Abstract
Purpose
We present a genetic and clinical analysis of two sisters, 3 and 4 years of age, with nanophthalmos and macular folds.
Methods
Ophthalmological examination, general pediatric examination and molecular genetic analysis of the MFRP gene were performed in both affected siblings.
Results
Clinical analysis showed high hyperopia (+11 and +12 D), short axial lengths (15 mm), and the presence of macular folds and optic nerve head drusen. Autofluorescence of the retina was generally normal with subtle macular abnormalities. Sequence analysis showed compound heterozygosity for severe MFRP mutations in both sisters: a previously reported p.Asn167fs (c.498dupC) and a novel stop codon mutation p.Gln91X (c.271C>T).
Conclusion
These are the youngest nanophthalmos patients in the literature identified with severe loss of MFRP function, showing already the known structural abnormalities for this disease. Adult patients affected by homozygous or compound heterozygous MFRP mutations generally show signs of retinal dystrophy, with ERG disturbances and RPE abnormalities on autofluorescence imaging. ERG examination could not be performed in these children, but extensensive RPE abnormalities were not seen at this young age.
Keywords: Membrane frizzled-related protein gene, MFRP, nanophthalmos, hyperopia, macular folds
Introduction
Nanophthalmos is a rare autosomal recessive condition with a very short axial length. This results in high hyperopia, ranging from +8.00 to +25.00. Additional characteristics are a narrow angle between iris and cornea, expansion of the choroidal vascular bed, and thickening of the sclera. Patients with nanophthalmos are especially prone to angle closure glaucoma and non-rhegmatogenous detachment. Decreased corrected visual acuity may be related to abnormal foveal structure and pronounced retinal folds in the macula (Serrano et al. 1998, Walsh & Goldberg 2007). Nanophthalmos can be an isolated finding, yet sometimes it is associated with other ocular or systemic malformations.
Autosomal recessive nanophthalmos is usually associated with severe mutations in the membrane-type Frizzled-related protein gene, MFRP (Sundin et al. 2005). This gene encodes a glycosylated transmembrane protein with an extracellular Frizzled-related cysteine-rich domain. It is specifically expressed in the retinal pigment epithelium and ciliary body. In humans, MFRP appears necessary for both, retinal maintenance in the adult, and normal ocular growth during childhood (Sundin et al. 2008). In the mouse, mutation of the MFRP gene results in slow onset photoreceptor degeneration, but does not appear to affect ocular growth.
In this report, we present a clinical and genetic analysis of two young sisters with nanophthalmos and macular folds caused by compound heterozygote mutations in the MFRP gene.
Methods
Clinical details
Ophthalmological examination included measurement of best-corrected visual acuity (BCVA), slit-lamp examination, funduscopy, cycloplegic refraction, B-mode ultrasonographic (US) examination and optical coherence tomography (OCT). Fundus autofluorescence imaging was performed using the OPTOS 200DX scanning laser camera. The clinical geneticist performed a general pediatric examination.
DNA Analysis
DNA was purified from whole blood of the proband, her sibling and each parent. All 13 coding exons of MFRP were PCR amplified in 3 large fragments, using the following primer pairs:
A (1.3 kb) Exons 1–5:
CCAAGAACTGGTCTAGCCTGGCAGCCTT,
TTAGCCCTTCTCCCTGCCACTCCCTGATTC.
B (1.4 kb) Exons 6–9:
CCAGTTTGGGGGTTGAGAAAATAGGACTGC,
GAGAATGGAATGTGCTGGGCCGACATGGAA.
C (1.6 kb) Exons 10–13:
AGGGCTGGTGCCCAGAACAGCTGTCTGCTT,
AGAGACCCTGCTGATGCTCCTTCCTTTGTT.
Touch-down PCR was carried out with Phusion DNA polymerase (New England Biolabs). An initial 8 minutes at 98°C activated the polymerase, and was followed by 64 cycles of: 20 seconds denaturation at 98°C, 30 seconds annealing (initially 72°C, decreased 0.5°C per cycle until 60°C), and 1 minute extension at 72°C. Amplimers were resolved by electrophoresis in 1% agarose, Tris-acetate EDTA gels, visualized with ethidium bromide and excised (Sambrook et al.). DNA was purified using a QuiaQuick gel extraction kit (Qiagen), and ABI dye-terminator sequenced (60°C annealing) using an ABI 3130 XL. Each template (A–C) was analyzed using each of its original PCR primers, plus the following internal primers:
A
ex3For GAGGAATACCCAACAGAAGGGGCTTTCAA
ex3Rev GAGAGCGGCTCATGGAGTTTCATTCCAA
ex4For AGCTCCTCTGAACGCCACCCTCCATCTTCT
ex4Rev GAGTTCAGAGGTCAAAAGGAGTGAGGTCCT
B
ex7Rev GGGCCAAAGAATGACTGAGCAGGAAATGCT
ex8For CCTAGCAAACTCATGAGCCCTTCCACCTCT
ex9Rev GAGAATGGAATGTGCTGGGCCGACATGGAA
C
ex11Rev ACTGTGCAGTACGGCAGTAGGGTTCTGTGA
ex12For CATTGGACCCATGTACACACAGGACCGA
Sequence assembly and display of the chromatogram data was carried out using Sequencher v4.10.1 software (Gene Codes Inc., Ann Arbor. MI).
Written informed consent was obtained from the parents for publication of medical data and figures. All research has followed the Tenets of Declaration of Helsinki.
Results
Ophthalmological examination
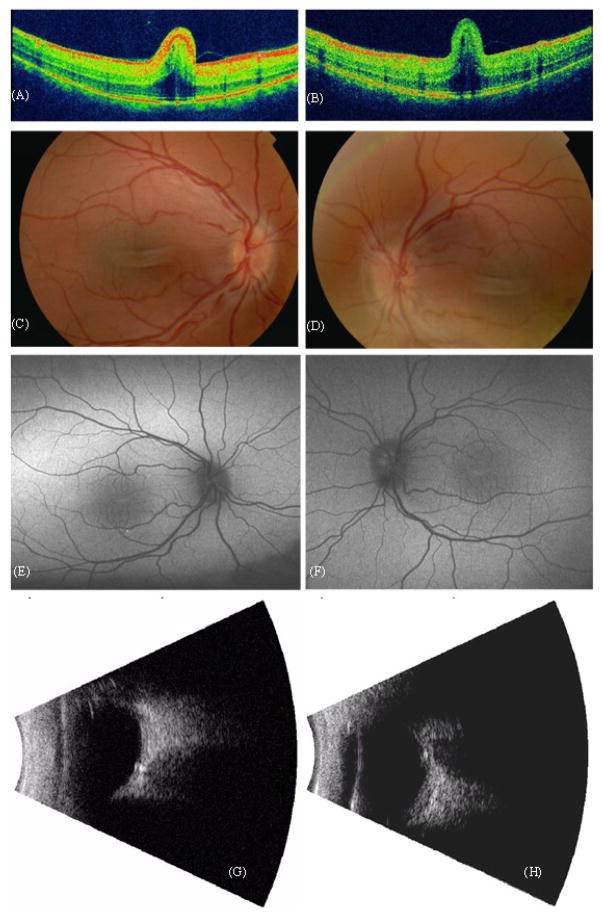
Case 1: A 4–year-old girl consulted our department because of high hyperopia. Cycloplegic examination showed a refractive error of S+11,50 D for both eyes, with a BCVA of 5/20. Examination of the fundus revealed a macular fold (Fig. 1A, B), a crowded and elevated optic disc (Fig 1C, D) and some vascular tortuositas. On ultrasonography, the axial length was 15 mm for each eye, further showing a thickened sclera and a prominent optic nerve with optic nerve drusen. Autofluorescence imaging was normal, with exception of a slightly abnormal macular pattern at the level of the macular fold, and some granular hyperreflectivity around the inferior macular vessels in both eyes.
Figure 1.
Case 1. OCT of the macular area of the right eye (A) and left eye (B). Fundus photography of the right (C) and left eye (D). Autofluorescence imaging of macular area of right (E) and left eye (F). Ultrasonography showing optic nerve head drusen in right (G) and left eye (H).
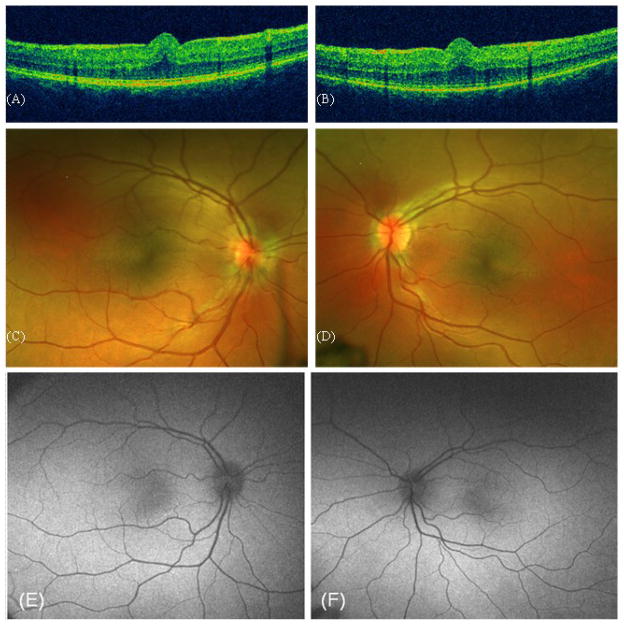
Case 2: Her 3-year-old sister presented with a refractive error of S+11 for both eyes. Her BCVA was 2/5 in her right eye and 5/10 in her left eye. Funduscopy showed an abnormal macular reflex, but no obvious macular fold. However, on OCT examination a small macular retinal fold was apparent (Fig. 2A, B). Meticulous examination of the OCT image in the youngest sisters reveals an intact fotoreceptor-layer, even underneath the macular fold. Autofluorescence imaging was normal in the youngest sister, except for some granular hyperreflectivity around the inferior macular vessels in the right eye.
Figure 2.
Case 2. OCT of the macular area of the right (A) and left eye (B). Fundus photography of the right (C) and left eye (D). Autofluorescence imaging of macular area of right (E) and left eye (F).
The parents did not consent to performing an ERG in the children. On inquiry both children showed signs of night blindness.
Further general physical examination of both girls showed no systemic symptoms. Both parents had no history of hypermetropic refractive errors or other ophthalmological abnormalities.
Molecular genetic analyses
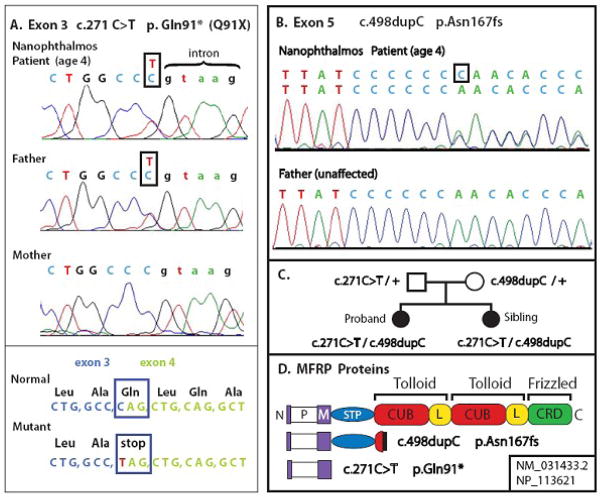
Protein-truncating mutations in exons 3 and 5 were identified in both the 4 year-old proband and her younger sister. DNA sequence of MFRP exon 3 (c.271C>T) revealed heterozygosity for a novel variant in which C is changed to T. Examination of the normal mRNA sequence and protein sequence encoded by this region indicated that the variant affects MFRP glutamine codon 91, which spans exons 3 and 4. This glutamine is converted to TAG, an amber stop codon, truncating MFRP at the end of the membrane spanning helix (p.Gln91X). The loss of Sau96I restriction endonuclease cleavage at the site of this mutation (not shown) confirmed the sequence data. Exon 5 revealed heterozygosity for a single C duplication in a run of 7 cytosines, designated c.498dupC, causing a frameshift in the codon of proline 166 and nonsense termination at codon 200 (p.Asn167fs). This frameshift mutation has been reported previously as 499insC (Ayala-Ramirez et al. 2006), causing a truncation of MFRP in the first cubilin domain.
Analysis of the parents revealed that the father is a carrier for the exon 3 mutation, while the mother carries the exon 5 mutation. In the patients, both maternally and paternally inherited copies of the MFRP gene are mutated (Fig. 3 A–C).
Figure 3.
Mutations identified in the MFRP gene.
A. Novel exon 3 stop codon mutation. Genomic amplimer sequences of proband and parents. Variant in box. Exon 3 in upper case, intron lower case.
B. Exon 5 mutation.
C. Pedigree of family, with genotypes.
D. Domain structure for the 579 amino acid primary sequence, showing mutant proteins. P: proline rich intracellular domain. M: single-pass transmembrane domain. STP: serine, threonine, proline rich domain. CUB: cubilin domain, L: LDL-A related domain pairing which is conserved in Tolloid family. Frizzled-homologous cysteine-rich domain. Diagrams of truncated proteins generated by each mutation.
Discussion
This case report presents two sisters with both having mutations in the MFRP gene and similar clinical findings including decreased visual acuity, high hyperopia, macular retinal folds. In the older sister, ultrasonography also revealed a thickened sclera and optic nerve head drusen. To our knowledge these sisters are the youngest patients reported in literature with confirmed MFRP mutations.
Recent descriptions of the phenotype in adult patients with a MFRP mutation report abnormal corrected acuity, extremely high hyperopia (in the range of +13 D until +20 D or even higher), foveal abnormalities like flattened fovea, foveal schisis, or macular folds and optic nerve head drusen. Fundus appearance may vary from hardly any pigmentary abnormalities to larger area’s with atrophy, with usual a preserved macular reflex. Also, autofluorescence may show a relative normal elliptical macular area in larger areas of RPE atrophy. ERGs usually show a rod-cone pattern dystrophy (Ayala-Ramirez et al. 2006, Mukhopadhyay et al. 2012, Sundin et al. 2005).
Nanophthalmos is a developmental defect that results from a reduction in growth of the eye. The axial length of nanophthalmic eyes ranges between 13–18.5 mm and is a major factor responsible for their high refractive error. Postnatal axial growth is the mechanism by which emmetropization corrects refractive error during the first 6 years of life. The MFRP gene is expressed in the RPE and ciliary body at 20 weeks of gestation and in the adult. Patients with a defective MFRP-gene appear incapable of correcting hyperopic refractive error during childhood, suggesting a possible role for this gene in normal emmetropization (Sundin et al. 2008).
The compound heterozygosity for mutations in the MFRP gene described in the present study should severely affect the function of MFRP by completely removing the cubilin, LDL-A and Frizzled-related CRD domains. Both mutations spare the intracellular and transmembrane domains (Fig. 3 D). It remains an open question whether these mutant proteins can persist, and whether they influence the disease phenotype. Since both mutations result in premature termination of translation by novel nonsense codons, nonsense-mediated is likely to substantially decrease mRNA levels (Kervestin and Jacobson, 2012). This would further decrease the amounts of any abnormal protein produced. Most likely, these are null mutations.
There are now 14 different MFRP mutations described in the literature (Table 1). Of these, 2 are single amino acid substitutions at extremely conserved sites, while 12 cause severe truncation of MFRP. While all exhibit severe hyperopia and appear fully recessive, the effect on rod function varies substantially between affected individuals. Patients affected by homozygous or compound heterozygous MFRP mutations generally show signs of photoreceptor dysfunction and retinal dystrophy later in life. Some patients as young as 18 years old complain of night blindness. This suggests that MFRP also plays a role in photoreceptor function maintenance, as appears to be the case in the mouse (Won et al. 2008). We could not perform an ERG in these children. However, the parents reported some sign of night blindness, hinting to loss of at least rod-function already at this young age. Visual acuity was already deficient at this early age, as indicated by the best corrected visual acuity of both children. Interference with high acuity retinal function could be related to both the macular fold or a less apparent cone dysfunction. In contrast to older patients (Ayala-Ramirez et al. 2006, Mukhopadhyay et al. 2010), in our patients there was no extensive RPE anomalies as autofluorescence imaging was almost normal, except for some granular staining in the macular area.
Table I. MFRP Mutations and their Phenotypic Features.
The 14 different known disease-associated mutations of MFRP are numbered in column 1 and defined at the DNA and protein level in columns 2 and 3 according to recent nomenclature conventions (Laros et al 2011). These were originally reported as either homozygous or compound heterozygotes, as indicated under the publication reference for the patient. Ages or age range are indicated in column 5. This is followed by refraction, comments on night blindness, dark adaptation kinetics, scotopic ERG (electroretinography) and the presence or absence of RP (retinitis pigmentosa).
| # | MFRP mutation (NM_031433.1) | MFRP Protein (13 coding exons) | Publication(s) | Age(s) | Refraction (spherical) | Night blind | ERG Dark adaptation | Fundus Features, RP (retinitis pigmentosa) |
|---|---|---|---|---|---|---|---|---|
| 1 | c.1149dupC (1143insC) | p.His384Pro fs8X truncation exon 10 |
Sundin et al. 2005. 1/1-homozygotes |
21 to 30 | Range OD, OS +11.90 to +19.00 |
No | Abnormal dark adaptation curves Rod ERG low Cone ERG normal |
Depigmented patches of RPE, No RP. Retinal folds. Macular edema, cysts. |
|
Mukhopadhyay et al. 2010. 1/1-homozygotes |
46 to 58 | Range OD,OS +13.00 to +20.00 |
Yes | Rod ERG absent Cone ERG moderately reduced, abnormal |
Depigmented patches of RPE, Retinitis Pigmentosa. | |||
| 2 | c.523T>C | p.Gln175X stop codon exon 5 |
Sundin et al. 2005. 2/2-homozygote |
9 | OD +13.40 OS +13.60 |
No | - | No RP |
| 3 | c.545T>C | p.Ile182Thr aa substitution at exon 5 highly conserved site. |
Sundin et al. 2005 3/4-heterozygotes |
25, 28 | OD +22.00 OS +22.25 |
No | - | No RP |
| 4 | c.498delC (492delC; c.492delC) | p.Asn167Thr fs25X truncation exon 5 |
Sundin et al. 2005. 3/4-heterozygotes |
|||||
|
Crespi et al. 2008. and Neri et al. 2012. 4/4-homozygotes |
40 to 60 | Range OD,OS +16.00 to +19.00 |
- | - | Retinitis Pigmentosa, Depigmented patches of RPE, Retinal folds, macular edema and cysts. | |||
|
Mukhopadhyay et al. 2010. 4/4-homozygote |
42 | OD +30.00 OS +27.75 |
Yes | Rod ERG absent Cone ERG moderately reduced, abnormal |
Retinitis Pigmentosa, Depigmented patches of RPE, Retinal folds, cysts. | |||
|
Kannabiran et al. 2012. 4/4-homozygotes |
21 to 29 | Range OD, OS +11.00 to +13.00 |
Mild | Rod ERG low Cone ERG normal |
Mild Retinitis Pigmentosa | |||
| 5 | c.498dupC (c.498_499insC) | p.Asn167Gln fs34X frameshift truncation exon 5 |
Ayala-Ramirez et al. 2006. 5/5-homozygotes |
39 to 49 | Range OD, OS +13.50 to +17.50 |
Yes | Rod ERG absent Cone ERG low |
Retinitis Pigmentosa, Depigmented patches of RPE, Retinal folds, cysts. |
| Wasmann et al. submitted 2012. 5/14-Heterozygotes |
3 to 4 | Range OD, OS +11.00 to +11.50 |
Mild | - | No RP Retinal folds, cysts. | |||
| 6 | c.951C>A | p.Y317X stop codon exon 8 |
Zenteno et al. 2009. 4/6-heterozygotes |
16 to 18 | +16.75 | Yes | Rod ERG absent Cone ERG low |
Retinitis Pigmentosa, Depigmented patches of RPE, Retinal folds, macular edema, cysts. |
| 7 | c.201 G>A | p.Trp67X stop codon exon 2 |
Mukhopadhyay et al. 2010. 7/8 heterozygotes |
47 to 50 | Range OD, OS +18.00 to +20.00 |
Yes | Rod ERG absent Cone ERG moderately low, abnormal |
Depigmented patches of RPE, Retinitis Pigmentosa, Retinal folds, cysts. |
| 8 | c.491_492insT | p.Asn167Gln fs34X truncation exon 5 | ||||||
| 9 | c.1622delTCTG | p.Val541Ala fs188X truncation exon 13 |
Mukhopadhyay et al. 2010. 4/9-heterozygote |
34 | OD +17.75 OS +18.00 |
Yes | Rod ERG absent Cone ERG reduced |
Depigmented patches of RPE, Retinitis Pigmentosa. Retinal folds, cysts. |
| 10 | c.1549C>T | p.R518W aa substitution exon 13 highly conserved site |
Aldahmesh et al. 2011. 10/10-homozygous |
- | high hyperopia (posterior microphthalmos) | - | - | - |
| 11 | c.367C>T | p.Q123X stop codon exon 4 |
Matsushita et al. 2012 11/12-heterozygous |
9 | OD +15.00 OS +13.0 |
No | Rod and Cone ERG within normal limits. | No RP. Absence of foveal pit, retinal folds, no macular cysts. |
| 12 | c.1328G>A | p.W443X stop codon exon 11 | ||||||
| 13 | G>T at 5′ splice donor of intron 9 | Protein truncation predicted after exon 9 | Dinculescu et al. 2012 | 19 | OD +11.00 OS +11.00 |
Yes | Cone ERG very low Rod ERG reduced |
Retinitis Pigmentosa. Absence of fovela pit, macular edema. |
| 14 | c.271C>T | p.Q91X (p.Q91*) Protein truncation after exon 3 | Wasmann et al. 2012 submitted 5/14-Heterozygotes |
3 to 4 | Range OD, OS +11.00 to +11.50 |
Yes-Mild | - | No RP, Retinal folds, cysts. |
A recent report by Dinculescu et al (2012) suggested that the retinal dystrophy in MFRP mutations might be a target for gene based therapy. The present case study may give further support to this suggestion with the description of the phenotype of two young patients with a MFRP mutation. We report that at this young age the structural macular anomalies are already well established. But irreversible RPE damage outside the macular area as seen on autofluorescence imaging in adult MFRP patients has not yet occurred. Furthermore, the OCT image of the retina of the younger child showed anatomical preservation of the fotoreceptor layer.
Acknowledgments
The authors would like to thank Dr. van Nouhuys, ophthalmologist in Nijmegen, the Netherlands, for his co-evaluation of the two patients presented in this manuscript. This research was funded in part by NIH grant EY013610 to OS.
References
- Aldahmesh MA, Nowilaty SR, Alzharani F, Al-Ebdi L, Mohamed JY, Rajab M, Khan AO, Alkuraya FS. Posterior microphthalmos as a genetically heterogeneous condition that can be allelic to nanophthalmos. Arch Ophthalmol. 2011;129:805–807. doi: 10.1001/archophthalmol.2011.129. [DOI] [PubMed] [Google Scholar]
- Ayala-Ramirez R, Graue-Wiechers F, Robredo V, Amato-Almanza M, Horta-Diez I, Zenteno JC. A new autosomal recessive syndrome consisting of posterior microphthalmos, retinitis pigmentosa, foveschisis, and optic disc drusen caused by a MFRP gene mutation. Mol Vis. 2006;12:1483–1489. [PubMed] [Google Scholar]
- Crespí J, Buil JA, Bassaganyas F, Vela-Segarra JI, Díaz-Cascajosa J, Ayala-Ramírez R, Zenteno JC. A novel mutation confirms MFRP as the gene causing the syndrome of nanophthalmos-renititis pigmentosa-foveoschisis-optic disk drusen. Am J Ophthalmol. 2008;146:323–328. doi: 10.1016/j.ajo.2008.04.029. [DOI] [PubMed] [Google Scholar]
- Dinculescu A, Estreicher J, Zenteno JC, Aleman TS, Schwartz SB, Huang WC, Roman AJ, Sumaroka A, Li Q, Deng WT, Min SH, Chiodo VA, Neeley A, Liu X, Shu X, Matias-Florentino M, Buentello-Volante B, Boye SL, Cideciyan AV, Hauswirth WW, Jacobson SG. Gene Therapy for Retinitis Pigmentosa Caused by MFRP Mutations: Human Phenotype and Preliminary Proof of Concept. Hum Gene Ther. 2012;23:367–376. doi: 10.1089/hum.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannabiran C, Singh H, Sahini N, Jalali S, Mohan G. Mutations in TULP1, NR2E3, and MFRP genes in Indian families with autosomal recessive retinitis pigmentosa. Mol Vis. 2012;18:1165–1174. [PMC free article] [PubMed] [Google Scholar]
- Kervestin S, Jacobson A. NMD, a multifaceted response to premature translational termination. Nature Reviews. 2012;13:700–712. doi: 10.1038/nrm3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laros JFJ, Blavier A, den Dunnen JT, Taschner PEM. A formalized description of the standard human variant nomenclature in extended Backus-Naur form. BMC bioinformatics. 2011;12(suppl 4):S5. doi: 10.1186/1471-2105-12-S4-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita I, Kondo H, Tawara A. Novel compound heterozygous mutations in the MFRP gene in a Japanese patient with posterior microphthalmos. Jpn J Ophthalmol. 2012;56:396–400. doi: 10.1007/s10384-012-0145-4. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay R, Sergouniotis PI, Mackay DS, Day AC, Wright G, Devery S, Leroy BP, Robson SG, Holder GE, Li Z, Webster AR. A detailed phenotypic assessment of individuals affected by MFRP-related oculopathy. Mol Vis. 2010;16:540–548. [PMC free article] [PubMed] [Google Scholar]
- Neri A, Leaci R, Zenteno JC, Casubolo C, Delfini E, Macaluso C. Membrane frizzled-related protein gene-related ophthalmological syndrome: 30 month follow-up of a sporadic case and review of genotype-phenotype correlation in the literature. Mol Vis. 2012;18:2623–2632. [PMC free article] [PubMed] [Google Scholar]
- Serrano JC, Hodgkins PR, Taylor DSI, Gole GA, Kriss A. The nanopthalmic macula. Br J Opthalmol. 1998;82:276–279. doi: 10.1136/bjo.82.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin OH, Leppert GS, Silva ED, Yang JM, Dhamaraj S, Maumenee IH, Santos LC, Parsa CF, Traboulsi EI, Broman KW, Dibernardo C, Sunness JS, Toy J, Weinberg EM. Extreme hyperopia is the result of null mutations in MFRP, which encodes a Frizzled-related protein. Proc Natl Acad Sci USA. 2005;102:9553–9558. doi: 10.1073/pnas.0501451102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin OH, Dharmaraj S, Bhutto IA, Hasegawa T, Mcleod DS, Merges CA, Silva ED, Maumenee IH, Lutty GA. Developmental basis of nanophthalmos: MFRP is required for both prenatal ocular growth and postnatal emmetropization. Ophthal Genet. 2008;29:1–9. doi: 10.1080/13816810701651241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MK, Goldberg MF. Abnormal foveal avascular zone in nanophthalmos. Am J Ophthalmol. 2007;143:1067–1068. doi: 10.1016/j.ajo.2007.01.051. [DOI] [PubMed] [Google Scholar]
- Won J, Smith RS, Peachey NS, Wu J, Hicks WL, Naggert JK, Nishina PM. Membrane frizzled-related protein is necessary for the normal development and maintenance of photoreceptor outer segments. Vis Neurosci. 2008;25:563–74. doi: 10.1017/S0952523808080723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenteno JC, Buentello-Volante B, Quiroz-Gonzalez MA, Quiroz-Reyes MA. Compound heterozygosity for a novel and recurrent MFRP gene mutation in a family with the nanophthalmos-retinitis pigmentosa complex. Mol Vis. 2009;15:1794–1798. [PMC free article] [PubMed] [Google Scholar]