Abstract
BDNF is the best-characterized neurotrophin in terms of its gene structure and modulation, secretion processing, and signaling cascades following its release. In addition to diverse features at the genetic and molecular levels, the abundant expression in several regions of the central nervous system has implicated BDNF as a potent modulator in many aspects of neuronal development, as well as synaptic transmission and plasticity. Impairments in any of these critical functions likely contribute to a wide array of neurodevelopmental, neurodegenerative, and neuropsychiatric diseases. In this review, we focus on a prevalent neurodevelopmental disorder, Rett syndrome (RTT), which afflicts 1:15,000 women world-wide. We describe the consequences of loss-of-function mutations in the gene encoding the transcription factor methyl-CpG binding protein 2 (MeCP2) in RTT, and then elaborate on the current understanding of how MeCP2 controls BDNF expression. Finally, we discuss the literature regarding alterations in BDNF levels in RTT individuals and MeCP2-based mouse models, as well as recent progress in searching for rational therapeutic interventions.
Keywords: BDNF, MeCP2, Rett syndrome, intellectual disability, mouse models
1. Overview: BDNF gene, secretion, and signaling
Among the neurotrophin family of growth factors, which includes nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5), BDNF is the best characterized for its gene structure and modulation, secretion processing, and signaling cascades following its release (Cunha et al., 2010; Greenberg et al., 2009; Reichardt, 2006).
The structure of the gene encoding BDNF in humans and rodents is quite unique in that it consists of a cohort of untranslated 5′ exons with different promoters (Aid et al., 2007; Liu et al, 2005, 2006). In the mouse and rat Bdnf, by means of alternative splicing unto the 3′ coding exon (exon IX) each of nine promoters yields many Bdnf transcripts. The number of transcripts can be further doubled, because exon IX contains two polyadenylation sites that can generate a short and a long splice variant of each transcript. All of these different Bdnf transcripts are translated into a single identical BDNF protein. Such seemingly redundant strategy for gene expression has proved necessary, as it allows for appropriate response to different conditions as well as fulfillment of region-specific demands. For instance, DNA demethylation activates one group of Bdnf promoters (exons I, IV, V, VIII, IX), whereas a different group (exons III, VII, IX) is induced after inhibition of histone deacetylase (HDAC) (Aid et al., 2007). Intriguingly, BDNF specifically targeted to distal dendrites to modulate synaptic plasticity is encoded from long 3′ UTRs mRNA variants, while BDNF retained in the cell body where it regulates neuronal survival is encoded from short 3′ UTR mRNA variants (An et al., 2008; Lau et al., 2010; Waterhouse et al., 2012).
BDNF is synthesized in the endoplasmic reticulum (ER) as a precursor protein proBDNF, which is then trafficked to the Golgi apparatus for proper folding of the mature domain (Lu et al., 2005). Binding of the sorting receptor carboxypeptidase E (CPE) to a motif in the mature domain aids to package proBDNF into large dense core vesicles, which are then targeted to the regulated secretory pathway (Chen et al., 2005; Lou et al., 2005). The VPS10 domain protein sortilin also takes part in sorting proBDNF to this pathway with a similar function to that of CPE, but by binding to the prodomain of proBDNF instead (Chen et al., 2005). Finally, proBDNF is cleaved either intracellularly or in the extracellular space near active synapses to produce the mature and active form of BDNF (Mowla et al., 2001; Pang et al., 2004; Yang et al., 2009). However, there is an intense debate whether proBDNF exists extracellularly in non-pathological conditions (Yang et al., 2009; but see Matsumoto et al., 2008). One view states that proBDNF has a physiological role through the activation of the low-affinity pan-neurotrophin p75 receptor (p75NTR) and functions as a negative regulator – e.g. promoting apoptosis, inhibiting dendritic complexity, inducing long-term depression (LTD) (Lu et al., 2005; Roux and Barker, 2002; Woo et al., 2005) – while mature BDNF has positive effects (e.g. neuronal survival, induction of long-term potentiation, LTP) by the activation of tropomyosin-related kinase B (TrkB) receptors (Bramham and Messaoudi, 2005). Furthermore, activity-dependent regulation of extracellular tissue plasminogen activator (tPA) followed by plasmin-mediated cleavage of proBDNF has been proposed to be necessary for the modulation of LTP by mature BDNF through TrkB receptor activation, as well as of LTD by proBDNF-p75NTR signaling (Pang et al., 2004).
TrkB receptor activation by BDNF sets in motion three major signaling transduction pathways: the mitogen-activated protein kinase (MAPK) pathway, the phosphatidyl-inositol 3-kinase (PI3K) pathway, and the phospholipase C (PLC) pathway. Each of these pathways confers distinctive BDNF actions, which have been comprehensively reviewed (Cunha et al., 2010; Huang and Reichardt, 2003; Reichardt, 2006; Segal, 2003). Here, we briefly describe the PLC signaling cascade because it is pertinent to later discussions on activation of ion channels of the transient receptor potential canonical (TRPC) subfamily. Autophosphorylation of TrkB subunits at Tyr785 recruits PLCγ to a specific docking site and its activation, causing the breakdown of phosphatidylinositol 4,5 bisphosphate (PIP2) into diacylglycerol (DAG), an activator of protein kinase C (PKC), and inositol trisphosphate (IP3), which activates specific Ca2+-permeable receptors (IP3R) on smooth ER cisterns, thus causing rises in the intracellular concentration of free Ca2+ ions. In addition to PKC, DAG activates Ca2+-permeable TRPC3/6/7 channels, which contribute to intracellular Ca2+ elevations (Amaral et al., 2007; Li et al., 1999, 2010). Thus, BDNF-TrkB signaling leads to Ca2+ signals initiated by intracellular store mobilization further amplified by influx, and could thus participate in any of the myriad of neuronal functions played by this ubiquitous second messenger (e.g. gene expression, neuronal excitability, neurotransmitter release, synaptic plasticity) (Berridge, 1998).
All these diverse features, together with abundant expression in several regions of the central nervous system have implicated BDNF as a potent modulator in many aspects of neuronal development (Huang and Reichardt, 2001), as well as synaptic transmission and plasticity (Chapleau et al., 2009; Greenberg et al., 2009; Lu, 2003; Lu et al., 2008; Poo, 2001; Tyler et al., 2002). Impairments of these critical functions in the brain are likely to be the origin of a variety of neurodevelopmental, neurodegenerative, and neuropsychiatric diseases (Autry and Monteggia, 2012; Zuccato and Cattaneo, 2009). In the following sections, we will discuss the neurodevelopmental disorder Rett syndrome (RTT), which afflicts 1:15,000 women world-wide (Chahrour and Zoghbi, 2007; Lauvick et al., 2006; Neul et al., 2010). We will describe the consequences of loss-of-function mutations in the gene encoding the transcription factor methyl-CpG binding protein 2 (MeCP2) in RTT individuals, and then elaborate on the current understanding of how MeCP2 controls BDNF gene expression. Finally, we will discuss the literature regarding alterations in BDNF levels in RTT individuals and MeCP2-based mouse models, as well as recent progress in the search for rational therapeutic interventions to alleviate RTT symptoms and improve the quality of life of the afflicted individuals and their caretakers.
2. MECP2 mutations cause Rett syndrome
RTT occurs sporadically and is the leading cause of intellectual disabilities in women world-wide (Percy and Lane, 2005). Individuals with RTT develop typically until 6-18 months when a host of neurological hallmarks appear, which include stereotypic hand movements with loss of purposeful hand use, impaired motor coordination, autonomic dysfunction, seizure, and loss of language skills (Hagberg et al., 1983; Neul et al., 2010). These behavioral traits have been traced to genetic phenotypes that, in a majority of cases, contain mutations in MECP2 (Amir et al., 1999). The spectrum of genetic alternations has been found to involve missense, nonsense and frame-shift mutations, as well as truncations due to premature STOP codons, with specific mutations correlating with clinical severity (Bebbington et al., 2008; Neul et al., 2008). MECP2 locates in chromosome Xq29 and encodes the MeCP2 protein, which binds to methylated cytosines in CpG islands, usually within gene promoter regions. MeCP2 function requires intact methyl-CpG binding (MBD) and transcriptional repression domains (TRD), critical for binding to regulatory gene regions and recruitment of cofactors, respectively.
MeCP2 levels in the brain are low during embryonic stages and increase steadily during the first few days after birth (Balmer et al., 2003; Kishi and Macklis, 2004; Shahbazian et al., 2002b), a postnatal period of intense synapse formation and maturation. The increase of MeCP2 in terms of spatial expression follows a pattern that starts in the posterior structures of the brain and spreads towards more rostral regions (Braunschweig et al., 2004; LaSalle et al., 2001; Shahbazian et al., 2002b). Even though MeCP2 expression is significant in early brain development, its function persists beyond this period extending to neuronal maintenance throughout the entire lifetime. Indeed, reactivation of the endogenous Mecp2 gene to normal levels in mature symptomatic Mecp2 knockout mice reverts most RTT-like features and extends their life span (Guy et al., 2007); consistently, Mecp2 inactivation in mature mice quickly causes characteristic RTT-like features and death (McGraw et al., 2011; Nguyen et al., 2012).
The availability of several mouse models of RTT based on MeCP2 dysfunction (Boggio et al., 2010; Calfa et al., 2011b; Li and Pozzo-Miller, 2012) allowed intense searches for MeCP2 target genes. These studies have raised two critical questions regarding this ubiquitously distributed transcription factor: does MeCP2 activate or inhibit gene expression? And, does MeCP2 control specific genes or does it have histone-like genome-wide functions? Initial studies indicated that MeCP2 functions as a transcriptional repressor by binding to methylated DNA and recruiting co-repressors and chromatin remodeling proteins, such as HDACs (Jones et al., 1998; Nan et al., 1998). Several genes (e.g. Sgk1 and Fkbp5, Crh) associated with specific RTT symptoms are indeed enhanced in mice with loss-of-function of MeCP2 (Chahrour and Zoghbi, 2007; LaSalle and Yasui, 2009; Nuber et al., 2005). However, this initial view of MeCP2 function had to be reconsidered due to the observation that the majority of genes modulated in mice with either MeCP2 loss- or gain-of-function are activated in MECP2 overexpressing mice and downregulated in Mecp2 knockout mice (Chahrour et al., 2008), which suggests that MeCP2 is an activator of gene transcription. Regardless of whether MeCP2 is a repressor or an activator, these findings brought forth the view that transcriptional deregulation takes place in a large set of genes when MeCP2 is dysfunctional, close to 1,300 genes. However, high-throughput profiling studies using either human postmortem tissue or whole brain tissue from Mecp2 knockout mice revealed only a few genes with altered transcription, with modest differences to control samples (Colantuoni et al., 2001; Tudor et al., 2002). Jordan and colleagues (2007) reasoned that significant changes in a region highly relevant to RTT could be diluted in the whole brain samples used for gene profiling studies. As such, they carried out microarray-based global gene expression studies only in the cerebellum of Mecp2 knockout mice (a region important for motor coordination and thus relevant to RTT symptoms) and found that several hundred genes were deregulated, with twice as many being increased rather than decreased (Jordan et al., 2007). Such widespread changes were later confirmed in the hypothalamus, which is responsible for autonomic phenotypes present in RTT (Chahrour et al., 2008). Nevertheless, the reality may not be this simple, as a study on transcriptional profiling of the granule cell body layer in the dentate gyrus of Mecp2 knockout mice revealed only a handful of genes with altered expression (Smrt et al., 2007). This scenario is further complicated by a recent study performing chromatin immunoprecipitation followed by sequencing (ChIP-Seq) to look for genome-wide DNA-protein associations followed by ChIP-qPCR to define specific MeCP2-gene associations (Cohen et al., 2011). Although MeCP2 was found to bind extensively to genes in cultured cortical neurons, its association with specific target genes was not altered upon neuronal stimulation, suggesting that MeCP2 might not function as a classical transcription factor, but rather as a fine-tuning regulator of gene transcription. Together with a report that suggests a compensatory role of histone H1 in Mecp2 knockout mice (Skene et al., 2010), the modest changes of gene expression in the study by Chahrour and colleagues (2008) may simply result from chromatin remodeling due to the loss or gain of a histone-like protein, i.e. MeCP2. These apparent discrepancies in how MeCP2 precisely regulates the expression of specific genes such as Bdnf (see below) call for future investigations.
3. Mechanisms of MeCP2 modulation of BDNF expression
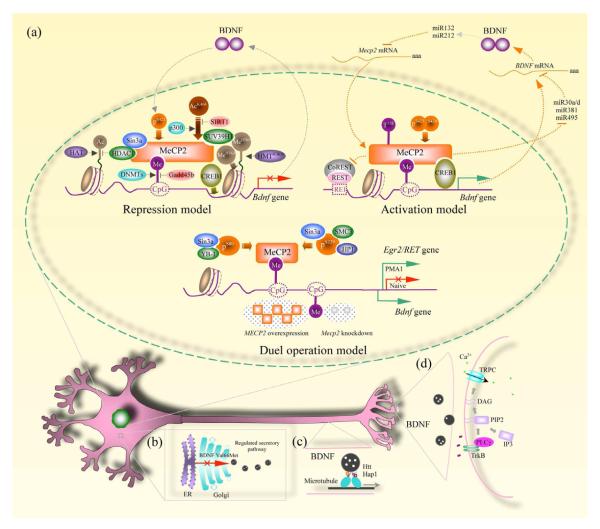
BDNF expression levels are low in the rodent brain during prenatal development, and rise dramatically during the postnatal period (Kolbeck et al., 1999; Maisonpierre et al., 1990), which coincides with the pattern of MeCP2 expression. More intriguingly, BDNF expression remains unaffected in the early presymptomatic stage of Mecp2 knockout mice, while it declines with the onset of RTT-like neuropathological and behavioral phenotypes (Chang et al., 2006; Wang et al., 2006). Furthermore, conditional deletion of Bdnf in postnatal forebrain excitatory neurons results in several phenotypes similar to those of Mecp2 knockout mice, such as hind limb clasping, decreased brain weight, and smaller olfactory and hippocampal neurons (Chang et al., 2006; Chen et al., 2001; Guy et al., 2001). Further evidence indicates that MeCP2 binds to Bdnf at methylated CpG sites adjacent to A/T runs (Klose et al., 2005). The functional and genetic relationship between BDNF and MeCP2 has intrigued and motivated neuroscientists to define the specific mechanism/s of MeCP2 modulation of BDNF expression. Two models were initially proposed: a repression model, and an activation model. More recently, these two seemingly opposite views have been integrated into a “dual operation” model (see Fig. 1a).
Figure 1.
BDNF deregulation in multiple regions of a neuron. a, Three models depict how MeCP2 regulate Bdnf transcription. For abbreviations and detailed description, see the text. b, BDNF is synthesized in the endoplasmic reticulum (ER) and transferred to the Golgi apparatus for proper folding. With the assistance of several proteins in the Golgi, BDNF is packaged into large dense core vesicles and targeted to the regulated secretory pathway. The Val66Met polymorphism with a valine substitution for methionine, results in the failure of proper BDNF maturation through this pathway. c, Huntingtin (Htt) and Huntingtin-associated protein 1 (Hap1) are involved in anterograde axonal transport of BDNF. Lack of these two proteins in Mecp2 knockout mice prevents BDNF from being targeted to synaptic terminals. d, BDNF released from presynaptic terminals binds to postsynaptic TrkB (tropomyosin-related kinase B) receptors and triggers their autophosphorylation. PLC is recruited to a docking site and breaks down phosphatidylinositol 4,5 bisphosphate (PIP2) into diacylglycerol (DAG) and inositol trisphosphate (IP3). DAG triggers activation of canonical transient receptor potential (TRPC) channels, resulting in membrane depolarization and Ca2+ influx. The amplitude of these postsynaptic membrane currents and Ca2+ signals are indirect estimators of the amount of BDNF released from presynaptic terminals.
3.1 Repression model
Earlier studies from different labs using primary neuronal cultures concluded that MeCP2 is a repressor of Bdnf transcription (Ballas et al., 2005; Chen et al., 2003; Martinowich et al., 2003; Zhou et al., 2006). Two of these studies reported that in the absence of membrane depolarization, MeCP2 is bound to Bdnf promoter IV (originally known as promoter III) and prevents transcription, while it is phosphorylated and released from that promoter 30 min after KCl-induced neuronal stimulation, resulting in the activation of Bdnf transcription (Chen et al., 2003; Ballas et al., 2005). Since BDNF secreted by neuronal activity can trigger MeCP2 phosphorylation and its release from Bdnf promoter IV, the ensuing Bdnf transcription was proposed to underlie the well-known positive feedback loop of BDNF-induced BDNF synthesis (Chen et al., 2003; Zhou et al., 2006). Interestingly enough, neuronal activity causes fast (as early as 1 min) phosphorylation of cAMP response element-binding protein (CREB) at Ser133, a requirement for BDNF expression, which indicates that the later relief of MeCP2 repression by unbinding from Bdnf promoter IV functions as a gating mechanism for early CREB activation-mediated Bdnf transcription (Chen et al., 2003). Such activity-dependent activation of CREB contributes significantly to BDNF expression, because BDNF levels in wildtype neurons are just 2-fold lower than Mecp2 knockout neurons in the absence of stimulation (i.e. naïve conditions), while neuronal depolarization induces a dramatic increase in BDNF expression that reaches ~100-fold in wildtype cells, which equalizes BDNF levels in wildtype and Mecp2 knockout neurons (Chen et al., 2003). It was later found that MeCP2 phosphorylation at Ser421 is critical for transcriptional regulation of Bdnf because hippocampal neurons expressing a point mutation in that site (Ser421 for Ala421, S421A) – rendering MeCP2 unable to dissociate from the Bdnf promoter IV – expressed approximately 50% less BDNF upon neuronal depolarization than untransfected neurons expressing wildtype MeCP2 (Zhou et al., 2006). In addition to MeCP2 phosphorylation, neuronal depolarization decreased methylation of CpG sites within Bdnf promoter IV (Martinowich et al., 2003). Thus, dissociation of MeCP2 and its co-repressors (e.g. Sin3a and HDAC1) from Bdnf promoter IV as result of such de-methylation reflects another gating mechanism because CREB’s affinity for this Bdnf regulatory region is correspondingly increased. Furthermore, de-acetylation of MeCP2 by SIRT1, a nicotinamide-adenine dinucleotide (NAD+)-dependent HDAC, increased transcription of Bdnf promoter IV (Zocchi and Sassone-Corsi, 2010, 2012). Intriguingly, SIRT1-mediated MeCP2 de-acetylation preceded its dissociation from the Bdnf exon IV promoter, and does not occur if MeCP2 is acetylated by p300.
The mechanisms underlying the enhancement of Bdnf transcription upon MeCP2 unbinding are DNA de-methylation and dissociation of co-repressors, which is consistent with a large literature on BDNF regulation prior to the discovery of MeCP2’s involvement. In mammalian cells, DNA methylation is performed by two general classes of DNA methyltransferases (DNMT), DNMT1 and DNMT3a/3b, which are responsible for both maintenance methylation and de novo methylation, respectively (Jaenisch and Bird, 2003). DNMTs, particularly DNMT1 and DNMT3a, are highly enriched in postmitotic neurons, and participate in multiple neuronal functions including synaptic transmission and plasticity, as well as learning and memory (Brooks et al., 1996; Feng et al., 2010; Kavalali et al., 2011). Regarding BDNF regulation, Bdnf promoter IV mRNA levels are 3 to 4 times higher in the brain of Dnmt1 knockout mice compared to wildtype littermates (Martinowich et al., 2003), which agrees with the general view of the negative correlation between gene methylation and gene activation. Consistent with this view, a pharmacological DNMT inhibitor differentially activated Bdnf regulatory regions: exons I and III in cultured Neuro2A mouse neuroblastoma cells (Aid et al., 2007), exons I, IV, and VI in rat hippocampus (Lubin et al., 2008), and exon I in cultured hippocampal neurons (Nelson et al., 2008). To keep proper levels of DNA methylation, neurons also express a group of DNA de-methylases, like growth arrest and DNA-damage-inducible beta (GADD45B), which is required for activity-dependent adult neurogenesis (Ma et al., 2009). Interestingly, Gadd45b knockout mice have normal levels of BDNF methylation under basal conditions, but they completely lack the enhancement of Bdnf IX and total mRNA levels caused by electroconvulsive treatment (ECT) in wildtype mice, which is accompanied by BDNF de-methylation (Ma et al., 2009).
Another line of evidence that supports the repression model comes from the simultaneous dissociation of MeCP2 and its associated repressors from Bdnf promoter regions (Martinowich et al., 2003). One family of these repressors, HDACs have been known to participate in gene silencing via de-acetylation of histones H3 and H4 (Jones et al., 1998; Nan et al., 1998). After HDAC dissociation, histone acetylation becomes more evident within Bdnf exon IV, which facilitates its transcription (Martinowich et al., 2003). Consistently, hyper-acetylation of histones H3 and H4 within Bdnf promoters (specially exon IV) accompanies enhanced BDNF expression after ECT (Tsankova et al., 2004), cocaine exposure (Kumar et al., 2005), antidepressant treatment (Tsankova et al., 2006), and extinction of conditioned fear memory (Bredy et al., 2007). Similar to the opposing but interdependent roles of DNMT and GADD45B in normal neuronal function, the counterpart of HDAC, histone acetyltransferase (HAT) is also necessary for proper brain function, like long-term memory formation (Korzus et al., 2004). The lower levels of acetylation of histones H3 and H4 in the aging brain (when BDNF levels decline) are associated not only with an upregulation of HDAC, but also with lower expression of HAT (Zeng et al., 2011). In addition to HDAC, MeCP2 also interacts with SUV39H, a histone methyltransferase that induces dimethylation of histone H3 on lysine 9 and provides an “inactivation code” for gene expression (Fuks et al., 2003). In the brain however, another histone methyltransferase that adds methyl groups to histone H3 on lysine 4 in fact activates gene expression (Bernstein et al., 2002). Consistent with these findings, neuronal activity decreases dimethylation of H3 on lysine 9 in the Bdnf promoter region, while increasing dimethylation on lysine 4 (Chen et al., 2003; Martinowich et al., 2003). It is worthwhile to note that, as with lysine 9, hypermethylation of histone H3 on lysine 27 is also associated with lower BDNF levels (Tsankova et al., 2006). Although its specific role remains to be determined, histone methylation at this site likely increases MeCP2 association with regulatory regions of the Bdnf gene.
3.2 Activation model
The repression model of MeCP2 control of Bdnf transcription described above (e.g. Chen et al., 2003) is consistent with the canonical mechanisms of epigenetic regulation (Boulle et al., 2012), but fails to explain the fact BDNF protein expression is lower in the brain of Mecp2 knockout mice (e.g. Chang et al., 2006; Li et al., 2012). This apparent discrepancy was pointed out early on, as MeCP2 overexpression increased Bdnf transcripts in cultured cortical neurons, while Mecp2 knockout neurons showed lower Bdnf exon IV levels (but not exon I) than control cells (Klein et al., 2007). Also, BDNF levels in hypothalamus correlated with MeCP2 levels, with lower levels in Mecp2 knockout mice and higher levels in MECP2 overexpressing mice than wildtype controls (Chahrour et al., 2008). Furthermore, conditional Mecp2 deletion in the hypothalamus resulted in BDNF downregulation only in that region, again suggesting that MeCP2 is an activator of Bdnf transcription (Fyffe et al., 2008). Consistently, double point mutations (S421A and S424A) increased the association between MeCP2 and Bdnf promoter IV, leading to higher levels of Bdnf IV transcripts, and overall BDNF expression in the hippocampus of Mecp2S421A;S424/y mice (Li et al., 2011), as opposed to lower BDNF exon IV transcripts in mice carrying a single point mutation (S421A) (Zhou et al., 2006). Similarly, the weaker association of MeCP2 carrying the RTT-associated T158A mutation with the Bdnf locus is thought to be responsible for impaired BDNF expression (Goffin et al., 2011). Finally, re-activation of Mecp2 expression by Cre-encoding lentiviruses increased BDNF levels to wildtype levels in neurons derived from Mecp2stop embryonic stem cells (Yazdani et al., 2012). The above observations provide strong evidence for MeCP2 to act as an activator of BDNF expression, but the specific mechanism remains to be defined. Chahrour et al. (2008) showed that the transcriptional activator CREB1 is co-localized together with MeCP2 on multiple activated target genes. While Bdnf was not directly tested for this interaction, it is likely to occur because CREB1 is a well-known activator of Bdnf transcription. Moreover, the targeted genes – irrespective of repressed or activated – showed a stronger association with MeCP2 in MECP2 overexpressing mice than in Mecp2 knockout mice (Chahrour et al., 2008). These findings suggest that the control of Bdnf transcription by MeCP2 is unlikely to be indirect. The fact that CpG islands within promoter regions of activated genes are less methylated than those in repressed genes raises the question as to whether basal methylation levels dictate the fate of genes as to be activated or repressed. However, the levels of neuronal activity may shift the methylation levels of individual genes and their subsequent transcriptional state, as observed by Martinowich and colleagues (2003).
In addition to a direct interaction between MeCP2 and the regulatory regions of the Bdnf gene to regulate its expression, several observations suggest an indirect mechanism. A brain-enriched microRNA, miR132, which represses MeCP2 translation and subsequently reduce BDNF expression, is probably responsible for the lower BDNF levels observed in Mecp2 knockout brains (Klein et al., 2007). Intriguingly, BDNF induces miR132 expression, which in turn downregulates MeCP2, forming a homeostatic mechanism (Vo et al., 2005). The interaction of MeCP2 with miR212 (although not in postnatal cortical neurons) (Vo et al., 2005), has a similar consequence on BDNF protein expression in the striatum of adult rats (Im et al., 2010). Other than the above two microRNAs that regulate BDNF expression indirectly, several microRNAs have been found that directly target Bdnf transcripts. For example, Wu and colleagues (2010) identified a total of 20 miRNA-binding sites in the 3′ UTR of the Bdnf transcript, which can be targeted by 16 upregulated microRNAs (e.g. miR30a/d, miR381, miR495) in the cerebellum of Mecp2 knockout mice, where they regulate Bdnf mRNA translation in a negative manner.
The fact that Mecp2 knockout mice show lower BDNF levels than wildtype mice evidently supports the activation model; however, the repression model cannot be uncompromisingly rejected if one considers additional several factors that may influence BDNF expression levels. The first factor to be considered is the activity state of the source neurons or brain tissue. Since neuronal activity in wildtype neurons led to BDNF levels much higher than the basal levels that result simply from the lack of a repressive protein (i.e. MeCP2) in Mecp2 knockout neurons (Chen et al., 2003), it follows that BDNF levels in vivo are mainly determined by ongoing neuronal activity. Indeed, presymptomatic Mecp2 knockout mice show normal BDNF levels (Chang et al., 2006) and neuronal activity (Kron et al., 2012). Spontaneous neuronal activity is reduced in the cerebral cortex of Mecp2 knockout mice (Dani et al., 2005; Chang et al., 2006; Wood et al., 2009), although neuronal networks in the hippocampus and the nucleus tractus solitarius (nTS) in the brainstem are hyperexcitable in MeCP2-deficient mice (Calfa et al., 2011a; Kline et al., 2010; Kron et al., 2012). Second, the studies that found higher BDNF levels in the absence of MeCP2 were carried out in primary cultures of embryonic or early postnatal neurons, which obviously lack the complex cellular and molecular interactions occurring during brain development, including the lack of miR132 regulation (Klein et al., 2007). In juvenile or adult mice, acute excision Mecp2 exons 3 and 4 by tamoxifen-induced Cre-lox recombination does not significantly decrease BDNF levels, at least at the time of RTT-like symptom onset (McGraw et al., 2011). In addition, Mecp2308 mice, which express a non-functional truncated protein and an RTT-like neurological syndrome (Shahbazian et al., 2002a), show even higher BDNF mRNA levels in the hippocampus than wildtype littermates (De Filippis et al., 2012). Also, viral-mediated Mecp2 deletion in the amygdala has minimal effect on BDNF levels (Adachi et al., 2009). Furthermore, higher Bdnf IV mRNA levels, as found in early studies (Chen et al., 2003), may not truly reflect BDNF protein levels, given that the mouse Bdnf gene has nine promoters with a highly complex differential regulation (Aid et al., 2007). Indeed, Bdnf IV is upregulated in Mecp2 knockout mice, but Bdnf II is downregulated, which supports the activation model (Abuhztzira et al., 2007). In this view, BDNF downregulation in Mecp2 knockout mice occurs because the gene repressor REST (RE1 silencing transcription factor) is upregulated in these mice, and its binding to Bdnf promoter II with the co-repressor CoREST overrides the enhancement derived from activation of Bdnf promoter IV. Similar to this dissociated regulation, Ogier and colleagues (2007) found that only Bdnf I mRNAs is upregulated in Mecp2 knockout mice, but all other promoters remain downregulated, resulting in overall low levels of Bdnf transcripts. Extending this concept further, even when Bdnf IX mRNA is measured (reflecting total mRNA), this amount of transcripts may still not represent total levels of BDNF protein because further modulation exists during mRNA translation, as well as during trafficking and processing of proBDNF before yielding mature BDNF within dense-core vesicles ready for Ca2+-dependent regulated release. As discussed above, proBDNF is thought to have negative effects on neuronal and synaptic function through the activation of p75NTR. Whether proBDNF is upregulated or its proteolytic cleavage is impaired in Mecp2 knockout mice remains to be clarified. Last but not least, BDNF protein levels have been measured mostly by Western immunoblotting and ELISA, which only assess global tissue levels and may not detect modest alterations and highly localized deficits, which could have significant consequences in neuronal and synaptic function.
3.3 Dual operation model
The higher levels of Bdnf transcripts detected in cultured cortical neurons from Mecp2 knockout mice (Chen et al., 2003) predict that MeCP2 overexpression will lead to lower BDNF levels. However, both Mecp2 knockdown and MECP2 overexpression enhanced BDNF protein levels in cultured hippocampal neurons (Larimore et al., 2009). These findings suggest that the control of BDNF expression by MeCP2 can dynamically switch between repression and activation. Indeed, a hypothetical “dual operation” mechanism seems to underlie MeCP2’s modulation of the expression of early growth response factor-2 (EGR2): MeCP2 represses EGR2 transcription in control SH-SY5Y neuroblastoma cells, while it activates EGR2 expression after cell differentiation with the phorbol ester 12-myristate 13-acetate (PMA). Intriguingly, prolonged exposure to PMA switches MeCP2 function back to repression of EGR2 expression. This dual operation is controlled by phosphorylation of Ser80 in MeCP2. A similar mechanism underlies MeCP2 regulation of the receptor tyrosine kinase gene RET, but involving phosphorylation at Ser229 (Gonzales et al., 2012). Surprisingly, all these different actions of MeCP2 occur without changes in the association of phosphorylated MeCP2 to the genes under regulation, which contrasts to the current model of MeCP2 control of gene transcription by reversible binding to specific promoter sites. Similarly, phosphorylation at Ser80 does not change MeCP2 association with target genes in cultured cortical neurons (Tao et al., 2009). Gonzales and colleagues (2012) proposed that, even though MeCP2 remains bound to target genes, its phosphorylation recruits different regulatory complexes to activate or repress gene expression. Indeed, phospho-Ser80 MeCP2 is known to associate with Sin3a and the RNA binding protein YB-1, while phospho-Ser229 MeCP2 with Sin3a, HP1 (heterochromatin protein 1) and SMC3, a component of the cohesin complex; however, the function of these associations is not clear yet. The above findings support a novel “dual operation” model of MeCP2 control of gene expression and warrant further research, especially with regards to MeCP2’s modulation of Bdnf transcription, which has significant implications for novel therapies for RTT individuals. Aside from phosphorylation of MeCP2, epigenetic modification of DNA may participate in a dual mechanism of transcriptional control of specific genes. Even though direct experimental evidence of this dual operation model is limited, it provides an intriguing new model of MeCP2 transcriptional regulation for future mechanistic investigations.
4. Altered BDNF expression, transport, and secretion in RTT patients and MeCP2- based mouse models
Much evidence has indicated a reduction in BDNF levels in MeCP2-based mouse models of RTT, which becomes significant with the appearance of RTT-like features. In the first 3-4 postnatal weeks, BDNF levels in most brain regions of male Mecp2 knockout mice are comparable to those in wildtype littermates (Chang et al., 2006; Wang et al., 2006). When behavioral impairments gradually start to manifest around 5-6 weeks, BDNF levels are lower in caudal parts of the brain, such as the brainstem and cerebellum (Kline et al., 2010; Ogier et al., 2007; Wang et al., 2006), without detectable changes in the cerebral cortex (Deogracias et al., 2012; Roux et al., 2012; Schaevitz et al., 2010; Want et al., 2006). By 7 weeks, BDNF levels are lower in male Mecp2 knockout mice throughout the entire brain (Chang et a., 2006; Li et al., 2012; Lonetti et al., 2010). Similar to male Mecp2 knockout mice, symptomatic female Mecp2 heterozygous mice express lower BDNF levels throughout the brain (Schmid et al., 2012). Due to technical limitations to measure its levels in humans, especially in brain, whether BDNF expression is impaired in RTT individuals is, so far, controversial. Two reports from the same group found that BDNF protein levels in cerebrospinal fluid and blood serum of RTT patients are comparable to unaffected individuals, as measured by ELISA (Vanhala et al., 1998; Riikonen, 2003). However, another two studies described lower Bdnf mRNA levels in autopsy brain samples from RTT individuals (Abuhatzira et al., 2007; Deng et al., 2007), which is reminiscent of the situation in Mecp2 mutant mice. Certainly, more studies are needed to resolve this apparent discrepancy, which will provide rational support for the development of clinic trials aimed to recover BDNF levels or boost its signaling through TrkB receptors in RTT individuals.
Whether all RTT individuals will benefit equally from increasing BDNF expression is still an open question, especially if one considers a single nucleotide polymorphism (SNP) in the human BDNF gene that affects its intracellular processing. The function of BDNF in neuronal development and synaptic plasticity relies on its proper trafficking to axons and dendrites, as well as its targeting to the regulated secretory pathway, which allows activity-dependent release (Fig. 1b). The substitution of valine for methionine at codon 66 (Val66Met) in a common BDNF SNP impairs intracellular trafficking and regulated secretion of the resulting mature BDNF protein (Egan et at., 2003), and has been involved in cognitive dysfunction in a wide range of neuropsychiatric disorders (Dincheva et al., 2012). However, studies on RTT individuals carrying the Met66 allele have been scarce to date. One report describes that RTT individuals carrying the Val66Met polymorphism showed delays in the onset of their seizures (Nectoux et al., 2008), while a later study indicated that Val66Met RTT patients tended to show more severe clinical symptoms, including the risk of seizures (Zeev et al., 2009). Additional studies involving more RTT individuals are needed to determine the distribution of the BDNF SNP in the RTT population and whether it is different than in typically developing women, as well as to characterize its impact on clinical progression of neurological symptoms depending on specific MECP2 mutations.
To reach its site for activity-dependent release, secretory granules containing BDNF are transported along axons and dendrites by microtubule-based motor systems (Fig. 1c) (Greenberg et al., 2009). Axonal transport of BDNF granules is slower in cultured cortical neurons from Mecp2 knockout mice, which results in lower cortical BDNF levels due to impaired transport by cortico-striatal axonal projections (Roux et al., 2012). This deficit in BDNF transport in Mecp2 mutant neurons can be reversed by Mecp2 re-expression, and is due to deregulation of Huntingtin (Htt) and Huntingtin-associated protein 1 (Hap1), known to be required for the anterograde transport of BDNF (Caviston and Holzbaur, 2009). Whether impaired BDNF trafficking in Mecp2 mutant neurons is due to deregulation of a direct MeCP2 control of the Htt and Hap1 genes requires further investigation.
Despite the low levels of BDNF expression and impaired BDNF transport in Mecp2 knockout neurons, some cells may express a compensatory mechanism to mobilize the most available BDNF releasable pool upon neuronal depolarization. For example, the total levels of BDNF protein is lower in cultured neurons of the nodose ganglion of presymptomatic Mecp2 mice than in those from wildtype mice, but the amount of BDNF released to the extracellular media upon depolarization is comparable in both genotypes (Wang et al., 2006). However, activity-dependent BDNF release from presynaptic mossy fibers onto CA3 pyramidal neurons is impaired in hippocampal slices from symptomatic Mecp2 knockout mice, evidenced by smaller membrane currents and Ca2+ signals initiated by TrkB activation and mediated by TRPC3 channels (Fig. 1d) (Li et al., 2012). Quite relevant to the fact that GABAergic interneurons require BDNF for their maturation (Abidin et al., 2008; Kohara et al., 2007; Marty et al., 2000), BDNF-evoked TRPC3 currents and Ca2+ signals are also smaller in CA3 interneurons of symptomatic Mecp2 knockout mice (WL, LP-M, unpublished data). Further research is needed to clarify the consequences of impaired BDNF release on GABAergic neuron and synapse development in Mecp2 knockout mice.
5. BDNF-related therapies in RTT mouse models
Improving BDNF expression and/or signaling has received much attention for the treatment of a variety of neurological disorders (Nagahara and Tuszynski, 2011; Zuccato and Cattaneo, 2009), and a great deal of progress has been achieved by the RTT research community (Table 1) (Gadalla et al., 2011; Katz et al., 2012). The proof-of-principle that restoring BDNF levels is beneficial to Mecp2-deficient mice comes from breeding female Mecp2 knockout mice expressing Cre recombinase under CamkII control (Mecp2+/− ;Cre93) with male mice that express a BDNF transgene under control of the synthetic CAGGS promoter preceded by a Cre-removable STOP cassette flanked by loxP sites. BDNF overexpression in postnatal excitatory forebrain neurons (i.e. those expressing CamkII-driven Cre) of Mecp2 knockout mice significantly extended their lifespan, improved locomotor function, increased brain weight, and reversed dampened spontaneous firing of cortical pyramidal neurons (Chang et al., 2006). In addition, BDNF overexpression in cultured hippocampal neurons reversed the impaired dendritic and axonal complexity caused by either shRNA-mediated Mecp2 knockdown or expression of RTT-associated MECP2 mutations (Larimore et al., 2009).
Table 1.
Strategies for improving BDNF signaling in Mecp2 knockout mice
For detailed description, see the text. Abbreviations: TrkB, tropomyosin-related kinase B; nTS, nucleus tractus solitarius; mEPSC, miniature excitatory postsynaptic current; mIPSC, minature inhibitory postsynaptic current; PSD, postsynaptic density; LTP, long-term potentiation.
| Approach | Effect | Evaluation | References | |
|---|---|---|---|---|
| Genetics |
Mecp2+/−;Cre93; CAGGS-BDNFstop |
BDNF overexpression in postmitotic forebrain neurons |
↑ lifespan; ↑ locomotor activity ↑ brain weight ↑ spontaneous firing (recorded in Lay V of somatosensory cortex) |
Chang et al., 2006 |
| Bdnf overexpression | BDNF overexpresison in neurons transfected with MECP2 shRNA or MECP2 R106W/TI58M |
↑ dentritic length; ← dendritic branch points | Larimore et al., 2009 | |
| Pharmacology | BDNF application | Increasing BDNF content | ↓ exaggerated EPSCs in nTS | Kline et al., 2010 |
| AMPKines | Increasing endogenous BDNF |
↓ episodes of high breathing frequency but not ↓ higher frequency variability ↓ minute volume/weight but not ↓ tidal volume/weight |
Ogier et al.,2007 | |
| LM22A-4 | Activating TrkB receptor | ↑ expiratory time; ↑ total breath duration | Schmid et al., 2012 | |
| Fingolimod | Increasing endogenous BDNF |
↑ locomotor activity; ↑ motor coordination ↑ straitum size ↑ frequency and amplitude of mEPSC; ↓ frequency and amplitude of mIPSC |
Deogracias et al., 2012 | |
| Cysteamine | Increasing BDNF transport and secretion |
↑ lifespan; ↑ locomotor activity | Roux et al, 2012 | |
| 7,8-dihydroxyflavone (7,8-DHF) |
Activating TrkB receptor | ↑ lifespan; ↑ locomotor activity ↓ tidal volume; ↓. irregular respiratory rhythms ↑ neuronal nuclei size |
Johnson et al., 2012 | |
| IGF-1 | Activating the same downstream signaling as BDNF |
↑ lifespan; ↑ locomotor activity ↓ cardiorespiratory irregularities ↑ brain size; ↑ spine density; ↑ PSD intensity ↑ synaptic transmission; ← cortical plasticity |
Tropea et al., 2009 | |
| Physiology | Environmental enrichment (EE) |
Increasing endogenous BDNF |
↑ slightly in lifespan; ↑ motor coordination; ↑ motor learning; ↑ spatial learning; ↓ anxiety ↑ excitatory but no inhibitory synaptic densities in primary somatosensory cortex; ↑ both excitatory and inhibitory synaptic densities in cerebellum ↑ LTP |
Lonetti et al., 2009 |
Pharmacological manipulations that involve delivery of recombinant mature BDNF, or enhancing endogenous BDNF expression or its downstream signaling pathways are more amenable alternatives for application in humans than gene therapy. For example, the respiratory dysfunction in Mecp2 knockout mice that phenocopies the irregular breathing suffered by RTT individuals is significantly improved by pharmacological manipulations of BDNF signaling due to its well-known role in the development and maintenance of synaptic and neuronal function within brainstem respiratory nuclei. Acute exposure to BDNF reverses neuronal hyperexcitability in brainstem slices containing the nTS, which is conveyed to central autonomic pathways and causes cardiorespiratory instability (Kline et al., 2010). While treatment with recombinant BDNF is theoretically the best option, its low blood-brain barrier penetration imposes severe limitations for systemic administration. AMPAkines, a family of nootropic agents that increase BDNF expression by preventing desensitization of AMPA-type glutamate receptors (Lynch and Gall, 2006), represent an exciting alternative to boost BDNF signaling in neurological disorders. Indeed, chronic treatment with the AMPAkine CX546 restored normal breathing frequency and minute volume/weight in Mecp2 knockout mice (Ogier et al., 2007). Another promising compound is the BDNF mimetic LM22A-4, which selectively activates TrkB but not other Trk family members (Massa et al., 2010). LM22A-4 administration to female Mecp2 heterozygous mice improved the levels of phosphorylated TrkB receptors and downstream Akt and ERK, as well as restored normal respiratory frequency by increasing expiratory time and total breath duration (Schmid et al., 2012).
In addition to specific reversal of cardiorespiratory deficits in Mecp2 knockout mice, other BDNF-related compounds have been examined for the amelioration of RTT-like neurological symptoms. Fingolimod, a compound with sufficient blood-barrier barrier permeability being used for the treatment of multiple sclerosis, stimulates the ERK signaling pathway and leads to enhanced BDNF expression, ultimately resulting in the improvement of RTT-like features in Mecp2 knockout mice (Deogracias et al., 2012). Likewise, oral treatment with cysteamine was found to extend the lifespan and improve locomotor activity in Mecp2 knockout mice (Roux et al., 2012), which may result from improved BDNF transport and secretion (Borrell-Pages et al., 2006). The TrkB receptor activator 7,8-dihydroxyflavone (7,8-DHF; Jang et al., 2010) was also reported to lengthen the lifespan and improve locomotor activity in Mecp2 knockout mice, as well as prevent weight loss and breathing pattern irregularities (Johnson et al., 2012). Finally, a clinical trial is currently underway testing insulin-like growth factor-1 (IGF-1) in RTT individuals based on the beneficial effects of the active tri-peptide in Mecp2 knockout mice (Tropea et al., 2009), thought to act by its similitude to the TrkB signaling pathway (Tropea et al., 2006).
Physical exercise and cognitive stimulation are also known to increase BDNF expression in rodents (Zuccato and Cattaneo, 2009). For example, environmental enrichment (EE) at presymptomatic stages (postnatal day 28) caused a slight improvement of motor coordination in female Mecp2 heterozygous mice, but not in male Mecp2 knockouts. Interestingly, brain BDNF levels correlated with the improvement of motor performance, with EE having an effect only in female Mecp2 heterozygous mice (Kondo et al., 2008). However, when EE was provided at pre-weaning age (postnatal day 10), male Mecp2 knockout mice showed significant improvements in motor coordination, motor learning, and anxiety as well as spatial learning. Furthermore, these behavioral reversals paralleled increased levels of BDNF in brain and restored LTP at cortical synapses (Lonetti et al., 2010).
Our present exploration for the treatment of RTT is still in its infancy, but with the growing knowledge of BDNF deregulation in Mecp2 deficient mice, the coming years are likely to see substantial advances in new therapeutic strategies tailored to target specific gene regions susceptible to detriment and aimed at the precise brain regions affected in RTT.
6. Conclusions and future directions
Ample evidence from mouse models indicates that the role of MeCP2 in brain development and function is different across various brain regions, suggesting that dysfunction in specific neuronal populations due to differential distribution of mutant MeCP2 contributes to phenotypic variability. The realization that MeCP2 acts as both a repressor and activator of potentially thousands of genes has increased the complexity of this once thought simple monogenetic disorder. Also, none of the experimental treatments tested in Mecp2 deficient mice fully reversed their RTT-like phenotypes, suggesting additional molecular and cellular deficits. In spite of these seemingly overwhelming limitations in our state of knowledge, we should remind ourselves that we have learned more in the last decade since the discovery that MeCP2 mutations cause RTT than in the preceding 30 years from the first description by Andreas Rett. Following this trajectory, it is likely that rational therapies grounded on basic scientific knowledge will be available for RTT individuals within the next decade.
We review the deregulation of BDNF function in Rett syndrome (RTT).
RTT affects 1:15,000 women world-wide and is caused by mutations in MeCP2.
MeCP2 controls BDNF expression.
RTT-like features in Mecp2 knockouts reflect impairments in BDNF function.
We discuss progress on therapeutic interventions based on improving BDNF function.
Acknowledgments
This work was supported by NIH grants NS-065027 and NS-40593 (LP-M), and by Postdoctoral Fellowship IRSF2824 (WL) from the International Rett Syndrome Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Wei Li, Department of Neurobiology, Civitan International Research Center, The University of Alabama at Birmingham, Birmingham, AL 35294, USA.
Lucas Pozzo-Miller, Department of Neurobiology, Civitan International Research Center, The University of Alabama at Birmingham, Birmingham, AL 35294, USA.
References
- Abidin I, Eysel UT, Lessmann V, Mittmann T. Impaired GABAergic inhibition in the visual cortex of brain-derived neurotrophic factor heterozygous knockout mice. J. Physiol. 2008;586:1885–1901. doi: 10.1113/jphysiol.2007.148627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuhatzira L, Makedonski K, Kaufman Y, Razin A, Shemer R. MeCP2 deficiency in the brain decreases BDNF levels by REST/CoREST-mediated repression and increases TRKB production. Epigenetics. 2007;2:214–222. doi: 10.4161/epi.2.4.5212. [DOI] [PubMed] [Google Scholar]
- Adachi M, Autry AE, Covington HE, III, Monteggia LM. MeCP2-mediated transcription repression in the basolateral amygdala may underlie heightened anxiety in a mouse model of Rett syndrome. J. Neurosci. 2009;29:4218–4227. doi: 10.1523/JNEUROSCI.4225-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J. Neurosci. Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral MD, Chapleau CA, Pozzo-Miller L. Transient receptor potential channels as novel effectors of brain-derived neurotrophic factor signaling: potential implications for Rett syndrome. Pharmacol. Ther. 2007;113:394–409. doi: 10.1016/j.pharmthera.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG binding protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, Xu B. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134:175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–657. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Balmer D, Goldstine J, Rao YM, LaSalle JM. Elevated methyl-CpG-binding protein 2 expression is acquired during postnatal human brain development and is correlated with alternative polyadenylation. J. Mol. Med. 2003;81:61–68. doi: 10.1007/s00109-002-0396-5. [DOI] [PubMed] [Google Scholar]
- Bebbington A, Anderson A, Ravine D, Fyfe S, Pineda M, de Klerk N, Ben-Zeev B, Yatawara N, Percy A, Kaufmann WE, Leonard H. Investigating genotype-phenotype relationships in Rett syndrome using an international data set. Neurology. 2008;70:868–875. doi: 10.1212/01.wnl.0000304752.50773.ec. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Humphrey EL, Erlich RL, Schneider R, Bouman P, Liu JS, Kouzarides T, Schreiber SL. Methylation of histone H3 Lys 4 in coding regions of active genes. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8695–8700. doi: 10.1073/pnas.082249499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Boggio EM, Lonetti G, Pizzorusso T, Giustetto M. Synaptic determinants of rett syndrome. Front. Synaptic. Neurosci. 2010;2:28. doi: 10.3389/fnsyn.2010.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell-Pagès M, Canals JM, Cordelières FP, Parker JA, Pineda JR, Grange G, Bryson EA, Guillermier M, Hirsch E, Hantraye P, Cheetham ME, Néri C, Alberch J, Brouillet E, Saudou F, Humbert S. Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. J. Clin. Invest. 2006;116:1410–1424. doi: 10.1172/JCI27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulle F, van den Hove DL, Jakob SB, Rutten BP, Hamon M, van Os J, Lesch KP, Lanfumey L, Steinbusch HW, Kenis G. Epigenetic regulation of the BDNF gene: implications for psychiatric disorders. Mol. Psychiatry. 2012;17:584–596. doi: 10.1038/mp.2011.107. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog. Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Braunschweig D, Simcox T, Samaco RC, LaSalle JM. X-Chromosome inactivation ratios affect wild-type MeCP2 expression within mosaic Rett syndrome and Mecp2−/+ mouse brain. Hum. Mol. Genet. 2004;13:1275–1286. doi: 10.1093/hmg/ddh142. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn. Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PJ, Marietta C, Goldman D. DNA mismatch repair and DNA methylation in adult brain neurons. J. Neurosci. 1996;16:939–945. doi: 10.1523/JNEUROSCI.16-03-00939.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfa G, Hablitz JJ, Pozzo-Miller L. Network hyperexcitability in hippocampal slices from Mecp2 mutant mice revealed by voltage-sensitive dye imaging. J. Neurophysiol. 2011a;105:1768–1784. doi: 10.1152/jn.00800.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfa G, Percy AK, Pozzo-Miller L. Experimental models of Rett syndrome based on Mecp2 dysfunction. Exp. Biol. Med. 2011b;236:3–19. doi: 10.1258/ebm.2010.010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston JP, Holzbaur EL. Huntingtin as an essential integrator of intracellular vesicular trafficking. Trends. Cell Biol. 2009;19:147–155. doi: 10.1016/j.tcb.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49:341–348. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Chapleau CA, Larimore JL, Theibert A, Pozzo-Miller L. Modulation of dendritic spine development and plasticity by BDNF and vesicular trafficking: fundamental roles in neurodevelopmental disorders associated with mental retardation and autism. J. Neurodev. Disord. 2009;1:185–196. doi: 10.1007/s11689-009-9027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, Nykjaer A, Hempstead BL, Lee FS. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J. Neurosci. 2005;25:6156–6166. doi: 10.1523/JNEUROSCI.1017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Cohen S, Gabel HW, Hemberg M, Hutchinson AN, Sadacca LA, Ebert DH, Harmin DA, Greenberg RS, Verdine VK, Zhou Z, Wetsel WC, West AE, Greenberg ME. Genome-wide activity-dependent MeCP2 phosphorylation regulates nervous system development and function. Neuron. 2011;72:72–85. doi: 10.1016/j.neuron.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuoni C, Jeon OH, Hyder K, Chenchik A, Khimani AH, Narayanan V, Hoffman EP, Kaufmann WE, Naidu S, Pevsner J. Gene expression profiling in postmortem Rett Syndrome brain: differential gene expression and patient classification. Neurobiol. Dis. 2001;8:847–865. doi: 10.1006/nbdi.2001.0428. [DOI] [PubMed] [Google Scholar]
- Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front. Mol. Neurosci. 2010;3:1–14. doi: 10.3389/neuro.02.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis B, Ricceri L, Fuso A, Laviola G. Neonatal exposure to low dose corticosterone persistently modulates hippocampal mineralocorticoid receptor expression and improves locomotor/exploratory behaviour in a mouse model of Rett syndrome. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.05.048. In press. [DOI] [PubMed] [Google Scholar]
- Deng V, Matagne V, Banine F, Frerking M, Ohliger P, Budden S, Pevsner J, Dissen GA, Sherman LS. Ojeda SR. 2007. FXYD1 is an MeCP2 target gene overexpressed in the brains of Rett syndrome patients and Mecp2 null mice. Hum. Mol. Genet. 2007;16:640–650. doi: 10.1093/hmg/ddm007. [DOI] [PubMed] [Google Scholar]
- Deogracias R, Yazdani M, Dekkers MP, Guy J, Ionescu MC, Vogt KE, Barde YA. Fingolimod, a sphingosine-1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. U. S. A. 2012;109:14230–14235. doi: 10.1073/pnas.1206093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincheva I, Glatt CE, Lee FS. Impact of the BDNF Val66Met polymorphism on cognition: implications for behavioral genetics. Neuroscientist. 2012;18:439–451. doi: 10.1177/1073858411431646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, Silva AJ, Fan G. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat. Neurosci. 2010;13:423–430. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuks F, Hurd PJ, Wolf D, Nan X, Bird AP, Kouzarides T. The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J. Biol. Chem. 2003;278:4035–4040. doi: 10.1074/jbc.M210256200. [DOI] [PubMed] [Google Scholar]
- Fyffe SL, Neul JL, Samaco RC, Chao HT, Ben-Shachar S, Moretti P, McGill BE, Goulding EH, Sullivan E, Tecott LH, Zoghbi HY. Deletion of Mecp2 in Sim1-expressing neurons reveals a critical role for MeCP2 in feeding behavior, aggression, and the response to stress. Neuron. 2008;59:947–958. doi: 10.1016/j.neuron.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadalla KK, Bailey ME, Cobb SR. MeCP2 and Rett syndrome: reversibility and potential avenues for therapy. Biochem. J. 2011;439:1–14. doi: 10.1042/BJ20110648. [DOI] [PubMed] [Google Scholar]
- Goffin D, Allen M, Zhang L, Amorim M, Wang IT, Reyes AR, Mercado-Berton A, Ong C, Cohen S, Hu L, Blendy JA, Carlson GC, Siegel SJ, Greenberg ME, Zhou Z. Rett syndrome mutation MeCP2 T158A disrupts DNA binding, protein stability and ERP responses. Nat. Neurosci. 2011;15:274–283. doi: 10.1038/nn.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales ML, Adams S, Dunaway KW, LaSalle JM. Phosphorylation of distinct sites in MeCP2 modifies cofactor associations and the dynamics of transcriptional regulation. Mol. Cell. Biol. 2012;32:2894–2903. doi: 10.1128/MCB.06728-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J. Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2 null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: report of 35 cases. Ann. Neurol. 1983;14:471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Im HI, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat. Neurosci. 2010;13:1120–1127. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Lam M, Punzo AM, Li H, Lin BR, Ye K, Mitchell GS, Chang Q. 7,8-dihydroxyflavone exhibits therapeutic efficacy in a mouse model of Rett syndrome. J. Appl. Physiol. 2012;112:704–710. doi: 10.1152/japplphysiol.01361.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- Jordan C, Li HH, Kwan HC, Francke U. Cerebellar gene expression profiles of mouse models for Rett syndrome reveal novel MeCP2 targets. BMC. Med. Genet. 2007;8:36. doi: 10.1186/1471-2350-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DM, Berger-Sweeney JE, Eubanks JH, Justice MJ, Neul JL, Pozzo-Miller L, Blue ME, Christian D, Crawley JN, Giustetto M, Guy J, Howell CJ, Kron M, Nelson SB, Samaco RC, Schaevitz LR, St. Hillaire-Clarke C, Young JL, Zoghbi HY, Mamounas LA. Preclinical research in Rett syndrome: setting the foundation for translational success. Dis. Model. Mech. 2012;5:733–745. doi: 10.1242/dmm.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET, Nelson ED, Monteggia LM. Role of MeCP2, DNA methylation, and HDACs in regulating synapse function. J. Neurodev. Disord. 2011;3:250–256. doi: 10.1007/s11689-011-9078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi N, Macklis JD. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol. Cell. Neurosci. 2004;27:306–321. doi: 10.1016/j.mcn.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Klein ME, Lioy DT, Ma L, Impey S, Mandel G, Goodman RH. Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat Neurosci. 2007;10:1513–1514. doi: 10.1038/nn2010. [DOI] [PubMed] [Google Scholar]
- Kline DD, Ogier M, Kunze DL, Katz DM. Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2 null mice. J. Neurosci. 2010;30:5303–5310. doi: 10.1523/JNEUROSCI.5503-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose RJ, Sarraf SA, Schmiedeberg L, McDermott SM, Stancheva I, Bird AP. DNA binding selectivity of MeCP2 due to a requirement for A/T sequences adjacent to methyl-CpG. Mol. Cell. 2005;19:667–678. doi: 10.1016/j.molcel.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Kohara K, Yasuda H, Huang Y, Adachi N, Sohya K, Tsumoto T. A local reduction in cortical GABAergic synapses after a loss of endogenous brain-derived neurotrophic factor, as revealed by single-cell gene knock-out method. J. Neurosci. 2007;27:7234–7244. doi: 10.1523/JNEUROSCI.1943-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbeck R, Bartke I, Eberle W, Barde YA. Brain-derived neurotrophic factor levels in the nervous system of wild-type and neurotrophin gene mutant mice. J. Neurochem. 1999;72:1930–1938. doi: 10.1046/j.1471-4159.1999.0721930.x. [DOI] [PubMed] [Google Scholar]
- Kondo M, Gray LJ, Pelka GJ, Christodoulou J, Tam PP, Hannan AJ. Environmental enrichment ameliorates a motor coordination deficit in a mouse model of Rett syndrome-Mecp2 gene dosage effects and BDNF expression. Eur. J. Neurosci. 2008;27:3342–3350. doi: 10.1111/j.1460-9568.2008.06305.x. [DOI] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron M, Howell CJ, Adams IT, Ransbottom M, Christian D, Ogier M, Katz DM. Brain activity mapping in Mecp2 mutant mice reveals functional deficits in forebrain circuits, including key nodes in the default mode network, that are reversed with ketamine treatment. J. Neurosci. 2012;32:13860–13872. doi: 10.1523/JNEUROSCI.2159-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Larimore JL, Chapleau CA, Kudo S, Theibert A, Percy AK, Pozzo-Miller L. Bdnf overexpression in hippocampal neurons prevents dendritic atrophy caused by Rett-associated MECP2 mutations. Neurobiol. Dis. 2009;34:199–211. doi: 10.1016/j.nbd.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaSalle JM, Goldstine J, Balmer D, Greco CM. Quantitative localization of heterogeneous methyl-CpG-binding protein 2 (MeCP2) expression phenotypes in normal and Rett syndrome brain by laser scanning cytometry. Hum. Mol. Genet. 2001;10:1729–40. doi: 10.1093/hmg/10.17.1729. [DOI] [PubMed] [Google Scholar]
- LaSalle JM, Yasui DH. Evolving role of MeCP2 in Rett syndrome and autism. Epigenomics. 2009;1:119–130. doi: 10.2217/epi.09.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AG, Irier HA, Gu J, Tian D, Ku L, Liu G, Xia M, Fritsch B, Zheng JQ, Dingledine R, Xu B, Lu B, Feng Y. Distinct 3’UTRs differentially regulate activity-dependent translation of brain-derived neurotrophic factor (BDNF) Proc. Natl. Acad. Sci. U. S. A. 2010;107:15945–15950. doi: 10.1073/pnas.1002929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurvick CL, de Klerk N, Bower C, Christodoulou J, Ravine D, Ellaway C, Williamson S, Leonard H. Rett syndrome in Australia: a review of the epidemiology. J. Pediatr. 2006;148:347–352. doi: 10.1016/j.jpeds.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Li H, Zhong X, Chau KF, Williams EC, Chang Q. Loss of activity-induced phosphorylation of MeCP2 enhances synaptogenesis, LTP and spatial memory. Nat. Neurosci. 2011;14:1001–1008. doi: 10.1038/nn.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HS, Xu XZ, Montell C. Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron. 1999;24:261–273. doi: 10.1016/s0896-6273(00)80838-7. [DOI] [PubMed] [Google Scholar]
- Li W, Calfa G, Larimore J, Pozzo-Miller L. Activity-dependent BDNF release and TRPC signaling is impaired in hippocampal neurons of Mecp2 mutant mice. Proc. Natl. Acad. Sci. U. S. A. 2012;109:17087–17092. doi: 10.1073/pnas.1205271109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Pozzo-Miller L. Beyond widespread Mecp2 deletions to model Rett syndrome: conditional spatio-temporal knockout, single-point mutations and transgenic rescue mice. Autism. 2012;S1:005. doi: 10.4172/2165-7890.S1-005. doi:10.4172/2165-7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Calfa G, Inoue T, Amaral MD, Pozzo-Miller L. Activity-dependent release of endogenous BDNF from mossy fibers evokes a TRPC3 current and Ca2+ elevations in CA3 pyramidal neurons. J. Neurophysiol. 2010;103:2846–2856. doi: 10.1152/jn.01140.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Lu L, Zhu XG, Gong JP, Shaham Y, Uhl GR. Rodent BDNF genes, novel promoters, novel splice variants, and regulation by cocaine. Brain Res. 2006;1067:1–12. doi: 10.1016/j.brainres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Liu QR, Walther D, Drgon T, Polesskaya O, Lesnick TG, Strain KJ, de Andrade M, Bower JH, Maraganore DM, Uhl GR. Human brain derived neurotrophic factor (BDNF) genes, splicing patterns, and assessments of associations with substance abuse and Parkinson’s Disease. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2005;134B:93–103. doi: 10.1002/ajmg.b.30109. [DOI] [PubMed] [Google Scholar]
- Lonetti G, Angelucci A, Morando L, Boggio EM, Giustetto M, Pizzorusso T. Early environmental enrichment moderates the behavioral and synaptic phenotype of MeCP2 null mice. Biol. Psychiatry. 2010;67:657–665. doi: 10.1016/j.biopsych.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase E. Neuron. 2005;45:245–255. doi: 10.1016/j.neuron.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn. Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol. Learn. Mem. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J. Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Gall CM. Ampakines and the threefold path to cognitive enhancement. Trends Neurosci. 2006;29:554–562. doi: 10.1016/j.tins.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- Marty S, Wehrlé R, Sotelo C. Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J. Neurosci. 2000;20:8087–8095. doi: 10.1523/JNEUROSCI.20-21-08087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa SM, Yang T, Xie Y, Shi J, Bilgen M, Joyce JN, Nehama D, Rajadas J, Longo FM. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J. Clin. Invest. 2010;120:1774–1785. doi: 10.1172/JCI41356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Rauskolb S, Polack M, Klose J, Kolbeck R, Korte M, Barde YA. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat. Neurosci. 2008;11:131–133. doi: 10.1038/nn2038. [DOI] [PubMed] [Google Scholar]
- McGraw CM, Samaco RC, Zoghbi HY. Adult neural function requires MeCP2. Science. 2011;333:186. doi: 10.1126/science.1206593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J. Biol. Chem. 2001;276:12660–12666. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug. Discov. 2011;10:209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- Nectoux J, Bahi-Buisson N, Guellec I, Coste J, De Roux N, Rosas H, Tardieu M, Chelly J, Bienvenu T. The p.Val66Met polymorphism in the BDNF gene protects against early seizures in Rett syndrome. Neurology. 2008;70:2145–2151. doi: 10.1212/01.wnl.0000304086.75913.b2. [DOI] [PubMed] [Google Scholar]
- Nelson ED, Kavalali ET, Monteggia LM. Activity-dependent suppression of miniature neurotransmission through the regulation of DNA methylation. J. Neurosci. 2008;28:395–406. doi: 10.1523/JNEUROSCI.3796-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neul JL, Fang P, Barrish J, Lane J, Caeg EB, Smith EO, Zoghbi H, Percy A, Glaze DG. Specific mutations in methyl-CpG-binding protein 2 confer different severity in Rett syndrome. Neurology. 2008;70:1313–1321. doi: 10.1212/01.wnl.0000291011.54508.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neul JL, Kaufmann WE, Glaze DG, Christodoulou J, Clarke AJ, Bahi-Buisson N, Leonard H, Bailey ME, Schanen NC, Zappella M, Renieri A, Huppke P, Percy AK, RettSearch Consortium Rett syndrome: revised diagnostic criteria and nomenclature. Ann. Neurol. 2010;68:944–950. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MV, Du F, Felice CA, Shan X, Nigam A, Mandel G, Robinson JK, Ballas N. MeCP2 is critical for maintaining mature neuronal networks and global brain anatomy during late stages of postnatal brain development and in the mature adult brain. J. Neurosci. 2012;32:10021–10034. doi: 10.1523/JNEUROSCI.1316-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuber UA, Kriaucionis S, Roloff TC, Guy J, Selfridge J, Steinhoff C, Schulz R, Lipkowitz B, Ropers HH, Holmes MC, Bird A. Up-regulation of glucocorticoid-regulated genes in a mouse model of Rett syndrome. Hum. Mol. Genet. 2005;14:2247–2256. doi: 10.1093/hmg/ddi229. [DOI] [PubMed] [Google Scholar]
- Ogier M, Wang H, Hong E, Wang Q, Greenberg ME, Katz DM. Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J. Neurosci. 2007;27:10912–10917. doi: 10.1523/JNEUROSCI.1869-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Percy AK, Lane JB. Rett syndrome: model of neurodevelopmental disorders. J. Child Neurol. 2005;20:718–721. doi: 10.1177/08830738050200090301. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riikonen R. Neurotrophic factors in the pathogenesis of Rett syndrome. J. Child Neurol. 2003;18:693–697. doi: 10.1177/08830738030180101101. [DOI] [PubMed] [Google Scholar]
- Roux JC, Zala D, Panayotis N, Borges-Correia A, Saudou F, Villard L. Modification of Mecp2 dosage alters axonal transport through the Huntingtin/Hap1 pathway. Neurobiol. Dis. 2012;45:786–795. doi: 10.1016/j.nbd.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog. Neurobiol. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Schaevitz LR, Moriuchi JM, Nag N, Mellot TJ, Berger-Sweeney J. Cognitive and social functions and growth factors in a mouse model of Rett syndrome. Physiol. Behav. 2010;100:255–263. doi: 10.1016/j.physbeh.2009.12.025. [DOI] [PubMed] [Google Scholar]
- Schmid DA, Yang T, Ogier M, Adams I, Mirakhur Y, Wang Q, Massa SM, Longo FM, Katz DM. A TrkB small molecule partial agonist rescues TrkB phosphorylation deficits and improves respiratory function in a mouse model of Rett syndrome. J. Neurosci. 2012;32:1803–1810. doi: 10.1523/JNEUROSCI.0865-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal RA. Selectivity in neurotrophin signaling: theme and variations. Annu. Rev. Neurosci. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, Armstrong D, Paylor R, Zoghbi H. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002a;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- Shahbazian MD, Antalffy B, Armstrong DL, Zoghbi HY. Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum. Mol. Genet. 2002b;11:115–124. doi: 10.1093/hmg/11.2.115. [DOI] [PubMed] [Google Scholar]
- Skene PJ, Illingworth RS, Webb S, Kerr AR, James KD, Turner DJ, Andrews R, Bird AP. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol. Cell. 2010;37:457–468. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrt RD, Eaves-Egenes J, Barkho BZ, Santistevan NJ, Zhao C, Aimone JB, Gage FH, Zhao X. Mecp2 deficiency leads to delayed maturation and altered gene expression in hippocampal neurons. Neurobiol. Dis. 2007;27:77–89. doi: 10.1016/j.nbd.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Hu K, Chang Q, Wu H, Sherman NE, Martinowich K, Klose RJ, Schanen C, Jaenisch R, Wang W, Sun YE. Phosphorylation of MeCP2 at Serine 80 regulates its chromatin association and neurological function. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4882–4887. doi: 10.1073/pnas.0811648106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D, Giacometti E, Wilson NR, Beard C, McCurry C, Fu DD, Flannery R, Jaenisch R, Sur M. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2029–2034. doi: 10.1073/pnas.0812394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D, Kreiman G, Lyckman A, Mukherjee S, Yu H, Horng S, Sur M. Gene expression changes and molecular pathways mediating activity-dependent plasticity in visual cortex. Nat. Neurosci. 2006;9:660–668. doi: 10.1038/nn1689. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J. Neurosci. 2004;24:5603–5610. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor M, Akbarian S, Chen RZ, Jaenisch R. Transcriptional profiling of a mouse model for Rett syndrome reveals subtle transcriptional changes in the brain. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15536–15541. doi: 10.1073/pnas.242566899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn. Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhala R, Korhonen L, Mikelsaar M, Lindholm D, Riikonen R. Neurotrophic factors in cerebrospinal fluid and serum of patients with Rett syndrome. J. Child Neurol. 1998;13:429–433. doi: 10.1177/088307389801300903. [DOI] [PubMed] [Google Scholar]
- Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH, Impey S. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16426–16431. doi: 10.1073/pnas.0508448102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Chan SA, Ogier M, Hellard D, Wang Q, Smith C, Katz DM. Dysregulation of brain-derived neurotrophic factor expression and neurosecretory function in Mecp2 null mice. J. Neurosci. 2006;26:10911–10915. doi: 10.1523/JNEUROSCI.1810-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse EG, An JJ, Orefice LL, Baydyuk M, Liao GY, Zheng K, Lu B, Xu B. BDNF promotes differentiation and maturation of adult-born neurons through GABAergic transmission. J. Neurosci. 2012;32:14318–14330. doi: 10.1523/JNEUROSCI.0709-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat. Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Wood L, Gray NW, Zhou Z, Greenberg ME, Shepherd GM. Synaptic circuit abnormalities of motor-frontal layer 2/3 pyramidal neurons in an RNA interference model of methyl-CpG-binding protein 2 deficiency. J. Neurosci. 2009;29:12440–12448. doi: 10.1523/JNEUROSCI.3321-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Tao J, Chen PJ, Shahab A, Ge W, Hart RP, Ruan X, Ruan Y, Sun YE. Genome-wide analysis reveals methyl-CpG-binding protein 2-dependent regulation of microRNAs in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. U. S. A. 2010;107:18161–18166. doi: 10.1073/pnas.1005595107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Siao CJ, Nagappan G, Marinic T, Jing D, McGrath K, Chen ZY, Mark W, Tessarollo L, Lee FS, Lu B, Hempstead BL. Neuronal release of proBDNF. Nat. Neurosci. 2009;12:113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani M, Deogracias R, Guy J, Poot RA, Bird A, Barde YA. Disease modeling using embryonic stem cells: MeCP2 regulates nuclear size and RNA synthesis in neurons. Stem Cells. 2012;30:2128–2139. doi: 10.1002/stem.1180. [DOI] [PubMed] [Google Scholar]
- Zeev BB, Bebbington A, Ho G, Leonard H, de Klerk N, Gak E, Vecsler M, Christodoulou J. The common BDNF polymorphism may be a modifier of disease severity in Rett syndrome. Neurology. 2009;72:1242–1247. doi: 10.1212/01.wnl.0000345664.72220.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Tan M, Kohyama J, Sneddon M, Watson JB, Sun YE, Xie CW. Epigenetic enhancement of BDNF signaling rescues synaptic plasticity in aging. J. Neurosci. 2011;31:17800–17810. doi: 10.1523/JNEUROSCI.3878-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, Hu L, Steen JA, Weitz CJ, Greenberg ME. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocchi L, Sassone-Corsi P. Joining the dots: from chromatin remodeling to neuronal plasticity. Curr. Opin. Neurobiol. 2010;20:432–440. doi: 10.1016/j.conb.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocchi L, Sassone-Corsi P. SIRT1-mediated deacetylation of MeCP2 contributes to BDNF expression. Epigenetics. 2012;7:695–700. doi: 10.4161/epi.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]