Abstract
Bacterial AB5 toxins are a clinically relevant class of exotoxins that includes several well-known members such as Shiga, cholera and pertussis toxins. Infections with toxin-producing bacteria cause devastating human diseases that affect millions of individuals each year and have no definitive medical treatment. The molecular targets of AB5 toxins reside in the cytosol of infected cells, and the toxins reach the cytosol by trafficking through the retrograde membrane transport pathway that avoids degradative late endosomes and lysosomes. Focusing on Shiga toxin as the archetype member, we review recent advances in understanding the molecular mechanisms involved in the retrograde trafficking of AB5 toxins and highlight how these basic science advances are leading to the development of a promising new therapeutic approach based on inhibiting toxin transport.
Introduction
AB5 toxins are a biomedically important class of bacterial exotoxins that cause devastating human diseases. Prominent members of this class include Shiga toxin (STx), which causes life threatening diarrhea, dysentery, hemorrhagic colitis and hemorrhagic uremic syndrome; cholera toxin (CTx) and E. coli heat labile enterotoxins, which cause endemic and epidemic diarrhea; and pertussis toxin (PTx), which is the causative agent for whooping cough [1, 2]. Each year, infections with these toxin producing bacteria affect millions of individuals and cause more than a million deaths [1].
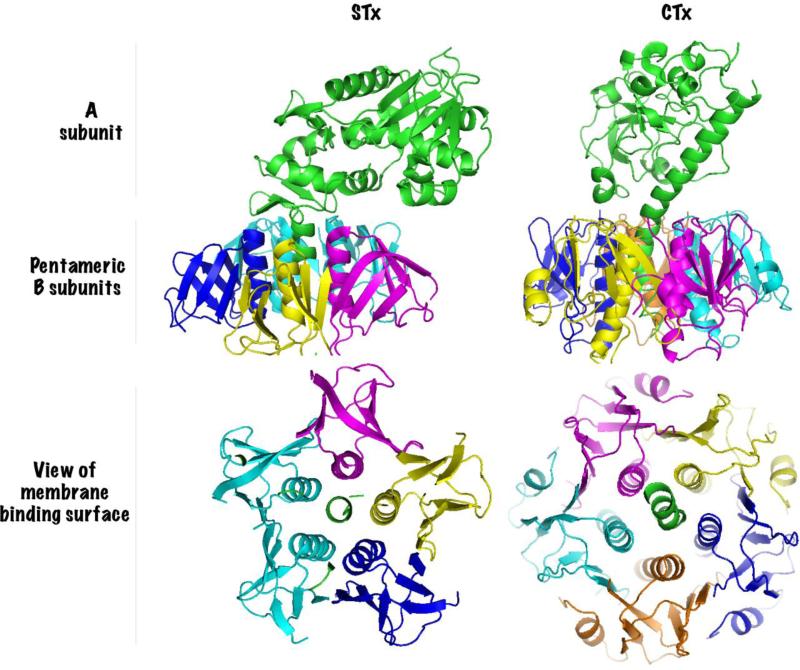
AB5 toxins are so-called because they are formed by the association of a single A subunit with a pentameric B-subunit (Fig.1) [1, 2]. The toxins exert their cytotoxic effect by altering the activity of specific molecular targets in the cytosol of infected cells. STx blocks protein synthesis by removing a single adenine residue from the 28S ribosomal RNA, and CTx and PTx increase cAMP levels by ADP-ribosylating the Gsα or Giα components of heterotrimeric G-proteins respectively [1, 2]. While the catalytic activity of the toxins reside in the A subunit, retrograde trafficking is mediated by the pentameric B-subunits [1]. As retrograde trafficking is a prerequisite for productive infections, there is significant interest in designing small molecule inhibitors of B subunit trafficking that may be therapeutically useful [3-5].
Figure 1.
Subunit structure of AB5 toxins. Shiga toxin (STx, PDB ID: 1R4Q, [88]) and Cholera toxin (CTx, PDB ID: 1XTC, [89]) are compared as representative members of the AB5 toxin family. Each subunit is distinctly colored. Catalytic A subunits have a single alpha helix penetrating the center of the pentameric B subunits. The basal surface of the B subunit pentamer contains binding sites for the glycolipid receptors and is shown in the lower panel.
Over the last two decades, the transport of STx has been more extensively studied than other AB5 toxins. Consequently, our understanding of STx transport is more advanced than that of other toxins. However, studies performed on other AB5 toxins suggest that there are common thematic and conceptual similarities in toxin transport although specific molecular factors may differ. Here we review the important steps in the retrograde trafficking of AB5 toxins with a specific focus on STx transport and summarize recent progress in developing small molecule inhibitors of toxin trafficking.
Retrograde trafficking to the endoplasmic reticulum
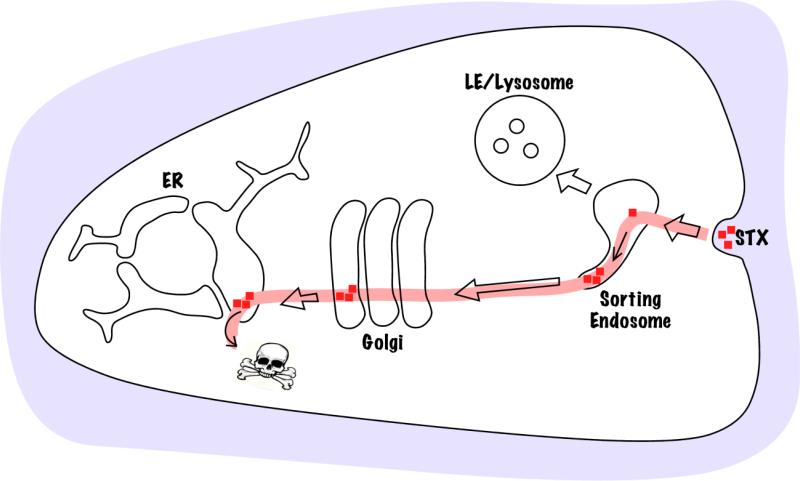
STx and other AB5 toxins follow an elaborate retrograde trafficking pathway to reach the cytosol from the cell exterior. Trafficking, mediated by the B-subunits, begins at the plasma membrane where the toxins bind cell surface receptors. After internalization, the toxins sequentially traffic through sorting endosomes and the Golgi apparatus to reach the endoplasmic reticulum from where the A-subunit is translocated to the cytosol (Fig.2) [6-11]. Studies on toxin trafficking have contributed immensely to our understanding of retrograde transport in general. Work on STx demonstrated, for the first time, that exogenous proteins internalized by endocytosis can be transported to the Golgi apparatus and the endoplasmic reticulum [12]. Later studies on STx led to the discovery of the direct transport pathway between sorting endosomes and the Golgi apparatus that bypasses degradative late endosomes [13]. Numerous endogenous proteins are now known to traffic via the retrograde pathway suggesting that the toxins co-opt a pre-existing endogenous pathway.
Figure 2.
Retrograde trafficking pathway of AB5 toxins. Toxins bind the cell surface, undergo endocytosis and enter sorting endosomes where they move into Golgi-directed tubular extensions. Trafficking to the Golgi bypasses degradative late endosomes (LE) and lysosomes. From the Golgi the toxins move to the endoplasmic reticulum and then the A-subunits are transported to the cytoplasm where they affect specific molecular targets.
Endocytosis
The glycolipid globotriaosylceramide (GB3) acts as the functional cell surface receptor for STx [14-16]. Receptor binding is essential for endocytosis. Mice depleted in the GB3 synthase gene are resistant to Shiga toxicosis [17], and in humans, endothelial cells of the microvasculature are primary targets of STx because they express high levels of GB3 [18, 19].
Clathrin-dependent endocytosis
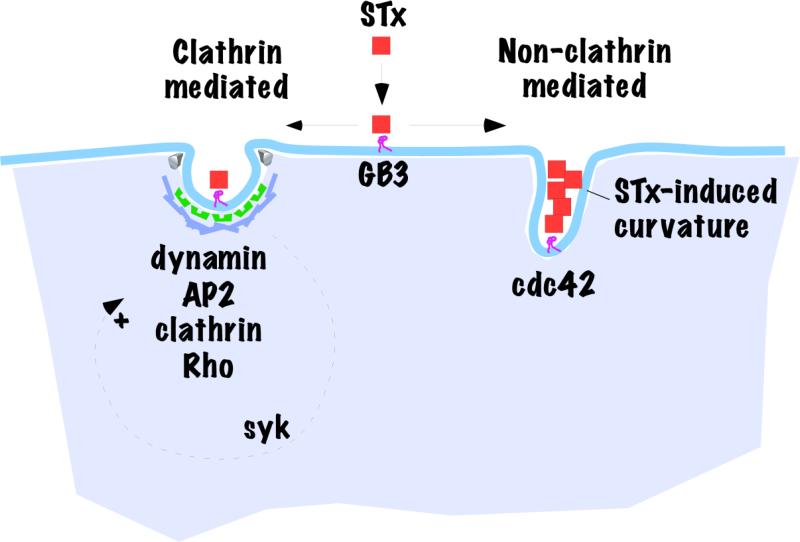
Studies show that both clathrin dependent and independent processes are involved in STx endocytosis (Fig.3). Ultrastructural analyses performed on HeLa cells incubated with STx reveal that at low temperatures (0°C), STx diffusely binds the plasma membrane but a short incubation at 37°C leads to accumulation of STx in coated pits, which are sites of clathrin-mediated endocytosis, as well as in uncoated pits [20]. When clathrin mediated endocytosis is blocked by depleting clathrin heavy chain using small interfering RNA (siRNA), STxB endocytosis decreases by ~40% while the same conditions reduce endocytosis of the clathrin pathway marker transferrin by ~80% [21]. Moreover, expression of dominant negative mutants of epsin or eps15 (proteins required for clathrin mediated endocytosis) reduce the endocytosis of the B-subunit of STx [(STxB), which shows similar transport kinetics to the STx holotoxin], by 40-50% but inhibit transferrin endocytosis by ~70% [22]. The fact that interfering with clathrin inhibits but does not abolish STxB endocytosis implies that clathrin independent pathways internalize a proportion of the toxin. Further, endocytosis assays performed over longer time frames suggest that clathrin independent pathways can compensate for the loss of clathrin function with time. Time course analysis performed in cells treated with anti-clathrin siRNA show that STxB endocytosis is ~30% less than control at 10 min but becomes equal to control by 40 min [23]. Additionally, when clathrin function is inhibited by K+ depletion or hypertonic treatment (these treatments disperse membrane associated clathrin lattices), there is no difference in STxB internalization into control or clathrin-inhibited cells at 60 min [24].
Figure 3.
Clathrin-dependent and -independent endocytosis of STx. The schematic diagram shows speculative relationship of two of the pathways by which STx gains access to the cell interior. Components known to be required in the respective pathways are highlighted. Also indicated are the upregulation of the clathrin-mediated pathway by STx-induced syk signaling and the ability of STx itself to induce membrane curvature leading to non-clathrin mediated internalization.
Clathrin-independent endocytosis
In comparison to clathrin mediated endocytosis, our understanding of the mechanisms involved in clathrin independent endocytosis is in its infancy. The pathways currently known have been classified into two major categories, dynamin dependent and independent, based on whether they require dynamin for membrane scission [25]. These categories have been further sub-divided according to known molecular requirements e.g. caveolae-mediated and RhoA-regulated in the dynamin dependent category, and Cdc42-regulated and Arf6-regulated in the dynamin independent category [25]. Further, the actin based endocytic processes of macropinocytosis and phagocytosis are also clathrin independent and not included in the above classification [25]. The role of individual clathrin independent pathways in STx endocytosis has not yet been rigorously investigated but, at the minimum, there is clear evidence that dynamin independent internalization can take place. One study reported that inhibition of dynamin activity by expression of a dominant negative mutant of dynamin (K44A) reduces STx endocytosis by only ~30-35% [21]. While the reduction in STx endocytosis is likely due to loss of clathrin-mediated internalization, which is also dynamin-dependent, the remaining STx endocytosis shows the participation of dynamin and clathrin independent endocytic pathways. Expression of dominant negative mutants of RhoA and Cdc42 also partially inhibits STx endocytosis, indicating that both RhoA and Cdc42 are required for optimal STx endocytosis [26]. Like dynamin, Rho GTPases play a role in clathrin-mediated endocytosis thus it is unclear whether the inhibitory effect is due to an effect on clathrin dependent or independent endocytosis. In contrast, dominant negative Cdc42 expression does not affect transferrin endocytosis indicating that the requirement of Cdc42 for STx endocytosis is independent of clathrin [26]. Remarkably, binding of STxB to GB3 containing cellular or model membranes induces narrow tubular invaginations [27]. This provides a mechanism for clathrin-independence since the toxin acting from the cell exterior is capable of inducing membrane curvature changes similar to those that clathrin promotes from the cell interior. While it is clear that STx utilizes both clathrin dependent and independent pathways to enter into cells, quantifying the exact contribution of individual endocytic pathways is difficult, if not impossible, because of cross talk between pathways and compensatory mechanisms that are activated when one or more pathways are blocked. Interestingly, work on CTx reveals that it also enters cells via clathrin dependent and independent mechanisms with the exact contribution of each pathway depending on the cell type studied and experimental system used [9, 28-30].
Modulation of internalization
In addition to exploiting endocytic pathways for uptake, recent work suggests that STx also modulates the activity of the cellular trafficking machinery to facilitate its own internalization and transport. Electron microscopy studies reveal that upon treatment with STx there is ~28-38% increase in the number of clathrin coated pits per unit plasma membrane [31]. Similarly, cells treated with STxB show ~60% increase in plasma membrane spots positive for the clathrin adaptor AP2 [31]. The mechanism by which STx induces clathrin recruitment to the plasma membrane is not fully understood but available data suggests that signaling via syk, a cytosolic tyrosine kinase, is important. Exposure of cells to STx for 5 min increases the level of phosphorylation of a variety of proteins including syk and clathrin heavy chain [32]. Phosphorylation of syk activates the kinase and inhibition of syk activity blocks both STx-induced clathrin phosphorylation and AP2 membrane recruitment indicating that syk is required for these events [31, 32]. Further, syk inhibition using siRNA or treatment with the small molecule inhibitor piceatannol also reduces STx endocytosis indicating that syk is required for optimal STx uptake [32]. The idea that clathrin phosphorylation enhances its recruitment to the plasma membrane is supported by the finding that epidermal growth factor induces tyrosine phosphorylation of clathrin heavy chain at a specific site required for epidermal growth factor endocytosis [33]. It is now important to identify the clathrin residues phosphorylated during STx endocytosis, determine the molecular effects of this phosphorylation on coated vesicle formation and test whether the block in STx endocytosis on syk inhibition is due to a block in clathrin heavy chain phosphorylation. Finally, in addition to clathrin mediated endocytosis, recent work suggests that STx increases microtubule assembly in a syk-independent manner, and the altered microtubule dynamics facilitate retrograde transport of the toxin [34]. Overall, STx uses multiple pre-existing endocytic routes and also activates diverse signaling pathways that enhance its cellular uptake.
Endosome-to-Golgi transport
After endocytosis, STx traffics to sorting endosomes from where the toxin is transported to the Golgi apparatus. Direct transport from the sorting endosome to the Golgi apparatus is essential for intoxication because it diverts the toxin away from late endosomes and lysosomes where degradative proteolytic enzymes are active. Over the past few years, a number of cytoplasmically-disposed molecular factors required for endosome-to-Golgi transport of STx have emerged (Table1). Briefly, at the level of the endosome, formation of Golgi directed transport intermediates requires clathrin, the clathrin adaptor epsinR, and the retromer complex (Fig.4) [21, 23, 35-37]. Excellent reviews describing how clathrin and retromer may co-ordinate endosome tubulation to mediate STx transport are available [10, 11]. Several other factors including dynamin are also required (see Table 1) [5, 21, 26, 34, 37-46]. Factors required for fusion with the trans Golgi network include the tethers golgin97, golgin245 and GCC185, and two SNARE complexes (syntaxin6, syntaxin16, Vti1a, Vamp3/4 and syntaxin5, Ykt6, GS15, GS28) [40, 47-50]. However, we do not yet have a complete picture of how these factors come together to sort STx to the Golgi. A particularly challenging problem is that STx and other AB5 toxins are completely contained within the lumenal compartment and do not directly communicate with the cytosolic trafficking machinery. Below, we discuss the challenges faced by lumenally restricted cargo in trafficking out of sorting endosomes and highlight possible mechanisms involved in STx sorting.
Table 1.
Factors required for sorting endosome-to-Golgi transport of STx.
| Factor | Function | Reference |
|---|---|---|
| Clathrin | Coat | [23], [21] |
| Retromer | Presumptive coat; senses/ induces membrane curvature | |
| SNX1 | [35] | |
| Vps26 | [36] | |
| Vps35 | [37] | |
| Epsin R | Clathrin adaptor | [23] |
| RME-8 | DnaJ protein; retromer and Hsc70-interacting partner; proposed to link retromer to Hsc70 | [37] |
| Hsc-70 | Clathrin uncoating ATPase; RME-8 interacting protein; proposed to uncoat clathrin from endosome tubules | [37] |
| Hrs | ESCRT-0 subunit; clathrin and retromer interacting protein; proposed to compete with RME-8 for retromer binding | [37] |
| Dynamin | Membrane scission | [21] |
| EHD3 | Eps15 homology domain protein; Knockdown alters SNX1 localization. | [38] |
| Rabenosyn-5 | EDH3 interacting protein; Rab4/5 effector; knockdown alters SNX1localization. | [38] |
| GPP130 | Endosomal receptor | [5] |
| OCRL1 | PI(4,5)P2 5-phosphatase; clathrin interacting protein | [39] |
| Rab6a’ | small GTPase | [40], [41] |
| Rab11 | small GTPase | [42] |
| Cdc42 | GTPase | [26] |
| ARHGAP21 | Cdc42 GTPase activating protein | [26] |
| Arl1 | small GTPase | [48] |
| Golgin97 | Tether, Arl1 effector | [48] |
| Golgin245 | Tether, Arl1 effector | [47] |
| GCC185 | Tether | [49] |
| Syntaxin6,Syntaxin16, Vti1a, Vamp3/4 | SNARE complex | [40] |
| Syntaxin5, Ykt6, GS15, GS28 | SNARE complex | [50] |
| Microtubules | Cytoskeletal component | [34] |
| Actin | Cytoskeletal component | [26] |
| Dynein | Motor | [34] |
| Cholesterol | Microdomain organization | [43] |
| V-ATPase | Proton pump | [44] |
| p38 | Kinase | [45] |
| PKCδ | Kinase | [46] |
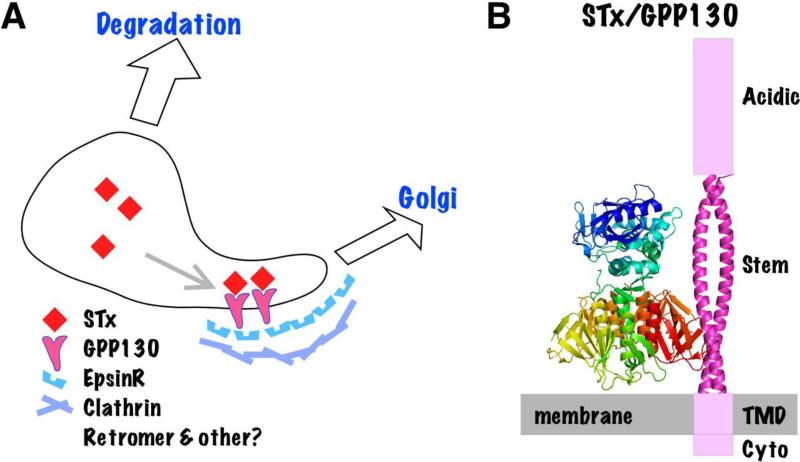
Figure 4.
Endosomal sorting of STx into Golgi-directed tubules. A. The schematic diagram shows STx binding the GPP130 ectodomain promoting its entry into Golgi-directed membrane tubules. Other membrane tubules participate in other pathways such as recycling to the plasma membrane. The remaining endosomal central cavity becomes part of degradative late endosomes and then lysosomes. Cytoplasmic sorting factors that mediate STx trafficking and may directly or indirectly interact with the GPP130 cytoplasmic domain are listed. B. Hypothetical sorting complex showing STx oriented with its basal surface binding the membrane and its B subunit sidewall binding the coiled-coil stem domain of GPP130. Other GPP130 domains indicated are its cytoplasmic (cyto), transmembrane (TMD) and acidic domains.
Constraints of geometric sorting
Transport of proteins from the sorting endosome to other subcellular destinations such as the recycling endosomal compartment, plasma membrane and the Golgi apparatus occurs via numerous narrow-diameter membrane tubules [51]. Cargo that is not specifically sorted into an endosomal membrane tubule reaches the late endosome due to endosome maturation. As lysosomal hydrolases are active in the late endosomal compartment, transport to the late endosome is generally coupled with cargo degradation. As much as 80% of the sorting endosome membrane surface area, but very little of its lumenal volume, is pinched off into membrane tubules [51]. Consequently, in the absence of specific targeting mechanisms, membrane proteins are abundantly present in tubules whereas lumenal cargo mostly distributes into the degradative path. Thus, the geometric nature of endosomal sorting provides a first order mechanism for delivering soluble lumenal cargo to late endosomes for degradation while protecting membrane receptors and other membrane bound cargo from the same fate. Current data suggest that AB5 toxins use protein- and lipid-based sorting mechanisms to sort to the Golgi and overcome what might otherwise be a geometric bias toward trafficking to late endosomes/lysosomes.
Protein-based sorting
Arguably, the most straightforward mechanism for AB5 toxin sorting into Golgi-directed membrane tubules is to interact with a cellular receptor undergoing endosome-to-Golgi transport. Surprisingly, it was only recently that a strong candidate receptor emerged and it appears to be highly specific for STx (Fig.4).
GPP130 is a dimeric transmembrane protein of unknown function that constitutively cycles between sorting endosomes and the Golgi apparatus [52-54]. STxB directly binds GPP130 at the lumenal side of its transmembrane domain in a region strongly predicted to form a long coiled-coil structure [5]. In the absence of GPP130, STxB fails to traffic to the Golgi [5, 55] and instead accumulates in lysosomes where it is degraded [5]. Binding of STxB to GPP130 and cycling of GPP130 are both required for STxB Golgi transport because transport is not supported by mutated versions of GPP130 that lack either the STxB binding site or a sequence stretch known to be critical for GPP130 cycling [5]. The identity of the cytosolic factors that mediate endosomal sorting of GPP130 remains unknown. There is a chance to elucidate the mechanism of STx sorting if it can be shown that the GPP130 cytoplasmic domain interacts with one or more of the cytosolic factors already implicated in STxB sorting (Fig.4 & Table 1).
Although other toxins may similarly co-opt host proteins that traffic from sorting endosomes to the Golgi apparatus, it is unlikely that GPP130 plays a role in their trafficking. CTx undergoes normal trafficking to the Golgi in the absence of GPP130 [5] and GPP130 fails to bind closely related variants of STxB (unpublished). Nevertheless, a detailed characterization of the GPP130-STxB binding interface may lead to the discovery of related receptors for other AB5 toxins.
Contribution of membrane lipids
In addition to protein based sorting, the properties of membrane lipids and the surface glycolipid receptors that the toxins bind may contribute to retrograde transport. Cholesterol depletion, which is employed to disrupt membrane microdomains, blocks the retrograde transport of STx [43]. Both STxB and its surface receptor GB3 are recovered with cholesterol enriched, detergent resistant membrane preparations [56-58]. However, interpretation of these findings is complicated. Positioning of transmembrane domain containing proteins such as GPP130 may also influence, and be influenced by, surrounding lipids and membrane curvature. While it is not yet known whether STx remains bound to GB3 after transport to the sorting endosome, GB3 may be involved in the endosomal sorting of STx in at least two ways. First, GB3 may increase concentration of the toxin in a microdomain that contains GPP130 thereby increasing likelihood of toxin-receptor interaction. Alternatively, GB3 and GPP130 may interact with the toxin co-operatively thereby increasing the retention of the toxin in the microdomain. That the transport of other AB5 toxins may similarly depend on membrane characteristics is suggested by the finding that CTxB retrograde transport is blocked by depletion of either cholesterol or the microdomain associated protein flotillin [28, 29, 59].
Golgi-to-endoplasmic reticulum transport
After reaching the Golgi apparatus, STx is transported to the endoplasmic reticulum. Studying the Golgi-to-endoplasmic reticulum transport of STx and other AB5 toxins has proved challenging in part because perturbation of trafficking at this step frequently alters transport from sorting endosomes. Endogenous markers of Golgi-to-endoplasmic reticulum transport mostly depend on the COPI vesicle coat complex. Best characterized is the recognition of a lumenally disposed KDEL motif in cargo by the transmembrane KDEL receptor, which in turn, interacts with the cytosolic COPI coat causing concentration of receptor-bound cargo complex in COPI vesicles that traffic to the endoplasmic reticulum [60-65]. However, neither STxB nor CTxB contain a KDEL motif and their transport is COP-I independent [66-68]. Note that the A subunit of CTx does have a KDEL motif which acts once the subunit has separated from the holotoxin. Instead of showing COP-I dependence, STxB transport is blocked by expression of a GDP-restricted dominant negative variant of the Rab6 GTPase [66, 67]. Rab6 has two isomers (Rab6a and Rab6a’) that differ in 3 amino acids, and Rab6a’ but not Rab6a is required for STx transport [41]. It is important to note that inhibition of Rab6a’ activity by siRNA-mediated depletion or dominant negative expression also has a strong inhibitory effect on endosome-to-Golgi transport (~70% block in STx transport to Golgi) [40, 41]. Therefore, conclusions about the role of Rab6a’ in STx transport at the Golgi-to-endoplasmic reticulum step are necessarily complicated. Further confusing matters, CTxB transport is Rab6 independent [69]. Overall, much remains to be learned concerning the presumably complicated process of toxin movement across the Golgi and into the endoplasmic reticulum. The identification of GPP130 as a receptor for STx transport to the Golgi raises the question of where and by what mechanism the toxin is released from GPP130 in preparation for its subsequent trafficking steps. Based on past difficulties, it may be that recent advances allowing acute and/or spatially-restricted inactivation of proteins [70] will be necessary to identify factors mediating post-Golgi transport of STx and other toxins.
Translocation to the cytosol
Once the toxins reach the endoplasmic reticulum, the active component of the A subunits are transported to the cytosol. Several lines of evidence suggest that the toxins exploit the endoplasmic reticulum associated degradation pathway to reach the cytosol, and that their retro-translocation occurs via the sec61 translocon [71-75]. Prior to the retro-translocation step, the catalytically active part of the A-subunit must separate from the rest of the toxin. We have a better understanding of the mechanisms involved in the retrotranslocation of CTx than STx. In CTx, the A subunit is present in the toxin particle as two cleaved but disulfide bonded chains A1, which is the catalytic moiety, and A2 [76]. In vitro studies show that the endoplasmic reticulum chaperone, protein disulfide isomerase, binds and unfolds A1, releasing A2 [71, 72]. The A1 subunit is subsequently translocated across the membrane by the sec61 translocon [73]. The A-subunit of STx is also proteolytically cleaved for optimal toxicity. Unlike CTx, which is cleaved after synthesis by a bacterial protease [76], STx cleavage is primarily mediated by the furin protease in the trans Golgi network and/or endosomes [77, 78]. Protein interaction studies show that STx can form a complex with several endoplasmic reticulum chaperones (HEDJ, a luminal Hsp40 chaperone; BiP and GRP94) and the sec61 translocon [74, 75]. While direct evidence is lacking, the above results suggest that, similar to CTxA, STxA is unfolded by endoplasmic reticulum chaperones and transported to the cytosol by the sec61 translocon. Mechanisms by which the active A-subunits interact with endoplasmic reticulum chaperones, functional relevance of toxin-chaperone interactions, and the process by which the toxin-chaperone interactions are linked with the sec61-mediated toxin retro-translocation are as yet unknown.
Blocking trafficking as a therapeutic strategy
Diseases caused by AB5 toxins do not have definitive treatments. As retrograde transport is essential for cytotoxicity, small molecules and drugs that block toxin transport represent a promising therapeutic strategy. Targeting the sorting endosome-to-Golgi transport step is particularly attractive because blocking transport at this step may re-route the toxin to degradative late endosomes and lysosomes.
Haslam and colleagues screened a chemical library of 14,400 small molecules and identified two inhibitors of STx transport [3]. Using inhibition of protein synthesis as a measure of toxicity, they observed that treatment of Vero cells with these compounds yields a protection factor (ratio of the IC50 of STx with drug treatment over that without drug treatment) range from 15 to 103 [3]. They further reported that these compounds also protect against ricin-induced block in protein synthesis in cell culture [3] (ricin is a plant toxin that traffics via the retrograde pathway and induces cell death by inhibiting protein synthesis [79]). However, the ability of these compounds to protect against STx or ricin-induced death in animal models is not known. Further, the molecular targets and mechanisms of action of these compounds are also not known.
A separate screen of 16,480 small molecules by Johannes and colleagues identified two inhibitors of toxin transport, named retro-1 and 2 [4]. Both these compounds effectively protect against STx1 and STx2-induced block in protein synthesis in cell culture. (Note that STx1 and STx2 are produced by E. coli. STx1 is 100% identical to STx, produced by Shigella, in the B-subunit and has a single conservative serine to threonine substitution in the A-subunit. STx2 is ~55% identical to STx [80]) The protection factors for retro-1 and -2 are 22 to 42 for STx1 and 65 to >100 for STx2 respectively. These compounds block STxB transport at the sorting endosome-to-Golgi step. The mechanism of action is still not clear but the localization of the SNARE proteins syntaxin5 and, to a lesser extent, syntaxin6 are altered in drug-treated cells [4]. Surprisingly, although syntaxin6 mediates the retrograde transport of various endogenous cargo proteins (e.g. TGN46) [81], and syntaxin5 is also required for endoplasmic reticulum-to-Golgi and intra-Golgi transport [82], treatment with retro-1 and 2 does not block retrograde transport of endogenous cargo including TGN46 [4]. However, treatment with these compounds blocks retrograde transport of CTxB and protects against ricin induced block in protein synthesis in cell culture suggesting that the compounds are somehow specific to exogenous toxins [4]. Importantly, treatment with retro-2 protects against ricin toxicity in a mouse model underlining the therapeutic promise of the compound [4]. The protective effect against STx and CTx in animal models remains to be tested. A recent attempt to modify retro-2 led to the identification of a dihydroquinazolinone derivative that exhibits ~200 % improvement in the protection factor in cell culture studies but the effectiveness of this compound in animal models has not yet been tested [83].
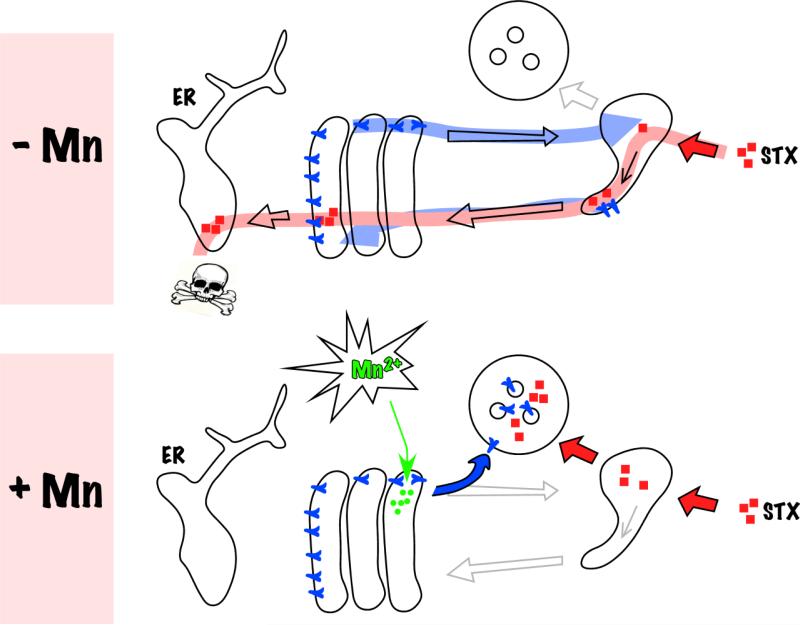
By what seems to be an unrelated but opportune occurrence, the physiological role of the STx receptor GPP130 may be related to manganese (Mn) homeostasis. Remarkably, exposure of cells to non-toxic doses of extracellular Mn induces rapid degradation of GPP130 leaving STx without its trafficking receptor (Fig.5) [5, 84, 85]. Mn-induced GPP130 downregulation requires a Golgi-localized Mn pump, a lumenal sequence stretch in GPP130, and Golgi-to-late endosome trafficking suggesting that a rise in Golgi Mn diverts GPP130 to late endosomes and then lysosomes [84, 85]. Mn blocks STx trafficking and the block is solely due to loss of GPP130 because STx trafficking is rescued in Mn-treated cells if they express a Mn-insensitive variant of GPP130 [5]. Further, treatment with Mn provides >3800 fold protection against STx1-induced cell death in culture and studies in mice reveal that Mn completely protects against STx1-induced death in vivo [5]. Although prolonged exposure to high levels of Mn can cause neurotoxicity [86], the duration and concentration of effective doses are non-toxic [5]. It is unlikely that Mn can be used to protect against STx2-induced disease because GPP130 appears to interact only with the identical B subunits of STx and STx1 (submitted manuscript under revision). However, a vast majority of all STx infections worldwide occur due to STx-producing Shigella infections in developing nations [87]. The prospect of using Mn as a treatment for these infections is appealing due to its low cost and easy availability. Further, the toxicology of Mn is well-studied, the molecular target is known and Mn can potentially be given prophylactically during epidemic outbreaks of S. dysenteriae.
Figure 5.
Mechanism of manganese (Mn) protection against STx (reprinted from [5], Supplemental Fig. 16). In control cells (-Mn), the Golgi-to-endosome cycling of GPP130 is depicted in blue and the GPP130-dependent retrograde trafficking of STx is depicted in red.Manganese addition (+Mn) diverts GPP130 to lysosomes leaving STx without a receptor for sorting into Golgi-directed endosome tubules. Consequently, STx is degraded in lysosomes.
While these studies are promising, further work is needed to develop the aforementioned small molecules and drugs. Animal studies with the retro compounds and Mn used pre-treatment protocols where the animals were given the drug prior to toxin exposure. Importantly, with STx, life-threatening complications develop several days after onset of enteric symptoms, providing an opportunity for treatment after diagnosis. Nevertheless, studies are now required to determine whether these drugs can protect against toxicity when given during or after toxin exposure. Further, the protective effect of Mn and the retro compounds in animals was evaluated using purified protein forms of the toxin but not bacterial infection models. While utilizing the purified toxin provides the most virulent form of toxicity, studies are now required to determine whether these drugs can protect against STx and other toxin-induced diseases when animals are infected with toxin producing bacteria.
Concluding remarks
In conclusion, advances made in understanding the cell biology of retrograde toxin transport are leading to the development of promising new therapeutic options. Further development of current experimental drugs retro-1, retro-2, and Mn needs to be aggressively pursued to develop approved treatments for infections caused by toxin-producing bacteria. Additionally, understanding the molecular basis of the endosomal sorting of AB5 toxins and determining whether endosomal receptors, like GPP130 for STxB, mediate the endosome-to-Golgi transport of other AB5 toxins is also essential. Such studies will enable us to generate new small molecules that can specifically block the ability of AB5 toxins to sort and traffic to the Golgi apparatus but leave endogenous cargo proteins unaffected. Blocking endosome-to-Golgi transport is therapeutically attractive because, as shown with Mn, inhibiting transport at this step can rapidly clear the toxin from infected cells due to degradation in late endosomes and lysosomes. Drugs that only selectively target toxin trafficking without affecting other cellular processes are likely to be safer and more effective alternatives in human patients.
Acknowledgements
Supported by National Institutes of Health grants R01 GM-084111 (to A.D.L.) and K99/R00 ES-020844 (to S.M.).
References
- 1.Beddoe T, Paton AW, Le Nours J, Rossjohn J, Paton JC. Structure, biological functions and applications of the AB5 toxins. Trends Biochem Sci. 2010;35:411–8. doi: 10.1016/j.tibs.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merritt EA, Hol WG. AB5 toxins. Curr Opin Struct Biol. 1995;5:165–71. doi: 10.1016/0959-440x(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 3.Saenz JB, Doggett TA, Haslam DB. Identification and characterization of small molecules that inhibit intracellular toxin transport. Infect Immun. 2007;75:4552–61. doi: 10.1128/IAI.00442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stechmann B, Bai SK, Gobbo E, Lopez R, Merer G, Pinchard S, Panigai L, Tenza D, Raposo G, Beaumelle B, Sauvaire D, Gillet D, Johannes L, Barbier J. Inhibition of retrograde transport protects mice from lethal ricin challenge. Cell. 2010;141:231–42. doi: 10.1016/j.cell.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 5.Mukhopadhyay S, Linstedt AD. Manganese blocks intracellular trafficking of Shiga toxin and protects against Shiga toxicosis. Science. 2012;335:332–5. doi: 10.1126/science.1215930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandvig K, Bergan J, Dyve AB, Skotland T, Torgersen ML. Endocytosis and retrograde transport of Shiga toxin. Toxicon. 2010;56:1181–5. doi: 10.1016/j.toxicon.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 7.Sandvig K, Spilsberg B, Lauvrak SU, Torgersen ML, Iversen TG, Van Deurs B. Pathways followed by protein toxins into cells. Int J Med Microbiol. 2004;293:483–90. doi: 10.1078/1438-4221-00294. [DOI] [PubMed] [Google Scholar]
- 8.Sandvig K, Van Deurs B. Delivery into cells: lessons learned from plant and bacterial toxins. Gene Ther. 2005;12:865–72. doi: 10.1038/sj.gt.3302525. [DOI] [PubMed] [Google Scholar]
- 9.Sandvig K, Van Deurs B. Membrane traffic exploited by protein toxins. Annu Rev Cell Dev Biol. 2002;18:1–24. doi: 10.1146/annurev.cellbio.18.011502.142107. [DOI] [PubMed] [Google Scholar]
- 10.Johannes L, Popoff V. Tracing the retrograde route in protein trafficking. Cell. 2008;135:1175–87. doi: 10.1016/j.cell.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Johannes L, Wunder C. Retrograde transport: two (or more) roads diverged in an endosomal tree? Traffic. 2011;12:956–62. doi: 10.1111/j.1600-0854.2011.01200.x. [DOI] [PubMed] [Google Scholar]
- 12.Sandvig K, Garred O, Prydz K, Kozlov JV, Hansen SH, Van Deurs B. Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature. 1992;358:510–2. doi: 10.1038/358510a0. [DOI] [PubMed] [Google Scholar]
- 13.Mallard F, Antony C, Tenza D, Salamero J, Goud B, Johannes L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J Cell Biol. 1998;143:973–90. doi: 10.1083/jcb.143.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacewicz M, Clausen H, Nudelman E, Donohue-Rolfe A, Keusch GT. Pathogenesis of shigella diarrhea. XI. Isolation of a shigella toxin-binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriaosylceramide. J Exp Med. 1986;163:1391–404. doi: 10.1084/jem.163.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lingwood CA, Law H, Richardson S, Petric M, Brunton JL, De Grandis S, Karmali M. Glycolipid binding of purified and recombinant Escherichia coli produced verotoxin in vitro. J Biol Chem. 1987;262:8834–9. [PubMed] [Google Scholar]
- 16.Lindberg AA, Brown JE, Stromberg N, Westling-Ryd M, Schultz JE, Karlsson KA. Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. J Biol Chem. 1987;262:1779–85. [PubMed] [Google Scholar]
- 17.Okuda T, Tokuda N, Numata S, Ito M, Ohta M, Kawamura K, Wiels J, Urano T, Tajima O, Furukawa K, Furukawa K. Targeted disruption of Gb3/CD77 synthase gene resulted in the complete deletion of globo-series glycosphingolipids and loss of sensitivity to verotoxins. J Biol Chem. 2006;281:10230–5. doi: 10.1074/jbc.M600057200. [DOI] [PubMed] [Google Scholar]
- 18.Obrig TG. Escherichia coli Shiga Toxin Mechanisms of Action in Renal Disease. Toxins (Basel) 2010;2:2769–2794. doi: 10.3390/toxins2122769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyd B, Lingwood C. Verotoxin receptor glycolipid in human renal tissue. Nephron. 1989;51:207–10. doi: 10.1159/000185286. [DOI] [PubMed] [Google Scholar]
- 20.Sandvig K, Olsnes S, Brown JE, Petersen OW, Van Deurs B. Endocytosis from coated pits of Shiga toxin: a glycolipid-binding protein from Shigella dysenteriae 1. J Cell Biol. 1989;108:1331–43. doi: 10.1083/jcb.108.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauvrak SU, Torgersen ML, Sandvig K. Efficient endosome-to-Golgi transport of Shiga toxin is dependent on dynamin and clathrin. J Cell Sci. 2004;117:2321–31. doi: 10.1242/jcs.01081. [DOI] [PubMed] [Google Scholar]
- 22.Nichols BJ, Kenworthy AK, Polishchuk RS, Lodge R, Roberts TH, Hirschberg K, Phair RD, Lippincott-Schwartz J. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J Cell Biol. 2001;153:529–41. doi: 10.1083/jcb.153.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saint-Pol A, Yelamos B, Amessou M, Mills IG, Dugast M, Tenza D, Schu P, Antony C, Mcmahon HT, Lamaze C, Johannes L. Clathrin adaptor epsinR is required for retrograde sorting on early endosomal membranes. Dev Cell. 2004;6:525–38. doi: 10.1016/s1534-5807(04)00100-5. [DOI] [PubMed] [Google Scholar]
- 24.Schapiro FB, Lingwood C, Furuya W, Grinstein S. pH-independent retrograde targeting of glycolipids to the Golgi complex. Am J Physiol. 1998;274:C319–32. doi: 10.1152/ajpcell.1998.274.2.C319. [DOI] [PubMed] [Google Scholar]
- 25.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–12. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hehnly H, Longhini KM, Chen JL, Stamnes M. Retrograde Shiga toxin trafficking is regulated by ARHGAP21 and Cdc42. Mol Biol Cell. 2009;20:4303–12. doi: 10.1091/mbc.E09-02-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romer W, Berland L, Chambon V, Gaus K, Windschiegl B, Tenza D, Aly MR, Fraisier V, Florent JC, Perrais D, Lamaze C, Raposo G, Steinem C, Sens P, Bassereau P, Johannes L. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature. 2007;450:670–5. doi: 10.1038/nature05996. [DOI] [PubMed] [Google Scholar]
- 28.Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- 29.Kirkham M, Fujita A, Chadda R, Nixon SJ, Kurzchalia TV, Sharma DK, Pagano RE, Hancock JF, Mayor S, Parton RG. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J Cell Biol. 2005;168:465–76. doi: 10.1083/jcb.200407078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torgersen ML, Skretting G, Van Deurs B, Sandvig K. Internalization of cholera toxin by different endocytic mechanisms. J Cell Sci. 2001;114:3737–47. doi: 10.1242/jcs.114.20.3737. [DOI] [PubMed] [Google Scholar]
- 31.Utskarpen A, Massol R, Van Deurs B, Lauvrak SU, Kirchhausen T, Sandvig K. Shiga toxin increases formation of clathrin-coated pits through Syk kinase. PLoS One. 2010;5:e10944. doi: 10.1371/journal.pone.0010944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lauvrak SU, Walchli S, Iversen TG, Slagsvold HH, Torgersen ML, Spilsberg B, Sandvig K. Shiga toxin regulates its entry in a Syk-dependent manner. Mol Biol Cell. 2006;17:1096–109. doi: 10.1091/mbc.E05-08-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilde A, Beattie EC, Lem L, Riethof DA, Liu SH, Mobley WC, Soriano P, Brodsky FM. EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell. 1999;96:677–87. doi: 10.1016/s0092-8674(00)80578-4. [DOI] [PubMed] [Google Scholar]
- 34.Hehnly H, Sheff D, Stamnes M. Shiga toxin facilitates its retrograde transport by modifying microtubule dynamics. Mol Biol Cell. 2006;17:4379–89. doi: 10.1091/mbc.E06-04-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bujny MV, Popoff V, Johannes L, Cullen PJ. The retromer component sorting nexin-1 is required for efficient retrograde transport of Shiga toxin from early endosome to the trans Golgi network. J Cell Sci. 2007;120:2010–21. doi: 10.1242/jcs.003111. [DOI] [PubMed] [Google Scholar]
- 36.Popoff V, Mardones GA, Tenza D, Rojas R, Lamaze C, Bonifacino JS, Raposo G, Johannes L. The retromer complex and clathrin define an early endosomal retrograde exit site. J Cell Sci. 2007;120:2022–31. doi: 10.1242/jcs.003020. [DOI] [PubMed] [Google Scholar]
- 37.Popoff V, Mardones GA, Bai SK, Chambon V, Tenza D, Burgos PV, Shi A, Benaroch P, Urbe S, Lamaze C, Grant BD, Raposo G, Johannes L. Analysis of articulation between clathrin and retromer in retrograde sorting on early endosomes. Traffic. 2009;10:1868–80. doi: 10.1111/j.1600-0854.2009.00993.x. [DOI] [PubMed] [Google Scholar]
- 38.Naslavsky N, Mckenzie J, Altan-Bonnet N, Sheff D, Caplan S. EHD3 regulates early-endosometo-Golgi transport and preserves Golgi morphology. J Cell Sci. 2009;122:389–400. doi: 10.1242/jcs.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choudhury R, Diao A, Zhang F, Eisenberg E, Saint-Pol A, Williams C, Konstantakopoulos A, Lucocq J, Johannes L, Rabouille C, Greene LE, Lowe M. Lowe syndrome protein OCRL1 interacts with clathrin and regulates protein trafficking between endosomes and the trans-Golgi network. Mol Biol Cell. 2005;16:3467–79. doi: 10.1091/mbc.E05-02-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mallard F, Tang BL, Galli T, Tenza D, Saint-Pol A, Yue X, Antony C, Hong W, Goud B, Johannes L. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J Cell Biol. 2002;156:653–64. doi: 10.1083/jcb.200110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Del Nery E, Miserey-Lenkei S, Falguieres T, Nizak C, Johannes L, Perez F, Goud B. Rab6A and Rab6A’ GTPases play non-overlapping roles in membrane trafficking. Traffic. 2006;7:394–407. doi: 10.1111/j.1600-0854.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 42.Wilcke M, Johannes L, Galli T, Mayau V, Goud B, Salamero J. Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-golgi network. J Cell Biol. 2000;151:1207–20. doi: 10.1083/jcb.151.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falguieres T, Mallard F, Baron C, Hanau D, Lingwood C, Goud B, Salamero J, Johannes L. Targeting of Shiga toxin B-subunit to retrograde transport route in association with detergent-resistant membranes. Mol Biol Cell. 2001;12:2453–68. doi: 10.1091/mbc.12.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dyve Lingelem AB, Bergan J, Sandvig K. Inhibitors of intravesicular acidification protect against Shiga toxin in a pH-independent manner. Traffic. 2012;13:443–54. doi: 10.1111/j.1600-0854.2011.01319.x. [DOI] [PubMed] [Google Scholar]
- 45.Walchli S, Skanland SS, Gregers TF, Lauvrak SU, Torgersen ML, Ying M, Kuroda S, Maturana A, Sandvig K. The Mitogen-activated protein kinase p38 links Shiga Toxin-dependent signaling and trafficking. Mol Biol Cell. 2008;19:95–104. doi: 10.1091/mbc.E07-06-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torgersen ML, Walchli S, Grimmer S, Skanland SS, Sandvig K. Protein kinase Cdelta is activated by Shiga toxin and regulates its transport. J Biol Chem. 2007;282:16317–28. doi: 10.1074/jbc.M610886200. [DOI] [PubMed] [Google Scholar]
- 47.Yoshino A, Setty SR, Poynton C, Whiteman EL, Saint-Pol A, Burd CG, Johannes L, Holzbaur EL, Koval M, Mccaffery JM, Marks MS. tGolgin-1 (p230, golgin-245) modulates Shiga-toxin transport to the Golgi and Golgi motility towards the microtubule-organizing centre. J Cell Sci. 2005;118:2279–93. doi: 10.1242/jcs.02358. [DOI] [PubMed] [Google Scholar]
- 48.Lu L, Tai G, Hong W. Autoantigen Golgin-97, an effector of Arl1 GTPase, participates in traffic from the endosome to the trans-golgi network. Mol Biol Cell. 2004;15:4426–43. doi: 10.1091/mbc.E03-12-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Derby MC, Lieu ZZ, Brown D, Stow JL, Goud B, Gleeson PA. The trans-Golgi network golgin, GCC185, is required for endosome-to-Golgi transport and maintenance of Golgi structure. Traffic. 2007;8:758–73. doi: 10.1111/j.1600-0854.2007.00563.x. [DOI] [PubMed] [Google Scholar]
- 50.Tai G, Lu L, Wang TL, Tang BL, Goud B, Johannes L, Hong W. Participation of the syntaxin 5/Ykt6/GS28/GS15 SNARE complex in transport from the early/recycling endosome to the trans-Golgi network. Mol Biol Cell. 2004;15:4011–22. doi: 10.1091/mbc.E03-12-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maxfield FR, Mcgraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–32. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 52.Puri S, Bachert C, Fimmel CJ, Linstedt AD. Cycling of early Golgi proteins via the cell surface and endosomes upon lumenal pH disruption. Traffic. 2002;3:641–53. doi: 10.1034/j.1600-0854.2002.30906.x. [DOI] [PubMed] [Google Scholar]
- 53.Linstedt AD, Mehta A, Suhan J, Reggio H, Hauri HP. Sequence and overexpression of GPP130/GIMPc: evidence for saturable pH-sensitive targeting of a type II early Golgi membrane protein. Mol Biol Cell. 1997;8:1073–87. doi: 10.1091/mbc.8.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bachert C, Lee TH, Linstedt AD. Lumenal endosomal and Golgi-retrieval determinants involved in pH-sensitive targeting of an early Golgi protein. Mol Biol Cell. 2001;12:3152–60. doi: 10.1091/mbc.12.10.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Natarajan R, Linstedt AD. A cycling cis-Golgi protein mediates endosome-to-Golgi traffic. Mol Biol Cell. 2004;15:4798–806. doi: 10.1091/mbc.E04-05-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith DC, Sillence DJ, Falguieres T, Jarvis RM, Johannes L, Lord JM, Platt FM, Roberts LM. The association of Shiga-like toxin with detergent-resistant membranes is modulated by glucosylceramide and is an essential requirement in the endoplasmic reticulum for a cytotoxic effect. Mol Biol Cell. 2006;17:1375–87. doi: 10.1091/mbc.E05-11-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nutikka A, Lingwood C. Generation of receptor-active, globotriaosyl ceramide/cholesterol lipid ‘rafts’ in vitro : A new assay to define factors affecting glycosphingolipid receptor activity. Glycoconj J. 2004;20:33–8. doi: 10.1023/B:GLYC.0000016740.69726.fb. [DOI] [PubMed] [Google Scholar]
- 58.Tam P, Mahfoud R, Nutikka A, Khine AA, Binnington B, Paroutis P, Lingwood C. Differential intracellular transport and binding of verotoxin 1 and verotoxin 2 to globotriaosylceramide-containing lipid assemblies. J Cell Physiol. 2008;216:750–63. doi: 10.1002/jcp.21456. [DOI] [PubMed] [Google Scholar]
- 59.Chinnapen DJ, Hsieh WT, Te Welscher YM, Saslowsky DE, Kaoutzani L, Brandsma E, D'auria L, Park H, Wagner JS, Drake KR, Kang M, Benjamin T, Ullman MD, Costello CE, Kenworthy AK, Baumgart T, Massol RH, Lencer WI. Lipid sorting by ceramide structure from plasma membrane to ER for the cholera toxin receptor ganglioside GM1. Dev Cell. 2012;23:573–86. doi: 10.1016/j.devcel.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 61.Nilsson T, Jackson M, Peterson PA. Short cytoplasmic sequences serve as retention signals for transmembrane proteins in the endoplasmic reticulum. Cell. 1989;58:707–18. doi: 10.1016/0092-8674(89)90105-0. [DOI] [PubMed] [Google Scholar]
- 62.Jackson MR, Nilsson T, Peterson PA. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990;9:3153–62. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Letourneur F, Gaynor EC, Hennecke S, Demolliere C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 64.Cosson P, Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994;263:1629–31. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- 65.Bremser M, Nickel W, Schweikert M, Ravazzola M, Amherdt M, Hughes CA, Sollner TH, Rothman JE, Wieland FT. Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell. 1999;96:495–506. doi: 10.1016/s0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]
- 66.Girod A, Storrie B, Simpson JC, Johannes L, Goud B, Roberts LM, Lord JM, Nilsson T, Pepperkok R. Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat Cell Biol. 1999;1:423–30. doi: 10.1038/15658. [DOI] [PubMed] [Google Scholar]
- 67.White J, Johannes L, Mallard F, Girod A, Grill S, Reinsch S, Keller P, Tzschaschel B, Echard A, Goud B, Stelzer EH. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J Cell Biol. 1999;147:743–60. doi: 10.1083/jcb.147.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen A, Hu T, Mikoryak C, Draper RK. Retrograde transport of protein toxins under conditions of COPI dysfunction. Biochim Biophys Acta. 2002;1589:124–39. doi: 10.1016/s0167-4889(02)00163-5. [DOI] [PubMed] [Google Scholar]
- 69.Morikawa RK, Aoki J, Kano F, Murata M, Yamamoto A, Tsujimoto M, Arai H. Intracellular phospholipase A1gamma (iPLA1gamma) is a novel factor involved in coat protein complex I- and Rab6-independent retrograde transport between the endoplasmic reticulum and the Golgi complex. J Biol Chem. 2009;284:26620–30. doi: 10.1074/jbc.M109.038869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jarvela T, Linstedt AD. Irradiation-induced protein inactivation reveals Golgi enzyme cycling to cell periphery. J Cell Sci. 2012;125:973–80. doi: 10.1242/jcs.094441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsai B, Rodighiero C, Lencer WI, Rapoport TA. Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell. 2001;104:937–48. doi: 10.1016/s0092-8674(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 72.Tsai B, Rapoport TA. Unfolded cholera toxin is transferred to the ER membrane and released from protein disulfide isomerase upon oxidation by Ero1. J Cell Biol. 2002;159:207–16. doi: 10.1083/jcb.200207120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmitz A, Herrgen H, Winkeler A, Herzog V. Cholera toxin is exported from microsomes by the Sec61p complex. J Cell Biol. 2000;148:1203–12. doi: 10.1083/jcb.148.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu M, Haslam DB. Shiga toxin is transported from the endoplasmic reticulum following interaction with the luminal chaperone HEDJ/ERdj3. Infect Immun. 2005;73:2524–32. doi: 10.1128/IAI.73.4.2524-2532.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Falguieres T, Johannes L. Shiga toxin B-subunit binds to the chaperone BiP and the nucleolar protein B23. Biol Cell. 2006;98:125–34. doi: 10.1042/BC20050001. [DOI] [PubMed] [Google Scholar]
- 76.Mekalanos JJ, Collier RJ, Romig WR. Enzymic activity of cholera toxin. II. Relationships to proteolytic processing, disulfide bond reduction, and subunit composition. J Biol Chem. 1979;254:5855–61. [PubMed] [Google Scholar]
- 77.Kurmanova A, Llorente A, Polesskaya A, Garred O, Olsnes S, Kozlov J, Sandvig K. Structural requirements for furin-induced cleavage and activation of Shiga toxin. Biochem Biophys Res Commun. 2007;357:144–9. doi: 10.1016/j.bbrc.2007.03.110. [DOI] [PubMed] [Google Scholar]
- 78.Garred O, Van Deurs B, Sandvig K. Furin-induced cleavage and activation of Shiga toxin. J Biol Chem. 1995;270:10817–21. doi: 10.1074/jbc.270.18.10817. [DOI] [PubMed] [Google Scholar]
- 79.Sandvig K, Grimmer S, Lauvrak SU, Torgersen ML, Skretting G, Van Deurs B, Iversen TG. Pathways followed by ricin and Shiga toxin into cells. Histochem Cell Biol. 2002;117:131–41. doi: 10.1007/s00418-001-0346-2. [DOI] [PubMed] [Google Scholar]
- 80.Strockbine NA, Jackson MP, Sung LM, Holmes RK, O'brien AD. Cloning and sequencing of the genes for Shiga toxin from Shigella dysenteriae type 1. J Bacteriol. 1988;170:1116–22. doi: 10.1128/jb.170.3.1116-1122.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ganley IG, Espinosa E, Pfeffer SR. A syntaxin 10-SNARE complex distinguishes two distinct transport routes from endosomes to the trans-Golgi in human cells. J Cell Biol. 2008;180:159–72. doi: 10.1083/jcb.200707136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu Y, Martin S, James DE, Hong W. GS15 forms a SNARE complex with syntaxin 5, GS28, and Ykt6 and is implicated in traffic in the early cisternae of the Golgi apparatus. Mol Biol Cell. 2002;13:3493–507. doi: 10.1091/mbc.E02-01-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noel R, Gupta N, Pons V, Goudet A, Garcia-Castillo MD, Michau A, Martinez J, Buisson DA, Johannes L, Gillet D, Barbier J, Cintrat JC. N-methyl dihydroquinazolinones derivatives of Retro-2 with enhanced efficacy against Shiga toxin. J Med Chem. 2013 doi: 10.1021/jm4002346. [DOI] [PubMed] [Google Scholar]
- 84.Mukhopadhyay S, Linstedt AD. Identification of a gain-of-function mutation in a Golgi P-type ATPase that enhances Mn2+ efflux and protects against toxicity. Proc Natl Acad Sci U S A. 2011;108:858–63. doi: 10.1073/pnas.1013642108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mukhopadhyay S, Bachert C, Smith DR, Linstedt AD. Manganese-induced trafficking and turnover of the cis-Golgi glycoprotein GPP130. Mol Biol Cell. 2010;21:1282–92. doi: 10.1091/mbc.E09-11-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aschner M, Erikson KM, Herrero Hernandez E, Tjalkens R. Manganese and its role in Parkinson's disease: from transport to neuropathology. Neuromolecular Med. 2009;11:252–66. doi: 10.1007/s12017-009-8083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999;77:651–66. [PMC free article] [PubMed] [Google Scholar]
- 88.Fraser ME, Fujinaga M, Cherney MM, Melton-Celsa AR, Twiddy EM, O'brien AD, James MN. Structure of shiga toxin type 2 (Stx2) from Escherichia coli O157:H7. J Biol Chem. 2004;279:27511–7. doi: 10.1074/jbc.M401939200. [DOI] [PubMed] [Google Scholar]
- 89.Zhang RG, Scott DL, Westbrook ML, Nance S, Spangler BD, Shipley GG, Westbrook EM. The three-dimensional crystal structure of cholera toxin. J Mol Biol. 1995;251:563–73. doi: 10.1006/jmbi.1995.0456. [DOI] [PubMed] [Google Scholar]