Abstract
Objective
To examine the autonomic nervous system and neurobehavioral response to a sustained visual attention challenge among 1-month old infants with prenatal substance exposure.
Study design
We measured heart rate (HR), respiratory sinus arrhythmia (RSA), and neurobehavior during sustained visual orientation tasks included in the NICU Network Neurobehavioral Scale (NNNS) in 1,129, 1-month infants with prenatal substance exposure. Four groups were compared: infants with prenatal cocaine and opiate exposure, infants with cocaine exposure, infants with opiate exposure, and infants with exposure to other substances (i.e. alcohol, marijuana, and tobacco).
Results
Infants with prenatal cocaine and opiate exposure had the highest HRs and lowest levels of RSA during a sustained visual attention procedure compared with the other three groups. Infants with prenatal cocaine and opiate exposure had poorer quality of movement and more hypertonicity during the NNNS exam compared with the other three exposure groups. Infants with prenatal cocaine and opiate exposure had more nonoptimal reflexes and stress/abstinence signs compared with infants with prenatal cocaine exposure only and infants with prenatal exposure to alcohol, tobacco, and marijuana.
Conclusions
Problems with arousal regulation were identified among infants with prenatal substance exposure. Autonomic dysregulation has been implicated as a mechanism by which these difficulties occur. Our results suggest that infants with both prenatal cocaine and opiate exposure have the greatest autonomic response to the challenge of a sustained visual attention task, which may place these infants at risk for developing problems associated with physiological and behavioral regulation, a necessary prerequisite for early learning.
Keywords: in utero drug exposure, physiology, neurobehavioral
Infants with prenatal cocaine exposure are more likely to exhibit difficulties with the regulation of arousal and attention at behavioral and physiological levels1 which may require repeated, or “hypervigilant” activation of the autonomic nervous system2. One measure of autonomic nervous system functioning is respiratory sinus arrhythmia (RSA). RSA is a measure of heart rate (HR) variability that coincides with breathing that reflects parasympathetic control of engagement with the environment3–5. Moderate decreases in RSA from baseline (termed RSA reactivity) occur during the stimulation that facilitates the ability to coordinate cognitive, behavioral, and emotional systems to flexibly respond to environmental demands6. A better understanding of how infants with prenatal substance exposure respond physiologically to challenge may elucidate possible mechanisms involved in the development of difficulties with regulation and attention in this high risk group of children. Here, we measured RSA in infants with prenatal substance exposure in response to a visual attention challenge from the NICU Network Neurobehavioral Scale, NNNS7.
In previous work, we reported neurobehavioral effects of prenatal cocaine or opiate exposure at 1 month using the NNNS8. Infants with cocaine exposure were less aroused, had poorer self-regulation, and were more excitable than non cocaine-exposed infants. Infants with opiate exposure only did not differ from cocaine exposed infants or non-exposed infants on the NNNS. However, infants with both cocaine and opiate exposure may be at greater risk for difficulties with arousal modulation at both the behavioral and physiological level than infants exposed to either substance alone. We examined NNNS scores and HR and RSA during visual orientation in infants with prenatal cocaine and opiate exposure to determine if these infants are at increased risk for difficulties with behavioral and physiological regulation in response to mild challenge.
Methods
This report is from the Maternal Lifestyle Study (MLS) multisite longitudinal cohort study on the evaluation of the long-term outcomes of children exposed to cocaine in utero. Enrollment and exclusion criteria for the MLS have been described in detail8. In brief, mothers with informed consent were enrolled at birth of an infant, at which time meconium and maternal report of drug use was obtained. The study had approval from the institutional review board at each site. Each site also had a certificate of confidentiality from the National Institute on Drug Abuse. Infants in the longitudinal study were selected to be in the exposed group (maternal report of cocaine or opiate use during pregnancy or gas chromatography–mass spectrometry confirmation of presumptive positive meconium screens for cocaine or opiate metabolites) or the comparison group (maternal denial of cocaine or opiate use during the pregnancy and a negative enzyme multiplied immunoassay meconium screen for cocaine and opiate metabolites). Because women who use cocaine and opiates frequently use other substances as well (ie, alcohol, tobacco, and marijuana), these substances were included in the exposed and comparison groups. Infants were also matched for race, sex, and gestational age. Mother-infant dyads (n =1388), 658 in the exposed group and 730 in the comparison group, were enrolled between 42–44 weeks post-menstrual age (PMA). For the present study the sample was further divided into 4 groups; cocaine exposed (n = 546), opiate exposed (n = 57), other substance exposed (n = 466) and cocaine and opiate exposed (n = 60) infants.
The NNNS was administered between 42–44 weeks PMA. The NNNS is a standardized comprehensive evaluation of the neurobehavioral performance of high-risk term and preterm infants that includes neurological and behavioral measures and signs of stress7. Psychometric properties of the exam have been established7. The NNNS was administered by certified psychometrists blinded to exposure status. Summary scores using the NNNS include: Attention, handling, self-regulation, arousal, excitability, lethargy, hypertonicity, hypotonicity, non-optimal reflexes, asymmetrical reflexes, quality of movement, and stress abstinence signs.
HR and RSA were measured for specific episodes during the administration of the NNNS and derived from the R-R time-series collected from digitized ECG recordings using Porges algorithm from MXEdit, Delta-Biometrics Inc, 1988–19939,10. ECG was recorded via three electrodes placed on the infant’s chest and abdomen. The ECG signal was sampled at 1kHz and stored on a computer for later analysis. Interbeat intervals were defined by detection of R-waves to the nearest ms.
Postprocessing of the data was conducted using a series of automated algorithms. R-R intervals outside of expected values were identified. Missed or spurious R-waves were flagged and corrected by linear interpolation. A 21-point moving polynomial was then applied to remove low frequency trends in the HR signal. A bandpass filter extracted the variance in heart period within the frequency band of spontaneous respiration in infants (0.24–1.04 Hz) by removing periodicities in the ECG signal that are outside the frequency range of the respiratory cycle. The resulting measure of RSA is within the frequency range of respiration. RSA was computed as the natural logarithm of heart period variance and reported in units of milliseconds squared, ln(ms)2. RSA data were calculated in 30 second overlapping windows and then averaged within each episode (baseline and attention task). The RSA data for an individual were used as long as there was a 30-s segment with less than 20% of segments identified with artifact11. Small amounts of artifact can be expected to have a minimal effect on measures of heart rate variability such as RSA3.
For the baseline period the infant was in a quiet awake state (state 4 on the NNNS) for at least 2 minutes (M = 2.23 minutes). HR and RSA were measured during the NNNS visual attention procedure. This procedure lasted, on average, 6.8 minutes (± 2.43 minutes) and includes infant visual tracking to animate and inanimate stimuli. This segment was chosen because tasks which challenge the infant’s attention are commonly used to assess HR and RSA12–14. During a mild challenge such as a visual orientation task, faster and shallower breathing gaits cardiac activity, typically resulting in increases in HR and decreases in RSA, reflecting decreased parasympathetic activity. Moderate decreases in RSA are considered functional, and reflect the infants’ ability to coordinate attention and behavior to “optimally” engage with the environment. On the other hand, large decreases of RSA to attention challenge may be indicative of behavioral dysregulation and risk for maladaptive fight/flight responding6,15. Estimates of HR and RSA were averaged across the attention episode. Higher values of HR and lower values of RSA indicate a greater physiological response to the visual orientation task.
Statistical Analyses
To evaluate whether infants in the different exposure groups varied with respect to behavior on the NNNS and HR and RSA during the orientation task we ran a general linear model univariate analysis of co-variance, controlling for the covariates listed below as well as baseline HR and RSA (in separate models). We used an ANCOVA to assess physiological response to visual orientation (e.g. including baseline levels as a covariate) as controlling for baseline physiology, which is more reliable than using a change score in which the unreliability of each variable is compounded. A four-level variable consisting of infants with cocaine exposure only, opiate exposure only, other substance exposure (alcohol, tobacco, marijuana), and cocaine and opiate exposure was entered as a predictor of HR and RSA (in separate models) during the attention procedure.
Covariates were included if correlated (p<.05) with HR during baseline, HR during the attention procedure or with exposure status. Variables that met these criteria for HR analysis and included as covariates were sex, birth weight, race, site, PMA, and maternal age. Variables that met these criteria for RSA analysis and included as covariates were SES, birth weight, race, site, PMA, and maternal age.
Results
Complete NNNS and exposure status data were available for 1,129 participants. Complete demographic and HR data were available for 886 of the original sample, and complete demographic and RSA data were available for 825 participants. Comparison of the characteristics between the included infants and those not included showed differences in maternal age, low SES and race (Table I; available at www.jpeds.com). The four study groups (Table I) differed in maternal age, birth weight, race, PMA, and prenatal exposure to alcohol, tobacco and marijuana.
Table 1.
Demographic characteristics by exposure group
| Other substance exposed n = 466 (Mean ± SD or %) |
Cocaine exposed n = 546 (Mean ± SD or %) |
Opiate exposed n = 57 (Mean ± SD or %) |
Cocaine + Opiate exposure n = 60 (Mean ± SD or %) |
p | |
|---|---|---|---|---|---|
| Maternal age | 26.9 ± 5.8 | 30.2 ± 4.8 | 29.7 ± 7.2 | 32.0 ± 5.6 | <.001 |
| Birth weight (g) | 2762.5 ± 812.5 | 2561.1 ± 739.7 | 2849.8 ± 820.8 | 2392.5 ± 806.8 | <.001 |
| Postmenstrual age (weeks) | 40.8 ± 3.7 | 40.1 ± 4.0 | 41.2 ± 3.6 | 40.0 ± 4.4 | .02 |
| SES (continuous) | .35 | ||||
| Low (IV–V) | 55.9 | 61.4 | 56.6 | 61.1 | |
| Sex | .22 | ||||
| Male | 52.4 | 53.1 | 61.4 | 35 | |
| Race | <.001 | ||||
| Caucasian | 17.4 | 12.5 | 43.9 | 38.3 | |
| African | 75.1 | 80.0 | 45.6 | 55.0 | |
| American | |||||
| Hispanic | 6.0 | 6.4 | 7.0 | 5.0 | |
| Other | 1.5 | 1.1 | 3.5 | 1.7 | |
| Prenatal alcohol exposure | 77.0 | 75.1 | 36.8 | 58.3 | <.001 |
| Prenatal tobacco exposure | 44.4 | 82.8 | 59.6 | 91.7 | <.001 |
| Prenatal marijuana exposure | 15.2 | 41.0 | 12.3 | 36.7 | <.001 |
Bivariate correlations revealed strong, significant negative correlations between HR and RSA, and small, though significant positive correlations between baseline HR and HR during orientation and arousal and excitability scores on the NNNS (Table II). Examination of scatterplots of HR/RSA during baseline and during the NNNS orientation task revealed linear associations. We also examined the possibility of suppressor effects of PMA and birth weight and no support was found; results were consistent regardless of whether PMA and birth weight were entered separately or in combination.
Table 2.
Correlations among study measures
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | 15. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Baseline HR | --- | ||||||||||||||
| 2. HR during orientation | .52*** | --- | |||||||||||||
| 3. Baseline RSA | −.41*** | −.32*** | --- | ||||||||||||
| 4. RSA during orientation | −.33*** | −.57*** | .62*** | --- | |||||||||||
| 5. Attention | .02 | −.04 | −.05 | −.02 | --- | ||||||||||
| 6. Arousal | .15*** | .10** | −.03 | −.07* | −.31*** | --- | |||||||||
| 7. Self-regulation | −.12*** | −.05 | .02 | .05 | .38*** | −.65*** | --- | ||||||||
| 8. Amount of Handling | .04 | .10*** | −.01 | −.06 | −.36*** | .52*** | −.45*** | --- | |||||||
| 9. Quality of movement | −.07* | −.02 | .02 | .02 | .10** | −.40*** | .58*** | −.22*** | --- | ||||||
| 10. Excitability | .13*** | .07* | −.05 | −.08* | −.26*** | .82*** | −.79*** | .45*** | −.59*** | --- | |||||
| 11. Lethargy | −.09** | .02 | .07* | .04 | −.63*** | −.24*** | .02 | −.04 | .18*** | −.26*** | --- | ||||
| 12. Non-optimal reflexes | .04 | −.05 | −.06 | −.04 | .07* | −.10** | .07* | −.13*** | −.04 | −.04 | .05 | --- | |||
| 13. Asymmetrical reflexes | −.04 | −.03 | .02 | −.01 | .09** | −.12*** | .12*** | −.10*** | .02 | −.09** | −.001 | .08** | --- | ||
| 14. Hypertonicity | .02 | −.06 | −.01 | .04 | .03 | .20*** | −.20*** | .06 | −.24*** | .28*** | −.16*** | .18*** | .04 --- | ||
| 15. Hypotonicity | −.03 | −.05 | .04 | .02 | .03 | −.11*** | .01 | −.09** | .003 | −.06* | .15*** | .18*** | −.03 | −.03 | --- |
| 16. Stress Abstinence | .06 | .04 | −.05 | −.08* | −.12*** | .39*** | −.48*** | .32*** | −.54*** | .46*** | −.11*** | .15*** | −.004 | .33*** | .03 |
Note. HR, Heart Rate; RSA, Respiratory Sinus Arrhythmia;
p <.001;
p <.01;
p <.05.
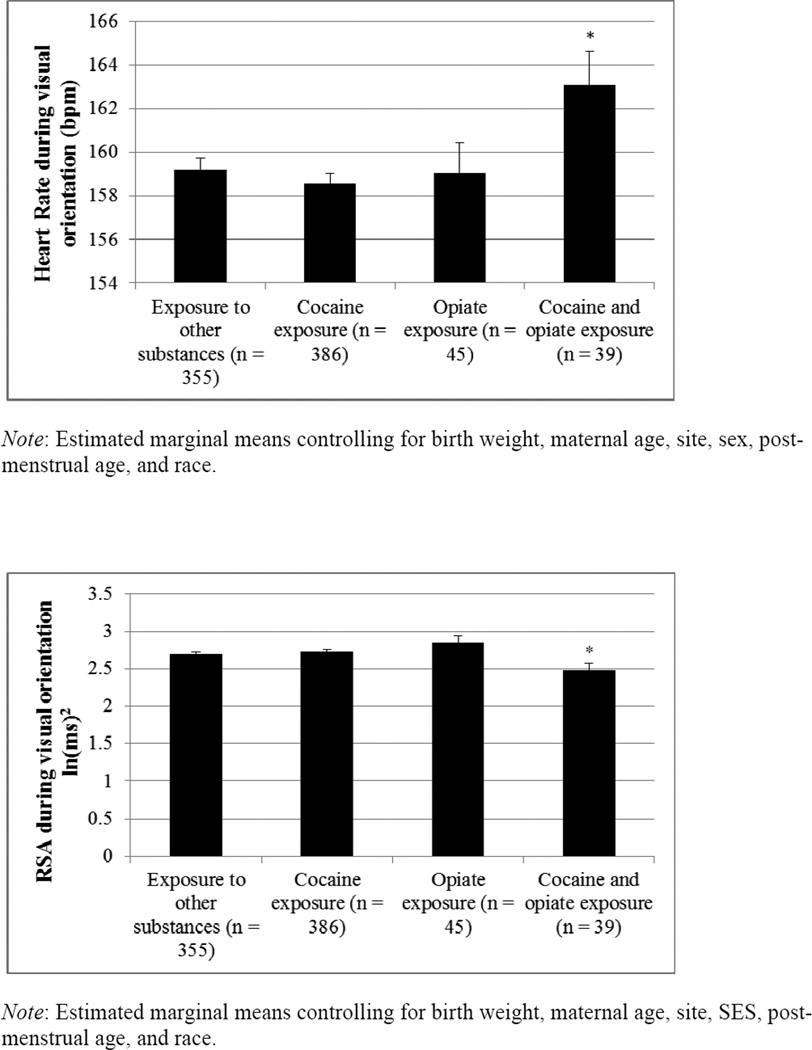
Prenatal substance exposure was a significant predictor of HR during the attention procedure, controlling for baseline HR and the other covariates, F (3, 874) = 2.83, p = .04 (Table III). Planned contrasts (Figure) showed that infants with prenatal cocaine and opiate exposure had higher HRs than infants with cocaine exposure only (p = .004), infants with opiate exposure only (p = .05), and infants with exposure to other substances (p = .02). There were no statistically significant differences in HR between infants in the other three groups.
Table 3.
Analysis of Covariance Predicting HR and RSA during orientation
| Dependent Variable: HR during orientation | |
| Parameter | F(1, 874) |
| SES | .41 |
| Site | .45 |
| Birth weight | 10.17*** |
| Maternal age | 1.15 |
| Race | .11 |
| Sex | .98 |
| PMA | 2.34 |
| Baseline HR | 304.08*** |
| Prenatal drug exposure | 2.83* |
| Dependent Variable: RSA during orientation | |
| Parameter | F(1, 814) |
| SES | 3.58 |
| Site | .49 |
| Birth weight | 4.12* |
| Maternal age | 3.80* |
| Race | 2.68 |
| PMA | .59 |
| Baseline RSA | 508.10*** |
| Prenatal drug exposure | 3.61** |
Note:
p <.001;
p <.01;
p <.05.
Figure 1.
HR (top panel) and RSA (bottom panel) during NNNS visual orientation procedure by exposure classification.
Prenatal substance exposure was also a significant predictor of RSA during the attention procedure, controlling for baseline RSA and the other covariates, F(3, 814) = 3.11, p = .03 (Table III). Planned contrasts (Figure) showed that infants with prenatal cocaine and opiate exposure had lower RSA compared with infants with cocaine exposure only (p = .01), infants with opiate exposure only (p = .004), and infants with exposure to other substances (p = .04). There were no statistically significant differences in RSA between infants in the other three groups.
On the NNNS (Table IV), infants with prenatal cocaine and opiate exposure had lower quality of movement, (F(3, 1069) = 2.64, p = 0.05), and more hypertonicity (F(3, 1076) = 2.92, p = 0.03), than infants in the other three exposure groups. Infants with prenatal cocaine and opiate exposure had more nonoptimal reflexes (F(3, 1079) = 2.61, p = 0.05), and stress/abstinence signs (F(3, 1079) = 6.60, p <.001) than infants with prenatal cocaine exposure only and prenatal exposure to alcohol, tobacco, and marijuana (but not compared with infants with prenatal opiate exposure only).
Table 4.
Physiological Measures and NNNS Scales in Substance-exposed Infants
| Other substance exposed n = 466 (Mean ± SE) |
Cocaine exposed n = 546 (Mean ± SE) |
Opiate exposed n = 57 (Mean ± SE) |
Cocaine + Opiate exposure n = 60 (Mean ± SE) |
p | |
|---|---|---|---|---|---|
| Baseline HR | 158.5 ± .8 | 158.0 ± .7 | 159.0 ± 2.1 | 163.5 ± 2.2 | .13 |
| Baseline RSA | 2.8 ± .9 | 2.9 ± .9 | 2.8 ± .8 | 2.7 ± .9 | .57 |
| HR during visual orientation | 158.9 ± .6 | 158.5 ± .5 | 158.8 ± 1.6 | 165.0 ± 1.7 | .003 |
| RSA during visual orientation | 2.67 ± .04 | 2.77 ± .04 | 2.84 ± .11 | 2.47 ± .11 | .03 |
| Attention | 5.35 ± 0.07 | 5.34 ± 0.06 | 5.58 ± 0.19 | 5.68 ± 0.19 | 0.23 |
| Arousal | 4.42 ± 0.03 | 4.38 ± 0.03 | 4.44 ± 0.10 | 4.45 ± 0.10 | 0.78 |
| Regulation | 5.02 ± 0.04 | 5.01 ± 0.04 | 4.91 ± 0.12 | 4.81 ± .12 | 0.34 |
| Handling | 0.56 ± 0.01 | 0.53 ± 0.01 | 0.58 ± 0.04 | 0.55 ± 0.04 | 0.46 |
| Quality of movement | 4.38 ± 0.04 | 4.39 ± 0.03 | 4.54 ± 0.10 | 4.14 ± 0.10 | 0.05 |
| Excitability | 4.60 ± 0.13 | 4.60 ± 0.12 | 4.49 ± 0.36 | 5.14 ± 0.36 | 0.53 |
| Lethargy | 3.32 ± 0.11 | 3.57 ± 0.10 | 3.13 ± 0.30 | 2.95 ± 0.30 | 0.08 |
| Nonoptimal reflexes | 4.47 ± 0.10 | 4.40 ± 0.10 | 4.73 ± 0.30 | 5.22 ± 0.30 | 0.05 |
| Asymmetrical reflexes | 0.84 ± 0.05 | 0.87 ± 0.05 | 0.67 ± 0.15 | 1.03 ± .015 | 0.40 |
| Hypertonicity | 0.57 ± 0.05 | 0.57 ± 0.04 | 0.47 ± 0.13 | 0.95 ± 0.13 | 0.03 |
| Hypotonicity | 0.24 ± 0.03 | 0.22 ± 0.03 | 0.29 ± 0.08 | 0.35 ± 0.08 | 0.38 |
| Stress/abstinence | 0.18 ± 0.004 | 0.17 ± 0.004 | 0.20 ± 0.01 | 0.22 ± 0.01 | <0.001 |
Note: Estimated marginal means controlling for birth weight, site, sex, and post-menstrual age.
Discussion
Infants with prenatal cocaine and opiate exposure exhibited the most neurobiological dysregulation in the NNNS assessment at both the behavioral (NNNS) and physiological (HR and RSA) levels compared with infants with prenatal cocaine or opiate exposure alone or infants with prenatal exposure to other substances. The majority of research on substance-exposed infants has been limited to the effects of a single substance, such as cocaine, or opiates, while controlling for the effects of other substances16. A small body of research, however, suggests that infants with cocaine and opiate exposure may be at greater risk for poor developmental outcomes8,17. Infants with prenatal cocaine1,8 and opiate18 exposure are at risk for difficulties with modulating arousal and attention, and subsequent problems with emotion regulation1,19 and learning20 by middle childhood. Yet unknown are the mechanisms by which infants with prenatal cocaine and opiate exposure may be at risk for these negative outcomes, though a promising avenue of research involves physiological aspects of regulatory systems.
Physiological systems subserving attention and arousal, specifically HR and RSA, have previously been studied among infants with prenatal cocaine or opiate exposure as both HR and RSA are involved in the control of visual attention14 and arousal regulation21. Findings from studies of HR and RSA in infants with prenatal cocaine or opiate exposure are mixed. In one study, infants who were cocaine and opiate exposed exhibited greater HR and lower RSA at baseline22 compared with infants with opiate exposure only, though this study did not covary the effects of demographic and medical variables that may have confounded results. In addition, baseline levels of HR or RSA are typically not used to index arousal regulation and excitability. A better index is RSA during challenge21. Infants with prenatal cocaine exposure typically do not exhibit RSA responses to challenge23,24,25 compared with non-exposed controls; however, these studies did not include infants with opiate exposure and did not adequately control for the effects of other substances. There are only two studies of RSA in children with prenatal opiate exposure. In one, opiate exposed 7–12 year-old boys did not exhibit RSA reactivity during an attention-demanding task18. In the other, RSA reactivity was greater in opiate and alcohol exposed 7–12 year-old children than controls26.
The inconsistent findings may be due to differences in age of assessment of RSA reactivity, lack of adequate control for the effects of polysubstance exposure, or exclusion of participants who are both cocaine and opiate-exposed. The tasks used to assess RSA reactivity also may not have been potent enough to tax the attention systems. We used a visual attention task that is analogous to visual exploration that is a necessary prerequisite for object exploration and subsequent early learning, and attention-demanding tasks have commonly been used to elicit RSA and HR responses21. Our results may be used to help identify infants who may need additional support in learning how to self-regulate in the context of interacting with attention-demanding stimuli. In addition, they point to a possible mechanism by which infants with prenatal cocaine and opiate exposure exhibit difficulties with attention and arousal modulation.
In typically developing infants HR increases and RSA decreases in response to attention demanding stimuli12,14. Moderate decreases in RSA are characteristic of focused attention and self-regulation13,14 as parasympathetic withdrawal provides the activation needed to respond to attention demands. Thus, one interpretation of our findings could simply be that the cocaine and opiate exposed infants in our study required more parasympathetic withdrawal to mount the response to the demands of attention stimulation. Although the mechanisms of action are different between cocaine and opiates27, they both affect reward circuitry in the brain; specifically, the ventral tegmental area and extended amygdala which are regions rich in monoamines and are involved in reactivity to stress and regulation27. The combined impact of cocaine and opiate exposure could require these infants to utilize greater neurobiological resources in order to meet environmental demands, thereby further taxing an already stressed central nervous system28,29.
A second interpretation is that the greater decreases in RSA among infants with prenatal cocaine and opiate exposure reflect a stress response6. Sympathetic arousal is part of the “flight or fight” reaction that also includes activation of the HPA system and release of cortisol. Thus, we suggest that the increased HR response observed in the infants exposed to both cocaine and opiates could indicate increased sympathetic activity as part of a stress response. These infants may have shown an acute response to stress rather than the ability to respond to the demands of attention stimulation. According to the framework of allostatic load30, repeated activation of physiological systems results in increased likelihood of psychological and medical morbidity due to “wear and tear” on these systems. Over time, the autonomic nervous system may become overly stressed, making it more likely for these infants to exhibit autonomic dysregulation in response to even mild attention-demanding stimuli.
Prenatal substance exposure can also alter physiological stress response systems through epigenetic mechanisms that increase fetal exposure to maternal cortisol affecting physiological and neurobehavioral stress reactivity in the infant31. Epigenetic changes in placental genes that regulate fetal exposure to maternal cortisol have been related to newborn neurobehavior on the NNNS, including infant attention32 and quality of movement33. The findings that cocaine and opiate exposed infants also show increased HR and RSA responses on the NNNS during an attention-demanding task, and poorer quality of movement and greater hypertonicity is consistent with the interpretation that these infants were exhibiting a stress response. Future research is needed to determine whether this response may be impacted via epigenetic processes. These results do not preclude the possibility, however, that exposure to drugs other than cocaine and opiates have teratogenic effects.
This study has limitations. An unexposed comparison group of infants with no prenatal substance exposure may have helped clarify our findings. For instance, we would have been able to determine whether infants with prenatal cocaine and opiate exposure were exhibiting significantly greater HR and lower RSA than unexposed infants. However, the fact that these differences were observed among groups of infants, all with some form of exposure to substances prenatally argues for the robust effects found in this study. Although prenatal exposure to alcohol, marijuana or tobacco did not met criteria for inclusion as covariates, there were some group differences in exposure to these other substances. However, because our criteria for inclusion of covariates was well established in the literature and defined a priori, we feel that our comparison groups were appropriate.
The first month of life represents a particularly vulnerable time for infants, characterized by high levels of cortisol secretion as the infant learns to become more physiologically and behaviorally organized. Infants with both prenatal cocaine and opiate exposure may be a more susceptible group given their high levels of sympathetic and parasympathetic responses to a sustained visual attention challenge, and because behaviorally they exhibited more motoric disorganization. As the autonomic nervous system is involved in the maintenance of coordination of attention and arousal, our results suggest that early difficulties with regulation of arousal, exacerbated by the increased likelihood of being reared in an adverse environment, may make the infant who is exposed to cocaine and opiates more vulnerable to difficulties with arousal regulation. An important developmental objective for the infant is to coordinate biological and behavioral attention systems with the goal of maintaining a calm state while visually inspecting new stimuli. This ability is also a necessary prerequisite for early learning. That infants with prenatal cocaine and opiate exposure had difficulties with biobehavioral regulation suggests that they may be at increased risk for problems with regulation later in development, in part due to heightened physiological responses identifiable by 1 month.
These findings could point to the importance of including physiological measures in addition to behavioral assessments to identify which infants may need extra support and intervention. The ability to differentiate infants who show a stress response from infants who show the expected response to environmental demands could have implications for preventive intervention; for example, to combat the well-known long term effects of chronic stress or allostatic load30,34. In clinical practice, our group uses the NNNS to help management of infants while in hospital. For example, some substance exposed infants benefit from soothing strategies and proprioceptive interventions to support infants’ motor organization. These interventions include containment, use of a pacifier, neutral warmth, and infant massage, preferably by the parent35. The goal is to capitalize on the malleability of the infant neurobiological stress response to support sensitive caregiving experiences and ultimately promote positive adaptation.
Acknowledgments
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Abbreviations
- HR
Heart Rate
- RSA
Respiratory Sinus Arrhythmia
- NNNS
NICU Network Neurobehavioral Scale
- ECG
Electrocardiogram
Appendix 1
The following individuals and federal funding grants contributed to this study and, in addition to the authors, are members of <>:
Brown University, Warren Alpert Medical School Women & Infants Hospital of Rhode Island (U10 HD27904, N01 HD23159) – Cynthia Miller-Loncar, PhD; Jean Twomey, PhD; Laura Dietz, MA; Melissa Kupchak, RN.
National Institute on Drug Abuse – Vincent L. Smeriglio, PhD; Nicolette Borek, PhD.
RTI International (U10 HD36790) – W. Kenneth Poole, PhD; Abhik Das, PhD; Debra Fleischmann, BS.
University of Miami Holtz Children's Hospital (GCRC M01 RR16587, U10 HD21397) – Ann L. Graziotti, MSN, ARNP; Tonya Barriere-Perez, MSW; Janine Closius, BS; Diedre Gallop, MSW; Edgar Garcia, RN ARNP; Susan Gauthier, BA; Wendy Griffin, RN; Elizabeth Jacque, RN; Jennifer Lewis; Daniel A. Messinger, PhD; Yamille Valdez.
University of Tennessee (U10 HD42638) – Charlotte Bursi, MSSW; Deloris Lee, MSSW; Lillie Hughey, MSSW.
Wayne State University Hutzel Women’s Hospital and Children’s Hospital of Michigan (U10 HD21385) – Eunice Woldt, RN MSN; Jay Ann Nelson, BSN; Catherine Bartholomay, BA; Lisa Sulkowski, BS; Nicole Walker, BA.
Appendix 2
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network and an interinstitute agreement with the National Institute on Drug Abuse (NIDA) through cooperative agreements (U10-DA-024117-01, U10-HD-21385 [to S.S.], I10-DA-024128-06, U10-HD-2786 [to H.B.], U10-DA-024119-01, U10-HD-27904 [to B.L.], U10-DA-024118-01, and U10-HD-21397 [to C.B.]), NICHD contract (N01-HD-2-3159 to B.L.), and a National Research Service Award from the National Institute on Drug Abuse (F32DA032175 to E.C.). Support for the Maternal Lifestyle Study was provided by the National Institutes of Health through the National Institute on Drug Abuse and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with supplemental funding from the National Institute of Mental Health, the Administration on Children, Youth, and Families, and the Center for Substance Abuse and Treatment, US Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, or the National Institutes of Health. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Funding and conflict of interest information is available at www.jpeds.com (Appendix 2).
References
- 1.Mayes LC, Grillon C, Granger R, Schottenfeld R. Regulation of arousal and attention in preschool children exposed to cocaine prenatally. Ann N Y Acad Sci. 1998;846:126–143. [PubMed] [Google Scholar]
- 2.Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosom Med. 2007;69:935–943. doi: 10.1097/PSY.0b013e31815a8f6b. [DOI] [PubMed] [Google Scholar]
- 3.Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 4.Grossman Respiration, stress, and cardiovascular function. Psychophysiology. 1983;20:284–300. doi: 10.1111/j.1469-8986.1983.tb02156.x. [DOI] [PubMed] [Google Scholar]
- 5.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beauchaine TP. Vagal tone, development, and Gray's motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- 7.Lester BM, Tronick EZ. History and description of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113:634–640. [PubMed] [Google Scholar]
- 8.Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, et al. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002;110:1182–1192. doi: 10.1542/peds.110.6.1182. [DOI] [PubMed] [Google Scholar]
- 9.Porges SW. Method and apparatus for evaluating rhythmic oscillations in aperiodic physiological response systems. US. 1985 [Google Scholar]
- 10.Porges SW. Respiratory sinus arrhythmia: Physiological basis, quantitative methods, and clinical implications. Cardiorespiratory and cardiosomatic psychophysiology. 1986;114:101–115. [Google Scholar]
- 11.Jennings JR, Berg WK, Hutcheson JS, Obrist P, Porges S, Turpin G. Committee report. Publication guidelines for heart rate studies in man. Psychophysiology. 1981;18:226–231. doi: 10.1111/j.1469-8986.1981.tb03023.x. [DOI] [PubMed] [Google Scholar]
- 12.Richards JE, Casey BJ. Heart rate variability during attention phases in young infants. Psychophysiology. 1991;28:43–53. doi: 10.1111/j.1469-8986.1991.tb03385.x. [DOI] [PubMed] [Google Scholar]
- 13.Bornstein MH, Suess PE. Child and mother cardiac vagal tone: Continuity, stability, and concordance across the first 5 years. Developmental Psychology. 2000;36:54–65. [PubMed] [Google Scholar]
- 14.Richards JE. Development and stability in visual sustained attention in 14 20, and 26 week old infants. Psychophysiology. 1989;26:422–430. doi: 10.1111/j.1469-8986.1989.tb01944.x. [DOI] [PubMed] [Google Scholar]
- 15.Beauchaine, Gatzke-Kopp L, Mead HK. Polyvagal Theory and developmental psychopathology: emotion dysregulation and conduct problems from preschool to adolescence. Biological psychology. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lester BM, Lagasse LL. Children of addicted women. J Addict Dis. 2010;29:259–276. doi: 10.1080/10550881003684921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair P, Rothblum S, Hebel R. Neonatal outcome in infants with evidence of fetal exposure to opiates, cocaine, and cannabinoids. Clin Pediatr. 1994;33:280–285. doi: 10.1177/000992289403300505. [DOI] [PubMed] [Google Scholar]
- 18.Hickey JE, Suess PE, Newlin DB, Spurgeon L, Porges SW. Vagal tone regulation during sustained attention in boys exposed to opiates in utero. Addictive Behaviors. 1995;20:43–59. doi: 10.1016/0306-4603(94)00044-y. [DOI] [PubMed] [Google Scholar]
- 19.Schuetze P, Eiden RD, Edwards EP. A Longitudinal Examination of Physiological Regulation in Cocaine-Exposed Infants Across the First 7 Months of Life. Infancy. 2009;14:19–43. doi: 10.1080/15250000802569660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine TP, Liu J, Das A, Lester BM, Lagasse L, Shankaran S, et al. Effects of prenatal cocaine exposure on special education in school-aged children. Pediatrics. 2008;122:e83–e91. doi: 10.1542/peds.2007-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beauchaine T. Vagal tone, development, and Gray's motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- 22.Jansson LM, Dipietro JA, Elko A, Velez M. Infant autonomic functioning and neonatal abstinence syndrome. Drug Alcohol Depend. 2010;109:198–204. doi: 10.1016/j.drugalcdep.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuetze P, Eiden RD, Coles CD. Prenatal cocaine and other substance exposure: effects on infant autonomic regulation at 7 months of age. Dev Psychobiol. 2007;49:276–289. doi: 10.1002/dev.20215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheinkopf SJ, Lagasse LL, Lester BM, Liu J, Seifer R, Bauer CR, et al. Vagal tone as a resilience factor in children with prenatal cocaine exposure. Dev Psychopathol. 2007;19:649–673. doi: 10.1017/S0954579407000338. [DOI] [PubMed] [Google Scholar]
- 25.DiPietro JA, Suess PE, Wheeler JS, Smouse PH, Newlin DB. Reactivity and regulation in cocaine-exposed neonates. Infant Behavior and Development. 1995;18:407–414. [Google Scholar]
- 26.Suess PE, Newlin DB, Porges SW. Motivation, sustained attention, and autonomic regulation in school-age boys exposed in utero to opiates and alcohol. Experimental and Clinical Psychopharmacology. 1997;5:375–387. doi: 10.1037//1064-1297.5.4.375. [DOI] [PubMed] [Google Scholar]
- 27.Koob G, Le Moal M. Neurobiology of Addiction. New York: Academic Press; 2006. [Google Scholar]
- 28.Fama R, Pfefferbaum A, Sullivan EV. Perceptual learning in detoxified alcoholic men: contributions from explicit memory, executive function, and age. Alcoholism, clinical and experimental research. 2004;28:1657–1665. doi: 10.1097/01.alc.0000145690.48510.da. [DOI] [PubMed] [Google Scholar]
- 29.Sheinkopf SJ, Lester BM, Sanes JN, Eliassen JC, Hutchison ER, Seifer R, et al. Functional MRI and response inhibition in children exposed to cocaine in utero. Preliminary findings. Dev Neurosci. 2009;31:159–166. doi: 10.1159/000207503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 31.Lester BM, Padbury JF. Third pathophysiology of prenatal cocaine exposure. Dev Neurosci. 2009;31:23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- 32.Bromer C, Marsit CJ, Armstrong DA, Padbury JF, Lester B. Genetic and epigenetic variation of the glucocorticoid receptor (NR3C1) in placenta and infant neurobehavior. Developmental psychobiology. 2012 doi: 10.1002/dev.21061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsit CJ, Maccani MA, Padbury JF, Lester BM. Placental 11-beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PLoS ONE. 2012;7:e33794. doi: 10.1371/journal.pone.0033794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA: The Journal of the American Medical Association. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 35.Bigsby R, Lee YJ. Neonatal screening and supportive interventions to promote neurobehavioral development. Medicine and Health in Rhode Island. 2010;93:139–141. [PubMed] [Google Scholar]