Abstract
Background
Pancreatic cancer cells exist in a hypoxic microenvironment containing numerous factors which impact tumor survival, proliferation and metastasis. MicroRNAs (miRs) are differentially expressed in cancer but also altered by hypoxia. We hypothesized that hypoxia could induce miR-21 expression, an oncomir in pancreatic cancer cells.
Materials and Methods
We examined how hypoxia regulates miR-21 expression in pancreatic cancer cell lines (BxPC-3, AsPC-1) by stem-loop RT-PCR. Chromatin immunoprecipitation (ChIP) assays were used to study how hypoxia alters HIF-1α binding to the hypoxia response element of miR-21. BxPC-3 and AsPC-1 cells were transfected with a constitutively stable HIF-1α subunit or vector control (pcDNA3.1) to determine the influence of miR-21 in normoxia. The effect of mature miR-21 sense and antisense oligonucleotides on proliferation and apoptosis in hypoxic and normoxic conditions was assessed via WST-1 assay and flow cytometry.
Results
MiR-21 levels increased in all cell lines grown in hypoxic conditions versus normoxia while siRNA targeting HIF-1α reduced miR-21 expression. Hypoxic conditions resulted in direct binding of HIF-1α to the predicted binding site in miR-21. Transfection with a constitutively stable HIF-1α expression plasmid in normoxia resulted in upregulated miR-21, similar to that seen in hypoxia. Cells transfected with antisense constructs targeting miR-21 had reduced proliferation and increased apoptosis in normoxia whereas miR-21 overexpression abrogated hypoxia-associated reductions in proliferation.
Conclusions
MiR-21 is induced by hypoxia in pancreatic cancer cells via HIF-1α upregulation. MiR-21 overexpression allows cells to avoid apoptosis in a hypoxic microenvironment. Inhibition of miR-21 expression may increase cellular susceptibility to hypoxia in pancreatic cancer.
Keywords: Pancreatic cancer, hypoxia, micro-RNA, HIF-1alpha
INTRODUCTION
Pancreatic cancer is the fourth most common cause of cancer-related death in the United States.1, 2 In the best of circumstances, when disease is confined to the pancreas without lymphatic or hematogenous spread, five-year overall survival remains less than 30% with a median survival of approximately 18 - 24 months.3 Unfortunately, the majority of patients are not amenable to resection due to locally advanced or metastatic disease. This dismal prognosis owes to the aggressive nature of the disease resulting in advanced stage at the time of presentation and its inherent resistance to chemotherapy and radiation. Much research has focused on identifying gene expression patterns that might explain its pathogenesis and aggressive nature. Thus far, results have been disappointing.
Hypoxia is an essential component of the neoplastic microenvironment often allowing a selective advantage for tumor cells over otherwise non-invasive cells more sensitive to a low oxygen state. The evidence for hypoxia in pancreatic cancer is in their characteristic avascular appearance on computed tomography4 and from intratumoral oxygen tension measurements.5 Hypoxic conditions in solid malignancies may confer resistance to conventional radiation and chemotherapy.6, 7 A functional link between hypoxia and microRNA expression in colon and breast cancer cell lines was shown by Kulshreshtha at. al8 and in several other cancers.9-11 Interestingly, the majority of hypoxia-associated microRNAs predicted in silico are differentially overexpressed in pancreatic cancer.
MicroRNA research holds the promise of providing molecular insight into carcinogenesis and disease progression. These small non-coding RNAs, when in mature form, are incorporated into the RNA-induced silencing complex (RISC) where they act by binding to a conserved sequence of the 3’ untranslated region of protein-coding target genes, leading to mRNA degradation or translational inhibition depending on perfect or imperfect complentarity, respectively.12 Target genes involved in apoptosis or cell cycle progression allow microRNAs to act as oncogenes and tumor suppressor genes.13-15 These oncomirs have been described in both hematologic and solid tumors. One such miR, miR-21, is overexpressed in multiple malignancies including pancreatic, esophageal, lung, breast and colon cancers.16-20 This oncogenic miR has been linked to tumor aggression and carcinogenesis, in part, by preventing apoptosis.21, 22 MiR-21 is significantly overexpressed in pancreatic cancer as detected by in situ hybridization and its strong expression predicts limited survival in patients with node-negative disease.23 Little is known, however, of the mechanism by which upregulation of miR-21 occurs.
In this study we explored the role of hypoxia in pancreatic cancer. Of particular interest was the hypoxic regulation of miR-21 and its relevance in tumor pathogenesis. We show that miR-21 is upregulated in pancreatic cancer cells in response to hypoxia through expression of hypoxia inducible factor (HIF)-1α, and that miR-21 expression allows for cell survival in the hypoxic environment.
MATERIALS AND METHODS
Cell lines and culture conditions
Human pancreatic cell lines (AsPC-1 and BxPC-3) were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in Dulbecco’s modified eagle’s media (DMEM) supplemented with 10% fetal bovine serum (FBS) in 21% O2 and 5% CO2 at 37°C. Hypoxia was induced in a hypoxic chamber (Billups-Rothenberg, Inc. Del Mar, CA) with 1% oxygen in parallel with cells maintained in normoxia. O2 and CO2 were measured and controlled using duel flow meters in order to monitor, measure, and keep concentrations consistent between experiments.
Real-time reverse transcription PCR (qRT-PCR)
RNA was extracted from cells using TRIzol (Invitrogen) according to manufacturer’s recommendations. RNA quality and quantity were determined by the Nanodrop ND-1000 Spectrophotometer (Nanodrop Products, Wilmington, DE). After treatment with DNAse (Invitrogen, CA, USA), 20ng of RNA was reverse transcribed into cDNA with Taqman Reverse Transcriptase Kit (Applied Biosystems, Inc, CA, USA). Real time RT-PCR was performed using microRNA-21-specific primers from the Taqman MicroRNA Assay using the Ct method and normalized with snoRNA U48.
Chromatin immunoprecipitation (ChIP) assay
Samples were cross-linked using 1% formaldehyde for 10 minutes and reactions stopped with 125mM glycine for 5 minutes. Cells were washed twice with 5ml of ice-cold phosphate-buffered saline (PBS) followed by centrifugation at 1500rpm 4°C for 10 minutes. Lysis buffer was supplemented with protease inhibitors (CalBiochem, CA, USA) and added to pellets followed by 10 minutes incubation on ice. Sonication was performed for DNA shearing to fragments between 200 bp and 1 kb. Supernatant was recovered and diluted in ChIP dilution buffer (Millipore Corp, MA, USA), followed by immunoprecipitation overnight at 4°C with polyclonal anti-HIF-1α antiserum (Abcam, Inc, MA, USA). Negative control beads (MOCK) were prepared in the absence of anti-HIF-1α antiserum. Immunocomplexes were recovered by addition of 50% slurry of salmon sperm DNA-protein A-agarose to samples and washed. The immunoprecipitated DNA was retrieved from the beads by incubation in elution buffer at 65°C for 3h. Cross-linking was reversed using 200mM NaCl at 65°C overnight followed by proteinase K digestion at 45°C for 2h. DNA was then purified using a PCR purification kit (Qiagen, Inc, CA, USA), and PCR was performed with primers designed to span the region of hypoxia response elements (HRE) predicted for HIF-1α in the 5’ direction of miR-21.8 CTGAGGCAAAGGGAAATG (forward); GAGACCAGCCTGGCTAACAC (reverse). PCR products were amplified from 1μg, resolved on an 1.5% agarose gel and visualized by ethidium bromide staining.
Transient Transfection
Cells were transfected with the precursor of miRNA-21 or negative Control#1 (Ambion, TX, USA) and antisense oligo of miR-21 (Exiqon, Denmark) at final concentrations of 100 nM using LipofectAMINE 2000 reagent (Invitrogen, CA, USA). The transfection mixture was dissolved in Opti-MEM serum free media (Invitrogen) and at the time of transfection cells were seeded in medium with 10% FBS and no antibiotics. Six hours after transfection, medium was exchanged for full growth medium.
Cell proliferation
For cell proliferation, cells were seeded in a 96-well plate (5000 cells/well). After transfection cell viability was determined at 48h using WST1 reagent (Roche, CA, USA) according to the manufacturer’s protocol. Absorbance at 490 nm was measured via kinetic microplate reader (Spectra Max 190; Molecular Devices, CA, USA). Experiments were done in triplicate and repeated three times.
Plasmids
Mutant versions of HIF-1α with double proline-to-alanine substitutions making it constitutively active have been described.24 The three-HRE–thymidine kinase (tk)–luciferase reporter is a HIF-responsive construct containing a tandem of hypoxia-responsive elements from the erythropoietin promoter. Plasmid was provided as a kind gift from Dr. Mircea Ivan (Indiana University).
Western blot analysis
Whole-cell extracts were prepared in 300μl of ice-cold lysis buffer (10mM TRIS-HCL, 150mM NaCl, 1% TRITON X-100, 2mM EDTA, 0.5% NP40) with protease inhibitors Cocktail II (Calbiochem, Germany), 48 hours after transfection. Cells were washed with ice cold PBS and lysed. 60 μg of protein was separated on 4-20% polyacrylamide gels (BioRad, CA, USA). Immunoblot analysis was performed using the following antibodies: anti-HIF-1α dilution 1:200 (BD Transduction Laboratories, CA, USA), and anti-vinculin – dilution 1:500 (Santa Cruz, CA, USA). Secondary horseradish peroxidase (HRP) antibody was detected using ECL Western Analysis System (Amersham Bioscience, NJ, USA).
Apoptosis Analysis
At 48h after transfection with anti miR-21, cells were collected, washed with cold PBS and stained with Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI). The BD Pharmingen Detection Kit for Annexin V was used according to manufacturer’s protocol. Cells that were FITC-/PI- were considered viable, FITC+/PI- cells were considered early apoptotic, and FITC+/PI+ cells were considered nonviable.
Statistics
All experiments were completed in triplicate and repeated. Continuous data were compared by Student’s T test. Data are presented as mean ± standard deviation unless otherwise stated. p<0.05 was considered significant.
RESULTS
MiR-21 expression is upregulated by hypoxia in vitro
Pancreatic cancer cell lines, BxPC-3 and AsPC-1, were cultured for 48 hours in normoxic or hypoxic conditions and miR-21 expression was assessed by RT-PCR. Cells cultured in hypoxic conditions for 48 hours resulted in significantly increased expression of HIF-1α (Fig. 1A, p=0.0015). Given our previous report of differential miR-21 overexpression in resected human pancreatic cancers,19 we first sought to define the impact of hypoxia on miR-21 expression by qRT-PCR in human pancreatic cancer cell lines. AspC-1 and BxPC3 cultured in hypoxic conditions for 48 hours showed increased miR-21 expression (Fig. 1B). Chromatin immunoprecipitation (ChIP) assay were used to assess recruitment of HIF-1α to the predicted hypoxia response element (HRE) region for miR-21 under normoxic and hypoxic conditions. The HIF-1α binding site within the predicted region of miR-21 was enriched, indicating direct binding of miR-21 by HIF-1α (Fig. 1C). The anti-HIF-1α antiserum (but not MOCK control) immunoprecipitated the miR-21 fragment in hypoxic AsPC-1 cells but very little in the normoxic cells.
Figure 1. Hypoxia induces MiR-21 and recruits HIF-1α to MiR-21 promoter in pancreatic cancer cell lines.
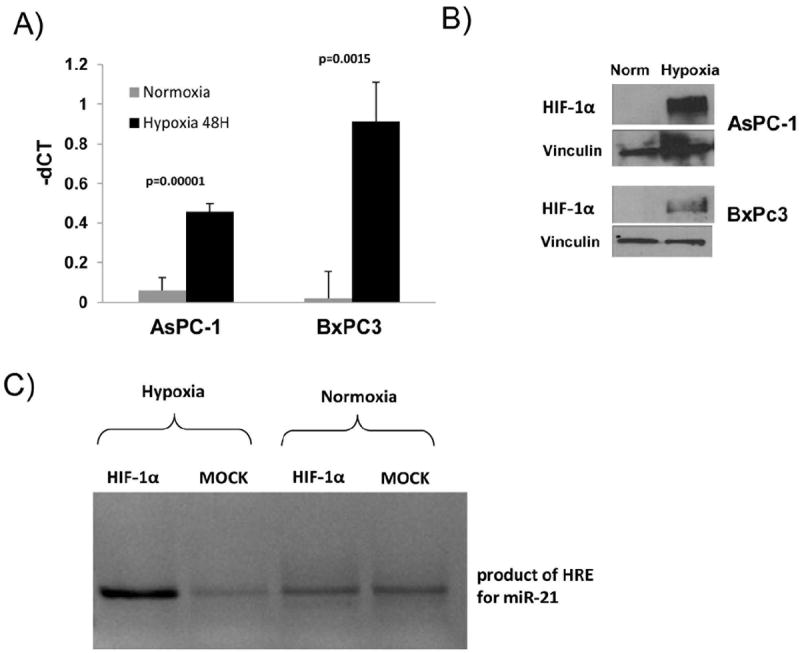
A) Cells were grown in 21% (normoxia) or 1% oxygen (hypoxia) for 48 hours. MiR-21 was assayed by qRT-PCR and normalized to snoRNA U48. B) Immunoblot of HIF-1 α from lysates of cells cultured at normoxic or hypoxic conditions. C) ChIP analyses of recruitment of HIF-1α to the predicted hypoxia response element (HRE) region of miR-21 following treatment of AsPC-1 cells grown at normoxic or hypoxic conditions for 72 hours.
MiR-21 induction in hypoxia is dependent on HIF-1α
The role of HIF-1α in hypoxia-regulated miR-21 expression was first tested by RNA interference. Under hypoxic conditions, silencing of HIF-1α in the BxPC-3 cell line attenuated the miR-21 overexpression (Fig. 2). A similar trend was observed in AsPC-1, but did not reach statistical significance. Next, we transduced BxPC-3 and AsPC-1cell lines with a constitutively stable HIF-1α subunit or vector-only control (pcDNA3.1) under normoxic conditions. HIF-1α stabilization was achieved by altering the two alpha subunits that are subjected to oxygen-dependent hydroxylation and proteasomal degradation via VHL-dependent ubiquitylation.24 The presence of exogenous HIF-1α after transfection was confirmed via immunoblot (Fig. 3A). Constitutively stable HIF-1α resulted in increased miR-21 expression in both pancreatic cancer cell lines (Fig 3B).
Figure 2. MiR-21 upregulation by hypoxia is attenuated by inhibition of HIF-1α with siRNA.
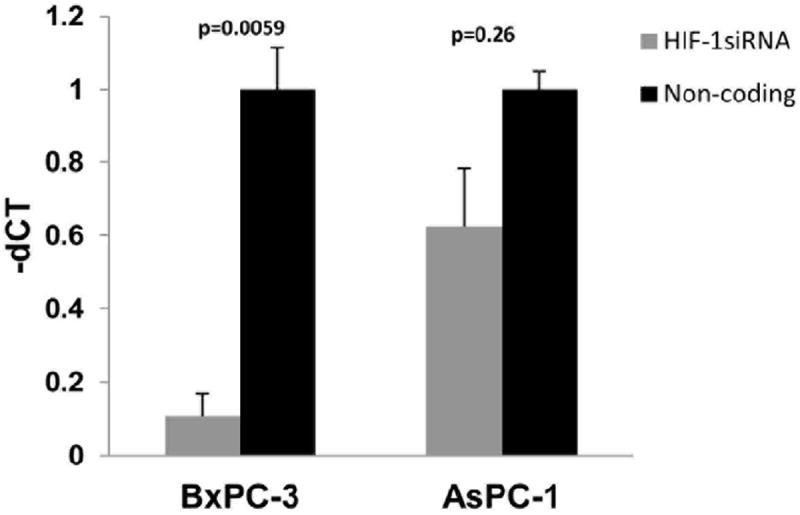
BxPC-3 and AsPC-1 cells were transfected with HIF-1 α siRNA and cultured in normoxic or hypoxic conditions for 48 hours and miRNA expression was assessed by RT-PCR.
Figure 3. HIF-1α overexpression in normoxia.
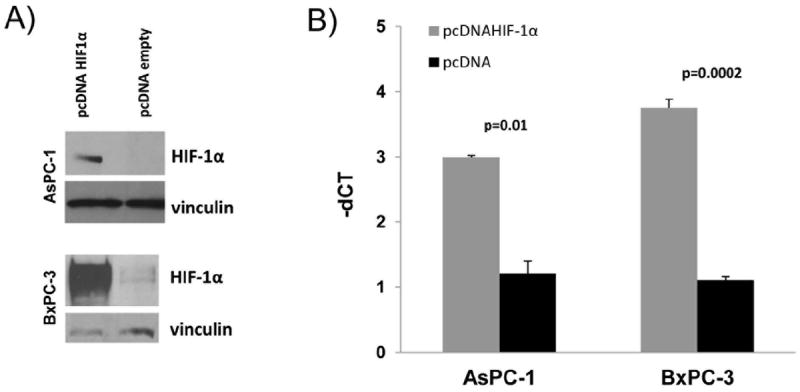
A) Western blot of HIF-1α expression 48 hours after transfection with constitutively stable pcDNA HIF-1α; B) MiR-21 overexpression through transfection of the cells with pcDNA HIF-1α by qRT-PCR in normoxia
miR-21 increases proliferation and overrides hypoxia-induced cell cycle arrest
Hypoxia treatment induces cell cycle arrest at the G1/S transition.25 In normoxia, when HIF-1α is destabilized and not functional, miR-21 overexpression did not significantly increase cell proliferation whereas inhibition of miR-21 did decrease proliferation. Conversely in hypoxia, miR-21 inhibition did not further reduce proliferation but increasing miR-21 expression resulted in increased proliferation (Fig. 4).
Figure 4. miR-21 increases proliferation and overrides hypoxia-induced cell cycle arrest.
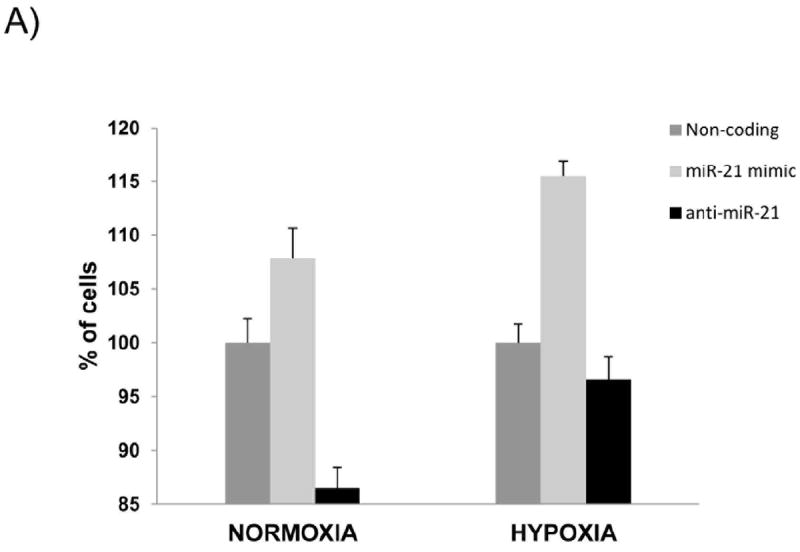
AsPC-1 cells were transfected with mature miR-21, antisense to miR-21 and NC-non coding and were cultured for 48 hours in normoxic or hypoxic conditions. Proliferation was measured by WST-1 assay.
miR-21 enhances pancreatic cancer cell survival
Transfection of cells with anti-miR-21 resulted in a modest decrease in cell proliferation. Thus we determined the role of miR-21 on cell survival. BxPC-3 (and AsPC-1 data not shown) transfected with anti-miR-21 had greater apoptosis as compared to controls either in normoxic or hypoxic conditions (Fig. 5).
Figure 5. Pancreatic cancer cell survival is attenuated by anti-miR21 siRNA.
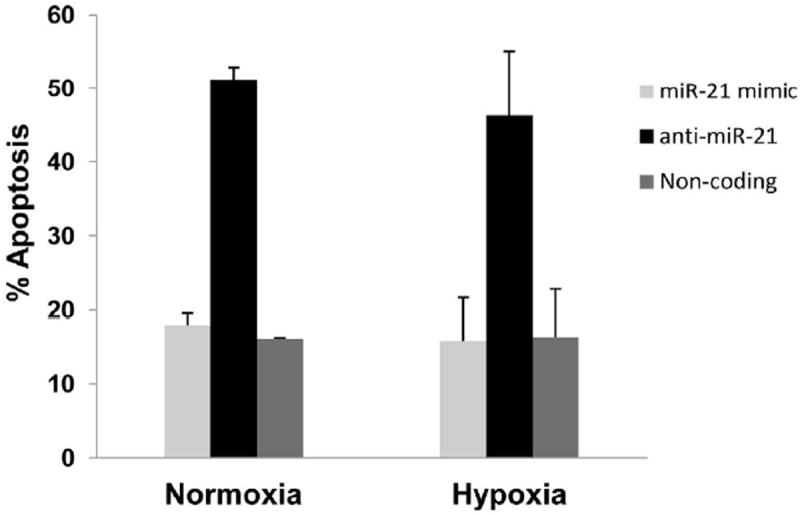
AsPC-1 cells were treated with 100nm anti-miR21 siRNA were harvested after 48 hours of incubation and using Annexin V-FITC was stained to assess apoptosis.
DISCUSSION
MicroRNA are often dysregulated in tumors and have therefore been classified as oncomirs, which modulate cell proliferation and survival.26, 27 miR-21 was among the first oncomirs identified, and is overexpressed in multiple histologic subtypes of cancer including glioblastoma, lung cancer and leukemia.28, 29 Increased miR-21 levels in pancreatic cyst fluid have been shown to be a predictive marker for ductal adenocarcinoma.30 Other recent studies also provide evidence that miR-21 can upregulate Bcl-2, leading to chemoresistance in pancreatic cancer cells.31, 32 Prior studies from our group have identified a microRNA expression profile unique for pancreatic cancer and identified that miR-21 expression inversely correlated with overall survival19 and was also expressed in human pancreatic cancer cell lines.23, 32 In the present study we demonstrate that miR-21 is regulated by hypoxia-induced HIF-1α expression in human pancreatic cancer cell lines. We found that hypoxia-induced regulation of miR-21 through expression of HIF-1α enhanced survival and decreased apoptosis in human pancreatic cancer cell lines. It is known that the majority of tumors from pancreatic cancer patients express mutated KRAS.19, 33 In the present study we found that even cell lines with wild-type KRAS (BxPC-3) showed an increase in miR-21 expression under hypoxic conditions and relied on miR-21 for survival. Future studies in both wild-type and KRAS mutant pancreatic tumors will be important to further characterize the relationship between miR expression and individual molecular subtypes of pancreatic cancer. Importantly these data uncover a novel role for miR-21 in pancreatic cancer and suggest it may be a relevant therapeutic target for this disease.
The tumor microenvironment is characterized by dysregulated blood flow, angiogenesis, and a lack of oxygen, which can modulate tumor gene expression and function.34, 35 Other groups have shown that oncomir expression in tumor cells can be modulated by expression of HIF-1α36, 37 Norman et al. provides evidence that hypoxia increases miR-210 in lung and melanoma cells resulting in silencing of genes that regulate immune recognition by cytotoxic T cells.38 Another study showed that hypoxic induction of miR-210 mediates the metastasis of hepatocellular carcinoma.39 miR-155 is another oncomir regulated by hypoxia in lung cancer cells and its increased expression protects cells from radiation damage.40 Evidence of hypoxia regulating oncomirs in other cancers has been well documented. Studies in cell lines from other solid tumors such as breast, colon, and renal have observed a role for miR-21 in regulating cell growth and survival.10, 41, 42 However, the role of hypoxic conditions in regulating oncomir expression in pancreatic cancer is poorly understood.
This study primarily focused on how hypoxia regulates miR-21 since this oncomir has a significant role in pancreatic cancer. Indeed, pancreatic cancer cell lines incubated in hypoxic conditions increased miR-21 expression. Also, attenuating HIF-1α expression via siRNA under hypoxic conditions abrogated induction of miR-21 expression. Finally, pancreatic cancer cells transfected with miR-21 siRNA cultured under both normoxic and hypoxic conditions had decreased proliferation and greater apoptosis compared to mock-transfected cells. These data support the role for basal expression of miR-21 as an essential regulator of pancreatic cancer cell survival under both normoxic and hypoxic conditions. Our data suggests that hypoxic conditions in the pancreatic tumor microenvironment can increase the HIF-1α expression, leading to increased miR-21 and tumor cell survival. This work has elucidated a novel and important role for miR-21 as an oncomir that is regulated by the hypoxic environment. The present study focused only on one important oncomir, miR-21, which has documented importance for pancreatic cancer cell survival. However we are aware that numerous additional oncomirs may be regulated by hypoxia. Indeed, using an array approach, a recent study in retinoblastoma demonstrated that hypoxia upregulates several miRs including miR-181, miR-125, and miR-491.43 Importantly, further studies will enhance our understanding of the redundancy across individual miRs that are regulated by hypoxia in pancreatic cancer.
MiR-21 is becoming recognized as an important oncomir that modulates various cellular functions including cell proliferation and survival when expressed in pancreatic cancer cells. This study showed that miR-21 expression is regulated by HIF-1α and provides rationale for treatment modalities that reduce hypoxia in the tumor microenvironment. This approach could reduce miR-21 expression, and potentially improve of targeted therapies for pancreatic cancer.
Acknowledgments
Funding: Research supported by the 2010 AACR-FNAB Fellows Grant for Translational Pancreatic Cancer Research Grant Number 10-30-14-COLL (ALC), NCI CA13325-01 (MB), and the Society of University Surgeons Junior Faculty Award (MB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baxter NN, Whitson BA, Tuttle TM. Trends in the treatment and outcome of pancreatic cancer in the United States. Ann Surg Oncol. 2007;14:1320–6. doi: 10.1245/s10434-006-9249-8. [DOI] [PubMed] [Google Scholar]
- 2.Bohnsack MT, Czaplinski K, Gorlich D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA. 2004;10:185–91. doi: 10.1261/rna.5167604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 4.Megibow AJ. Pancreatic adenocarcinoma: designing the examination to evaluate the clinical questions. Radiology. 1992;183:297–303. doi: 10.1148/radiology.183.2.1561324. [DOI] [PubMed] [Google Scholar]
- 5.Koong AC, Mehta VK, Le QT, et al. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys. 2000;48:919–22. doi: 10.1016/s0360-3016(00)00803-8. [DOI] [PubMed] [Google Scholar]
- 6.Yokoi K, Fidler IJ. Hypoxia increases resistance of human pancreatic cancer cells to apoptosis induced by gemcitabine. Clin Cancer Res. 2004;10:2299–306. doi: 10.1158/1078-0432.ccr-03-0488. [DOI] [PubMed] [Google Scholar]
- 7.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–16. [PubMed] [Google Scholar]
- 8.Kulshreshtha R, Ferracin M, Wojcik SE, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–67. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei Z, Li B, Yang Z, et al. Regulation of HIF-1alpha and VEGF by miR-20b tunes tumor cells to adapt to the alteration of oxygen concentration. PLoS One. 2009;4:e7629. doi: 10.1371/journal.pone.0007629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Oliveira PE, Zhang L, Wang Z, et al. Hypoxia-mediated regulation of Cdc25A phosphatase by p21 and miR-21. Cell Cycle. 2009;8:3157–64. doi: 10.4161/cc.8.19.9704. [DOI] [PubMed] [Google Scholar]
- 11.Gee HE, Camps C, Buffa FM, et al. hsa-miR-210 is a marker of tumor hypoxia and a prognostic factor in head and neck cancer. Cancer. doi: 10.1002/cncr.25009. [DOI] [PubMed] [Google Scholar]
- 12.Lee YS, Nakahara K, Pham JW, et al. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- 13.Johnson SM, Grosshans H, Shingara J, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–47. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102:13944–9. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He L, Thomson JM, Hemann MT, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–33. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–61. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J, Jahng WJ, Di Vizio D, et al. The phosphoinositide kinase PIKfyve mediates epidermal growth factor receptor trafficking to the nucleus. Cancer Res. 2007;67:9229–37. doi: 10.1158/0008-5472.CAN-07-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma WJ, Lv GD, Tuersun A, et al. Role of microRNA-21 and effect on PTEN in Kazakh’s esophageal squamous cell carcinoma. Mol Biol Rep. doi: 10.1007/s11033-010-0480-9. [DOI] [PubMed] [Google Scholar]
- 19.Bloomston M, Frankel WL, Petrocca F, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–8. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen BS, Jorgensen S, Fog JU, et al. High levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patients. Clin Exp Metastasis. 28:27–38. doi: 10.1007/s10585-010-9355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng F, Henson R, Lang M, et al. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology. 2006;130:2113–29. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 22.Talotta F, Cimmino A, Matarazzo MR, et al. An autoregulatory loop mediated by miR-21 and PDCD4 controls the AP-1 activity in RAS transformation. Oncogene. 2009;28:73–84. doi: 10.1038/onc.2008.370. [DOI] [PubMed] [Google Scholar]
- 23.Dillhoff M, Liu J, Frankel W, et al. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J Gastrointest Surg. 2008;12:2171–6. doi: 10.1007/s11605-008-0584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivan M, Kondo K, Yang H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–8. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 25.Gardner LB, Li Q, Park MS, et al. Hypoxia inhibits G1/S transition through regulation of p27 expression. J Biol Chem. 2001;276:7919–26. doi: 10.1074/jbc.M010189200. [DOI] [PubMed] [Google Scholar]
- 26.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 27.Kasinski AL, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer. 2011;11:849–64. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–33. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 29.Landgraf P, Rusu M, Sheridan R, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–14. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryu JK, Matthaei H, Dal Molin M, et al. Elevated microRNA miR-21 levels in pancreatic cyst fluid are predictive of mucinous precursor lesions of ductal adenocarcinoma. Pancreatology. 2011;11:343–50. doi: 10.1159/000329183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong J, Zhao YP, Zhou L, et al. Bcl-2 upregulation induced by miR-21 via a direct interaction is associated with apoptosis and chemoresistance in MIA PaCa-2 pancreatic cancer cells. Arch Med Res. 2011;42:8–14. doi: 10.1016/j.arcmed.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Moriyama T, Ohuchida K, Mizumoto K, et al. MicroRNA-21 modulates biological functions of pancreatic cancer cells including their proliferation, invasion, and chemoresistance. Mol Cancer Ther. 2009;8:1067–74. doi: 10.1158/1535-7163.MCT-08-0592. [DOI] [PubMed] [Google Scholar]
- 33.Kim ST, Lim do H, Jang KT, et al. Impact of KRAS mutations on clinical outcomes in pancreatic cancer patients treated with first-line gemcitabine-based chemotherapy. Mol Cancer Ther. 2011;10:1993–9. doi: 10.1158/1535-7163.MCT-11-0269. [DOI] [PubMed] [Google Scholar]
- 34.Cassavaugh J, Lounsbury KM. Hypoxia-mediated biological control. J Cell Biochem. 2011;112:735–44. doi: 10.1002/jcb.22956. [DOI] [PubMed] [Google Scholar]
- 35.Bussolati B, Grange C, Camussi G. Tumor exploits alternative strategies to achieve vascularization. FASEB J. 2011;25:2874–82. doi: 10.1096/fj.10-180323. [DOI] [PubMed] [Google Scholar]
- 36.Chan SY, Loscalzo J. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle. 2010;9:1072–83. doi: 10.4161/cc.9.6.11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ivan M, Harris AL, Martelli F, et al. Hypoxia response and microRNAs: no longer two separate worlds. J Cell Mol Med. 2008;12:1426–31. doi: 10.1111/j.1582-4934.2008.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noman MZ, Buart S, Romero P, et al. Hypoxia-Inducible miR-210 Regulates the Susceptibility of Tumor Cells to Lysis by Cytotoxic T Cells. Cancer Res. 2012;72:4629–4641. doi: 10.1158/0008-5472.CAN-12-1383. [DOI] [PubMed] [Google Scholar]
- 39.Ying Q, Liang L, Guo W, et al. Hypoxia-inducible microRNA-210 augments the metastatic potential of tumor cells by targeting vacuole membrane protein 1 in hepatocellular carcinoma. Hepatology. 2011;54:2064–75. doi: 10.1002/hep.24614. [DOI] [PubMed] [Google Scholar]
- 40.Babar IA, Czochor J, Steinmetz A, et al. Inhibition of hypoxia-induced miR-155 radiosensitizes hypoxic lung cancer cells. Cancer Biol Ther. 2011;12:908–14. doi: 10.4161/cbt.12.10.17681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han M, Wang Y, Liu M, et al. MiR-21 regulates epithelial-mesenchymal transition phenotype and hypoxia-inducible factor-1alpha expression in third-sphere forming breast cancer stem cell-like cells. Cancer Sci. 2012;10:1058–64. doi: 10.1111/j.1349-7006.2012.02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu H, Brannon AR, Reddy AR, et al. Identifying mRNA targets of microRNA dysregulated in cancer: with application to clear cell Renal Cell Carcinoma. BMC Syst Biol. 2010;4:51. doi: 10.1186/1752-0509-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu X, Jia R, Zhou Y, et al. Microarray-based analysis: identification of hypoxia-regulated microRNAs in retinoblastoma cells. Int J Oncol. 2011;38:1385–93. doi: 10.3892/ijo.2011.961. [DOI] [PubMed] [Google Scholar]


