Abstract
Cell-based therapies to restore heart function after infarction have been tested in pre-clinical models and clinical trials with mixed results, and will likely require both contractile cells and a vascular network to support them. We and others have shown that human endothelial colony forming cells (ECFC) combined with mesenchymal progenitor cells (MPC) can be used to “bio-engineer” functional human blood vessels. Here we investigated whether ECFC + MPC form functional vessels in ischemic myocardium and whether this affects cardiac function or remodeling. Myocardial ischemia/reperfusion injury (IRI) was induced in 12-week-old immunodeficient rats by ligation of the left anterior descending coronary artery. After 40 min, myocardium was reperfused and ECFC + MPC (2 × 106 cells, 2:3 ratio) or PBS was injected. Luciferase assays after injection of luciferase-labeled ECFC + MPC showed that 1,500 ECFC were present at day 14. Human ECFC-lined perfused vessels were directly visualized by femoral vein injection of a fluorescently-tagged human-specific lectin in hearts injected with ECFC + MPC but not PBS alone. While infarct size at day 1 was no different, LV dimensions and heart weight to tibia length ratios were lower in cell-treated hearts compared with PBS at 4 months, suggesting post-infarction remodeling was ameliorated by local cell injection. Fractional shortening, LV wall motion score, and fibrotic area were not different between groups at 4 months. However, pressure–volume loops demonstrated improved cardiac function and reduced volumes in cell-treated animals. These data suggest that myocardial delivery of ECFC + MPC at reperfusion may provide a therapeutic strategy to mitigate LV remodeling and cardiac dysfunction after IRI.
Keywords: Ischemic myocardium, ECFC, MPC, Angiogenesis, Cardiac function
Introduction
Myocardial infarction remains a major cause of morbidity and mortality throughout the world. Although acute mortality rates for infarction have fallen dramatically, a significant number of patients go on to develop late sequelae including heart failure and arrhythmia [1]. Over the last decade, many investigators have tested cell-based therapies aimed to prevent adverse ventricular remodeling and to promote recovery of the myocardium in rodents and in patients with acute myocardial infarction. Due to the risks of teratoma formation [2] and host immune response to allogeneic embryonic stem cells [3], autologous adult stem/progenitor cells have emerged as a feasible strategy to treat ischemic myocardium. Examples of adult stem/progenitor cells that have been tested are bone marrow-derived cells, including mononuclear cells (MNC) [4–7], mesenchymal stem cells (MSC) [8], common myeloid cells [9], granulocyte–macrophage progenitors [9], endothelial progenitor cells (EPC) [10], blood-derived CD34+ cells [11, 12], adipose-derived stem cells [13], and saphenous vein-derived pericyte progenitors [14]. In contrast to these hematopoietic and mesenchymal populations, cardiosphere-derived cells (CDC) isolated from human heart biopsies have been tested in pre-clinical and clinical models. CDCs show myogenic differentiation potential, secrete angiogenic factors, and improved cardiac function when injected into infarcted mouse hearts [15]. Intracoronary delivery of autologous CDCs after myocardial infarction was shown to be safe in a prospective randomized trial [16]. Delivery of autologous heart-derived c-kit positive cells in the SCIPIO trial also appeared safe and beneficial after 4 months [17]. In aggregate, these cell-based approaches have shown some promising effects in ischemic myocardium but the underlying mechanism(s) are not well-understood.
Some studies have focused on the ability of the injected cells to build new blood vessels de novo and/or stimulate angiogenesis, based on the concept that rapid restoration of microvascular networks within the ischemic myocardium would mitigate adverse remodeling and promote functional recovery. The consensus now is that ‘early’ EPCs isolated from peripheral blood MNC fractions increase neovascularization by secreting pro-angiogenic factors rather than differentiating into endothelium [18]. Therefore, it is still an open question whether adult stem/progenitor cells with vasculogenic capability in vitro can assemble into neo-vascular networks that connect with host vessels in ischemic myocardium.
Our approach is to use well-defined cell populations that have been shown to assemble vascular networks in vivo to build neo-vascular networks in ischemic myocardium. We use a two cell strategy—consisting of human endothelial colony forming cells (ECFC) and human mesenchymal progenitor cells (MPC)—to build functional, perfused vascular networks [19–21]. Human ECFC co-implanted with MPCs, which take on perivascular cell phenotype when in contact with ECFCs, form functional blood vessels within 7 days in immunodeficient mice [19, 22]. The newly formed human vascular networks can be transplanted to secondary recipients and reconnect to the new host vasculature within 3 days [21]. Finally, ECFC and MPC form vascular networks rapidly in vivo when implanted in a variety of extracellular matrices [23]. This versatility led us to propose ECFC and MPC will form neo-vessels that integrate with existing host vessels in ischemic myocardium. We used luciferase-labeled ECFCs to quantify cellular retention in a rat myocardial ischemia/reperfusion injury (IRI) model and in vivo labeling by femoral vein injection of a mixture of human- and rodent-specific fluorescently-conjugated lectins [21] to detect perfused human and rat vessels at 1, 2 week and 3 months after myocardial IRI. We assessed cardiac function by echocardiography and pressure–volume (PV) loop analyses 4–5 months after myocardial IRI. To the best of our knowledge, no prior studies have directly demonstrated formation of perfused vessels lined with human endothelial cells in ischemic myocardium. The reduction in adverse ventricular remodeling and modest improvements in cardiac function that occurred in rats injected with ECFC + MPC suggest these cells could provide a vascular network to support the recovery of contractile cells.
Methods
Isolation and culture of ECFC, MPC
Human umbilical cord blood was obtained from the Brigham and Women’s Hospital in accordance with an Institutional Review Board-approved protocol. ECFC were isolated from the adherent MNC fraction using CD31-coated magnetic beads (Invitrogen, CA) as described [24]. ECFCs were expanded on fibronectin (FN)-coated plates (1 μg/cm2; Millipore, MA) using EGM-2 (without hydrocortisone; Lonza, MD) supplemented with 20 % FBS (Hyclone, UT) and 1× glutamine–penicillin–streptomycin (GPS; Cellgro, VA). ECFC isolated from one cord blood donor were used between passages 5 and 8 for all experiments.
MPC were isolated from the MNC fraction of human adult bone marrow (Lonza) as described [24]. MNC were seeded on 1 % gelatin-coated plates using MPC-medium (EGM-2 without hydrocortisone, VEGF, bFGF, and heparin and supplemented to 20 % FBS, 1× GPS) with 15 % autologous bone marrow plasma. Unbound cells were removed at 48 h, and the adherent cell fraction maintained in culture until 70 % confluence. MPC from one bone marrow donor, expanded on FN-coated plates in MPC-medium, were used between passages 5 and 8.
IRI model
Animal experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center in an AAALAC-approved facility. 12 week old immunodeficient (nude) rats (Taconic, NY) were anaesthetized with ketamine (80–100 mg/kg) and xylazine (12 mg/kg) intraperitoneally. Rats were intubated and mechanically ventilated using small animal ventilator (SAR-830, CWE Inc., PA). A left thoracotomy was performed, and the left anterior descending (LAD) was ligated using a slip-knot with 6–0 silk suture. Ischemia was confirmed by myocardial blanching. After 5 min, fluorescent microspheres (400 μl of 10-μm FluoSpheres, Molecular Probes) were injected into the LV cavity to distinguish ischemic and non-ischemic regions. After 40 min, the LAD ligature was released and reperfusion visually confirmed. ECFC + MPC (2:3 ratio; total 2 × 106 cells suspended in 50 μl of PBS) or PBS alone were injected into two different sites with a 29 gauge syringe (TERUMO, Japan) within 5 min after reperfusion: one in the border zone adjacent to the ischemic area and one within the ischemic area. During surgery, body temperature was monitored and kept at 37 °C with a thermostatically-controlled heating pad connected to a rectal probe during whole experiment (Physitemp Instruments Inc., NJ).
Infarct size and area-at-risk analysis
Rats were euthanized 24 h after reperfusion, and LVs were sectioned transversely into 6–7 sections with thickness of 2 mm. To delineate the infarct, sections were incubated in 1 % 2, 3, 5-triphenyltetrazolium chloride (TTC)-containing Tris–HCl buffer (pH 7.8) at 37 °C for 10 min and then were fixed in 10 % formalin-phosphate buffered saline for 30 min. Each slice was weighed and photographed from both sides by using a microscope equipped with a high-resolution digital camera. The area-at-risk (AAR) delineated by absence of fluorescent microspheres and the infarct area delineated by TTC were measured from enlarged digital micrographs with NIH Image as described [25].
Luciferase assay for cell retention
ECFCs were infected with Lenti-pUbiquitin (Ub)-firefly luciferase (fluc)-GFP at a multiplicity of infection of 10 [26]. Infectivity was determined by GFP expression and luciferase/GFP-expressing ECFCs were sorted by FACS and expanded under the ECFC culture condition [19].
Hearts were harvested 1 day, 1, and 2 weeks after luciferase-labeled ECFC and MPC were injected into ischemic myocardium. Hearts were homogenized in lysis buffer (Promega Corporation, WI) and luciferase activity measured (Promega Corporation). To calculate the percentage of cells retained in the heart, a standard curve of cell number versus luciferase activity was generated with serial dilutions of cell lysate prepared from cultured luciferase-labeled ECFC. Cell lysate was diluted into a normal heart homogenate to account for any effects the heart homogenates might have on luciferase activity. The luciferase activity of each experimental heart homogenate was converted to ECFC number using the standard curve.
In vivo labeling by femoral vein injection of UEA-I and GS-IB4 lectins
Rhodamine-conjugated UEA-I and FITC-conjugated GS-IB4 (Vector, CA) were mixed in 1 mM CaCl2 in saline. The lectin mixture (200 μg of UEA-I and 300 μg of GS-IB4/500 μL/rat) was injected into the femoral vein 10 min before rats were euthanized. Hearts were harvested, fixed in 10 % buffered formalin overnight, incubated in 30 % sucrose in a second overnight step, embedded in OCT, frozen, and cryosectioned [21].
Microvessel density analysis
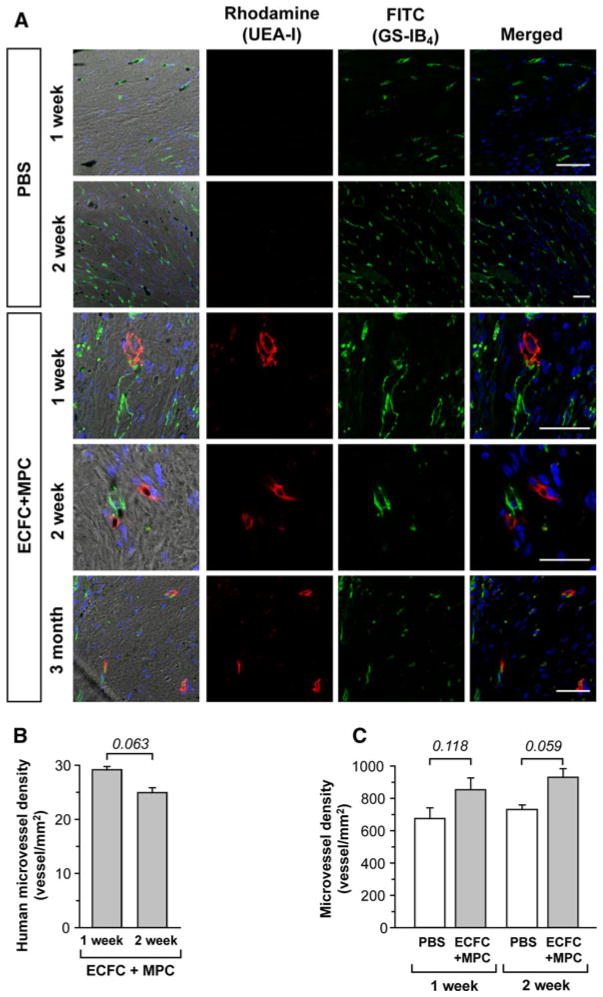
Twelve micrometer-thick frozen sections of heart were mounted with Vectashield with DAPI (Vector). Perfused human or rat microvessels were identified as human by rhodamine fluorescence or rat by FITC fluorescence. Fluorescently labeled vessels were counted using Leica TCS SP2 Acousto-Optical Beam Splitter confocal system equipped with a DMIRE2 inverted microscope (Diode 405 nm, Argon 488 nm, HeNe 594 nm; Leica Microsystems, Wetzlar, Germany) at room temperature. Image files were exported in 8 bit format. A 40x/1.25 oil objective with Zoom 1, 2, or 3 was used (Fig. 2a). Human microvessel density was analyzed with the images showing rhodamine-positive human vessels of each heart and reported as vessels/mm2. Total microvessel density was analyzed by counting rhodamine- and/or FITC-positive vessels in 12 randomly taken images of each heart and reported as vessels/mm2.
Fig. 2.
Microvessel density analysis of ischemic myocardium. Hearts were harvested at multiple time points after IRI following PBS or ECFC + MPC injection. Perfused human and rat vessels were identified by tail-vein injection of a mixture of rhodamine (red)-conjugated UEA-I and FITC (green)-conjugated GS-IB4. a Representative confocal images of lectin-labeled vessels in LV at 1, 2 week, and 3 month (scale bars represent 50 μm). b Graph of human microvessel density in LV of post-IRI with ECFC + MPC injection (n = 3; mean ± SEM.). c Graph of total microvessel density in LV of post-IRI with PBS or ECFC + MPC injection (n = 3; mean ± SEM). Numbers above bars are P values. (Color figure online)
Fibrosis analysis
At the time of sacrifice, hearts were excised, weighed, and placed in 10 % formalin. The fixed heart was cut in short-axis slices with thickness of 4 mm. The tissue slices corresponding to the basal, mid-ventricular, and apical left ventricle, as well as the apical cap, were embedded in paraffin and sectioned. Myocardial tissue sections were stained with trichrome. Digital images of the stained slides were acquired using a light microscope at 20× magnification. The digital images of each section were manually assembled into a composite short-axis image (Adobe Photoshop). From each composite image, the area of fibrosis was measured using ImageJ software. For each heart, the sum of fibrosis area for all four short-axis levels was expressed as a fraction of the sum of LV area.
Echocardiography
Echocardiography was performed at 1, 2 weeks, and 4 months following IRI. Rats were sedated using 1–2 % inhaled isoflurane and positioned supine on a warming pad. Sedation was monitored to avoid excessive bradycardia. An ultrasound machine equipped with a high-frequency linear-array transducer (i13L transducer; Vivid Five, GE Healthcare) was used to obtain two-dimensional echocardiographic images in the LV short-axis plane at the basal, mid-ventricular, and apical levels. Images were obtained in the parasternal long-axis orientation for assessment of the true apex. M-mode recordings were made in the short-axis mid-ventricular level for measurement of LV wall thickness and chamber dimensions at end-diastole and end-systole, and for calculation of fractional shortening (FS). The regional wall motion score (WMS) was determined as described previously [27] by assigning a score of 1 (normal), 2 (hypokinetic), 3 (akinetic), or 4 (dyskinetic) to each of 13 LV segments: 4 segments per short-axis plane, one additional segment for the apical cap on long-axis images.
PV loop
Rats were anesthetized with inhaled isoflurane, intubated, and mechanically ventilated (SAR-830, CWE Inc. PA). Body temperature was monitored and kept at 37 °C with a thermostatically-controlled heating pad connected to a rectal probe (Physitemp Instruments Inc., NJ). A 1.9 French ADVantage PV catheter (Scisense, Canada) was then inserted into the right carotid artery and advanced into the LV to measure pressure and volume. The inferior vena cava was transiently occluded using blunt forceps to generate a family of pressure–volume curves from which load-independent parameters were derived as described previously [28]. Data were acquired and analyzed using Labscribe 2 software (iWorx 125 Systems Inc., NH).
Statistical analysis
Values are expressed as mean ± SEM. Values were analyzed by ANOVA followed by a Fisher least significant difference posthoc test for multiple comparisons or compared using Student’s t test for paired comparisons (STATISTICA). P ≤ 0.050 was considered statistically significant.
Results
Infarct size and ECFC retention in ischemic myocardium
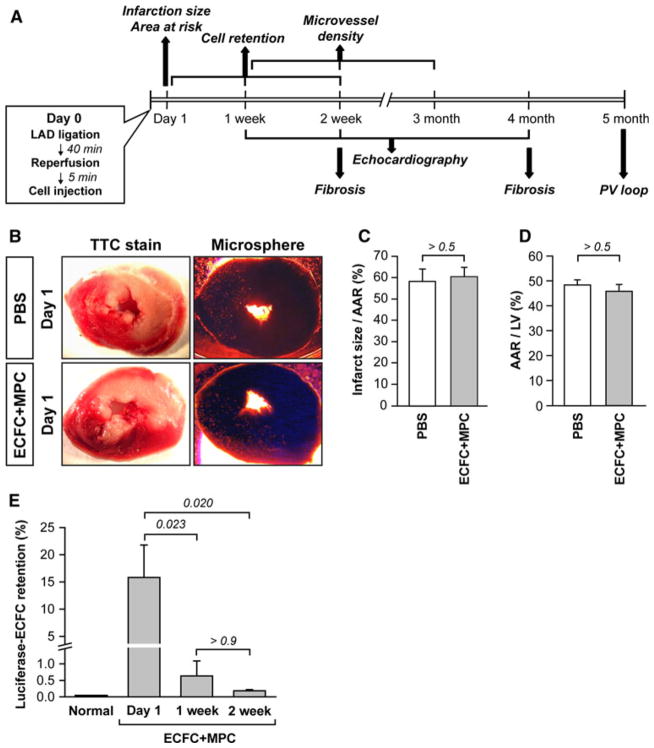
An overview of the analyses performed over time in different groups of animals is shown in Fig. 1a. On day 0, IRI was induced by ligation of the LAD for 40 min followed by reperfusion. ECFC + MPC suspended in PBS or PBS alone were injected within 5 min of reperfusion. Infarct size and AAR were measured on day 1 and found to be similar between the two groups (Fig. 1b–d).
Fig. 1.
Initial infarction size and human ECFC retention rate in rat ischemic myocardium. a Diagram of experimental schedule. b–d Initial infarction size and AAR 1 day post IRI with PBS or ECFC + MPC injection (n = 5; mean ± SEM). e Luciferase-labeled ECFC retention rate at 1 day, 1, 2 week post-IRI with PBS or ECFC + MPC injection (n = 4; mean ± SEM). Normal data from healthy rat hearts served as a negative control (n= 3; mean ± SEM). Numbers above bars are P values
We quantified the number of ECFC retained in hearts 1 day, 1, and 2 weeks after IRI in a subset of animals that were injected with luciferase-labeled ECFC + non-labeled MPC. Heart homogenates were assayed for luciferase activity, and compared to a standard curve generated with cultured luciferase-ECFC diluted into a normal cardiac homogenate. At day 1, 15.81 ± 5.97 % of the injected ECFC (1.26 × 105 cells) remained in the heart. The percentage fell to 0.63 ± 0.46 % at day 7 and further decreased to 0.18 ± 0.04 % at day 14 (Fig. 1e). The retention rate was low, but detectable and quantifiable, corresponding to about 1500 ECFC retained at 14 days in the ischemic, beating heart. The number of MPC retained was not determined.
Detection of perfused human and rat vessels in vivo
Human ECFC-lined perfused vessels in rat myocardium were directly visualized by femoral vein injection of a mixture of rhodamine-conjugated Ulex europaeus agglutinin I (UEA-I), a lectin specific for human endothelium, and FITC-conjugated Griffonia simplifolia isolectin B4(GS-IB4), a lectin specific for rodent endothelium. Three distinct patterns were observed: UEA-I-positive human vessels (red), GS-IB4-positive rat vessels (green), and UEA-I/GS-IB4-positive chimeric vessels (Fig. 2a). This in vivo labeling technique has several advantages: (1) identifying functional perfused vessels in contrast to dead-end tubular structures, (2) distinguishing human from rat vessels, and (3) visualizing the site of anastomosis.
Perfused human vessels were seen in myocardial tissue injected with ECFC + MPC but not PBS at 1 and 2 weeks after injection (Fig. 2a). Furthermore, ECFC-derived human vessels were detected at 3 months, indicating the human vessels integrated with the host myocardial vessels and maintained integrity and functionality for 3 months. The number of UEA-labeled fluorescent vessels was 29.10 ± 0.65 per mm2 at 1 week, and 24.94 ± 0.88 per mm2 at 2 week (Fig. 2b). The total number of perfused vessels (red and green fluorescence) was also quantified. There was a trend towards higher number of perfused vessels in the cell-injected myocardium compared to PBS-injected myocardium at 2 week (Fig. 2c). However, the number of human vessels formed in ECFC + MPC injected hearts cannot fully account for the increase in total vessels/mm2, suggesting that rat vessels were also increased in ECFC + MPC injected hearts.
Cardiac functional analyses
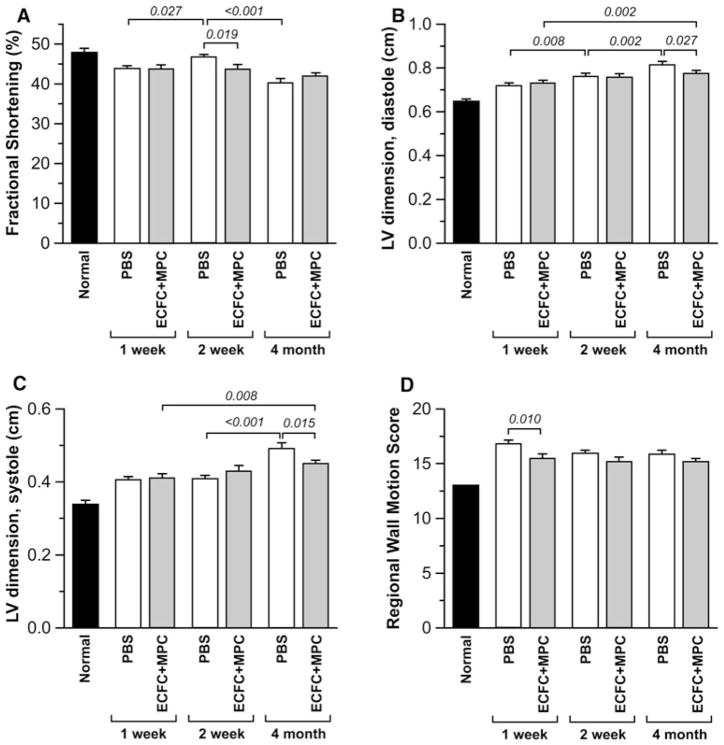
We used echocardiography to evaluate the effect of ECFC + MPC injection on the cardiac function after IRI. Echocardiography revealed that FS, a measure of global LV function, was less at 2 weeks in the ECFC + MPC group compared to PBS group (46.7 ± 0.7 % vs. 43.7 ± 1.2 %; P = 0.019), but not different after 4 months between groups (40.2 ± 1.1 % vs. 42.2 ± 0.6 %; P > 0.1; Fig. 3a). LV internal dimensions at end-diastole and end-systole increased in both groups over time, but less in ECFC + MPC group than PBS group at 4 month post-IRI (Fig. 3b, c), indicating that ECFC + MPC injection can attenuate long-term adverse ventricular remodeling. A composite LV regional WMS was calculated by assessing wall motion of 13 segments of each heart (normal WMS = 13.0). WMS was better preserved in the ECFC + MPC group compared to PBS group at 1 week post-IRI (15.5 ± 0.4 vs. 16.8 ± 0.4; P = 0.010; Fig. 3d) but not different at later time points.
Fig. 3.
Echocardiography analysis for cardiac function. Echocardiography was performed on isoflurane-anesthetized rats at 1, 2 week, and 4 month post-IRI following PBS or ECFC + MPC injection (n = 11–17). Normal data were obtained from healthy rats (n = 2; average from repeated measurements at 3 different time points). a Fractional Shortening (FS). b LV internal dimension at end-diastole. c LV internal dimension at end-systole. d Regional WMS. Data present mean ± SEM. Numbers above bars are P values
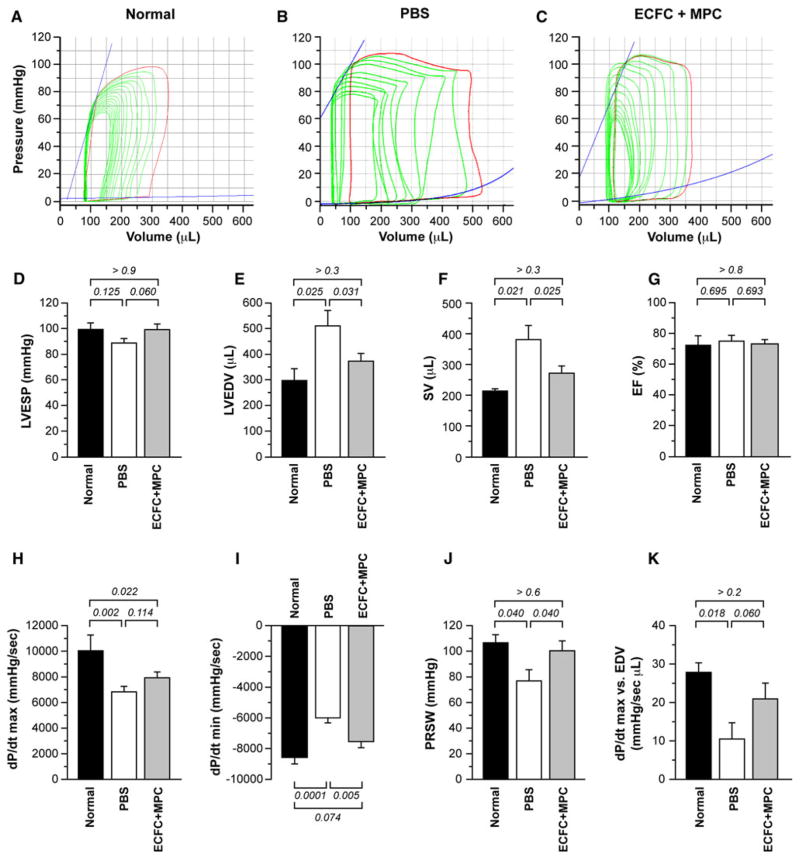
For more precise analysis of cardiac function, we performed invasive hemodynamics with PV loops 5 months after IRI. Overall cardiac function declined after IRI followed by PBS injection compared with normal condition as shown in Fig. 4. A characteristic right shift in the PV loops is seen in both experimental groups compared to the normal heart (Fig. 4a–c) indicating greater LV volumes due to chamber dilation and decreased contractility following IRI.
Fig. 4.
Pressure–volume (PV) loop analysis of rat hearts. PV loop measurement was performed on anesthetized rats at 5 month post-IRI with PBS or ECFC + MPC injection (n = 6–7). Normal data were obtained from healthy rats (n = 3–4). a–c Representative PV loops from normal rats, PBS-, and ECFC + MPC-injected rats at 5 month post-IRI. d LVESP. e LVEDV. f Stroke volume (SV). g Ejection fraction (EF). h Maximum rate of pressure change in the LV (dP/dt max). i Minimum rate of pressure change in the LV (dP/dt min). j Preload recruitable stroke work (PRSW). k dP/dt max versus EDV. Data present mean ± SEM. Numbers above bars are P values
LV end-systolic pressure (LVESP) was not significantly different among the groups but showed a non-significant trend toward improvement after ECFC + MPC compared with PBS injection (Fig. 4d). Similarly there were no significant differences in LV end-diastolic pressure (LVEDP) among the groups (Table 2). LV end-diastolic volume (LVEDV) was significantly higher in PBS group and reduced in the ECFC + MPC group to a volume not significantly different from normal hearts (Fig. 4e). LV end-systolic volume (ESV) was not different among the groups (Table 2). Reflecting the LV dilation, stroke volume (SV) and cardiac outputs (CO) were increased in PBS-treated hearts while ejection fraction (SV divided by LVEDV) was similar among the groups (SV: Fig. 4f; CO: Table 2). Ejection fraction (EF (%) = SV divided by LVEDV) was similar among the groups (Fig. 4G).
Table 2.
Parameters of cardiac function from PV loops at 5 months post-IRI
| Parameter\group | Normal | PBS | ECFC + MPC | P value normal versus PBS | P value PBS versus ECFC + MPC | P value normal versus ECFC + MPC |
|---|---|---|---|---|---|---|
| HW/TL (mg/mm) | 21.57 ± 0.88 | 33.17 ± 2.87 | 27.31 ± 1.12 | 0.001 | 0.028 | 0.052 |
| HW/BW (mg/g) | 3.24 ± 0.16 | 3.68 ± 0.32 | 3.30 ± 0.11 | 0.185 | 0.182 | 0.852 |
| HR (beats/min) | 443.4 ± 17.3 | 429.4 ± 16.6 | 449.3 ± 13.1 | 0.560 | 0.298 | 0.798 |
| LVESP (mmHg) | 99.32 ± 5.05 | 88.74 ± 3.54 | 99.21 ± 4.31 | 0.125 | 0.060 | 0.986 |
| LVEDP (mmHg) | 6.84 ± 1.66 | 6.09 ± 1.38 | 7.53 ± 0.89 | 0.689 | 0.336 | 0.706 |
| dP/dt max (mmHg/s) | 10,043 ± 1,225 | 6,826 ± 439 | 7,934 ± 447 | 0.002 | 0.114 | 0.022 |
| dP/dt min (mmHg/s) | −8,650 ± 404 | −6,076 ± 272 | −7,574 ± 404 | 0.0001 | 0.005 | 0.074 |
| LVESV (μL) | 84.3 ± 38.4 | 129.9 ± 30.6 | 101.2 ± 16.2 | 0.317 | 0.355 | 0.700 |
| LVEDV (μL) | 297.4 ± 46.0 | 510.8 ± 59.8 | 372.5 ± 30.6 | 0.025 | 0.031 | 0.377 |
| SV (μL) | 213.0 ± 7.6 | 381.0 ± 45.4 | 271.3 ± 23.7 | 0.021 | 0.025 | 0.366 |
| CO (mL/min) | 92.35 ± 6.03 | 161.71 ± 20.78 | 121.41 ± 10.24 | 0.031 | 0.060 | 0.319 |
| EF (%) | 72.30 ± 8.70 | 74.97 ± 4.07 | 73.12 ± 3.00 | 0.695 | 0.693 | 0.899 |
| Tau (Weiss) (ms) | 6.21 ± 0.65 | 7.75 ± 0.45 | 7.28 ± 0.27 | 0.025 | 0.345 | 0.093 |
| Tau (Glantz) (ms) | 6.18 ± 0.44 | 8.65 ± 0.70 | 7.94 ± 0.14 | 0.004 | 0.228 | 0.027 |
| ESPVR (mmHg/μL) | 1.311 ± 0.478 | 0.945 ± 0.291 | 1.492 ± 0.389 | 0.532 | 0.244 | 0.751 |
| EDPVR (mmHg/μL) | 6.590 ± 4.984 | 3.924 ± 0.917 | 6.468 ± 3.733 | 0.514 | 0.469 | 0.977 |
| Stiffness | 0.003 ± 0.001 | 0.002 ± 0.001 | 0.003 ± 0.001 | 1.000 | 0.822 | 0.854 |
| PRSW (mmHg) | 106.58 ± 6.21 | 76.85 ± 8.67 | 100.32 ± 7.75 | 0.040 | 0.040 | 0.622 |
| dP/dt max versus EDV (mmHg/s·μL) | 27.87 ± 2.44 | 10.50 ± 4.25 | 20.92 ± 4.10 | 0.018 | 0.060 | 0.289 |
Values are mean ± SEM. (n = 6–7 for PBS and ECFC + MPC groups; n = 3–4 for normal group)
dP/dt max and dP/dt min, maximum and minimum rates of pressure development in the LV, showed a significant deterioration in PBS group. ECFC + MPC injection did not significantly change dP/dt max compared with PBS injection but significantly improved dP/dt min, suggesting cell injection had a greater effect on myocardial relaxation than contraction (Fig. 4h, i).
Preload recruitable stroke work (PRSW) and dP/dt max versus EDV, principle load-independent parameters, were reduced in the PBS group, but not significantly reduced in the ECFC + MPC group (Fig. 3j, k). However, other load-independent measures of intrinsic contractility (the end-systolic pressure volume relationship [ESPVR]) or relaxation (the end-diastolic pressure volume relationship [EDPVR]) did not differ from normal in either of the groups (Table 2).
To relate global cardiac function to LV hypertrophy, the heart weight-to-body weight ratio (HW/BW) and heart weight-to-tibia length (HW/TL) were calculated as an index of cardiac hypertrophy. HW/BW was lower in the ECFC + MPC group than the PBS group at 1 week post-IRI (3.6 ± 0.1 vs. 3.4 ± 0.1; P < 0.05) but was no different between the groups at 5 months post-IRI (Table 1). HW/TL was lower in ECFC + MPC group than PBS group 5 months post-IRI, further supporting mitigation of LV remodeling by ECFC + MPC injection. The area affected by fibrosis, expressed as a percent of the total LV area, was reduced at 4 months compared to 2 weeks in both PBS- and ECFC + MPC-injected hearts likely reflecting scar contraction, but no difference between the two groups was seen at either time point (Supplemental Fig. 1).
Table 1.
Selected abbreviations for cardiac function data
| Abbreviation | Terminology |
|---|---|
| IRI | Myocardial ischemia/reperfusion injury |
| HW | Heart weight |
| TL | Tibia length |
| BW | Body weight |
| HR | Heart rate |
| LVESP | Left ventricular end-systolic pressure |
| LVEDP | Left ventricular end-diastolic pressure |
| dP/dt max | Maximum rate of pressure change in the left ventricle |
| dP/dt min | Minimum rate of pressure change in the left ventricle |
| LVESV | Left ventricular end-systolic volume |
| LVEDV | Left ventricular end-diastolic volume |
| SV | Stroke volume |
| CO | Cardiac output |
| EF | Ejection fraction |
| Tau | Time constant of left ventricular pressure fall |
| ESPVR | End-systolic pressure volume relationship |
| EDPVR | End-diastolic pressure volume relationship |
| PRSW | Preload recruitable stroke work |
Discussion
Here we show the feasibility of building new blood vessels in ischemic myocardium. Highly purified, human ECFC combined with human bone marrow-derived MPC were injected into the boundary and ischemic area of the myocardium immediately after perfusion was restored. A small percentage of the ECFC was retained in the heart and formed blood vessels connected to the host circulation. This is the first demonstration of integration of perfused human endothelial cell-lined vessels with the host vasculature in a setting of myocardial ischemia.
The motivation for this study was to determine if an endothelial cell–based strategy specifically designed to increase the microvessel density in the ischemic myocardium would promote functional recovery of the heart. Perfused vessel density was increased in hearts injected with ECFC + MPC suspended in PBS, however the increased number of vessels did not reach statistical significance, perhaps due to the limited number of sections, representing only a small fraction of the left ventricle, that were analyzed. Human vessels, detected by femoral-vein injection of rhodamine-conjugated UEA-I, comprised only a small fraction of total perfused vessels in the 12-μm thick frozen sections examined.
The ECFC + MPC and PBS groups, along with normal controls, were analyzed over a period of 5 months (Fig. 1a). Echocardiography, pressure–volume hemodynamic parameters, and heart weight to tibia length ratios demonstrated that injection of ECFC + MPC provided a modest therapeutic benefit. LV dimensions in systole and diastole increased over 4 months in both groups. However, LV dimensions were significantly decreased in the ECFC + MPC treated group compared to the PBS group 4 months after IRI. Consistent with this, heart weight to tibia length ratios were increased >50 % in PBS injected rats (P = 0.001) and significantly improved in ECFC + MPC injected rats. Noninvasive measurement of overall LV function (e.g. FS and a regional WMS) showed no sustainable differences between ECFC + MPC- and PBS-injected hearts. Taken together these data suggest that cell therapy reduced adverse ventricular remodeling after IRI.
Five months after IRI, we performed invasive hemodynamic measurements with pressure volume loops to assess cardiac performance in the PBS- and ECFC + MPC-injected rats in comparison to age- and strain-matched normal rats. In Fig. 4, a pattern emerges in which ECFC + MPC treated hearts are more similar to normal hearts than PBS-treated hearts in several important measures of cardiac performance. Particularly dramatic are the volume changes. For example, PBS-treated hearts showed an increase in LVEDV compared to normal, reflecting adverse remodeling which was substantially better (and no different from normal) in ECFC + MPC-injected hearts. Measures of myocardial relaxation showed mixed results. dP/dt min was impaired in the PBS-treated compared to normal and cell-treated hearts, while Tau was similarly increased in both IRI groups. Similarly, there were mixed effects on measures of myocardial contractility. EF, LVESP, LVEDP, LVESV, ESPVR, and EDPVR were all similar between the two groups and also indistinguishable from normal hearts (Table 2), suggesting relatively modest effects of the ischemic injury overall, and thus making it more difficult to demonstrate benefits of cell delivery. In contrast, two of the principal load-independent measures of LV contractile function, PRSW and dP/dt max versus EDV, were better after cell injection. PRSW was reduced in the PBS hearts compared to normal and ECFC + MPC hearts. The increased SV and CO seen the PBS-treated hearts may be a function of the increased LVEDV and relatively preserved overall ejection fraction. These combined data again suggest an attenuation of adverse ventricular remodeling in the ECFC + MPC treated hearts, which is not due to reduction in infarct size. Whether this effect is due to the human blood vessels per se or due to the presence of human ECFC and MPC within the myocardium cannot be discerned from this study. However, regenerating the vasculature and improving blood flow to the myocardium is certainly the most likely mechanism to explain the improved cardiac performance in the ECFC + MPC injected hearts.
Our lab and others have shown the feasibility of building bio-engineered human vascular networks in immune-deficient mice in a variety of ectopic settings. These involve for the most part subcutaneous implantation of cells suspended in or embedded within an extracellular matrix or scaffold material [29–31]. Further, many studies have shown robust vessel formation when a mesenchymal support cell that can surround the abluminal surface of the human endothelial lined vessels is included. Here we show for the first time that ECFC + MPC can form perfused human vessels within 1 week, the earliest time point we examined, in ischemic myocardium. Importantly, the femoral vein infusion of a human-specific lectin demonstrates the vessels are perfused and functional. This provides critical information compared to more conventional immunostaining with anti-human CD31 antibodies, which can detect individual cells and/or tubular structures but does not verify that the endothelial tube has anastomosed with the circulatory system. The presence of red blood cells within the lumen could be due to dead-end vessels or vessels that were only briefly connected to the host before thrombotic occlusion as described by White et al. [32].
Many approaches have been taken or are underway to increase vascularity post-myocardial infarction. Therapeutic angiogenesis in the heart was first tested by delivery of angiogenic peptides such as VEGF-A [33] or basic FGF [34]. More recently, a self-assembling nanofiber containing VEGF-A showed promising benefits in rats when injected into the ischemic myocardium immediately after infarction [35]. Other studies have delivered angiogenic factors and cytokines using microencapsulated human adipose stem cells [36] or skeletal muscle cells engineered to over-express angiogenic factors [37]. It is widely appreciated that precisely controlled activity levels of angiogenic factors will be necessary for both sustained efficacy and safety of these approaches. A cell-based approach to construct vascularized myocardium has been developed in the Murry lab in which human embryonic stem cell-derived cardiomyocytes, human umbilical vein ECs, and clones of human MSCs expressing high levels of the proteoglycan versican were assembled into a scaffold free patch and implanted into non-injured myocardium in athymic rats [38].
Specific populations of cardiac progenitor cells have been shown to differentiate into cardiomyocytes, endothelial cells and smooth muscle cells, and thus such cells may provide a means to increase vascularity in ischemic myocardium as well as replace cardiomyocytes. As of yet, there are no definitive studies showing that cardiac progenitor cells can form functional blood vessels in vivo [39]. CDCs may increase vascularity by a paracrine mechanism based on the balanced set of angiogenic factors secreted by cardiosphere derived cells [15]. Bone marrow-derived MSCs, MNCs, EPCs and CD34+ cells may also stimulate angiogenesis by secreting pro-angiogenic factors and thereby increasing vascularity and perfusion of ischemic myocardium.
Our study is clearly delineated from this body of work in that we used two highly-purified human cell populations to refurbish the microvascular network in a setting of ischemia reperfusion injury in athymic nude rats. The cells were suspended as single cells in PBS prior to injection, free of growth factors, serum or extracellular matrix. We chose this experimental design to focus solely on the ability of the endothelial and mesenchymal cells to assemble and connect with host myocardial vessels. The cells were delivered into the ischemic area, immediately after IRI, by injection to avoid the need to introduce a cell targeting strategy. IRI is characterized by LV remodeling [40] and microvascular dysfunction [41], and complicates myocardial infarction by causing myocardial damage and loss of cardiac function, thereby increasing risk of mortality.
We hypothesize that maximizing the benefits of cardiac cell therapy will ultimately require optimization of both cardiomyogenic and vasculogenic potential. Future studies will focus on two areas to advance the vasculogenic strategy explored here toward clinical application. Cell retention, survival and vessel formation may be improved by increasing cell number, providing nanofibers with angiogenic factors, and using MPCs with high levels of versican [38]. Then, the strategy should be transitioned to intracoronary or intravenous infusion of ECFC and MPCs after myocardial infarction and/or IRI. Several human clinical trials have relied on homing of stem/progenitor cells delivered by intracoronary or intravenous infusion to ischemic myocardium weeks to months after the initial injury. The current study suggests that an optimized vasculogenic treatment could substantially mitigate adverse remodeling and may even have some beneficial effects on cardiac function. However, it seems likely that restoration of contractile function will require delivery of contractile cardiomyogenic cells as well. In that context, we anticipate that an optimized strategy for establishing a vascular supply to regenerated myocardium could have synergistic benefits with co-delivered cardiomyocyte progenitors.
Supplementary Material
Acknowledgments
Supported by HL09262 (JB, TR) and K08-HL098569 (MC). We thank Dr. David Zurokowski, Boston Children’s Hospital, for advice on the statistical analyses. We thank Thomas Neufeld for the excellent technical assistance on fibrosis analysis.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10456-013-9354-9) contains supplementary material, which is available to authorized users.
Conflict of interest The authors have no conflicts of interest to disclose.
Contributor Information
Kyu-Tae Kang, Vascular Biology Program and Department of Surgery, Boston Children’s Hospital, Harvard Medical School, 300 Longwood Ave, Boston, MA 02115, USA. College of Pharmacy, Duksung Women’s University, Seoul, Republic of Korea.
Matthew Coggins, Cardiovascular Division, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
Chunyang Xiao, Cardiovascular Division, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
Anthony Rosenzweig, Cardiovascular Division, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
Joyce Bischoff, Email: joyce.bischoff@childrens.harvard.edu, Vascular Biology Program and Department of Surgery, Boston Children’s Hospital, Harvard Medical School, 300 Longwood Ave, Boston, MA 02115, USA.
References
- 1.Roger VL, Go AS, et al. Executive summary: heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Cao F, Lin S, et al. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113(7):1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bifari F, Pacelli L, Krampera M. Immunological properties of embryonic and adult stem cells. World J Stem Cells. 2010;2(3):50–60. doi: 10.4252/wjsc.v2.i3.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamihata H, Matsubara H, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104(9):1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 5.Dohmann HF, Perin EC, et al. Transendocardial autologous bone marrow mononuclear cell injection in ischemic heart failure: postmortem anatomicopathologic and immunohistochemical findings. Circulation. 2005;112(4):521–526. doi: 10.1161/CIRCULATIONAHA.104.499178. [DOI] [PubMed] [Google Scholar]
- 6.Traverse JH, Henry TD, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA. 2012;308(22):2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Traverse JH, Henry TD, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011;306(19):2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva GV, Litovsky S, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111(2):150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 9.Wara AK, Croce K, et al. Bone marrow-derived CMPs and GMPs represent highly functional proangiogenic cells: implications for ischemic cardiovascular disease. Blood. 2011;118(24):6461–6464. doi: 10.1182/blood-2011-06-363457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawamoto A, Gwon HC, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103(5):634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 11.Yeh ET, Zhang S, et al. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108(17):2070–2073. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 12.Losordo DW, Schatz RA, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115(25):3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 13.Ii M, Horii M, et al. Synergistic effect of adipose-derived stem cell therapy and bone marrow progenitor recruitment in ischemic heart. Lab Invest. 2011;91(4):539–552. doi: 10.1038/labinvest.2010.191. [DOI] [PubMed] [Google Scholar]
- 14.Katare R, Riu F, et al. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ Res. 2011;109(8):894–906. doi: 10.1161/CIRCRESAHA.111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li TS, Cheng K, et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59(10):942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makkar RR, Smith RR, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379(9819):895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolli R, Chugh AR, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378(9806):1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110(4):624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melero-Martin JM, De Obaldia ME, et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103(2):194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foubert P, Matrone G, et al. Coadministration of endothelial and smooth muscle progenitor cells enhances the efficiency of proangiogenic cell-based therapy. Circ Res. 2008;103(7):751–760. doi: 10.1161/CIRCRESAHA.108.175083. [DOI] [PubMed] [Google Scholar]
- 21.Kang KT, Allen P, Bischoff J. Bioengineered human vascular networks transplanted into secondary mice reconnect with the host vasculature and re-establish perfusion. Blood. 2011;118(25):6718–6721. doi: 10.1182/blood-2011-08-375188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melero-Martin JM, Khan ZA, et al. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109(11):4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 23.Allen P, Melero-Martin J, Bischoff J. Type I collagen, fibrin and PuraMatrix matrices provide permissive environments for human endothelial and mesenchymal progenitor cells to form neovascular networks. J Tissue Eng Regen Med. 2011;5(4):e74–e86. doi: 10.1002/term.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melero-Martin JM, Bischoff J. Chapter 13. An in vivo experimental model for postnatal vasculogenesis. Methods Enzymol. 2008;445:303–329. doi: 10.1016/S0076-6879(08)03013-9. [DOI] [PubMed] [Google Scholar]
- 25.Matsui T, Tao J, et al. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104(3):330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 26.Wu JC, Cao F, et al. Proteomic analysis of reporter genes for molecular imaging of transplanted embryonic stem cells. Proteomics. 2006;6(23):6234–6249. doi: 10.1002/pmic.200600150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang RM, Bierig M, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Das S, Aiba T, et al. Pathological role of serum- and glucocorticoid-regulated kinase 1 in adverse ventricular remodeling. Circulation. 2012;126(18):2208–2219. doi: 10.1161/CIRCULATIONAHA.112.115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Au P, Tam J, Fukumura D, Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111(9):4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Aledia AS, et al. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng Part A. 2009;15(6):1363–1371. doi: 10.1089/ten.tea.2008.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Aledia AS, et al. Rapid anastomosis of endothelial progenitor cell-derived vessels with host vasculature is promoted by a high density of cotransplanted fibroblasts. Tissue Eng Part A. 2010;16(2):585–594. doi: 10.1089/ten.tea.2009.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White SM, Hingorani R, et al. Longitudinal in vivo imaging to assess blood flow and oxygenation in implantable engineered tissues. Tissue Eng Part C Methods. 2012;18(9):697–709. doi: 10.1089/ten.tec.2011.0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henry TD, Annex BH, et al. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107(10):1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 34.Laham RJ, Chronos NA, et al. Intracoronary basic fibroblast growth factor (FGF-2) in patients with severe ischemic heart disease: results of a phase I open-label dose escalation study. J Am Coll Cardiol. 2000;36(7):2132–2139. doi: 10.1016/s0735-1097(00)00988-8. [DOI] [PubMed] [Google Scholar]
- 35.Lin YD, Luo CY, et al. Instructive nanofiber scaffolds with VEGF create a microenvironment for arteriogenesis and cardiac repair. Sci Transl Med. 2012;4(146):146ra109. doi: 10.1126/scitranslmed.3003841. [DOI] [PubMed] [Google Scholar]
- 36.Paul A, Chen G, et al. Genipin-crosslinked microencapsulated human adipose stem cells augment transplant retention resulting in attenuation of chronically infarcted rat heart fibrosis and cardiac dysfunction. Cell Transpl. 2012;21(12):2735–2751. doi: 10.3727/096368912X637497. [DOI] [PubMed] [Google Scholar]
- 37.Konoplyannikov M, Haider KH, et al. Activation of diverse signaling pathways by ex vivo delivery of multiple cytokines for myocardial repair. Stem Cells Dev. 2012;22(2):204–215. doi: 10.1089/scd.2011.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kreutziger KL, Muskheli V, et al. Developing vasculature and stroma in engineered human myocardium. Tissue Eng Part A. 2011;17(9–10):1219–1228. doi: 10.1089/ten.tea.2010.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chong JJ. Cell therapy for left ventricular dysfunction: an overview for cardiac clinicians. Heart Lung Circ. 2012;21(9):532–542. doi: 10.1016/j.hlc.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 40.Vilahur G, Juan-Babot O, et al. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J Mol Cell Cardiol. 2011;50(3):522–533. doi: 10.1016/j.yjmcc.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 41.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190(3):255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.