control.
References
- 1.Mountziaris PM, Spicer PP, Kasper FK, Mikos AG. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng Part B Rev. 2011;17(6):393–402. doi: 10.1089/ten.teb.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–84. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 3.Kolar P, Schmidt-Bleek K, Schell H, Gaber T, Toben D, Schmidmaier G, Perka C, Buttgereit F, Duda GN. The early fracture hematoma and its potential role in fracture healing. Tissue Eng Part B Rev. 2010;16(4):427–34. doi: 10.1089/ten.TEB.2009.0687. [DOI] [PubMed] [Google Scholar]
- 4.Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF-α promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A. 2011;108(4):1585–90. doi: 10.1073/pnas.1018501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pape H-C, Marcucio R, Humphrey C, Colnot C, Knobe M, Harvey EJ. Trauma-induced inflammation and fracture healing. J Orthop Trauma. 2010;24(9):522–5. doi: 10.1097/BOT.0b013e3181ed1361. [DOI] [PubMed] [Google Scholar]
- 6.Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol. 2009;5(12):667–76. doi: 10.1038/nrrheum.2009.217. [DOI] [PubMed] [Google Scholar]
- 7.Kon T, Cho T-J, Aizawa T, Yamazaki M, Nooh N, Graves DT, Gerstenfeld LC, Einhorn TA. Expression of osteoprotegerin, receptor activator of NF-κB ligand (osteoprotegrin ligand) and related pro-inflammatory cytokines during fracture healing. J Bone Miner Res. 2001;16(6):1004–14. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- 8.Mountziaris PM, Mikos AG. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev. 2008;14(2):179–86. doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerstenfeld LC, Cho T-J, Kon T, Aizawa T, Tsay A, Fitch J, Barnes GL, Graves DT, Einhorn TA. Impaired fracture healing in the absence of TNF-α signaling: The role of TNF-α in endochondral cartilage resorption. J Bone Miner Res. 2003;18(9):1584–92. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 10.Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Cruceta J, Graves BD, Einhorn TA. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells Tissues Organs. 2001;169(3):285–94. doi: 10.1159/000047893. [DOI] [PubMed] [Google Scholar]
- 11.Bocker W, Docheva D, Prall W, Egea V, Pappou E, Rossmann O, Popov C, Mutschler W, Ries C, Schieker M. IKK-2 is required for TNF-α-induced invasion and proliferation of human mesenchymal stem cells. J Mol Med. 2008;86(10):1183–92. doi: 10.1007/s00109-008-0378-3. [DOI] [PubMed] [Google Scholar]
- 12.Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. Human mesenchymal stem cells stimulated by TNF-{alpha}, LPS, or hypoxia produce growth factors by an NF{kappa}B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008 Mar 1;294(3):C675–82. doi: 10.1152/ajpcell.00437.2007. 2008. [DOI] [PubMed] [Google Scholar]
- 13.Hess K, Ushmorov A, Fiedler J, Brenner RE, Wirth T. TNF[alpha] promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-[kappa]B signaling pathway. Bone. 2009;45(2):367–76. doi: 10.1016/j.bone.2009.04.252. [DOI] [PubMed] [Google Scholar]
- 14.Ding J, Ghali O, Lencel P, Broux O, Chauveau C, Devedjian JC, Hardouin P, Magne D. TNF-[alpha] and IL-1[beta] inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 2009;84(15-16):499–504. doi: 10.1016/j.lfs.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Paula-Silva FWG, Ghosh A, Silva LAB, Kapila YL. TNF-{alpha} promotes an odontoblastic phenotype in dental pulp cells. J Dent Res. 2009;88(4):339–44. doi: 10.1177/0022034509334070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mountziaris PM, Tzouanas SN, Mikos AG. Dose effect of tumor necrosis factor-[alpha] on in vitro osteogenic differentiation of mesenchymal stem cells on biodegradable polymeric microfiber scaffolds. Biomaterials. 2010;31(7):1666–75. doi: 10.1016/j.biomaterials.2009.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacey DC, Simmons PJ, Graves SE, Hamilton JA. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: implications for bone repair during inflammation. Osteoarthritis Cartilage. 2009;17(6):735–42. doi: 10.1016/j.joca.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Lin C-C, Metters AT, Anseth KS. Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNF[alpha]. Biomaterials. 2009;30(28):4907–14. doi: 10.1016/j.biomaterials.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balga R, Wetterwald A, Portenier J, Dolder S, Mueller C, Hofstetter W. Tumor necrosis factor-alpha: Alternative role as an inhibitor of osteoclast formation in vitro. Bone. 2006;39(2):325–35. doi: 10.1016/j.bone.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 20.Iqbal J, Sun L, Kumar TR, Blair HC, Zaidi M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc Natl Acad Sci U S A. 2006;103(40):14925–30. doi: 10.1073/pnas.0606805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mountziaris PM, Tzouanas SN, Mikos AG. The interplay of bone-like extracellular matrix and TNF-α signaling on in vitro osteogenic differentiation of mesenchymal stem cells. J Biomed Mater Res A. 2012;100A(5):1097–106. doi: 10.1002/jbm.a.34058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12(5):1197–211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 23.Pham QP, Sharma U, Mikos AG. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7(10):2796–805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 24.Datta N, Holtorf HL, Sikavitsas VI, Jansen JA, Mikos AG. Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials. 2005;26(9):971–7. doi: 10.1016/j.biomaterials.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Pham QP, Kasper FK, Baggett LS, Raphael RM, Jansen JA, Mikos AG. The influence of the in vitro generated bone-like extracellular matrix on osteoblastic gene expression of marrow stromal cells. Biomaterials. 2008;29(18):2729–39. doi: 10.1016/j.biomaterials.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 27.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 28.Merchant FA, Periasamy A. Correction Using Calibration Images. In: Wu Q, Merchant FA, Castleman KR, editors. Microscope Image Processing. Elsevier; Burlington: 2008. p. 258. [Google Scholar]
- 29.Lu Z, Wang G, Dunstan CR, Chen Y, Lu WY-R, Davies B, Zreiqat H. Activation and promotion of adipose tissue-derived mesenchymal stem cells by tumour necrosis factor-alpha preconditioning for bone tissue engineering. J Cell Physiol. 2013 doi: 10.1002/jcp.24330. Epub ahead of print (doi: 10.1002/jcp.24330) [DOI] [PubMed] [Google Scholar]
- 30.Briolay A, Lencel P, Bessueille L, Caverzasio J, Buchet R, Magne D. Autocrine stimulation of osteoblast activity by Wnt5a in response to TNF-alpha in human mesenchymal stem cells. Biochem Biophys Res Commun. 2013;430(3):1072–7. doi: 10.1016/j.bbrc.2012.12.036. [DOI] [PubMed] [Google Scholar]
- 31.Lu Z, Wang G, Dunstan CR, Zreiqat H. Short-term exposure to tumor necrosis factor-alpha enables human osteoblasts to direct adipose tissue-derived mesenchymal stem cells into osteogenic differentiation. Stem Cells Dev. 2012;21(13):2420–9. doi: 10.1089/scd.2011.0589. [DOI] [PubMed] [Google Scholar]
- 32.Martins AM, Pham QP, Malafaya PB, Sousa RA, Gomes ME, Raphael RM, Kasper FK, Reis RL, Mikos AG. The role of lipase and a-amylase in both the degradation of starch/poly(e-caprolactone) fiber meshes and the osteogenic differentiation of cultured marrow stromal cells. Tissue Eng Part A. 2009;15(2):295–305. doi: 10.1089/ten.tea.2008.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang W, Yang F, Wang Y, Both SK, Jansen JA. In vivo bone generation via the endochondral pathway on three-dimensional electrospun fibers. Acta biomaterialia. 2013;9(1):4505–12. doi: 10.1016/j.actbio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Peter SJ, Liang CR, Kim DJ, Widmer MS, Mikos AG. Osteoblastic phenotype of rat marrow stromal cells cultured in the presence of dexamethasone, beta-glycerolphosphate, and L-ascorbic acid. J Cell Biochem. 1998;71:55–62. doi: 10.1002/(sici)1097-4644(19981001)71:1<55::aid-jcb6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 35.Cheng SL, Yang JW, Rifas L, Zhang SF, Avioli LV. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134(1):277–86. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- 36.Rundle CH, Wang H, Yu H, Chadwick RB, Davis EI, Wegedal JE, Lau K-HW, Mohan S, Ryaby JT, Baylink DJ. Microarray analysis of gene expression during the inflammation and endochondral bone formation stages of rat femur fracture repair. Bone. 2006;38:521–9. doi: 10.1016/j.bone.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Hecht J, Kuhl H, Haas SA, Bauer S, Poustka AJ, Lienau J, Schell H, Stiege AC, Seitz V, Reinhardt R, Duda GN, Mundolos S, Robinson PN. Gene identification and analysis of transcripts differentially regulated in fracture healing by EST sequencing in the domestic sheep. BMC Genomics. 2006;7:172. doi: 10.1186/1471-2164-7-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singhatanadgit W, Salih V, Olsen I. Bone morphogenetic protein receptors and bone morphogenetic protein signaling are controlled by tumor necrosis factor-[alpha] in human bone cells. Int J Biochem Cell Biol. 2006;38(10):1794–807. doi: 10.1016/j.biocel.2006.05.005. [DOI] [PubMed] [Google Scholar]