Abstract
Objective
Short-term exercise training improves glycemic control, but the effect of short-term training on postprandial satiety peptide responses or perceived satiety remains unknown. We tested the hypothesis that short-term aerobic exercise training (15 days) would alter postprandial pancreatic and gut peptide [pancreatic polypeptide (PP) and peptide YY (PYY)] responses and perceived appetite and satiety in obese individuals.
Subjects
Thirteen healthy obese men and women (age: 42±2 y; BMI: 30-45 kg/m2)
Measurements
Subjects were studied before and after 15 days of training (walking 1 h at 70-75% VO2peak). On the study day, subjects consumed 1500 kcal as 6 meals (250 kcal: 9 g protein, 40 g CHO, 6 g fat) while blood samples and satiety measurements were taken at baseline and every 20 min for 12 h. Blood was analyzed for pancreatic polypeptide (PP), peptide YY (PYY), glucose, and insulin levels. Appetite and satiety was assessed with a visual analog scale throughout the day.
Results
Incremental area under the curve (iAUC) for PP increased significantly with training (pre 2788±753; post 3845±830 pg/ml·min for 12-h, p<0.001), but there was no difference in the PP response to each meal. The initial PP response to the first meal increased (ΔPPmin 20-0: pre 86±25; post 140±36 pg/ml, p<0.05) with training. PYY iAUC showed no significant changes with training but showed a significant main effect of time across meals, with the largest response being to the first meal (P<0.005). There were no changes in satiety, glucose, or insulin levels with training.
Conclusion
Short-term exercise training increases postprandial PP concentrations in obese individuals; however, PYY levels and glycemic control remain unaffected. Both PP and PYY show meal-induced increases at all meals but PYY has a greater response at the first meal with reduced responses at subsequent meals.
Keywords: gut hormones, hunger, satiety, autonegative feedback, short term training
Introduction
Regulation of energy balance has been the focus of considerable research over the past 20 years as the obesity epidemic has escalated. Energy homeostasis is a tightly regulated process involving hormone signaling from the periphery via vagal afferents to the hindbrain and the hypothalamus (1). These incoming signals from the peripheral organs such as the stomach, intestine, pancreas, and adipose tissue (2) are integrated with information from other brain regions. More recently, the regulation of appetite and the gut-brain interaction has been of interest as a strategy to help in weight control. Two hormones that have been of interest are pancreatic polypeptide (PP) and peptide YY (PYY).
Pancreatic polypeptide is under vagal control and secreted from pancreatic cells in proportion to food intake where plasma levels can be elevated for up to 6-h (3). Individuals classified as morbidly obese and individuals with Prader-Willi syndrome show a blunted PP response following a meal (4), while administration of PP to individuals with Prader-Willi syndrome decreases food intake (5). Food ingestion is the main stimulus for PP release although other factors alter circulating levels. Exercise, which creates a energy deficit, leads to an increase in PP levels (6).
Peptide YY (PYY) is also implicated in energy balance. It is part of a family of peptides that includes neuropeptide Y, and is known to alter food intake and body weight gain. PYY is secreted primarily from L cells within the intestinal mucosa of the ileum (7) in response to nutrient ingestion, and can remain elevated for ~120 min after the meal (8). PYY has been reported to be reduced or unaltered with obesity (9) and increased with anorexia nervosa (10). PYY exists in human blood in two forms; PYY1-36 and PYY3-36, the latter of which preferentially binds to the inhibitory presynaptic Y2 receptor subtypes expressed in the appetite regulatory center of the arcuate nucleus within the hypothalamus. In both lean and obese subjects, infusion of PYY3-36 reduced food consumption (11-13), although there are conflicting results (14) suggesting that PYY infusion may need to be administered in a specific infusion pattern to exert its anorexigenic effects.
The negative energy balance that is created with an acute exercise bout is known to stimulate PYY and PP responses (15, 16). Studies employing an acute exercise bout have shown increases in PYY concentrations, either during exercise (15), or in the immediate post-exercise period (15-18). However, only a few studies (19-22) have examined the effect of exercise training on the postprandial PYY and PP response. In one study, Kelly et al (21) used diet and exercise training in older adults and demonstrated an increase in the change to peak PYY levels in response to an oral glucose load, but PYY area under the curve (AUC) did not change. In another study (23), adolescents with severe obesity showed no change in fasting PYY concentrations following a long-term combination of supervised aerobic exercises and a balanced diet causing weight reduction.
These previous studies however, only examined fasting PYY concentrations or postprandial responses to a single OGTT after weight loss, and did not examine the hormone responses over the course of an entire day when multiple meals are consumed. Given that the glucose and insulin response to the first meal are not replicated in subsequent meals (known as the second-meal phenomenon) (24-26), and that there is evidence that PP (27) and PYY (28) exhibit clear diurnal patterns, it is possible that postprandial PP and PYY responses may also demonstrate altered responses with subsequent meals, and that this response may be altered through exercise. It has not been established how short-term aerobic exercise training without weight loss alters the postprandial PP and PYY response in obese individuals consuming frequent mixed meals over the course of an entire day. Thus the purpose of the present study was to establish the effect of short-term exercise training on postprandial PYY and PP over the course of a 12-h study day when multiple small meals are consumed in obese individuals. We hypothesized that exercise training would increase PYY and PP responses to the first meal but these responses would not continue throughout the day when additional meals are provided. Further, the short term training model is unique, in that subjects experience an energy deficit with the exercise training, but no weight loss is expected, thus allowing us to examine the impact of an exercise induced energy deficit without the confounding factor of weight loss (29-32).
Methods
Experimental Design
The University of Missouri Institutional Review Board approved the study, and all subjects read and signed an informed consent form prior to participation. Thirteen obese subjects completed two study days including one study day before and one study day after a 15 day exercise training program. During the study day, blood was sampled every 20 min for 12 h, and subjects consumed a 250 kcal shake every 2 h (14% protein, 21% fat, and 65% carbohydrate, Walgreens Co., Deerfield, IL, USA). Subjects remained sedentary throughout the 12 h day. Approximately a one month period was allotted between study days with the exercise training occurring during this period.
Subjects
Obese men and women (ages of 25-50 y) were recruited for this study. Body mass index (BMI) of the subjects was in the range of 30-45 kg/m2, they had a normal fasting blood glucose level (< 100 mg/dL), and subjects were not taking any glucose lowering medications. Subjects were included in the study if they were sedentary, had no prior history of heart, lung, kidney, endocrine, or liver disease, and women had to be pre-menopausal, and not pregnant or lactating. The BodPod (Cosmed USA, Chicago, IL) was used to measure body density and percent body fat was estimated using the Siri equation.
Study day
At 0700 h following an overnight fast, a venous catheter was placed in the antecubital vein and kept patent with a saline drip. Blood samples and perceived appetite and satiety (assessed via a visual analogue scale) were taken at baseline and every 20 min thereafter for 12-h. After the first blood sample, the subjects consumed a liquid meal (within 5 min) and additional meals were provided every 2-h until the end of the study (6 meals total). All subjects consumed 1500 kcal over the course of the 12-h. Subjects rested quietly throughout the day. The post training exercise study days were 24-36 h after the last exercise training bout to minimize an acute exercise bout effect on the meal response of PP and PYY.
Three days prior to the study day, subjects kept a dietary record. Energy intake and regular meal frequency patterns were assessed. Food records were analyzed for total energy and macro/micronutrient content using Food Processor SQL, version 10.8 (ESHA Research, Salem, OR, USA). These records were also used so subjects could repeat their dietary intake on their subsequent visit. Subjects were requested to eat their usual diet, and to consume at least 200 g of carbohydrates the day prior to the study day.
Exercise Stress Test- Exercise Intervention
Peak oxygen consumption (VO2peak) was assessed from expired gases (Parvomedics, Salt Lake City, UT) using a continuous treadmill protocol (22). Briefly, the subjects started walking at 2.5 mph and 0% grade and every 2 min thereafter the treadmill speed was increased by 0.5 mph. At 3.5 mph, the speed was held constant and the grade was increased by 2% every 2 min until exhaustion. VO2 peak was selected as the highest O2 consumption attained according to procedures previously described (33). During this test, a 12-lead EKG was monitored at each stage of exercise and evaluated by a cardiologist. The VO2 peak was utilized to determine the initial training load and the effectiveness of the exercise program. Subjects completed a VO2 peak test pre and post exercise training.
The duration of the exercise training program was 15 days, which had to be completed in a 3 week period. The training regimen required subjects to walk for 60 min/day at 70% of their respective VO2 peak. Subjects were allowed to take a few minutes of rest every 15-20 min if needed, but 60 min of work had to be completed. The exercise was completed in a period lasting ~60-70 min long. These subjects were supervised in each exercise session by study personnel for all visits. Both time and intensity were monitored throughout all workouts. All subjects completed all 15 days of exercise.
Blood Analysis
Three ml blood samples were collected and immediately transferred into serum separator tubes. All samples were assayed for PP, total PYY, glucose, and insulin concentrations. Serum PP and PYY concentrations were assayed with kits from Millipore (Billerica, MA) using Luminex xMap Technology (Linco Research, St. Charles, MO) on a Luminex 100/200 platform (Luminex Corporation, Austin, TX). Serum insulin concentrations were determined using an Immulite 1000 Immunoassay System (Siemens Healthcare Diagnostics, Inc., Deerfield, IL, USA). All procedures followed manufacturer’s instructions, with quality controls within expected ranges for each assay. Inter-assay coefficients of variation (CV) for insulin, PP, and total PYY were 8.3%, 11% and 14%, and intra-assay CV for insulin, PP, and total PYY were 6.9%, 10% and 9%, respectively. Glucose concentrations were determined by colorimetric assays (Fischer Scientific, Inc., Houston, TX, USA). The CV for glucose was 1.9%. All samples for a given subject were run in the same assay.
Statistical Analysis
The data from this project are part of a larger study which has been reported in part previously (34). For that project sample size requirements were based on a Type I error rate of 0.05, two-tailed testing, and a minimal power level of .80. All analyses were performed using Statistical Package for the Social Sciences statistical software, version 19.0 (IBM SPSS Inc, Chicago, IL, USA). A 2 (pre/post) X 37 (time) analysis of variance (ANOVA) with repeated measures was used to test for significant differences due to the intervention and across the 12-h day. A paired t-test was used to establish differences in the incremental area under the curve (iAUC) pre and post intervention. A 2 (pre/post) X 6 (time) repeated measures ANOVA was employed to examine differences in the 2-h AUC between each meal. Post-hoc analyses were run with the Bonferroni correction to control for Type I error. Paired samples t-tests were used to compare descriptive variables pre and post training. The Pearson Product Moment Correlation was used to make associations between iAUC for PP, PYY, insulin, glucose, hunger and satiety. Statistical significance was set at P ≤ 0.05 and values are reported as means ± standard error.
Results
Thirteen men and women (2 men, 11 women) participated in this study. The mean age, weight, body mass index (BMI), and body fat percent were 41.2 ± 1.8 y, 224.0 ± 8.2 kg, 35.5 ± 1.1 kg/m2, and 45.9%, respectively, while fasting blood glucose was normal (80.8 ± 2.3 mg/dL). Weight, BMI, and percent body fat did not significantly change with training (P = NS). Aerobic fitness (VO2) did not change with training (pre: 25.1 ± 1.3, post: 24.9 ± 1.2 ml/kg/min, P = NS). The subjects reported a usual meal frequency of 3-4 meals per day with an occasional snack between meals. Average daily energy consumed during the three days prior to testing was not different (P > 0.05). There was no change in their diet composition pre to post study days. Fasting glucose or insulin concentrations were not significantly different with the exercise training (glucose: pre = 88.9 ± 2.6, post = 92.1 ± 3.0 mg/dL; insulin: pre = 10.6 ± 1.5, post = 10.1 ± 1.7 pg/ml, P=NS). Likewise the glucose and insulin iAUC over the 12-h did not change with exercise training (glucose: pre = 10,494±112, post = 9,752 ± 868 mg/dL·min for 12 h, P = NS; insulin: pre = 18,794 ± 2,004, post = 16,197 ± 1,444 pg/ml·min for 12 h, P < 0.097).
Hormone concentrations
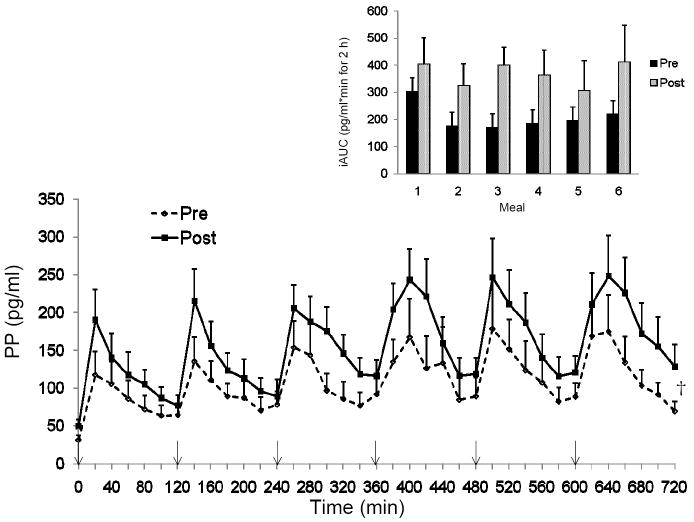
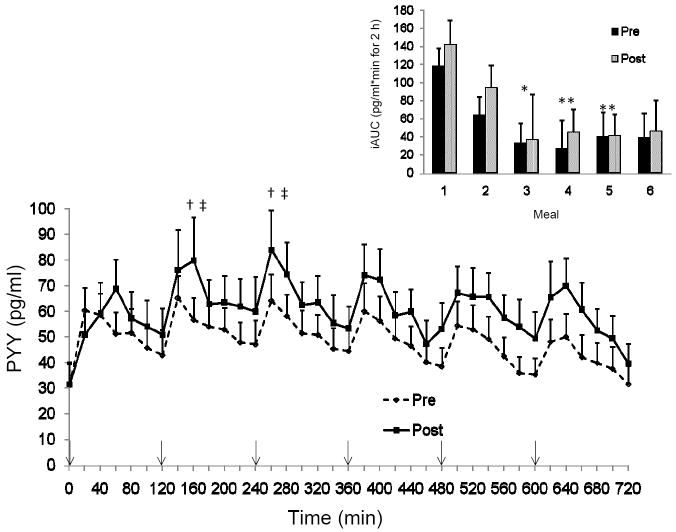
Fasting PP levels were significantly higher following the 15 days of training (PP: pre = 31.6 ± 6.3, post = 50 ± 8.3 pg/ml, P < 0.05), while the fasting PYY values did not change from pre to post exercise (PYY: pre = 31.1 ± 8.3, post = 31.5 ± 8.6 pg/ml). The pattern of PP response was similar pre and post training but the peak PP values were significantly greater post training (pre training = 166.9 ± 43.1 vs. post training = 236.2 ± 39.5 pg/ml; main effect of training, P < 0.01). Total and incremental PP AUC increased with the exercise training (tAUC pre = 3927 ± 935, post = 5646 ± 1023 pg/ml·min for 12-h; P < 0.005) (Figure 1). We examined the 2-h response after each meal to determine if there was an effect of each meal on the PP response and found no difference in iAUC pre or post training in the PP response between the six meals (Figure 1 inset). Total and incremental PYY AUC were not altered with exercise training (tAUC pre = 1760 ± 260, post = 2196 ± 343 pg/ml·min for 12-h; P = NS) (Figure 2). However, the PYY response across the various meals showed a significant main effect of time (P < 0.005). The iAUC of the first meal, although not different pre to post training, was greater than the PYY response to meals during the middle of the day (meal 3 P < 0.05; meal 4 P < 0.001 and meal 5 P <0.01; Figure 2 inset).
Figure 1.
The pattern of response for pancreatic polypeptide (PP) over 12-h pre and post 15 days of training. Inset: Incremental area under the curve (AUC) for each 2-h block around each meal. †P<0.05 time effect -pre to post training; n=13. ↓ indicates a meal.
Figure 2.
The pattern of response for peptide YY (PYY) over 12-h pre and post 15 days of training. Inset: Incremental area under the curve (AUC) for each 2-h block around each meal.*P<0.05 vs iAUC meal 1,**P<0.01 vs iAUC meal 1,†P<0.05 vs peak meal 1, ‡P<0.05 vs peak meal 5; n=13. ↓ indicates a meal.
Hunger/Fullness
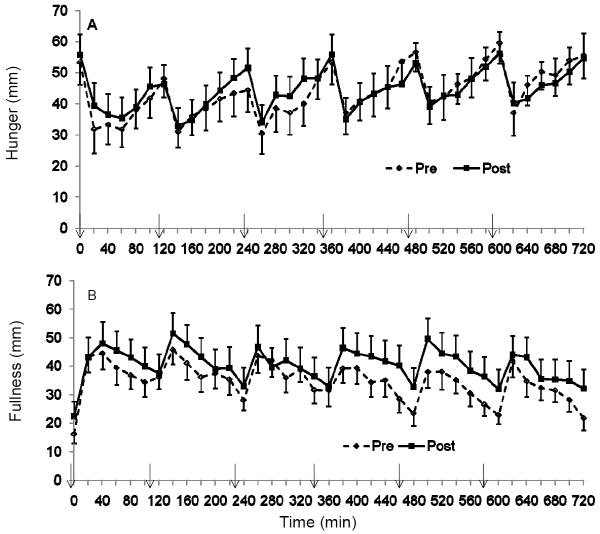
Each meal stimulated changes in both hunger and fullness but over the course of 12-h we found no differences in hunger or fullness pre to post training (P = NS, Figure 3). The tAUC was not different pre to post training for hunger (pre: 1589 ± 212, post: 1591 ± 214 mm*min for 12-h) or for fullness (pre: 1264 ± 166, post 1463 ± 243 mm*min for 12-h; P = NS). Dividing the day into 2-h intervals, we found that 2-h fullness iAUC was greatest following the first meal as compared to subsequent meals (P<0.001) and there were no differences between the other meals. Hunger 2-h AUC was also lower after the initial meal compared to following meals 2, 3, 4 and 5 (P < 0.001).
Figure 3.
The pattern of response for (A) hunger and (B) satiety over 12-h pre and post 15 days of training; n=13. ↓ indicates a meal.
Correlations
Pre-training the baseline PP concentration was correlated with both PP tAUC over the 12-h (r = 0.84, P < 0.001) and with the iAUC over 12-h (r = 0.74, P = 0.004), while baseline PPY concentrations were correlated with PYY tAUC only (r = 0.82, P = 0.001). Post training similar relationships between fasting PP and PYY concentrations and tAUC and iAUC (P<0.05) still existed.
Hunger was correlated with pre training PYY iAUC (r = -0.61, P = 0.03) but not post training, while fullness was not correlated with PYY. PP was not correlated with either hunger or fullness pre or post training. We examined if there were any relationships between PP or PYY and hunger or fullness at the respective meals, but no associations were found. We also examined if PP or PYY iAUC were correlated with glucose or insulin AUC at each meal throughout the day. PP iAUC was not associated with glucose or insulin iAUC at any meal, however, PYY iAUC at meals 4 and 6 were correlated with glucose at meals 4 (r = 0.634, P = 0.027) and 6 (r = 0.658, P = 0.02). The PYY iAUC for meal 4 was associated with the insulin iAUC for meal 4.
Discussion
Energy balance is a tightly regulated process involving hormone signaling from the periphery (1), and exercise is a potent physiological stimulus that is able to induce an acute negative energy balance and alter appetite-regulating signals from the periphery. Depending on the time frame of the response, different mechanisms may operate in this complex system which regulates appetite. We found that short term exercise training without weight loss, or changes in glucose and insulin levels, increased postprandial PP responses over a 12-h period, and this response was elevated not only to the first meal but to each subsequent meal. In contrast to some reports (20, 21), but supporting others (19, 22), we observed no change in the PYY response over the 12-h day post training. Although exercise training had no effect on postprandial PYY responses, the PYY response to the first meal was greater than observed at subsequent meals, particularly those responses in the middle of the day.
Martins et al. (15) were the first to observe an increase in postprandial PP concentrations when exercise was performed in the postprandial state, and these elevated PP levels were maintained through the post exercise period and were associated with decreased hunger sensations. Recently Balaguera-Cortes et al (35) reported that an acute bout of resistance exercise transiently increased PP concentrations, which is similar to the findings of Martins et al (15). Longer-term studies examined PP changes during periods of weight loss. Kahleova et al (36) observed decreased fasting PP concentrations following 12 wk of dieting, and an additional 12 wk of diet-and-exercise had no impact on fasting PP concentrations. While previous work (15, 35) primarily showed increased PP concentrations post exercise in response to acute changes in energy balance, the findings from the current study adds to the existing literature by showing that short-term training increases the meal-driven PP response by ~44% on a subsequent day with no exercise, and this effect is independent of weight loss. Moreover, the 12-h study day has allowed us to assess the second-meal effect under strict laboratory conditions. Since the prior exercise bout was 24-36 h prior to the study day, this PP response reflects a chronic effect of the training that may have been due to changes in energy balance, but were not associated with changes in glucose or insulin levels. Interestingly, we did not find any association between PP and hunger or fullness in our study most likely because our last exercise bout was 24-36 h prior to the study day; whereas, previous studies (15) who have reported an association were examining hunger and fullness immediately following exercise.
The underlying mechanism for the elevated postprandial PP levels after exercise training is unclear. PP release from the pancreatic neuroendocrine cells is predominately driven by meal stimuli, and its release is stimulated during all phases of digestion (3). The function of PP is to self-regulate pancreatic secretions (endocrine and exocrine) and to provide satiety signals to the brain. PP and mechanosensory information concerning satiety and hunger are transmitted from the viscera to the brain via the vagus nerve or blood, aiding food intake regulation (3). In particular, PP has a high affinity for Y4 receptors which are highly expressed in the area postrema which mediates PP actions (37), and binding to the Y4 receptor modulates the vasovagal reflex arc resulting in inhibition of food intake (38). PP involvement of afferent vagal activity may play a crucial role in the anorexigenic actions of PP and the regulation of energy balance. Morbidly obese individuals have blunted postprandial responses of PP, while anorexic subjects have an exaggerated postprandial release (3, 10). It is possible the increase in PP observed on a non-exercise day following training may be in response to the energy imbalance created, allowing for more anorexigenic behavior or decreased food intake.
This study also shows that PP responds to multiple meals, with a pattern demonstrating a sharp increase following meal ingestion and then a fall in PP levels between meals. There was no difference in the peak PP levels with the subsequent meals. We observed a very similar response to each meal over the course of the day, which is unlike the findings of Johns et al (27) who reported greater postprandial circulating PP levels following a meal in the evening than in the morning. The lack of diurnal response seen in our study may be due to our subjects being obese and pathological conditions such as obesity may disturb the circadian patterns normally observed or that Johns et al. (27) only collected blood samples every 2-h in response to 2 meals over 24-h, while we collected blood samples every 20 min in response to 6 meals over a 12-h period. It is also possible that the energy imbalance from exercise training disrupts these circadian signals.
In the current study, PYY concentrations did not increase with exercise training. With weight loss through diet and exercise, Gueugnon et al (20) reported an increase in fasting PYY levels in obese adolescents but did not see changes postprandially; while Jones et al (39) reported an increase in fasting PYY levels following 32 weeks of training. Kelly et al (21) reported increases in the change in PYY from baseline to 30 min in response to an OGTT, but reported no differences in PYY area under the curve in response to this test. Also, they may have observed a greater change in PYY with their meal as it had a slightly greater caloric load (300 kcal) compared with our mixed meal (250 kcal). Our data extends these previous findings demonstrating that short term exercise training does not alter the PYY response to meals that are consumed throughout the day on a subsequent non-exercise day in obese individuals.
The present study also highlights the effects of subsequent meals on hormonal responses over a 12-h period. Our data clearly shows that the incremental PP concentrations in response to each meal were tightly regulated throughout the day, and this regulation does not seem to be due to glucose or insulin levels as we did not see any association between these variables and PP levels. This implies there is no limitation in PP production and secretion with this small meal challenge or no autonegative feedback of PP on the pancreatic cells following the first meal.
Unlike the findings of Hill et al. (28), who noted that PYY concentrations increased after breakfast until about 1-h after lunch, we observed peak PYY concentrations between 20-80 min after every meal which then gradually declined until the onset of the next meal. This difference in response may in part be explained by differences in the meals provided. The meals in the current study were liquid, and consisted of 250 kcal, whereas the meals provided in the study of Hill et al. (28) were solid, higher in protein and fat, and averaged 416 kcal or more. However, the highest absolute peak concentrations of PYY occurred after the 2nd and 3rd meal (equating to 2-4-h after the 1st meal), which is in agreement with Hill et al. (28) who observed the highest peak PYY concentrations after the lunch meal (4-h after the 1st meal). Thus, the meal driven diurnal response of PYY was similar between studies despite different study designs. However, when we quantified responses using the iAUC to examine the PYY response to each meal, we observed a greater PYY iAUC after the first meal than at subsequent meals, which was significantly (72%; P < 0.05) attenuated by the third meal. This attenuation in iAUC across the meals suggests that there is either an autonegative feedback onto the L cells with subsequent meals or that L cells become desensitized or depleted in response to repeated stimulation with frequent meals in obese individuals.
Studies have suggested that increased meal frequency throughout the day may allow for beneficial metabolic effects as well as greater 24-h satiety (40, 41). Both hunger and fullness were measured over the course of the study day yet there were no differences in perceived hunger and fullness despite smaller meal driven changes in PYY after the first meal. This lack of change in satiety could be linked to the stable PP levels. Asakawa et al (42) demonstrated in mice that the potency of feeding inhibition was greater with PP than with PYY1-36 and PYY3-36. In the present study, the PP levels increased dramatically post training yet neither hunger nor fullness were altered pre to post training. Dividing the day into 2-h intervals revealed that both hunger and fullness were greater at the beginning of the day than later in the day, when the subjects had just completed a 12-h fast. This also coincided with higher PP and PYY AUC, although we found no association between hormonal values and perceived hunger and fullness. Only a few studies have examined the changes in PYY and/or PP with exercise training and measured satiety. Guelfi et al (19) reported that 12 wk of aerobic training increased satiety but had no effect on hunger, while resistance training had no impact on either satiety or hunger. Further, they reported no change in PP or PYY levels in these overweight men. Likewise, Martins et al (22) found no increase in PYY levels with 12 wk of aerobic training, but did observe significant increases in hunger, fullness, and the desire to eat. Taken together, these findings, as well as our findings, imply there may be a disconnect between PP and PYY and hunger and fullness with exercise training. Earlier studies have reported decreased food intake with PP (12) or PYY infusions (13), and for PYY these effects were only observed at pharmacological doses. In our study with the small meal size, the changes in PYY and PP most likely were not substantial enough to have a consistent effect on hunger and satiety, as were seen with peptide infusion.
This study has some potential limitations that should be considered when interpreting the results. This study was originally designed to examine the effect of meal frequency on hormonal responses, thus we used a small liquid meal as our meal challenge to ensure it was consumed fast and cleared from the gut before the onset of the next meal. Using a solid meal with more calories which has a longer transit time in the gut may provide a very different response. Additionally, if the meals were further apart (e.g. every 4 h) a difference in the meal response may also have occurred. However the strength of our study is that we have studied the subjects during the waking hours and across multiple meals, thus they would have been still postprandial when the subsequent meal was consumed providing us with insight to the changes in these hormones to repeated feedings. The training regimen may also have impacted the response seen. This was 1-h of exercise at a moderate to vigorous pace. Less intense exercise for a shorter duration, more similar to what many obese individuals may partake in, may not provide the training stimulus for the changes that we observed. Further we used aerobic exercise (walking) and resistance exercise or a combination of aerobic and resistance exercise may provide a very different stimulus. Lastly, we measured total PYY concentrations as opposed to PYY3-36. While PYY3-36 preferentially targets the presynaptic Y2 inhibitory receptors, PYY1-36 is known to bind and activate multiple NPY receptor subtypes (Y1, Y2 and Y5).
Similar to previous reports (28) small frequent, liquid meals result in a meal driven PYY and PP response. This study demonstrates for the first time that post aerobic exercise training meal consumption will result in a greater meal-driven PP, but not PYY responses. The PP response to the meal appears to be tightly modulated over subsequent meals while PYY demonstrates autonegative feedback at the subsequent meals. This highlights that only sampling appetite hormones at the first meal may not reflect what is happening throughout the remainder of the day. Lastly, short term aerobic exercise training may induce changes in PP concentrations earlier than is observed in PYY concentrations and before changes in hunger and fullness occur.
Acknowledgments
This study was supported in part by a NIH R21DK084467-01 grant. We would like to thank the nurses of the Clinical Research Unit for helping with catheter insertion and monitoring the subjects.
Footnotes
Disclosure
We have no conflicts of interest to declare.
References
- 1.Maier C, Riedl M, Vila G, Nowotny P, Wolzt M, Clodi M, et al. Cholinergic regulation of ghrelin and peptide YY release may be impaired in obesity. Diabetes. 2008 Sep;57(9):2332–40. doi: 10.2337/db07-0758. Epub 2008/06/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007 May;132(6):2116–30. doi: 10.1053/j.gastro.2007.03.048. Epub 2007/05/15. eng. [DOI] [PubMed] [Google Scholar]
- 3.Kojima S, Ueno N, Asakawa A, Sagiyama K, Naruo T, Mizuno S, et al. A role for pancreatic polypeptide in feeding and body weight regulation. Peptides. 2007 Feb;28(2):459–63. doi: 10.1016/j.peptides.2006.09.024. Epub 2007/01/09. eng. [DOI] [PubMed] [Google Scholar]
- 4.Zipf WB, O’Dorisio TM, Cataland S, Sotos J. Blunted pancreatic polypeptide responses in children with obesity of Prader-Willi syndrome. J Clin Endocrinol Metab. 1981 Jun;52(6):1264–6. doi: 10.1210/jcem-52-6-1264. Epub 1981/06/01. eng. [DOI] [PubMed] [Google Scholar]
- 5.Berntson GG, Zipf WB, O’Dorisio TM, Hoffman JA, Chance RE. Pancreatic polypeptide infusions reduce food intake in Prader-Willi syndrome. Peptides. 1993 May-Jun;14(3):497–503. doi: 10.1016/0196-9781(93)90138-7. Epub 1993/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 6.Gingerich RL, Hickson RC, Hagberg JM, Winder WW. Effect of endurance exercise training on plasma pancreatic polypeptide concentration during exercise. Metabolism. 1979 Dec;28(12):1179–82. doi: 10.1016/0026-0495(79)90129-x. Epub 1979/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 7.Li JB, Asakawa A, Li Y, Cheng K, Inui A. Effects of exercise on the levels of peptide YY and ghrelin. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2011 Mar;119(3):163–6. doi: 10.1055/s-0030-1262790. Epub 2010/08/07. eng. [DOI] [PubMed] [Google Scholar]
- 8.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985 Nov;89(5):1070–7. doi: 10.1016/0016-5085(85)90211-2. Epub 1985/11/01. eng. [DOI] [PubMed] [Google Scholar]
- 9.Ashby D, Bloom SR. Recent progress in PYY research--an update report for 8th NPY meeting. Peptides. 2007 Feb;28(2):198–202. doi: 10.1016/j.peptides.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Alderdice JT, Dinsmore WW, Buchanan KD, Adams C. Gastrointestinal hormones in anorexia nervosa. Journal of psychiatric research. 1985;19(2-3):207–13. doi: 10.1016/0022-3956(85)90019-6. Epub 1985/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 11.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003 Sep 4;349(10):941–8. doi: 10.1056/NEJMoa030204. Epub 2003/09/05. eng. [DOI] [PubMed] [Google Scholar]
- 12.Batterham RL, Le Roux CW, Cohen MA, Park AJ, Ellis SM, Patterson M, et al. Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab. 2003 Aug;88(8):3989–92. doi: 10.1210/jc.2003-030630. [DOI] [PubMed] [Google Scholar]
- 13.Degen L, Oesch S, Casanova M, Graf S, Ketterer S, Drewe J, et al. Effect of peptide YY3-36 on food intake in humans. Gastroenterology. 2005 Nov;129(5):1430–6. doi: 10.1053/j.gastro.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Tschop M, Castaneda TR, Joost HG, Thone-Reineke C, Ortmann S, Klaus S, et al. Physiology: does gut hormone PYY3-36 decrease food intake in rodents? Nature. 2004 Jul 8;430(6996):1–165. 2. doi: 10.1038/nature02665. Epub 2004/07/13. eng. [DOI] [PubMed] [Google Scholar]
- 15.Martins C, Morgan LM, Bloom SR, Robertson MD. Effects of exercise on gut peptides, energy intake and appetite. J Endocrinol. 2007 May;193(2):251–8. doi: 10.1677/JOE-06-0030. [DOI] [PubMed] [Google Scholar]
- 16.Ueda SY, Yoshikawa T, Katsura Y, Usui T, Nakao H, Fujimoto S. Changes in gut hormone levels and negative energy balance during aerobic exercise in obese young males. J Endocrinol. 2009 Apr;201(1):151–9. doi: 10.1677/JOE-08-0500. [DOI] [PubMed] [Google Scholar]
- 17.Broom DR, Batterham RL, King JA, Stensel DJ. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. Am J Physiol Regul Integr Comp Physiol. 2009 Jan;296(1):R29–35. doi: 10.1152/ajpregu.90706.2008. Epub 2008/11/07. eng. [DOI] [PubMed] [Google Scholar]
- 18.Hagobian TA, Sharoff CG, Stephens BR, Wade GN, Silva JE, Chipkin SR, et al. Effects of exercise on energy-regulating hormones and appetite in men and women. Am J Physiol Regul Integr Comp Physiol. 2009 Feb;296(2):R233–42. doi: 10.1152/ajpregu.90671.2008. Epub 2008/12/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guelfi KJ, Donges CE, Duffield R. Beneficial effects of 12 weeks of aerobic compared with resistance exercise training on perceived appetite in previously sedentary overweight and obese men. Metabolism. 2012 Sep 6; doi: 10.1016/j.metabol.2012.08.002. Epub 2012/09/11. Eng. [DOI] [PubMed] [Google Scholar]
- 20.Gueugnon C, Mougin F, Nguyen NU, Bouhaddi M, Nicolet-Guénat M, Dumoulin G. Ghrelin and PYY levels in adolescents with severe obesity: effects of weight loss induced by long-term exercise training and modified food habits. Eur J Appl Physiol. 2012;12(5):1797–805. doi: 10.1007/s00421-011-2154-2. [DOI] [PubMed] [Google Scholar]
- 21.Kelly KR, Brooks LM, Solomon TP, Kashyap SR, O’Leary VB, Kirwan JP. The glucose-dependent insulinotropic polypeptide and glucose-stimulated insulin response to exercise training and diet in obesity. Am J Physiol Endocrinol Metab. 2009 Jun;296(6):E1269–74. doi: 10.1152/ajpendo.00112.2009. Epub 2009/04/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martins C, Kulseng B, King NA, Holst JJ, Blundell JE. The effects of exercise-induced weight loss on appetite-related peptides and motivation to eat. J Clin Endocrinol Metab. 2010 Apr;95(4):1609–16. doi: 10.1210/jc.2009-2082. [DOI] [PubMed] [Google Scholar]
- 23.Gueugnon C, Mougin F, Nguyen NU, Bouhaddi M, Nicolet-Guenat M, Dumoulin G. Ghrelin and PYY levels in adolescents with severe obesity: effects of weight loss induced by long-term exercise training and modified food habits. Eur J Appl Physiol. 2012 May;112(5):1797–805. doi: 10.1007/s00421-011-2154-2. Epub 2011/09/13. eng. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins DJ, Wolever TM, Buckley G, Lam KY, Giudici S, Kalmusky J, et al. Low-glycemic-index starchy foods in the diabetic diet. Am J Clin Nutr. 1988 Aug;48(2):248–54. doi: 10.1093/ajcn/48.2.248. Epub 1988/08/01. eng. [DOI] [PubMed] [Google Scholar]
- 25.Jovanovic A, Gerrard J, Taylor R. The second-meal phenomenon in type 2 diabetes. Diabetes Care. 2009 Jul;32(7):1199–201. doi: 10.2337/dc08-2196. Epub 2009/04/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolever TMS, jenkins DJA, Vuksan V, Jenkins AL, Bucklen GC, Wong GS. Beneficial effect of a low glycemic index diet in type II diabetes. Diabetic med. 1992;9:451–8. doi: 10.1111/j.1464-5491.1992.tb01816.x. [DOI] [PubMed] [Google Scholar]
- 27.Johns CE, Newton JL, Westley BR, May FE. Human pancreatic polypeptide has a marked diurnal rhythm that is affected by ageing and is associated with the gastric TFF2 circadian rhythm. Peptides. 2006 Jun;27(6):1341–8. doi: 10.1016/j.peptides.2005.11.002. Epub 2005/12/20. eng. [DOI] [PubMed] [Google Scholar]
- 28.Hill BR, De Souza MJ, Williams NI. Characterization of the diurnal rhythm of peptide YY and its association with energy balance parameters in normal-weight premenopausal women. Am J Physiol Endocrinol Metab. 2011 Aug;301(2):E409–15. doi: 10.1152/ajpendo.00171.2011. Epub 2011/05/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Battaglia GM, Zheng D, Hickner RC, Houmard JA. Effect of exercise training on metabolic flexibility in response to a high-fat diet in obese individuals. Am J Physiol Endocrinol Metab. 2012 Dec 15;303(12) doi: 10.1152/ajpendo.00355.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black SE, Mitchell E, Freedson PS, Chipkin SR, Braun B. Improved insulin action following short-term exercise training: role of energy and carbohydrate balance. J Appl Physiol. 2005 Dec;99(6):2285–93. doi: 10.1152/japplphysiol.00291.2005. [DOI] [PubMed] [Google Scholar]
- 31.Houmard JA, Cox JH, MacLean PS, Barakat HA. Effect of short-term exercise training on leptin and insulin action. Metabolism. 2000 Jul;49(7):858–61. doi: 10.1053/meta.2000.6751. [DOI] [PubMed] [Google Scholar]
- 32.Houmard JA, Hickey MS, Tyndall GL, Gavigan KE, Dohm GL. Seven days of exercise increase GLUT-4 protein content in human skeletal muscle. J Appl Physiol. 1995 Dec;79(6):1936–8. doi: 10.1152/jappl.1995.79.6.1936. [DOI] [PubMed] [Google Scholar]
- 33.McArdle WD, Katch FI, Katch VL. Exercise Physiology: Energy, Nutrition, and Human Performance. 4. Philadelphia: Williams & Wilkins; 2001. [Google Scholar]
- 34.Heden TD, Liu Y, Sims LJ, Whaley-Connell AT, Chockalingam A, Dellsperger KC, et al. Meal Frequency Differentially Alters Postprandial Triacylglycerol and Insulin Concentrations in Obese Women. Obesity (Silver Spring) 2012 Jun 22; doi: 10.1002/oby.20247. Epub 2012/07/28. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balaguera-Cortes L, Wallman KE, Fairchild TJ, Guelfi KJ. Energy intake and appetite-related hormones following acute aerobic and resistance exercise. Appl Physiol Nutr Metab. 2011 Dec;36(6):958–66. doi: 10.1139/h11-121. Epub 2011/11/25. eng. [DOI] [PubMed] [Google Scholar]
- 36.Kahleova H, Mari A, Nofrate V, Matoulek M, Kazdova L, Hill M, et al. Improvement in beta-cell function after diet-induced weight loss is associated with decrease in pancreatic polypeptide in subjects with type 2 diabetes. Journal of diabetes and its complications. 2012 Sep;26(5):442–9. doi: 10.1016/j.jdiacomp.2012.05.003. Epub 2012/06/08. eng. [DOI] [PubMed] [Google Scholar]
- 37.Whitcomb DC, Puccio AM, Vigna SR, Taylor IL, Hoffman GE. Distribution of pancreatic polypeptide receptors in the rat brain. Brain research. 1997 Jun 20;760(1-2):137–49. doi: 10.1016/s0006-8993(97)00295-3. Epub 1997/06/20. eng. [DOI] [PubMed] [Google Scholar]
- 38.Simpson K, Parker J, Plumer J, Bloom S. CCK, PYY and PP: the control of energy balance. Handbook of experimental pharmacology. 2012;(209):209–30. doi: 10.1007/978-3-642-24716-3_9. Epub 2012/01/18. eng. [DOI] [PubMed] [Google Scholar]
- 39.Jones TE, Basilio JL, Brophy PM, McCammon MR, Hickner RC. Long-term exercise training in overweight adolescents improves plasma peptide YY and resistin. Obesity (Silver Spring) 2009 Jun;17(6):1189–95. doi: 10.1038/oby.2009.11. Epub 2009/02/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Speechly DP, Buffenstein R. Greater appetite control associated with an increased frequency of eating in lean males. Appetite. 1999 Dec;33(3):285–97. doi: 10.1006/appe.1999.0265. [DOI] [PubMed] [Google Scholar]
- 41.Speechly DP, Rogers GG, Buffenstein R. Acute appetite reduction associated with an increased frequency of eating in obese males. Int J Obes Relat Metab Disord. 1999 Nov;23(11):1151–9. doi: 10.1038/sj.ijo.0801046. [DOI] [PubMed] [Google Scholar]
- 42.Asakawa A, Inui A, Yuzuriha H, Ueno N, Katsuura G, Fujimiya M, et al. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology. 2003 May;124(5):1325–36. doi: 10.1016/s0016-5085(03)00216-6. Epub 2003/05/06. eng. [DOI] [PubMed] [Google Scholar]