Abstract
E-cadherin, a member of the cadherin family of transmembrane adhesion receptors, is critical for cutaneous barrier function as it promotes keratinocyte and Langerhans cell adhesion in the epidermis. Recent murine models of chronic inflammation identified new E-cadherin-expressing subsets of mononuclear phagocytes, including alternatively activated macrophages and selected inflammatory dendritic cells. It has been shown in vitro that expression of E-cadherin by murine macrophages promotes their homotypic aggregation and fusion to multinucleated giant cells, a signature cell type of granulomatous inflammation. The purpose of our study was to assess E-cadherin expression on histiocytes and giant cells in cutaneous granulomas in human. E-cadherin expression was evaluated by immunohistochemistry of formalin fixed paraffin-embedded skin biopsies of foreign body granulomas (n=21) and sarcoidosis (n=21). The results showed consistent membranous E-cadherin staining pattern on mononucleated histiocytes and multinucleated giant cells in both granuloma types. These E-cadherin expressing histiocytes are distinct from dermal Langerhans cells as they lacked CD1a expression. Our findings suggest that E-cadherin expressing mononuclear histiocytes are likely precursors for multinucleated giant cells in cutaneous granulomas and may play a critical role in disease pathogenesis.
Keywords: E-cadherin, granuloma, histiocyte, multinucleated giant cells, sarcoidosis, foreign body
Introduction
Chronic granulomatous inflammation in the skin is characterized by the tight aggregation of dermal mononuclear phagocytes and multinucleated giant cells, which are thought to encapsulate and prevent the systemic spread of hard to eradicate microorganisms such as mycobacteria, selected fungi or helminths(1). Granuloma formation is also a hallmark of non-infectious cutaneous disorders. Foreign body granulomatous reaction (FBGR) is designed to isolate foreign particles including splinters, suture and keratin fragments released from ruptured cysts or hair follicles. Sarcoidosis is a multisystem inflammatory disorder in which epithelioid macrophages and dendritic cells aggregate into granulomas, likely in response to poorly characterized antigens(2).
Multiple innate and adaptive immune pathways have been implicated in the pathogenesis of granulomatous inflammation. Most granulomas, including sarcoidosis, are driven by proinflammatory cytokines such as TNFα and TH1-type cytokines including IFN-γ (3). In the pathogenesis of sarcoidal granulomas, IFN-γ activated macrophages (classically activated) and dendritic cells (DCs) are thought to play a central role. More recently, increased levels of IL17A, IL-22 and IL-23 have been observed in the blood and tissue of sarcoidosis patients indicating a potential role for the TH17 immune pathway in disease pathogenesis (4). In contrast to sarcoidal granulomas, FBGR is dependent on innate cell derived IL-4 as evidenced by the impairment of FBGR by blocking IL-4 antibodies in vivo but preservation of this response in T-cell deficient animals (5, 6). Based on these observations, it is inferred that FBGR is dependent on IL-4-activated macrophages and/or dendritic cells, which are also referred to as alternatively activated macrophages or dendritic cells (AAMacs and AADCs) (7, 8).
Multinucleated giant cells (MNGCs) are the hallmark feature of granulomas. In FBGR, foreign-body-type giant cells are formed by IL-4 dependent fusion of AAMacs and/or AADCs (9). In sarcoidal granulomas, the presence of FB-type giant cells are likely the products of IL-4 or IL-13 dependent cell-cell fusion, while other multinucleated giant cells are possibly formed through IFN-γ/IL17-dependent fusion pathways (4, 10).
E-cadherin, an epithelial cadherin, is critical in epidermal barrier function by regulating the formation of tight junctions between keratinocytes (11). E-cadherin expression also has been observed in CD1a+ Langerhans cells (LCs) in the epidermis where it is thought to enhance heterotypic adhesion between LCs and keratinotyes (12). E-cadherin is upregulated in IL-4/IL-13 activated macrophages and promotes the homotypic aggregation and fusion of these cells (13, 14). Furthermore, a novel subset of murine inflammatory DCs expressing E-cadherin has been recently identified with TH17 promoting potential in inflammatory colitis(15). The contribution of E-cadherin expressing mononuclear phagocytes to human inflammatory conditions including granulomatous inflammation is not known. Here we evaluated whether E-cadherin is expressed by histiocytes within cutaneous foreign body and sarcoidal granulomas.
Materials and Methods
Patient selection
Following Institutional Review Board approval, archived skin biopsies representing cutaneous foreign body granuloma (n=21) and sarcoidosis (n=21) were reviewed and selected for our study by two board certified dermatopathologists at the Penn Cutaneous Pathology Service.
Immunohistochemical staining
Immunohistochemistry of formalin fixed paraffin embedded tissue sections (4mm) was performed to determine E-cadherin expression on a Leica Bond™ immunostaining platform using a brown chromogen indicator (Diaminobenzidine) against a hematoxylin counterstain using the Bond Polymer Refine Detection System. Samples were rehydrated in xylene (3 times), 95% ethanol, and 75% ethanol. Heat induced epitope retrieval was done for 20 minutes with ER2 solution (Leica Microsystems AR9640). Antibodies against E-cadherin (Dako; Carpinteria, CA; M3612) were used at a 1:10 dilution. Antibodies against CD1a (Dako; Carpinteria, CA; M3571) at 1:50 dilution, pan-CK (BioGenex; Freemont, CA; MU357-UC) at 1:75 dilution, and AE1/AE3 (Leica; Buffalo Grove, IL; #NCL-L-AE1/AE3) at 1:100 dilution were used. For negative controls, either an isotyped-matched control primary antibody was used or the primary antibody was omitted from the staining protocol.
Results
Hematoxylin and eosin (H&E) stained sections demonstrated FBGR at sites of follicular rupture (n=2, Figures 1A and B). epidermal inclusion cyst rupture (n=6, not shown), scar and granulomatous inflammation (n=9, not shown), reactions to non-suture polarizable foreign material (n=3, not shown), and suture granuloma (n=1, not shown). Immunohistochemical staining for E-cadherin showed strong staining in the epidermis, hair follicles, eccrine ducts and cyst wall epithelium (Figures 1C and D). At low power, there was weaker but significant E-cadherin staining on granuloma-associated histiocytes and MNGCs in 20/21 (95%) of the cases studied (Figures 1C, E, G and I highlights representative cases). At high power magnification, E-cadherin staining showed specific membranous staining pattern (Figures 1D, F, H and J). H& E sections of cutaneous sarcoidosis demonstrated naked granulomas composed of epithelioid histiocytes and MNGCs (Figures 2A and B demonstrate a representative case). There was no foreign material observed in our cases of sarcoidosis by polarized microscopy. The E-cadherin staining pattern of mononuclear and multinuclear histiocytes was similar to the pattern in FBGR, with 21/21 (100%) of the sarcoidal cases demonstrating specific membranous staining pattern for E-cadherin (Figures 2C–J). Additionally, in both FBGR and sarcoidosis cases, MNGCs were rimmed by adherent E-cadherin positive mononuclear cells (Figures 1 and 2, D, F, H, and J panels). The number of E-cadherin expressing mono- and multinucleated histiocytes varied among cases in both types of granulomas. Notably, granuloma-associated histiocytes did not stain with CD1a or AE1/AE3 immunostains (not shown). The specificity of the E-cadherin staining was further confirmed by negative staining with isotype-matched control antibody (not shown).
Figure 1.
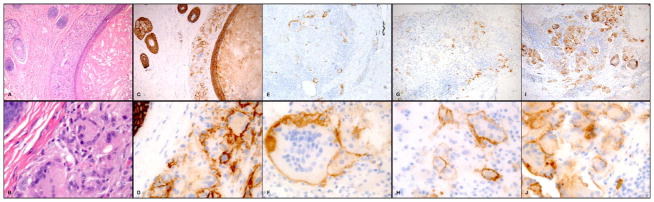
E-cadherin staining of foreign body granulomas. Hematoxylin and eosin (H&E) stained sections demonstrated FBGR at sites of follicular cyst rupture (A, 100x magnification, and B, 600x magnification). Immunohistochemical staining for E-cadherin demonstrated specific membranous staining on granuloma-associated histiocytes and MNGCs on low power (100x magnification) (C, E, G and I) and high power magnification (600x) (D, F, H and J). ). Foreign body reactions were to site of follicular rupture (A–D, G–H) and granulomatous reaction to scar (E–F, I–J).
Figure 2.
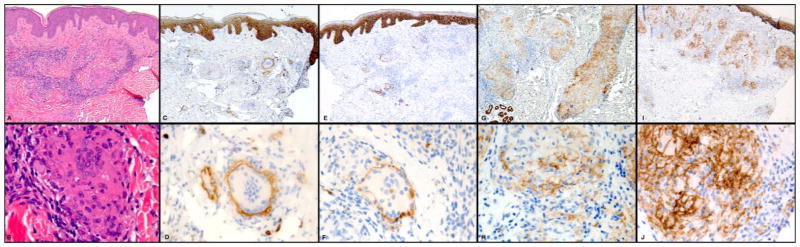
E-cadherin staining of cutaneous sarcoidosis. Hematoxylin and eosin (H&E) stained sections demonstrated naked granulomas composed of epithelioid histiocytes and MNGCs (A and B). E-cadherin staining pattern of mononuclear and multinuclear histiocytes demonstrated membranous staining. Low power magnification, 100x (C, E, G, and I) and high power magnification, 600x (2D, F, H and J).
Discussion
Histiocyte is originally a histological description referring to large phagocytic cells with abundant cytoplasm and one or more pale oval or kidney-shaped nuclei in secondary lymphoid organs.(16) Currently, we loosely use this term in reference to monocyte-derived tissue phagocytes that include macrophages and dendritic cells. The boundaries of this term are further stretched by growing number of new phenotypic and functional subsets of tissue phagocytes.(17) The role of these new histiocyte subsets in human disease is poorly understood. Here we demonstrate that significant number of E-cadherin expressing mononuclear histiocytes and MNGCs are present in two immunologically distinct cutaneous granulomas, FBGR and sarcoidosis.
E-cadherin expression has been previously described in several myeloid-derived innate phagocytes in mice, including Langerhans cells, AAMacs, AADCs, and IL-23 producing inflammatory dendritic cells. The exact lineage derivation of the E-cadherin+ mononuclear cells in cutaneous granulomas is currently unclear, due to the lack of appropriate surface markers that could discriminate between various human macrophage and dendritic cell subsets. Nonetheless, the lack of CD1a staining on granuloma-associated histiocytes indicates that the E-cadherin+ cells observed in this study are not Langerhans cells. The lack of staining for high molecular weight keratin (AE1/3) demonstrated that the E-cadherin expressing cells in granulomas are not epithelial in origin and not stained artifactually due to phagocytosed keratin debris. In mice, E-cadherin expressing AAMacs emerge upon stimulation by IL-4 or IL-13 and have been associated with tissue remodeling and repair, as well as IL-4-dependent macrophage fusion (7). Thus E-cadherin expressing histiocytes could potentially correspond to AAMacs in foreign body granulomas (Figure 3A). As opposed to AAMacs, E-cadherin+ AADCs maturing at inflammatory tissue foci from circulating monocytes are potent IL-12 producing cells that can augment IFN-γ producing TH1 responses (8). Thus, it is conceivable that in the early, initiating phase of sarcoidal granuloma formation, an IL-4/IL-13-driven innate reaction composed of AADCs initiates and augments the characteristic TH1 milieu observed in well-formed sarcoidal granulomas (Figure 3B). Similar immune regulatory loops between TH1 and TH2 responses have been observed in infectious animal models (18). Alternatively, the E-cadherin+ mononuclear histiocytes in sarcoidal samples can represent IL-23 producing inflammatory DCs that had been described in a mouse model of inflammatory colitis.(15). These cells could potentiate a TH17 response that had been associated with sarcoidosis.(4, 10). Thus, E-cadherin+ histiocytes in sarcoidosis could promote both TH1 and TH17 responses, which would synergistically activate IFN-γ and IL-17A-dependent myeloid fusion pathways leading to MNGC formation (Fig. 3B).(19) The presence of E-cadherin expressing histiocytes rimming e-cadherin+ MNGCs also suggests that E-cadherin+ mononuclear cells correspond to fusogenic precursors for MNGCs in both FBGR and sarcoidosis (Fig. 3A and B). The critical role for E-cadherin-dependent cell-cell adhesion in macrophage fusion also supports this notion (13).
Figure 3.
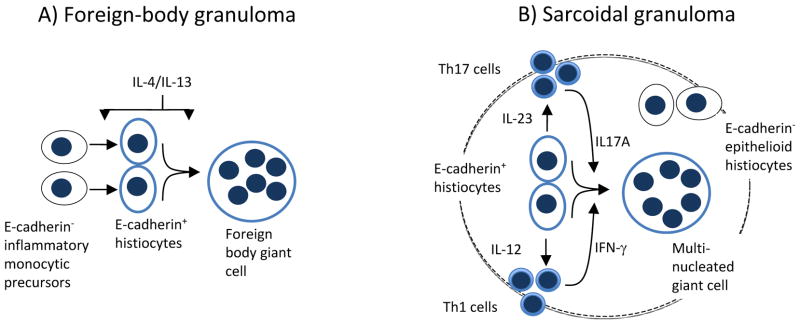
Potential immune function of E-cadherin+ histiocytes in foreign body and sarcoidal granulomas. (A) IL-4/IL-13 promotes the differentiation of E-cadherin+ AAMacs from E-cadherin− monocytic precursors. Fusion of E-cadherin+ AAMacs results in MNGC formation. (B) Presumed potentiation of TH1 and TH17 responses in sarcoidosis by E-cadherin+ AADCs and IL-23-producing inflammatory DCs, respectively. IFN-γ and IL-17A promote the fusion of E-cadherin+ mononuclear precursors to form E-cadherin+ MNGCs.
Overall, our data suggest that E-cadherin expressing histiocytes may play an essential role in cutaneous granuloma formation. Since FBGR and sarcoidosis represent distinct immune processes, E-cadherin+ histiocytes could be important in a broader spectrum of granulomatous reactions. Our ongoing efforts are aimed to assess the presence of these cells in both infectious and non-infectious granulomas including granuloma annulare, necrobiosis lipoidica and necrobiotic xanthogranuloma. Better understanding of the role of these innate cells in cutaneous granulomatous disorders, may provide further understanding of the disease pathogenesis and could lead to alternative therapeutic interventions.
Acknowledgments
Funding sources: This was supported in part by grants received from the Penn Skin Disease Research Center (NIAMS P30-AR057217) (to A.S. and M.R.), Center for Human Appearance at the University of Pennsylvania (to A.S. and M.R.), Career Development Awards from the National Institute of Health (NIAID, K08AI080138 to AS) and Dermatology Foundation (to M.R.).
Abbreviations
- FBGR
foreign body granulomatous reaction
- DCs
dendritic cells
- AAMacs
alternatively activated macrophages
- AADCs
alternatively activated dendritic cells
- MNGCs
multi-nucleated giant cells
Footnotes
Conflict of Interest: None
References
- 1.Boros DL. In: The Cellular Immunological Aspects of the Granulomatous Response. Boros DL, editor. Washington: ASM Press; 2003. pp. 1–28. [Google Scholar]
- 2.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153–65. doi: 10.1056/NEJMra071714. Epub 2007/11/23. [DOI] [PubMed] [Google Scholar]
- 3.Bergeron A, Bonay M, Kambouchner M, et al. Cytokine patterns in tuberculous and sarcoid granulomas: correlations with histopathologic features of the granulomatous response. Journal of immunology. 1997;159(6):3034–43. Epub 1997/09/23. [PubMed] [Google Scholar]
- 4.Ten Berge B, Paats MS, Bergen IM, et al. Increased IL-17A expression in granulomas and in circulating memory T cells in sarcoidosis. Rheumatology (Oxford) 2012;51(1):37–46. doi: 10.1093/rheumatology/ker316. Epub 2011/11/15. [DOI] [PubMed] [Google Scholar]
- 5.Kao WJ, McNally AK, Hiltner A, Anderson JM. Role for interleukin-4 in foreign-body giant cell formation on a poly(etherurethane urea) in vivo. J Biomed Mater Res. 1995;29(10):1267–75. doi: 10.1002/jbm.820291014. Epub 1995/10/01. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez A, Macewan SR, Meyerson H, Kirk JT, Anderson JM. The foreign body reaction in T-cell-deficient mice. J Biomed Mater Res A. 2009;90(1):106–13. doi: 10.1002/jbm.a.32050. Epub 2008/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. Epub 2010/06/01. [DOI] [PubMed] [Google Scholar]
- 8.Cook PC, Jones LH, Jenkins SJ, Wynn TA, Allen JE, Macdonald AS. Alternatively activated dendritic cells regulate CD4+ T-cell polarization in vitro and in vivo. Proc Natl Acad Sci U S A. 2012;109(25):9977–82. doi: 10.1073/pnas.1121231109. Epub 2012/06/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helming L, Gordon S. Macrophage fusion induced by IL-4 alternative activation is a multistage process involving multiple target molecules. Eur J Immunol. 2007;37(1):33–42. doi: 10.1002/eji.200636788. Epub 2006/12/13. [DOI] [PubMed] [Google Scholar]
- 10.Facco M, Cabrelle A, Teramo A, et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax. 2011;66(2):144–50. doi: 10.1136/thx.2010.140319. [DOI] [PubMed] [Google Scholar]
- 11.Tunggal JA, Helfrich I, Schmitz A, et al. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J. 2005;24(6):1146–56. doi: 10.1038/sj.emboj.7600605. Epub 2005/03/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang A, Amagai M, Granger LG, Stanley JR, Udey MC. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature. 1993;361(6407):82–5. doi: 10.1038/361082a0. Epub 1993/01/07. [DOI] [PubMed] [Google Scholar]
- 13.Van den Bossche J, Bogaert P, van Hengel J, et al. Alternatively activated macrophages engage in homotypic and heterotypic interactions through IL-4 and polyamine-induced E-cadherin/catenin complexes. Blood. 2009;114(21):4664–74. doi: 10.1182/blood-2009-05-221598. Epub 2009/09/04. [DOI] [PubMed] [Google Scholar]
- 14.Van den Bossche J, Malissen B, Mantovani A, De Baetselier P, Van Ginderachter JA. Regulation and function of the E-cadherin/catenin complex in cells of the monocyte-macrophage lineage and DCs. Blood. 2012;119(7):1623–33. doi: 10.1182/blood-2011-10-384289. Epub 2011/12/17. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui KR, Laffont S, Powrie F. E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity. 2010;32(4):557–67. doi: 10.1016/j.immuni.2010.03.017. Epub 2010/04/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.BKW The origin of the white blood cell. J Am Med Assoc. 1934;103(1523) [Google Scholar]
- 17.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–64. doi: 10.1038/nri1733. Epub 2005/12/03. [DOI] [PubMed] [Google Scholar]
- 18.Biedermann T, Zimmermann S, Himmelrich H, et al. IL-4 instructs TH1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nat Immunol. 2001;2(11):1054–60. doi: 10.1038/ni725. Epub 2001/10/16. [DOI] [PubMed] [Google Scholar]
- 19.Coury F, Annels N, Rivollier A, et al. Langerhans cell histiocytosis reveals a new IL-17A-dependent pathway of dendritic cell fusion. Nat Med. 2008;14(1):81–7. doi: 10.1038/nm1694. Epub 2007/12/25. [DOI] [PubMed] [Google Scholar]


