Abstract
Background
Studies have demonstrated an enhanced dynorphin/kappa-opioid receptor (KOR) system following repeated cocaine exposure, but few reports have focused on neuroadaptations within the central amygdala (CeA).
Methods
We identified KOR-related physiological changes in the CeA following escalation of cocaine self-administration in rats. We used in vitro slice electrophysiological (intracellular and whole-cell recordings) methods to assess whether differential cocaine access in either 1h (short access, ShA) or 6h (long access, LgA) sessions induced plasticity at CeA GABAergic synapses, or altered the sensitivity of these synapses to KOR agonism (U50488) or antagonism (nor-BNI). We then determined the functional effects of CeA KOR blockade in cocaine-related behaviors.
Results
Baseline evoked GABAergic transmission was enhanced in the CeA from ShA and LgA rats compared to cocaine-naïve rats. Acute cocaine (1 uM) application significantly decreased GABA release in all groups (naïve, ShA, and LgA rats). Application of U50488 (1 uM) significantly decreased GABAergic transmission in the CeA from naïve rats, but increased it in LgA rats. Conversely, nor-BNI (200 nM) significantly increased GABAergic transmission in the CeA from naïve rats, but decreased it in LgA rats. Nor-BNI did not alter the acute cocaine-induced inhibition of GABAergic responses. Finally, CeA microinfusion of nor-BNI blocked cocaine-induced locomotor sensitization and attenuated the heightened anxiety-like behavior observed during withdrawal from chronic cocaine exposure in the defensive burying paradigm.
Conclusion
Together these data demonstrate that CeA dynorphin/KOR systems are dysregulated following excessive cocaine exposure and suggest KOR antagonism as a viable therapeutic strategy for cocaine addiction.
Keywords: Addiction, Anxiety, Central Amygdala, Cocaine, GABA, Kappa-Opioid Receptor
Introduction
Cocaine addiction is a chronic relapsing disorder characterized by compulsive cocaine intake, dysregulation of brain reward and stress systems, and persistent cocaine-seeking behavior (1, 2). Chronic cocaine exposure produces multiple neuroadaptations in limbic brain circuitry implicated in distinct aspects of the addiction cycle, while a recruitment of brain stress (or anti-reward) circuitry following extensive cocaine experience is thought to mediate a shift to negative reinforcement mechanisms underlying excessive cocaine use (3). Moreover, this transition may further drive compulsive drug-seeking behavior by facilitating the intersection of positive and negative motivational circuitry (4), including a potentiation of extended amygdala signaling (2, 5). For example, extended access to cocaine self-administration in rats produces profound motivational deficits (6) along with a recruitment of corticotropin-releasing factor (CRF) signaling in the central amygdala (CeA), a neuroadaptation thought to contribute to the production of withdrawal-induced negative affective states during withdrawal (7).
Extended withdrawal from cocaine exposure also potentiates CRF-mediated neurotransmission at lateral amygdala-to-CeA synapses (8), suggesting that CeA activity and drug-seeking behavior may be facilitated by stress-associated neuropeptide systems in the CeA. In this regard, there is substantial CeA co-localization of CRF and the endogenous opioid dynorphin (9), a neuropeptide hypothesized to drive negative reinforcement mechanisms in addiction (10, 11). With regard to their actions at neuronal opioid receptors, dynorphins, particularly dynorphin A, are preferential kappa opioid receptor (KOR) agonists (12). Mansour and colleges (13) found that KOR binding, while not as widespread as both mu and delta opioid receptor binding, was densely distributed in the nucleus accumbens (NAc) and CeA. Consistent with this observation, the KOR agonist salvinorin A increases Fos protein levels in both the NAc shell and CeA (14). Chronic KOR blockade can be achieved via a single administration of the long-acting antagonist nor-binaltorphimine (nor-BNI, 15). Importantly, nor-BNI attenuates progressive-ratio responding for cocaine in LgA animals (16), suggesting that dynorphin signaling mediates compulsive cocaine seeking during withdrawal, although the neural circuitry mediating this effect is currently unknown. It is believed that the influence of the dynorphin system on the rewarding properties of drugs of abuse is primarily mediated by its action in the NAc (17), where KOR activation inhibits dopamine (18) and glutamate (19) release. Here, we hypothesize that dysregulated KOR activity in the CeA, possibly in cooperation with CRF signaling, may contribute to the development of negative motivational states closely associated with withdrawal and protracted abstinence associated with cocaine addiction.
Little direct information is available regarding interactions between the dynorphin/KOR system and GABAergic transmission in the CeA in animals with extended access to cocaine self-administration. To address this question, using in vitro electrophysiological studies we compared basal GABAergic transmission in short access (ShA-1 hour cocaine access per day) and long access (LgA-6 hours access to cocaine per day) animals with cocaine-naïve controls to determine whether excessive cocaine administration might produce differential neuroadaptations in CeA GABAergic plasticity. We also examined the acute effects of applied cocaine and KOR ligands on evoked and spontaneous GABAergic transmission in CeA slices from cocaine-experienced versus naive animals. Finally, to link these neuroplastic changes to functional neuroadaptations, we determined the in vivo effects of intra-CeA KOR antagonism on two distinct and complementary models of cocaine addiction symptomatology: cocaine-induced locomotor sensitization and withdrawal-induced anxiety-like behavior. We found that acute cocaine significantly decreased GABAergic responses via decreasing GABA release in both naïve and LgA rats but not ShA rats. The application of U50488, a KOR agonist, significantly decreased, while nor-BNI, a KOR antagonist, significantly increased CeA GABAergic transmission in the CeA from naïve rats. Notably, application of U50488 significantly increased, while nor-BNI significantly decreased, CeA GABAergic transmission in LgA rats. In naïve rats, application of nor-BNI did not alter the acute cocaine-induced inhibition of GABAergic transmission, ruling out a direct mediation of cocaine effects by KORs. Finally, CeA microinfusion of nor-BNI blocked cocaine-induced locomotor sensitization and attenuated the heightened anxiety-like behavior observed during withdrawal from chronic cocaine exposure in the defensive burying paradigm. These combined electrophysiological and behavioral results demonstrate that the CeA dynorphin/KOR pathway is dysregulated following excessive cocaine exposure, and support KOR antagonism as a potential therapeutic strategy for cocaine addiction.
Methods and Materials
Detailed methods are provided in the Supplemental Information.
In vitro brain slice preparation
We prepared in vitro brain CeA slices (300–400 μm thick) as previously described (20–22) from male Wistar rats (556.9 ± 16 g) that were anesthetized with isoflurane (1–3%) and decapitated during withdrawal from cocaine self-administration (methods described in the Supplemental Information).
Intracellular recordings
We recorded from CeA neurons (primarily from the medial subdivision of the CeA) with sharp micropipettes filled with 3M KCl using discontinuous current-clamp mode (21). We held most neurons near their resting membrane potential (RMP), acquired the data with an Axoclamp-2A preamplifier (Axon Instruments, Foster City, CA) and analyzed using pClamp software (Axon Instruments, Foster City, CA). We evoked pharmacologically isolated GABAA receptor-mediated inhibitory postsynaptic potentials (IPSPs) by stimulating locally within the CeA through a bipolar stimulating electrode while superfusing the slices with the glutamate receptor blockers 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 μM) and DL-2-amino-5-phosphonovalerate (APV; 30 μM), and the GABAB receptor antagonist (CGP 55845A; 1 μM) (21) (20).
Whole-cell patch-clamp recording of miniature IPSCs
We recorded from CeA neurons visualized in brain slices using infrared differential interference contrast (IR-DIC) optics and a CCD camera (EXi Aqua, QImaging) (22–23). Whole-cell voltage-clamp recordings were made with a Multiclamp 700B amplifier (Molecular Devices), low-pass filtered at 2–5kHz, digitized (Digidata 1440A; Molecular Devices), and stored on a PC using pClamp 10 software (Axon Instruments). Patch pipettes (4–8MΩ) were pulled from borosilicate glass (Warner Instruments) and filled with an internal solution composed of (in mM): 145 KCl; 0.5 EGTA; 2 MgCl2; 10 HEPES; 2 Na-ATP; 0.2 Na-GTP. GABAergic miniature IPSCs (mIPSCs) were recorded in the presence of 10 μM CNQX, 30 μM APV, 1 μM CGP 55845A, and 1 μM tetrodotoxin (TTX). Frequency, amplitude, and kinetics of miniature IPSCs were analyzed using a semi-automated, threshold-based mini detection software (Mini Analysis, Synaptosoft Inc., Fort Lee, NJ).
Cocaine-induced locomotor sensitization and withdrawal-related behaviors
Two separate cohorts of animals were injected chronically with cocaine (20 mg/kg, IP) following intra-CeA administration of the KOR antagonist nor-BNI. Measures of locomotion and defensive burying were recorded as described in the Supplemental Information.
Statistical analysis
Self-administration data were analyzed using repeated measures one-way analysis of variance (ANOVA) with Dunnett’s post-hoc test. We used t-test analyses for individual mean comparisons and within-subject one-way repeated measures (RM) ANOVA to compare IPSPs within a group. When appropriate, the Student Newman-Keuls post hoc test was used to assess significance between treatments. To assess differences resulting from cocaine treatment and drug interaction between groups, we used two-way repeated-measures ANOVA. Statistical significance was set at p < 0.05. Averaged values are presented as mean ± SEM.
Results
Baseline evoked CeA GABAergic responses are elevated in cocaine self-administering animals
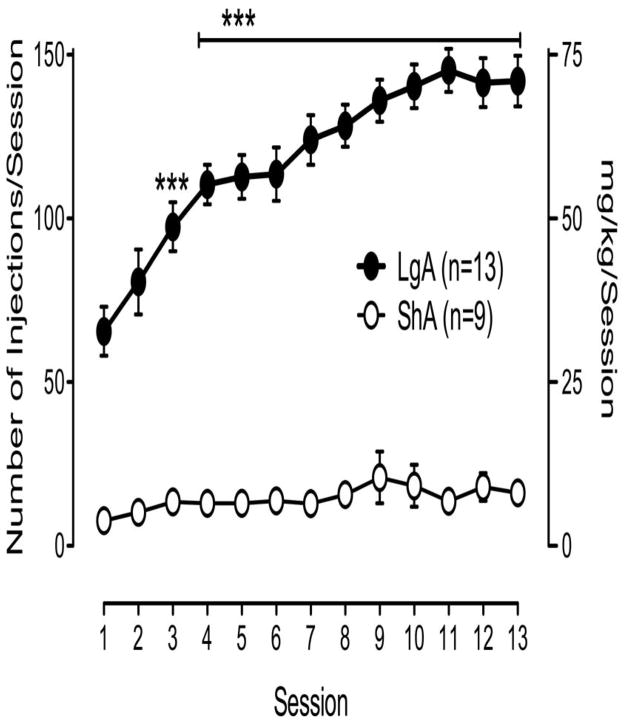
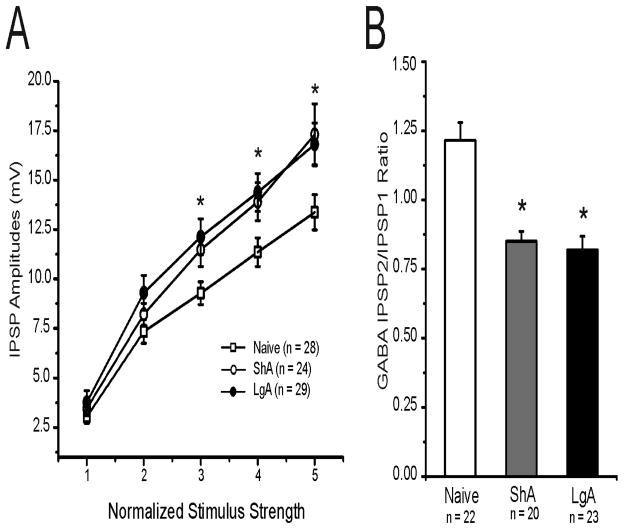
Cocaine self-administration in LgA rats significantly escalated with extended access and reached the maximum within 11 sessions [F (17,233)=25, p < 0.001] (Fig. 1). ShA rats did not display a significant increase in cocaine self-administration [F (12,96)=1.8, p > 0.05]. One day following the last cocaine self-administration, animals were sacrificed for electrophysiological analysis. We first examined whether there were any differences in baseline GABA release in animals with differential experience with cocaine self-administration. We recorded intracellularly from a total 94 CeA neurons with a mean RMP of −78.5 ± 0.9 mV and a mean input resistance of 115.3 ± 3.9 MΩ. We did not observe significant differences in membrane properties (see Supplement: Table S1), and there were also no significant differences in voltage–current relationships (not shown). We evoked pharmacologically isolated GABAA-IPSPs (IPSPs) by stimulating locally within the CeA. Baseline IPSP input–output curves generated by equivalent stimulus intensities were significantly higher [F (2,78) = 1.81, p < 0.05] in slices from both self-administering groups compared to those from cocaine-naive animals (Fig. 2A), suggesting an increased efficiency of GABAergic transmission after cocaine self-administration. We examined paired-pulse facilitation (PPF) of the IPSPs to assess pre- versus postsynaptic mechanisms. One-way ANOVA revealed a significant difference in the baseline PPF ratios from both ShA and LgA groups compared to cocaine-naive animals [(F (2,62) = 19.37, p < 0.001)] (Fig. 2B), suggesting an increased baseline evoked GABA release.
Figure 1.
Cocaine self-administration in LgA (6h/d) and ShA (1h/d) rats. LgA rats displayed a significant escalation of intake over repeated cocaine self-administration sessions (***p < 0.001). Data are expressed as mean ± SEM of the number of cocaine infusions/session on the left axis and mg/kg cocaine intake on the right axis.
Figure 2.
Basal evoked GABAergic transmission is enhanced in ShA and LgA rats. A: Input-output curves of mean GABAA-IPSP amplitudes. Mean baseline GABAergic transmission is significantly (*p < 0.05) increased in neurons from ShA (n = 24) and LgA (n = 29) animals compared with naive rats (n = 28). B: Histograms plotting the baseline PPF ratio of IPSPs in CeA neurons from naïve, ShA, and LgA rats. In the ShA (n = 22) and LgA (n = 20) groups, baseline PPF ratios were significantly (*p < 0.05) lower versus naïve (n = 23).
Acute cocaine superfusion decreases CeA GABAergic transmission
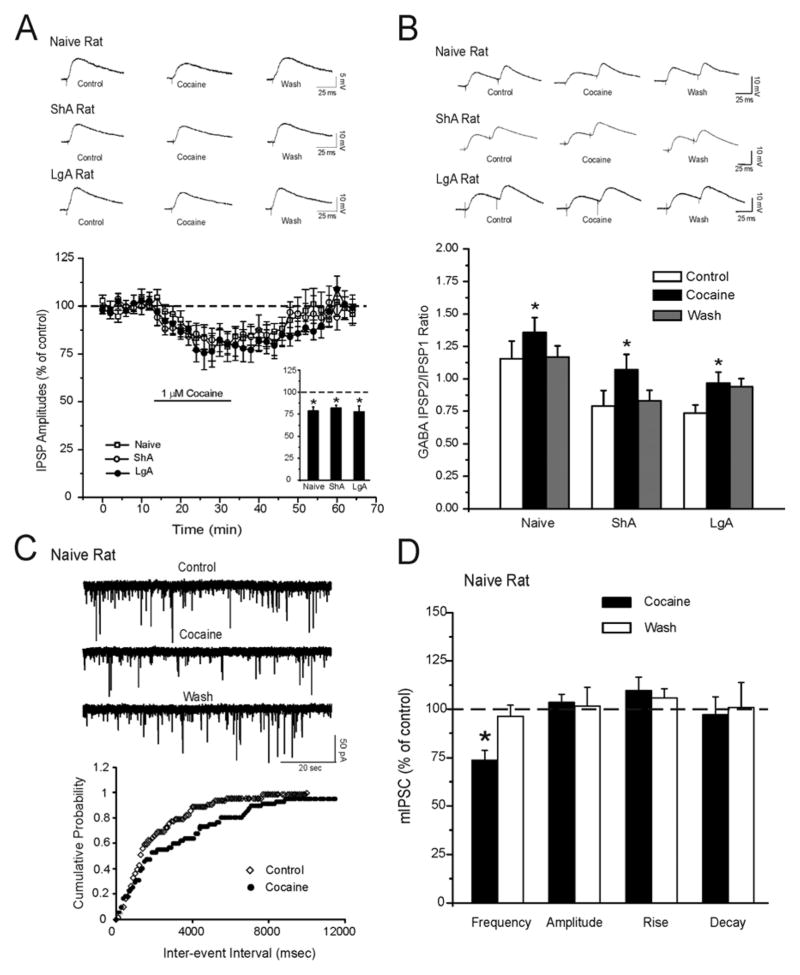
We next determined the effect of acute superfusion (10–20 min) of 1 uM cocaine (24–26) on GABAergic transmission in CeA neurons from naïve, ShA, and LgA animals. We used two protocols: 1) input-output (I/O) relationships, and 2) averages of 4 consecutive responses at 50% maximal amplitude determined from the I/O relationship (Fig. 3A). In cocaine-naïve rats, we found that acute cocaine superfusion significantly (p < 0.05; n=7) decreased by 20% the mean evoked CeA IPSP amplitude measured over all stimulus strengths with recovery upon 20–25 minutes of washout (Fig. 3A). In CeA neurons from naïve rats, 10 uM or 500 nM cocaine induced an irreversible (by 50%; n = 5) or a slight (by 10 %; n = 6) decrease in GABAergic transmission, respectively (data not shown). In 11 and 10 CeA neurons from ShA and LgA rats, similar to naïve rats, cocaine significantly (p < 0.05) decreased evoked IPSPs by 20% (Fig. 3A). In all animal groups, acute cocaine also significantly (p < 0.05) increased the 50 msec PPF ratios of IPSPs in the majority of CeA neurons tested (Fig. 3B), suggesting decreased GABA release. To confirm that cocaine decreases presynaptic release of GABA in the CeA, we recorded spontaneous miniature IPSCs (mIPSCs) using whole-cell patch-clamp in the presence of 1 μM TTX to eliminate action potential-dependent release of neurotransmitters. In CeA neurons from a naïve rat, cocaine superfusion significantly (p < 0.01) decreased the mean mIPSC frequency to 73.8 ± 5.1% of control (Fig. 3C and D) and shifted the cumulative frequency distribution to longer inter-event intervals, further indicating decreased presynaptic GABA release. Cocaine had no effect on mean mIPSC amplitudes (p > 0.05; control: 83.5 ± 7 pA; cocaine: 87.8 ± 10 pA; Fig. 3D), decay (p > 0.05; control: 2.04 ± 0.2 msec; cocaine: 2.16 ± 0.2 msec), or rise (p > 0.05; control: 1.30 ± 0.2 msec; cocaine: 1.22 ± 0.2 msec) time of mIPSCs (Fig. 3D). In 10 CeA neurons from ShA rats, the decrease induced by acute cocaine in mean mIPSC frequency was similar to that obtained in cocaine-naïve rats (Supplement: Figure S1).
Figure 3.
Acute application of cocaine decreases baseline evoked and spontaneous GABAergic transmission. A: Top Panel: Representative evoked IPSPs recorded before, during cocaine (1 μM) application, and washout in naïve, ShA, and LgA rats. Bottom Panel: Pooled data of the experiments from A: time course depicting changes in evoked IPSP amplitude upon cocaine application and washout in the three groups. Insert: Histograms representing maximal percent decrease in mean (± SEM) evoked IPSP amplitudes averaged over the middle three stimulus strength intensities tested with cocaine application. *Indicates p < 0.05. B: Top Panel: Representative recordings of evoked PPF of IPSPs in CeA neurons from naïve, ShA, and LgA rats. Bottom Panel: Cocaine significantly (*p < 0.05) increases the PPF ratio of IPSPs in the CeA from naïve and LgA rats. C: Top Panel: Representative mIPSC recordings in CeA neurons from a cocaine-naïve rat during control, application of 1 uM cocaine, and washout. Cocaine decreased mIPSC frequency and (bottom panel) shifted the cumulative frequency histogram to the right, indicating a longer inter-event interval (lower frequencies) during cocaine application. D: Mean ± SEM frequency, amplitude, rise, and decay time of mIPSCs for CeA neurons. Cocaine significantly (*p < 0.05) decreases the mean mIPSC frequency but does not change mean mIPSC amplitude, rise, or decay time.
KOR antagonism does not block the decrease of GABAergic transmission induced by acute cocaine application
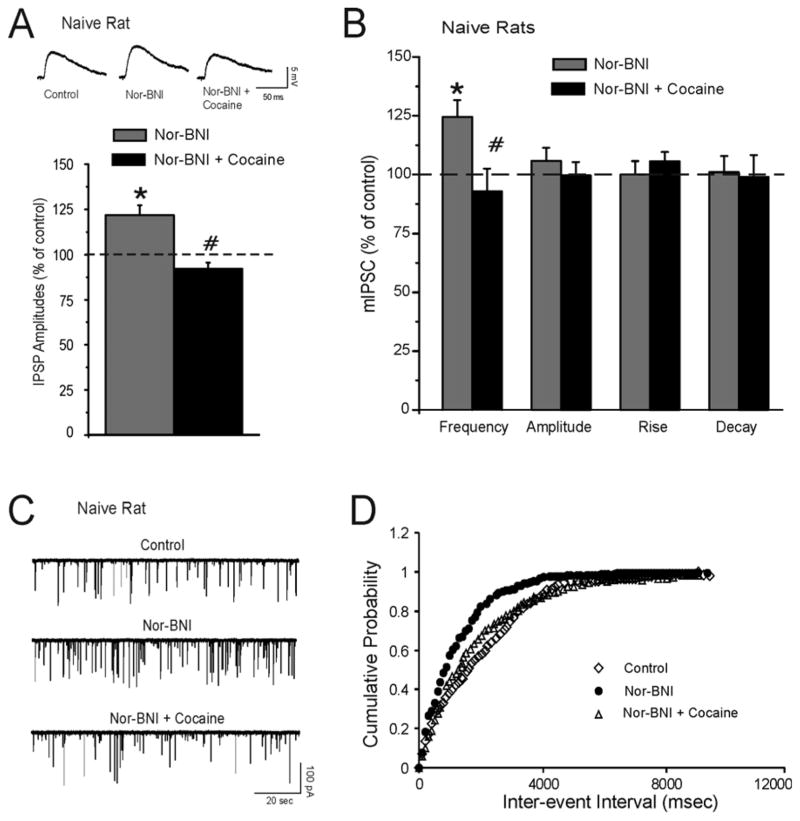
In this set of experiments, we tested whether blockade of KOR with 200 nM nor-BNI would affect cocaine-induced decreases in GABAergic responses. We found that nor-BNI alone significantly (p < 0.05; n = 7) increased evoked IPSPs to 121.7 ± 5.9% of control (averaged over all stimulus strengths; Fig. 4A), and the addition of cocaine significantly (p < 0.05) decreased IPSP amplitudes to 92.0 ± 9.7% of control (Fig. 4A). In another 7 CeA neurons, nor-BNI (200 nM) significantly (p < 0.05) increased the frequency of mIPSCs to 124.8 ± 5.1% of control (Fig. 4B, C and D) and shifted the cumulative frequency distribution to shorter inter-event intervals, indicating increased presynaptic GABA release. Cocaine (1uM) in the presence of nor-BNI significantly (p < 0.05) decreased mIPSCs frequency (Fig. 4B and C) and shifted the cumulative frequency to longer inter-event intervals (Fig. 4D). Neither nor-BNI nor cocaine altered the amplitudes (p > 0.05; control: 55.7 ± 9.5 pA; nor-BNI: 61.8 ± 14.0 pA; cocaine in nor-BNI: 57.7± 6.7), decay (p > 0.05; control: 2.01 ± 0.15 msec; nor-BNI: 1.97 ± 0.11 msec; cocaine in nor-BNI: 2.09 ± 0.18), or rise (p > 0.05; control: 3.3 ± 0.9 msec; nor-BNI: 3.6 ± 1.2 msec; cocaine in nor-BNI: 3.2 ± 0.9 Fig. 4B) time of mIPSCs. The cocaine-induced decrease (by 20–25%) in GABAergic responses was comparable in the presence or absence of nor-BNI. Thus, application of nor-BNI did not alter cocaine-induced inhibition of IPSPs, ruling out a direct mediation of cocaine effects by KORs.
Figure 4.
KOR antagonism does not block the decrease of GABAergic transmission induced by acute cocaine application. A: Top Panel: Representative evoked IPSPs recorded in control, nor-BNI (200 nM), and cocaine (1 μM) plus nor-BNI in the CeA of a cocaine-naïve rat. Bottom Panel: Histograms representing maximal percent decrease in mean (± SEM) evoked IPSP amplitudes averaged over the middle three stimulus strength intensities tested during the experimental protocol. Nor-BNI increased evoked IPSP amplitudes while cocaine reduced them (n = 7). *Indicates p < 0.05 between nor-BNI and control; # Indicates p < 0.05 between cocaine plus nor-BNI and nor-BNI alone. B: Mean ± SEM frequency, amplitude, rise, and decay time of mIPSCs for CeA neurons. Nor-BNI significantly (*p < 0.05) increased the mean mIPSC frequency while cocaine in the presence of nor-BNI significantly (#p < 0.05) decreased it. No changes in mean mIPSC amplitude, rise, or decay time were observed. C: Top Panel: Representative mIPSC recordings in CeA neurons from a cocaine-naïve rat during control, application of 200 nM nor-BNI, and 1 uM cocaine plus nor-BNI. D: Nor-BNI shifted the cumulative frequency histogram to the left, indicating a shorter inter-event interval (higher frequencies) during nor-BNI application. Cocaine decreased mIPSC frequency and shifted the cumulative frequency histogram to the right, indicating a longer inter-event interval (lower frequencies) during cocaine application.
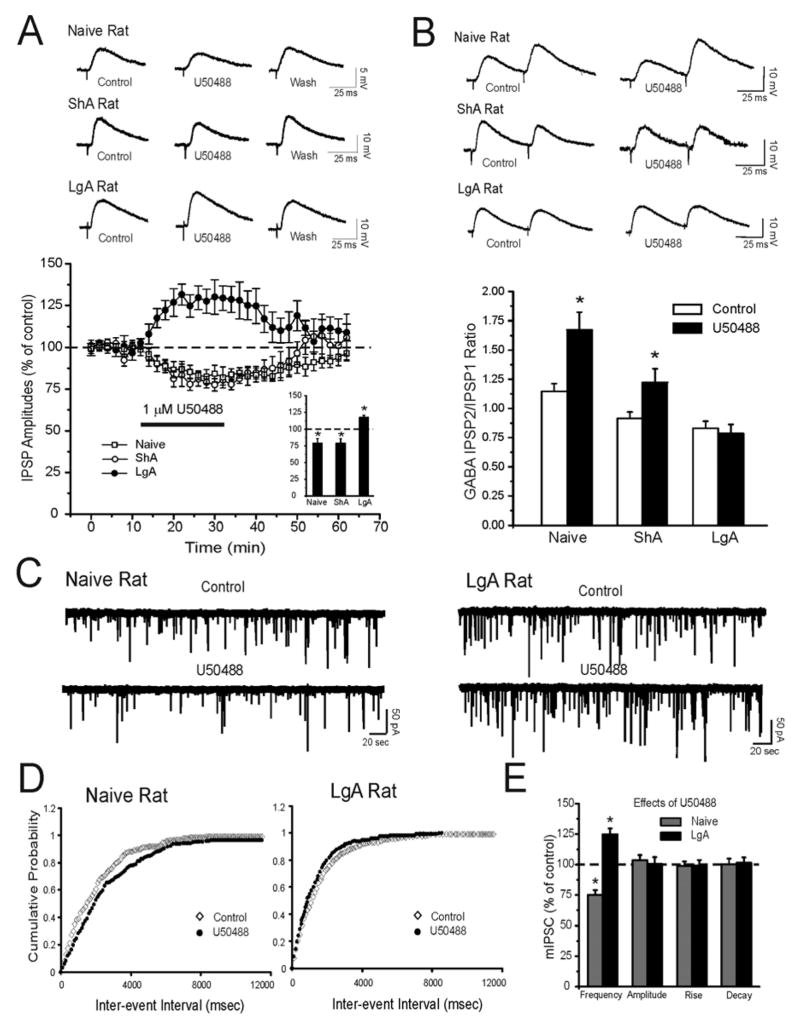
KOR activation produces a differential effect on GABAergic transmission in cocaine-naïve and ShA vs. LgA animals
We next assessed the impact of the KOR selective agonist U50488 on CeA GABAergic transmission. In CeA cells tested from cocaine-naïve rats, we found that bath application of U50488 (1 uM for 15–20 min) significantly (p < 0.05; n = 8) decreased the peak amplitude of the evoked IPSP to 80 ± 6% of baseline (Fig. 5A). Similar to naïve rats, in 8 CeA neurons from ShA rats, U50488 significantly (p<0.05) decreased evoked IPSPs (Fig. 5A). Remarkably, the typical U50488-induced inhibition of GABAergic transmission switched to a facilitation of GABAergic transmission in LgA rats (Fig. 5A). Thus, in CeA neurons from LgA rats, U50488 significantly (p < 0.05) increased evoked IPSPs. These effects were reversible upon 30 minutes of washout (Fig. 5A). In both cocaine-naïve and ShA rats, U50488-induced decreases of evoked IPSPs correlated with significant (p < 0.05) increases in PPF ratios, suggesting decreased GABA release (Fig. 5B). In CeA neurons from LgA animals, the KOR agonist did not alter the PPF ratios of IPSPs (Fig. 5B). A presynaptic inhibition of vesicular GABA release as a predominant effect of KOR activation was further confirmed by our mIPSC results. The mean frequency of mIPSCs was significantly (p < 0.01; n =11) reduced by 25% ± 4% of the baseline in CeA neurons from naïve rats. U50488 had no effect on mean mIPSC amplitudes (p > 0.05; control: 67.5 ± 3.1 pA; U50488: 64.0 ± 2.4 pA; Fig. 5C and E), decay (p > 0.05; control: 2.06 ± 0.1 msec; U50488: 2.04 ± 0.12 msec), or rise (p > 0.05; control: 4.46 ± 0.4 msec; U50488: 4.40 ± 0.4 msec) time of mIPSCs (Fig. 5C and E). The application of U50488 significantly shifted the cumulative frequency distribution to longer inter-event intervals in CeA neurons of naïve rats (Fig. 5D, Left Panel). We also found that U50488 significantly (p < 0.05) increased the frequency of mIPSCs in CeA cells from LgA rats. The cumulative frequency distribution was shifted to the right in CeA neurons of LgA rats (Fig. 5D, Right Panel). The KOR agonist did not affect the mean mIPSC amplitudes (p > 0.05; control: 76.2 ± 9.0 pA; U 50488: 75.6 ± 8.1 pA; Fig. 5C and E), decay (p > 0.05; control: 2.12 ± 0.2 msec; U50488: 2.11 ± 0.2 msec), or rise (p > 0.05; control: 4.85 ± 0.8 msec; U50488: 4.96 ± 0.7 msec) time of mIPSCs (Fig. 5C and E).
Figure 5.
Extended access to cocaine self-administration inverts the effects of a KOR agonist (U50488) on evoked and spontaneous CeA GABAergic transmission. A: Top Panel: Representative evoked IPSPs recorded before and during U50488 (1 uM) superfusion and washout in naïve, ShA, and LgA rats. Bottom Panel: Pooled data of the experiments from A: time course depicting changes in evoked IPSP amplitude upon U50488 application and washout in the three groups. In naive and ShA rats, U50488 significantly decreased evoked IPSPs. In contrast, U50488 increases evoked IPSPs in neurons from LgA rats. Insert: Histograms representing percent decrease in mean (± SEM) evoked IPSP amplitudes averaged over three stimulus strength intensities tested with U50488 application. *Indicates p < 0.05. B: Top Panel: Representative evoked PPF of IPSPs from CeA neurons of naïve, ShA, and LgA rats. Bottom Panel: U50488 significantly (*p < 0.05) increases the PPF ratio in CeA from naïve and ShA, but not LgA rats. C: Representative mIPSC recordings in CeA neurons from naïve and LgA rats. U50488 decreases the mIPSC frequency in the CeA from naïve rats but increased frequencies in LgA rats. D: Upon U50488 application the cumulative frequency histogram for the same neuron of C was shifted to the right, indicating lower frequencies in CeA neurons of naïve rats. U50488 shifted to the left the cumulative frequency histogram, indicating higher frequencies in CeA neurons of LgA rats. E: Mean ± SEM frequency, amplitude, rise, and decay time of mIPSCs for 9 and 7 CeA neurons from naïve and LgA rats, respectively. In naive rats, U50488 significantly (*p < 0.05) decreased the mean mIPSC frequency. In LgA rats, U50488 significantly (*p < 0.05) increased the mean mIPSC frequency. U50488 did not alter the mean mIPSC amplitude, rise, or decay time in the two groups. Also see Supplement: Figure S2.
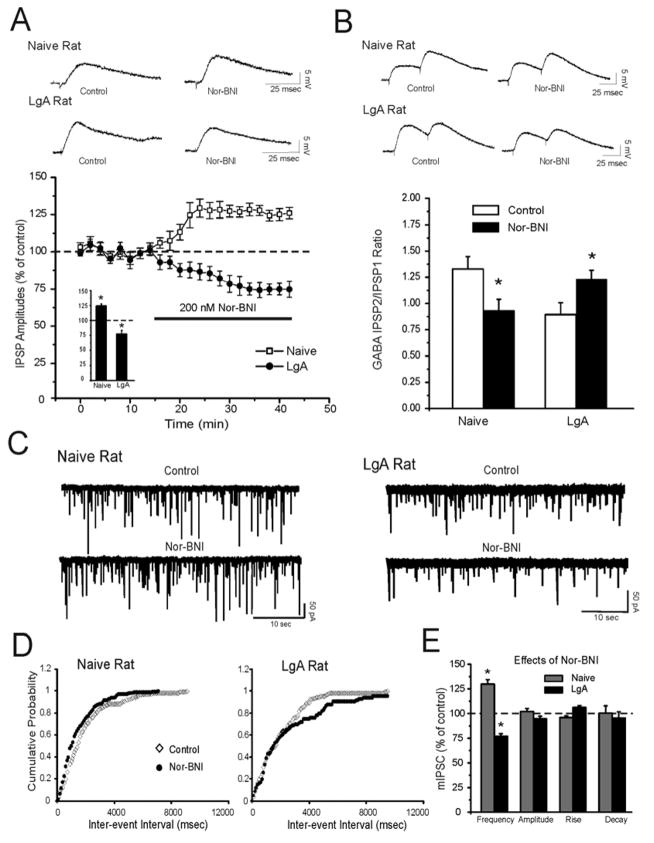
KOR antagonism leads to a presynaptic activation of CeA GABAergic transmission in cocaine-naïve rats, but to an inhibition in cocaine LgA rats
To assess tonic KOR activation we applied 200 nM nor-BNI for 20 minutes onto CeA neurons from naïve rats. We found that nor-BNI significantly increased the evoked basal IPSP amplitude (124.4 ± 3% of the baseline; n=10; Fig. 6A) in CeA neurons. This increase in evoked basal IPSP amplitude was associated with a significant (p < 0.05) decrease in PPF ratios, suggesting a tonic inhibition of GABA release (Fig. 6B). Nor-BNI also significantly (p < 0.05) increased mIPSC frequencies (Fig. 6C and E; n=12) and shifted the cumulative frequency to shorter inter-event intervals (Fig. 6D, Left Panel). Nor-BNI did not alter the amplitudes (p > 0.05; control: 64.5 ± 3.7 pA; nor-BNI: 67.4 ± 4.2 pA), decay (p > 0.05; control: 2.23 ± 0.16 msec; nor-BNI: 2.14 ± 0.16 msec), or rise (p > 0.05; control: 3.7 ± 0.6 msec; nor-BNI: 3.8 ± 0.6 msec Fig. 6C and E) time of mIPSCs. In contrast, in 8 CeA neurons from LgA cocaine rats, nor-BNI significantly (p < 0.05) decreased by 20–25% the amplitude of evoked IPSPs (Fig. 6A) and significantly (p < 0.05) increased the PPF ratio of IPSPs (Fig. 6B). Nor-BNI also significantly (p < 0.05; n=12) decreased the frequency of mIPSCs in CeA from LgA rats and shifted the cumulative frequency to longer inter-event intervals (Fig. 6D, Right Panel). The amplitudes (p > 0.05; control: 72.4 ± 5.1 pA; nor-BNI: 67.4 ± 4.2 pA), decay (p > 0.05; control: 2.27 ± 0.11 msec; nor-BNI: 2. 4 ± 0.14 msec), or rise (p > 0.05; control: 4.98 ± 0.4 msec; nor-BNI: 4.87 ± 0.5 msec) time of mIPSCs was not altered by nor-BNI (Fig. 6C and E).
Figure 6.
Long access cocaine administration inverts the effects of a KOR antagonist (nor-BNI) on evoked and spontaneous GABAergic transmission in the CeA. A: Top Panel: Representative evoked IPSPs recorded before and during superfusion of nor-BNI (200 nM) and washout in naïve and LgA rats. Bottom Panel: Pooled data of the experiments from A: time course depicting changes in evoked IPSP amplitude upon nor-BNI application and washout in the two groups. In naive rats, nor-BNI significantly (p < 0.05) increases evoked IPSP amplitudes. In contrast, nor-BNI significantly (p < 0.05) decreases evoked IPSP amplitudes in neurons from LgA rats. Insert: Histograms representing percent decrease in mean (± SEM) evoked IPSP amplitudes during nor-BNI application. *Indicates p < 0.05. B: Top Panel: Representative recordings of evoked 50 msec paired-pulse IPSPs in representative CeA neurons from naïve and LgA rats. Bottom Panel: nor-BNI significantly (*p < 0.05) decreases the 50 msec PPF ratio of IPSPs in CeA neurons from naïve rats, but increases ratios in 8 CeA neurons from LgA rats. C: Representative mIPSC recordings in CeA neurons from naïve and LgA rats. Superfusion of nor-BNI increases the mIPSC frequency in the CeA of naïve rats and decreases frequency in LgA rats. D: Upon nor-BNI application, the cumulative frequency histogram for the same neuron of C was shifted to the left, indicating higher frequencies in neurons of naïve rats (Left Panel). Nor-BNI shifted the cumulative frequency histogram to the right, indicating lower frequencies in neurons of LgA rats (Right Panel). E: Mean ± SEM frequency, amplitude, rise, and decay time of mIPSCs for 9 and 7 CeA neurons from naïve and LgA rats, respectively. In naive rats, nor-BNI significantly (*p < 0.05) increased mean mIPSC frequency, while in LgA rats, nor-BNI significantly (*p < 0.05) decreased mean mIPSC frequency. Nor-BNI did not alter the mean mIPSC amplitude, rise, or decay time in the two groups. Also see Supplement: Figure S2.
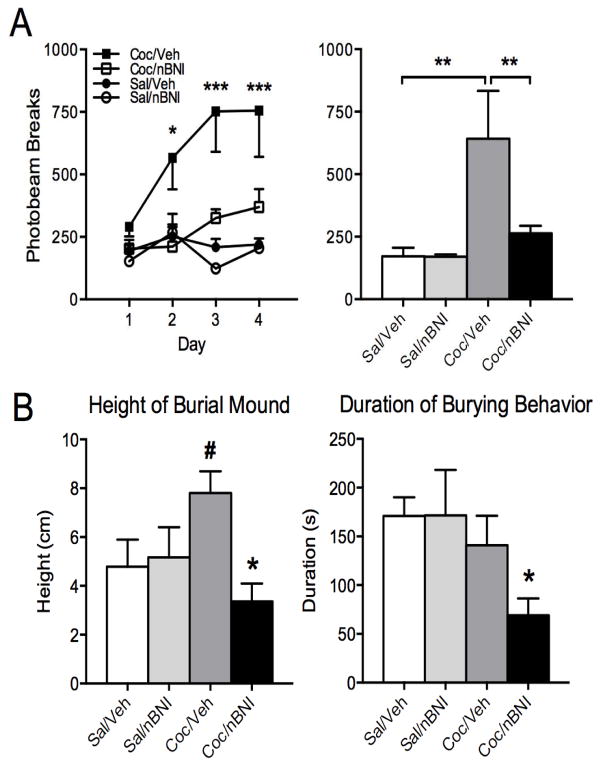
Intra-CeA injection of nor-BNI attenuates both cocaine-induced locomotor sensitization and cocaine withdrawal-induced anxiety-like behavior
Repeated cocaine produced progressively greater increases in locomotor activity over the consecutive days of testing in comparison to chronic saline (Fig. 7A left panel, group x day interaction, F (9,51)=2.159, p=0.041), while intra-CeA nor-BNI markedly abolished these behavioral effects of cocaine (Also see Supplement: Figure S3). Even after the fifth challenge with 20 mg/kg cocaine, increases in cocaine-stimulated locomotor activation were still prevented in the cocaine/nor-BNI group (Fig. 7A right panel, group effect, F (3,17)=6.271, p=0.005). Since withdrawal from chronic cocaine exposure produces anxiety-like behavior as revealed by the defensive burying procedure, we next determined whether the CeA dynorphin/KOR system plays a role in mediating this behavior. Forty-eight hours after the last saline or cocaine injection, animals were subjected to the defensive burying test to evaluate the anxiogenic-like effects of a shock-paired stimulus following the treatment regimens. ANOVA revealed an overall treatment effect on the height of the bedding material [F (3,36)=3.48, p < 0.05 (0.025)]. Neuman-Keuls analysis indicated that cocaine/vehicle-treated animals displayed more anxiety-like behavior (indicated by a significantly increased height of burial mound) compared to control animals (p < 0.05). Neuman-Keuls testing also revealed that intra-CeA nor-BNI attenuated the anxiogenic-like effects of cocaine withdrawal compared to intra-CeA vehicle (p < 0.05) (Fig. 7B). Furthermore, ANOVA revealed an overall group effect on the total duration of burying [F (3,36) =3.18; p < 0.05 (=0.036)], with cocaine/nor-BNI-treated animals displaying a significantly attenuated total time burying (i.e., an anxiolytic-like effect) compared to the cocaine/saline-treated group (p < 0.05) (Fig. 7B).
Figure 7.
Blockade of cocaine sensitization and withdrawal-related behaviors by prior CeA kappa-opioid receptor (KOR) antagonism. A: Repeated cocaine produced progressively greater increases in locomotor activity in comparison to chronic saline (Bonferroni tests, *p < 0.05, ** p <0.001), while intra-CeA nor-BNI markedly abolished these effects of cocaine. Even after the fifth challenge, the single nor-BNI pretreatment still prevented increases in cocaine-induced locomotion (Neuman-Keuls tests, **p < 0.01). B: Forty-eight hours after the last saline or cocaine injection, animals were subjected to the defensive burying test to evaluate the anxiogenic-like effects of a shock-paired stimulus following the treatment regimens. Cocaine/vehicle-treated animals displayed more anxiety-like behavior (indicated by a significantly increased height of burial mound) compared to control animals (# p < 0.05). Intra-CeA nor-BNI administered prior to cocaine exposure attenuated the anxiogenic effects of cocaine withdrawal compared to intra-CeA vehicle (*p < 0.05, Neuman-Keuls test). Cocaine/nor-BNI-treated animals also displayed a significantly attenuated total time burying (i.e., an anxiolytic-like effect) compared to the cocaine/saline-treated group (*p < 0.05, Neuman-Keuls test).
Discussion
Chronic cocaine dysregulates CeA GABAergic transmission and KOR modulation of CeA synapses
We examined neuroadaptations occurring in the CeA, a principal component of extended amygdala circuitry well known for its functional position at the interface of negative reinforcement and drug addiction (3). In particular, the GABAergic system in the CeA is thought to play an important role in the manifestation of anxiety-like behaviors associated with drug dependence. This is the first study to demonstrate that acute cocaine application decreases both basal evoked and spontaneous GABA transmission primarily via a presynaptic decrease in GABA release. We found increased baseline evoked GABAergic responses in CeA neurons from both ShA and LgA cocaine self-administering rats compared to naïve controls. The increased GABAergic transmission was associated with a significant increase in PPF ratios of evoked GABA IPSPs, suggesting increased GABA release. Thus, similar to ethanol-dependent rats (21, 27), rats displaying compulsive increases in cocaine self-administration exhibit increased GABAergic tone in the CeA. Despite the enhanced baseline evoked GABAergic responses, we did not observe enhanced spontaneous GABAergic transmission in the CeA of cocaine-exposed rats. These data suggest that while the basal probability of evoked GABA release was affected by cocaine exposure, the processes involved in action potential-independent release of GABA were not altered. For example, in the action potential-independent recording configuration, vesicular release could be affected by a readily releasable pool of transmitter but not by circuit network activity. Our data are in agreement with the growing body of literature suggesting that the processes leading to mIPSCs and evoked IPSPs are not always coordinately regulated (28–30).
Our data suggest dramatic neuroadaptations occurring with prolonged cocaine intake at CeA GABA synapses and that the observed “switch” in the effects of KOR ligands most likely occurs at the presynaptic level. There are several possible mechanisms that could underlie this opposing KOR modulation of GABAergic responses. We speculate that in the CeA of cocaine-experienced animals, increased dynorphin levels might strongly and persistently stimulate KORs, resulting in substantial alterations in KOR-coupled signaling (31) involved in presynaptic GABA release mechanisms. One possible target may be synapsin I, a presynaptic phosphoprotein that regulates the mobilization of synaptic vesicles, and synapsin I phosphorylation is increased in the CeA of cocaine self-administering animals (32). Heightened CeA dynorphin activity acting through our observed neuroadaptation in KOR signaling would appear to facilitate GABAergic transmission similar to, and possibly additive with, CRF activation of CeA GABAergic signaling (27), aligning these two brain stress neuropeptide systems to possibly synergize in the production of negative motivational states driving compulsive cocaine intake (Supplement: Figure S4). In contrast, blockade of this KOR signal via nor-BNI appears to blunt CeA GABAergic signaling, which may not only preclude the effects of dynorphin but may also dampen the effects of a potentiated CRF system on GABA activity and facilitation of negative motivational processes. This process may be facilitated due to parallel actions of CRF and dynorphin, or as a result of CRF driving dynorphin release/activity as occurs in multiple brain regions (33–35).
Importantly, we found that nor-BNI does not block acute cocaine-induced decreases in evoked and spontaneous GABA responses, ruling out a direct involvement of KORs in acute cocaine actions. We speculate that these acute cocaine actions are complex and may involve multisynaptic circuit actions. Future studies will shed light on the precise cellular mechanisms of acute cocaine physiology in the CeA. Interestingly, a recent study found that central KOR antagonism attenuated cocaine withdrawal-associated anhedonia-like behavior only if administered prior to, but not during, a binge cocaine regimen (36), suggesting an important role for dynorphin/KOR signaling in early neuroplastic mechanisms that eventually foster the development of cocaine intake escalation and addiction. This hypothesis is further supported by our current experimental design and results (Figure 7). However, Wee et al. (16) also demonstrated the efficacy of nor-BNI to block progressive (but not fixed) ratio responding for cocaine in escalated animals, suggesting that KOR blockade may remain capable of attenuating motivation for cocaine even after the establishment of the addicted state. Altogether, these findings suggest a more complex role for KOR signaling in addiction beyond a simple interaction with acute cocaine pharmacology.
Role of central amygdala dynorphin/KOR activity in cocaine addiction-related symptomatology
Several lines of evidence have suggested a potential recruitment of endogenous dynorphin/KOR signaling in cocaine addiction (35, 37). Increases in prodynorphin gene expression (38–40), dynorphin immunoreactivity (41–42), KOR gene expression (43), and KOR density (44) are found following a history of cocaine administration, primarily in the striatum. Increases in dynorphin levels and function have been proposed to underlie the negative emotional states (45–47) and dysregulated motivational processes (10) driven by excessive cocaine exposure. Kreek and colleagues (11) have recently proposed roles for magnified KOR signaling throughout distinct stages of the addiction cycle along with proposed therapeutic strategies of KOR antagonism during early withdrawal (to alleviate dysphoria and anxiety) and KOR partial agonism during protracted withdrawal (to diminish the propensity for future relapse or re-escalation of intake via buffering of both KOR and dopamine signaling).
Our results suggest that CeA KOR signaling may substantially influence amygdalo-striatal circuitry to alter cocaine sensitization processes. Our findings, at first glance, appear to conflict with studies showing that KOR agonism also blocks cocaine locomotor sensitization (see 48). However, both KOR agonists (49) and antagonists (16) modify cocaine reward, although KOR antagonism specifically blocks progressive ratio responding in LgA (but not ShA) animals while, in contrast, KOR agonism only reduces low-dose (i.e., ascending limb) cocaine self-administration. Thus, KOR-mediated regulation of addiction-like behaviors would appear to depend heavily on the extent and context of cocaine exposure, with KOR stimulation capable of blocking the rewarding effects of limited cocaine experiences and KOR antagonism becoming effective only in the context of excessive motivation for cocaine typically associated with addicted phenotypes (50–52). The latter scenario may reflect a greater or more sustained activation of dynorphin systems, and it is known that chronic KOR stimulation can potentiate cocaine’s locomotor effects in distinct environments (14). The fact that both KOR agonists and antagonists can differentially block cocaine-associated behaviors depending on magnitude of exposure and context suggests a dynamic balance of limbic KOR signaling that may become dysregulated as the addiction timeline progresses (10, 31).
Supplementary Material
Acknowledgments
This research was supported by the following grants from NIAAA and NIDA: DA025785 (SW), AA020839 (SE), DA033726 (TW), DA004398 (GFK), AA016895 (MR), and by The Pearson Center for Alcoholism and Addiction Research at TSRI. This is manuscript number 22054 from The Scripps Research Institute.
Abbreviations
- LgA
long access
- ShA
short access
- CeA
central amygdala
- ACSF
artificial cerebrospinal fluid
- U50488
kappa opioid receptor agonist
- norBNI
kappa opioid receptor antagonist
- IPSPs
inhibitory postsynaptic potentials
- mIPSCs
miniature inhibitory postsynaptic currents
- PPF
paired-pulse facilitation
- TTX
tetrodotoxin
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Self DW, Nestler EJ. Molecular mechanisms of drug reinforcement and addiction. Annu Rev Neurosci. 1995;18:463–495. doi: 10.1146/annurev.ne.18.030195.002335. [DOI] [PubMed] [Google Scholar]
- 2.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards S, Koob GF. Neurobiology of dysregulated motivational systems in drug addiction. Future Neurol. 2010;5:393–401. doi: 10.2217/fnl.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- 7.Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, et al. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- 8.Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, et al. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci. 2006;24:1733–1743. doi: 10.1111/j.1460-9568.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- 9.Marchant NJ, Densmore VS, Osborne PB. Coexpression of prodynorphin and corticotrophin-releasing hormone in the rat central amygdala: evidence of two distinct endogenous opioid systems in the lateral division. J Comp Neurol. 2007;504:702–715. doi: 10.1002/cne.21464. [DOI] [PubMed] [Google Scholar]
- 10.Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butelman ER, Yuferov V, Kreek MJ. kappa-opioid receptor/dynorphin system: genetic and pharmacotherapeutic implications for addiction. Trends Neurosci. 2012;35:587–596. doi: 10.1016/j.tins.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- 13.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- 14.Chartoff EH, Potter D, Damez-Werno D, Cohen BM, Carlezon WA., Jr Exposure to the selective kappa-opioid receptor agonist salvinorin A modulates the behavioral and molecular effects of cocaine in rats. Neuropsychopharmacology. 2008;33:2676–2687. doi: 10.1038/sj.npp.1301659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Portoghese AS, Lipkowski AW, Takemori AE. Bimorphinans as highly selective, potent kappa opioid receptor antagonists. J Med Chem. 1987;30:238–239. doi: 10.1021/jm00385a002. [DOI] [PubMed] [Google Scholar]
- 16.Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology (Berl) 2009;205:565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 18.Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- 19.Hjelmstad GO, Fields HL. Kappa opioid receptor inhibition of glutamatergic transmission in the nucleus accumbens shell. J Neurophysiol. 2001;85:1153–1158. doi: 10.1152/jn.2001.85.3.1153. [DOI] [PubMed] [Google Scholar]
- 20.Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruz MT, Herman MA, Cote DM, Ryabinin AE, Roberto M. Ghrelin Increases GABAergic Transmission and Interacts with Ethanol Actions in the Rat Central Nucleus of the Amygdala. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilpin NW, Misra K, Herman MA, Cruz MT, Koob GF, Roberto M. Neuropeptide Y opposes alcohol effects on gamma-aminobutyric acid release in amygdala and blocks the transition to alcohol dependence. Biol Psychiatry. 2011;69:1091–1099. doi: 10.1016/j.biopsych.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schilstrom B, Yaka R, Argilli E, Suvarna N, Schumann J, Chen BT, et al. Cocaine enhances NMDA receptor-mediated currents in ventral tegmental area cells via dopamine D5 receptor-dependent redistribution of NMDA receptors. J Neurosci. 2006;26:8549–8558. doi: 10.1523/JNEUROSCI.5179-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci. 2008;28:9092–9100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oleskevich S, Williams JT. Cocaine prolongs norepinephrine synaptic potentials in rat dorsal raphe. J Neurophysiol. 1995;73:687–692. doi: 10.1152/jn.1995.73.2.687. [DOI] [PubMed] [Google Scholar]
- 27.Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fredj NB, Burrone J. A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat Neurosci. 2009;12:751–758. doi: 10.1038/nn.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wasser CR, Kavalali ET. Leaky synapses: regulation of spontaneous neurotransmission in central synapses. Neuroscience. 2009;158:177–188. doi: 10.1016/j.neuroscience.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herman MA, Kallupi M, Luu G, Oleata CS, Heilig M, Koob GF, et al. Enhanced GABAergic transmission in the central nucleus of the amygdala of genetically selected Marchigian Sardinian rats: Alcohol and CRF effects. Neuropharmacology. 2013;67:337–348. doi: 10.1016/j.neuropharm.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruchas MR, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl) 2010;210:137–147. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards S, Graham DL, Bachtell RK, Self DW. Region-specific tolerance to cocaine-regulated cAMP-dependent protein phosphorylation following chronic self-administration. Eur J Neurosci. 2007;25:2201–2213. doi: 10.1111/j.1460-9568.2007.05473.x. [DOI] [PubMed] [Google Scholar]
- 33.Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruchas MR, Land BB, Lemos JC, Chavkin C. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PloS one. 2009;4:e8528. doi: 10.1371/journal.pone.0008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62:167–176. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurd YL, Svensson P, Ponten M. The role of dopamine, dynorphin, and CART systems in the ventral striatum and amygdala in cocaine abuse. Ann N Y Acad Sci. 1999;877:499–506. doi: 10.1111/j.1749-6632.1999.tb09285.x. [DOI] [PubMed] [Google Scholar]
- 38.Daunais JB, Roberts DC, McGinty JF. Cocaine self-administration increases preprodynorphin, but not c-fos, mRNA in rat striatum. Neuroreport. 1993;4:543–546. doi: 10.1097/00001756-199305000-00020. [DOI] [PubMed] [Google Scholar]
- 39.Spangler R, Unterwald EM, Kreek MJ. ‘Binge’ cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Brain Res Mol Brain Res. 1993;19:323–327. doi: 10.1016/0169-328x(93)90133-a. [DOI] [PubMed] [Google Scholar]
- 40.Fagergren P, Smith HR, Daunais JB, Nader MA, Porrino LJ, Hurd YL. Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. Eur J Neurosci. 2003;17:2212–2218. doi: 10.1046/j.1460-9568.2003.02636.x. [DOI] [PubMed] [Google Scholar]
- 41.Sivam SP. Cocaine selectively increases striatonigral dynorphin levels by a dopaminergic mechanism. J Pharmacol Exp Ther. 1989;250:818–824. [PubMed] [Google Scholar]
- 42.Smiley PL, Johnson M, Bush L, Gibb JW, Hanson GR. Effects of cocaine on extrapyramidal and limbic dynorphin systems. J Pharmacol Exp Ther. 1990;253:938–943. [PubMed] [Google Scholar]
- 43.Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- 44.Unterwald EM, Rubenfeld JM, Kreek MJ. Repeated cocaine administration upregulates kappa and mu, but not delta, opioid receptors. Neuroreport. 1994;5:1613–1616. doi: 10.1097/00001756-199408150-00018. [DOI] [PubMed] [Google Scholar]
- 45.Shippenberg TS, Herz A. Differential effects of mu and kappa opioid systems on motivational processes. NIDA Res Monogr. 1986;75:563–566. [PubMed] [Google Scholar]
- 46.McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shippenberg TS, Rea W. Sensitization to the behavioral effects of cocaine: modulation by dynorphin and kappa-opioid receptor agonists. Pharmacol Biochem Behav. 1997;57:449–455. doi: 10.1016/s0091-3057(96)00450-9. [DOI] [PubMed] [Google Scholar]
- 49.Schenk S, Partridge B, Shippenberg TS. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology (Berl) 1999;144:339–346. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- 50.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 51.Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edwards S, Whisler KN, Fuller DC, Orsulak PJ, Self DW. Addiction-related alterations in D1 and D2 dopamine receptor behavioral responses following chronic cocaine self-administration. Neuropsychopharmacology. 2007;32:354–366. doi: 10.1038/sj.npp.1301062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.