Abstract
Little is known about the decisions and perspectives of participants undergoing direct-to-consumer genetic testing (DTCGT). The aims of this study were to examine the views, attitudes and decision-making factors of primary care patients regarding DTCGT. Their experience of and reactions to testing also emerged during the study. In this longitudinal, qualitative study, 20 primary care patients participated in DTCGT and individual interviews: (1) prior to testing after the informed consent session, (2) after receiving results, (3) 3 months post-test, and (4) 12 months post-test. Interviews included open-ended questions and all transcripts were analyzed using grounded theory, constant comparison methods. Five key themes emerged from data analysis as participants underwent DTCGT and reflected on their decision over time: (1) limited concerns about DTCGT, (2) motivations for testing, (3) expectations of testing, (4) understanding of results, and (5) impact of testing and results. While a few participants expressed concerns before testing, participants were motivated to test by curiosity, gaining actionable knowledge, and altruism. Most were uncertain of what to expect from DTCGT and needed assistance in understanding results. While many reported testing had no significant impact on them, being relieved or pleased after testing was the most common emotional effect. Notably, a few participants made positive health changes in response to testing. Given the paucity of information about primary care patients and DTCGT, this study adds more in-depth information to the emerging research on how such participants’ view, make decisions about, experience and react to DTCGT over time. Because uncertainty remains about the accuracy of DTCGT, the response of primary care patients to this testing requires further investigation.
Keywords: Direct-to-consumer, Genetic testing, Primary care patients, Decision making, Ethical issues
Introduction
Direct-to-consumer genetic testing (DTCGT) is a relatively new and developing service which enables individuals to acquire genetic information outside of their doctor’s office through a few clicks of the mouse and a saliva sample sent to a DTCGT company. These tests seek to identify rare single-gene mutations and more common single-nucleotide polymorphisms, and offer risk estimates based on these findings. DTC genetic tests exist for ancestry and paternity, while others return results for a range of diseases, e.g., diabetes, cancers, heart disease, other non-health-related traits such as hair/eye color, baldness, and drug sensitivities, e.g., Warfarin. Some companies also include carrier screening for single gene disorders, e.g., cystic fibrosis and sickle cell anemia, though the majority of results focus on disease risk information and prediction (Borry et al. 2010).
Scientists, policy makers, health professionals, and bioethicists have raised concerns about DTCGT (Rogowski et al. 2010; Wasson et al. 2006). Some argue that science is in its infancy and that results are not valid or accurate enough to warrant mass marketing of the tests (American College of Medicine Genetics Board of Directors 2004; Committee on Genetics, American College of Obstetricians and Gynecologists 2008; Federal Trade Commission 2006; Hudson et al. 2007). Others’ concerns lie with the potential burden these tests could have on the limited resources of the medical system (Brett et al. 2012; McGuire and Burke 2008, 2011; White et al. 1999). If consumers used these tests and received unfavorable results, they might overwhelm their physicians’ offices requesting help with understanding and interpreting DTCGT results and/or additional testing or screenings for conditions for which they may or may not be at high risk (Giovanni et al. 2010).
A significant concern for practitioners in genetics and public health is the clinical validity and utility of the data from DTCGT (Edelman and Eng 2009; Khoury et al. 2009; Stack et al. 2011). If the test results are not valid, they may provide false-positive or false-negative risk results to individuals (Wasson et al. 2006; Wasson 2008). Bloss et al. (2012) found that in only 5 of the 15 conditions they examined did the DTCGT risk estimates align with the medical histories of the consumer (Bloss et al. 2012). Decisions made based on inaccurate genetic results could be harmful for individuals, particularly if health-related decisions are effected, such as preventive health screening or regular physician visits.
The ability of a lay public not trained in genetics or related fields to understand DTCGT results without the assistance of a qualified health professional is also queried (Wasson et al. 2006). Not only can DTCGT results be dense with medical and scientific jargon, but accurately interpreting risk of disease can be challenging. Leighton et al. (2012) found significant differences between the lay public’s interpretation of results of four sample tests (colorectal cancer, heart disease, and skin cancer) when compared to genetic counselors (Leighton et al. 2012). Even when lay respondents reported that the results were easy to understand, often they still misinterpreted the results.
Limited research has been published on how results from DTCGT and other genetic tests have affected the health behavior of users (Bloss et al. 2012; Gordon et al. 2012; McBride et al. 2010a). Kaufman et al. 2012 is one of the few studies to assess the impact of DTCGT on health behaviors of actual users (Kaufman et al. 2012). Via an online survey, they explored how consumers interpreted risk information and how their interpretations influenced their healthcare. Nine percent of their participants sought additional testing in relation to results they received from a DTCGT company, 16 % changed a medication or supplement, and 33 % reported paying more attention to their diet. McGowan et al. (2010) interviewed 23 early users of this testing and most did not report making changes based on their results or speak to their physicians (McGowan et al. 2010). Bloss et al. found no change in dietary fat intake, exercise, and anxiety levels among users of DTCGT recruited from health and technology companies (Bloss et al. 2010b). Results from these studies are varied and no clear picture of the impact of DTCGT has yet emerged.
Methods
Participants
This exploratory qualitative study was reviewed and approved by the Institutional Review Board. The study aims were: (1) to explore primary care patients’ views and attitudes about DTCGT and (2) to examine factors, including ethical considerations, that influenced their decisions about whether or not to undergo DTCGT. There were two phases to the study. Phase 1 involved four focus groups of patients recruited from a primary care waiting room at an urban academic medical center. The specifics of phase 1 are reported elsewhere (Wasson et al. 2012).
We targeted a primary care patient population because: (1) There was at the beginning of the study (August 2009), and remains, a lack of published research on primary care patient decision making about and reactions to DTCGT; (2) They offered insight into the decision making of a potentially more diverse sample than early studies of social networkers and early adopters; (3) Primary care patients constitute a broader audience for this testing than other early studies; (4) They may use their primary care physician as the gateway contact with the medical field, particularly when receiving DTCGT results; and (5) This sample already had an identified physician in case of medically relevant findings.
In phase 2, the study aims were explored longitudinally. In addition, primary care patients’ experience of and reactions to DTCGT emerged during the course of the study and are captured in this analysis. Twenty participants underwent informed consent, DTCGT, receipt of results and follow-up between December 2009 and June 2011. Eligibility criteria included being 18 years old, English-speaking, and not having prior genetic testing, except for standard pre-natal genetic testing which is widespread. Sixteen participants (55 %) were recruited from phase 1 to participate in phase 2 and four additional participants were recruited directly from the primary care waiting room. We hypothesized that 20 participants would capture a range of views, attitudes, and experience based on other qualitative studies (Madsen et al. 2007; Schmidt 2010). Because almost half of the individuals who participated in phase 1 did not choose to undergo DTCGT, data for both phases are reported separately.
Data collection
Data were gathered through four individual interviews over approximately 12 months during the: (1) informed consent session, where the saliva sample was provided for DTCGT; (2) receipt of results 4–6 weeks after the first interview; (3) 3 months post-results, and (4) 12 months post-results. Before deciding whether to undergo testing, a genetic counselor and study investigators discussed the range of possible results and limits of this testing with participants as part of the informed consent process. Participants also completed the 23andMe consent form via their online account in order to proceed with testing. A genetic counselor reviewed results with and answered questions for participants during interview 2.
All 20 participants provided a saliva sample for analysis after their individual informed consent session. The 23andMe test kit was used because it had a very broad range of test types: carrier status for known single-gene conditions, common disease associations, drug susceptibility, and physical traits. Further, these tests have widely varying clinical specificity and a wide range of expected psychosocial and medical impact from clinically actionable, e.g., BRCA mutations or drug sensitivities, to potentially interesting but medically irrelevant, e.g., eye-color or ear wax type. Lastly, the 23andMe kit was the least expensive DTCGT available. The cost of testing was funded through the study.
At each interview, participants were asked open-ended questions about reasons for participating in the testing, their decision to test, their views of DTCGT, expectations, hesitations or concerns about testing, and with whom they had or would discuss their participation and/or results. Interviews 3 and 4 (3 and 12 months post-results) also included questions on participants’ understanding of results, overall reaction to the testing process, whether testing had had any impact on them physically, emotionally, or psychologically, and if there was any impact on their relationships/family, or on their lifestyle/behavior. Their willingness to pay for and/or endorse this type of genetic testing was queried. Interview 4 included additional close-ended questions about the return of genetic research results. All 20 participants completed the first three face to face interviews and 17/20 completed the 12-month interview in person or by telephone as three were lost to follow-up. All interviews were audio recorded and transcribed verbatim. Participants received $50 for each session and a parking pass.
Data analysis
Two of the researchers analyzed and coded the data using standard grounded theory, constant comparison methods. Grounded theory is an inductive method of data analysis used when little is known about a phenomenon (Glaser and Strauss 1967; Glaser 1999). Two researchers read all the transcripts and made notes of potential categories or codes. They then analyzed each interview line by line to determine which category or categories were most relevant for the specific text. Data and codes were reviewed repeatedly as the overarching categories, or themes, emerged to ensure agreement. A third researcher provided conceptual guidance and analyzed a sampling of the interviews to check for inter-rater consistency. No major discrepancies were identified.
Results
Participants in this phase of the study had an average age of 49.5 years (range 29–63). Sixty percent were female, 50 % were African American and 50 % were White. All had graduated from high school, 25 % had undergraduate degrees and 5 % had graduate degrees. There was a mixture of employment and income levels and the majority was married (65 %) and had private or government health insurance (95 %) (Table 1).
Table 1.
Participant demographics
| Individual testing | |
|---|---|
| Age | |
| Range | 29–63 |
| Mean/average | 49.5 |
| Income | |
| Less than 5,000 | 10.00 % |
| 5,000–9,999 | 0.0 % |
| 10,000–24,000 | 30.0 % |
| 25,000–49,999 | 25.0 % |
| 50,000–74,999 | 15.0 % |
| 75,000–above | 20.0 % |
| Sex | |
| Female | 60.0 % |
| Male | 40.0 % |
| Education | |
| Less than HS | 0.0 % |
| High school graduate | 30.0 % |
| Some college | 40.0 % |
| College graduate | 25.0 % |
| Post graduate | 5.0 % |
| Marital status | |
| Never married | 15.0 % |
| Married | 65.0 % |
| Separated/divorced | 10.0 % |
| Widowed | 10.0 % |
| Race/ethnicity | |
| Black | 50.0 % |
| White | 50.0 % |
| Insurance | |
| Private | 55.0 % |
| Medicare/Medicaid | 40.0 % |
| None/uninsured | 5.0 % |
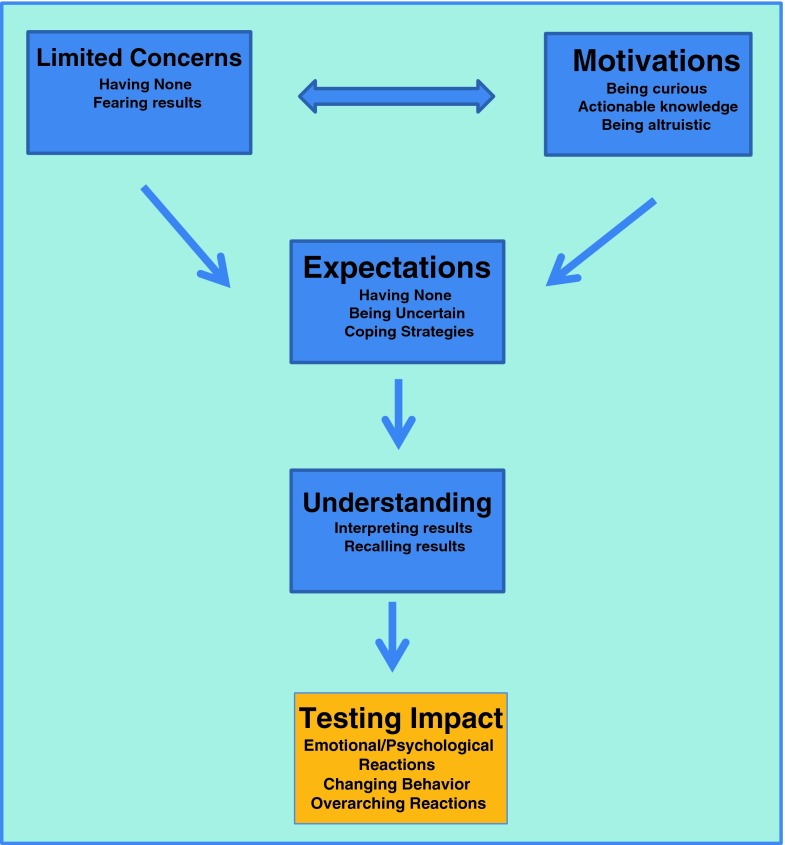
Qualitative data analysis revealed five main categories which capture participants’ views of, decision making about, experience of, and reactions to DTCGT from the pre-test session through 1 year after testing: (1) limited concerns about DTCGT; (2) motivations for testing; (3) expectations of testing; (4) understanding of results; (5) impact of testing and results (Fig. 1).
Fig 1.
Primary care patients’ decision making about, experience of and reactions to direct-to-consumer genetic testing
Limited concerns about DTCGT
When articulating their decision to undergo testing, some participants expressed limited concerns, fears, and hesitations.
All participants who agreed to the first interview, which included the informed consent process, completed testing. While a majority of participants stated they had no concerns or hesitations about testing, a notable minority expressed limited concerns. A repeated concern was the confidentiality of results, specifically that genetic results could affect health insurance or lead to denial of coverage.
The only real concern I had about it was where the information would go and if there would be a risk of being labeled where health insurance would be an issue, so I would be pegged as having something and somebody finding out that I have that risk and therefore insurance would be an issue…. (P15:I2)1
A few others expressed a fear of knowing, especially unwanted or “bad” results, though none declined testing.
I mean my first concern was the disturbing results that might come back with this. I mean sometimes people want to know but they don’t want to know. You know like, okay, that’s fine, okay, just keep it closed, just keep it closed, you know, or let me take a peek. I mean it’s almost like that type of situation. I mean you want to know but then you don’t want to know. (P13:I3)
Motivations for testing
Participants articulated a range of reasons why they decided to undergo DTCGT including curiosity, a desire to gain actionable knowledge and altruism.
- Being curious: Curiosity as a motivation for testing was expressed by a majority of participants. They were keen to discover what DTCGT would highlight about their own genetic make-up and experience this new type of testing personally.Well intellectual curiosity I think. Just to see how it was done, how the results would present, or how they would be presented, and how much they could see, how much could be shown from that. And as I say, I don’t have a scientific background, so a lot of it is just fascinating to see how all of that can spin out. It’s really; I mean I can’t believe that they can even figure this much out at this point with the millions and millions of things that they can track, so it’s just interesting. (P4:I3)
-
(b)Gaining actionable knowledge: Many participants repeatedly articulated a desire to gain knowledge and act on it as a key motivation for deciding to test. Some sought genetic knowledge because they had a known family history, while others did not, and a few revealed a personal history of disease as a motivating factor for testing.I was just really hoping to find out if I am at risk for any diseases that run in my family. Like my mother had diabetes, not my mother, but my grandmother had diabetes and my mother had heart problems, my father had cancer, and I was just hoping to see if any of these things, you know, if I had any genetic strain of these types of diseases. (P14:I2)Primarily because I have a health history that would lend itself to genetic testing. Being a breast cancer survivor and having two teenage daughters I thought that any information that I could glean from the testing would be important, and that was primarily the reason why I did it. (P15:I3)A key part of actionable knowledge was wanting to use this information to prepare for the future. Some articulated a belief that knowing was better than not knowing because they could seek ways of intervening early and preventing disease. They held a strong belief that the knowledge gained would be actionable and if they discovered increased risks of disease they would take preventive steps.Just because I think it’s better if you have more information that you can be more prepared and equipped in life. So if you know that there’s something going on you can go ahead and fix it now and not have to try to fix it later when it’s already unfixable. (P2:I4)
-
(c)Being altruistic: A few participants expressed a desire to help their families or the next generation by undergoing DTCGT. Part of their decision was motivated by wanting to learn what they might “pass on” to their children or share genetically with other family members.…not only just for yourself, you know, but you have to think about family, and then you got to think about future, and then you got to think about the next generations, I mean, so, you know, not being selfish and just thinking about yourself. I’m saying think about your family and the knowledge that they can gain from this type of testing. I mean, you know, you would also see that, you know, it will be beneficial for us all. (P13:I4)For a few others, altruism meant contributing to research and medical science more broadly, which was a key motivation in their decision to participate in testing.Because you go through life just thinking that you are born, you grow, you grow old and then you die. But, there is things that you can do to help others and yourself. Like this, studies and things where people, they can come up with cures for this and cures for that, preventive measurement, you know. That can help too. (P11:I2)
Expectations of testing
In contrast to ‘motivations’ which describes the “why” of the decision to test, this category captures the “what” regarding testing, i.e., what participants expected of the testing process and results. Participants’ expectations of DTCGT included having none to being uncertain to speculating on more specific ideas about results that they might receive.
- Being uncertain: During the first and second interviews, approximately half of participants stated that they were unsure or had no expectations of DTCGT and others expected to receive disease risk or health status information.I don’t know what to expect. I don’t know what to expect from it, so I just figure you be prepared to expect whatever the unexpected is. (P7:I1)
-
(b)Coping strategies: While only a few participants explicitly stated they expected to receive “bad results” before testing, after receiving results over half of participants described a coping strategy they would have employed if results had been “bad” or worrying.I didn’t know what to expect and my thing was, okay, if it was something bad I was going to see or get, oh well, I was just going to try to handle it and go see my doctor, you know and just try to work with it. Work with my doctor; work with whatever…. (P11:I2)
Understanding of results
At multiple points, participants were asked to reflect on their understanding of results and, during that process, their recollection of results and their decision to test was revealed over time.
The majority of participants thought their DTCGT results were fairly easy to understand with the help of a genetic counselor. Only one participant indicated that the genetic counselor was not needed to grasp results. Less than half thought they might have been able to understand the results if they had spent enough time working through them on their own and a similar proportion explicitly stated they would not have understood results without the genetic counselor’s assistance.
In discussing their results 1 year later, approximately half of participants recalled only a general impression of test results, i.e., being pleased with results or having no “bad” results. The others recalled at least one specific condition or disease risk, such as diabetes or a drug sensitivity.
I remember the results weren’t bad. I don’t really remember too much about it. I really don’t. (P7:I4)
Okay, I remember results that directly affected me in terms of health concerns or things that I might be at risk for - Coumadin sensitivity, restless leg syndrome. I think it was vein thrombosis problems and I think those were the main ones. Those were the main ones that I remember anyway. And, I thought it was interesting because I am affected by restless leg syndrome and I have what I think is the beginning of varicose veins, so, it sort of matched with some of the things I actually have. (P18:I4)
Impact of testing and results
Participants discussed their immediate reactions to DTCGT results and, over time, reflected on the impact DTCGT had on them and any changes they made or felt after testing.
-
Emotional and psychological reactions: Participants’ emotional and psychological reaction to DTCGT and results varied and altered over time for some while others remained unaffected.
Directly after receiving their results (interview 2), participants’ reactions to their results were diverse with no single dominant response. The most common response was feeling happy or pleased, especially if participants had not received many high risk results. Some explicitly noted they were relieved they had not received “bad” results.Well no, it’s fabulous because this is pretty much what I was hoping for, and I got what I wanted, so that’s why I’m really pleased, I’m really happy with it…. Well like I said, I was anticipating a lot of bad results, but since I did not get any I’m really pleased. I mean this makes me feel great. I know I’ll be around at least another year or two. (P17:I2)Others said they were not surprised by results or found results interesting. A few participants noted there was less or different information included in results than they expected and a few were simply neutral about results.I guess kind of a neutral reaction I guess; I don’t have a strong reaction one way or the other. There may be a few pieces of information in there that would be useful for me going forward, but I don’t have a strong reaction in either direction. (P19:12)Three months after receiving DTCGT results, participants’ reactions to testing were more uniform. Most expressed positive reactions to testing and results, claiming participation was a “good decision”. The primary reason given was that they gained knowledge or learned something. A few participants reinforced their altruistic motivations for testing, and a few remained neutral about testing and results.I think it was really good. I really think it’s good to have knowledge about yourself because whatever you know already you gonna always learn something new as you go deeper into it… (P14:I3)One year after receiving results, participants reflected retrospectively on the emotional and/or psychological impact of DTCGT. Half of participants completing this interview stated DTCGT had no effect on them. While a few indicated they had been anxious before receiving results, nearly half noted that they were relieved or not worried by their actual results.No, no worries or anything like that because once again those tests aren’t quite founded yet, so there was no worries or problems that I might have had or anything. (P12:I4)A few participants articulated the specific psychological effect DTCGT had on them noting how worried they were about various disease risks before testing. They were reassured and relieved by their “good” DTCGT results and indicated their mindset about their health and well-being altered after receiving these results.I just said it had a positive impact on me. I feel better about myself. You know, like I said, you read things, you hear things, there’s always something in the news that’s put there to scare you, to frighten you and you’re like, “Oh.” The test pretty much took care of that, in my own mind that is. In my own mind it pretty much relieved me of a lot of those worries, fears, and whatnot. Like I said, I enjoyed the test, I liked it, and I just can’t emphasize the positive impact it did have for both of us, to be honest with you. I think it would probably have the same impact too with others and their families. In my opinion, it’s best to know the truth about things. I wasn’t in any high risk areas, if you remember; which is good. (P17:I4)
-
(b)
Changing behavior: Throughout the testing process and beyond, participants described whether and why they made lifestyle or behavioral changes after testing.
During the final interview, approximately half of participants indicated they had made no changes in response to results, mainly because there was nothing on which to act. They viewed results as neither containing worrying nor actionable information nor providing any new health information.Well I just read them and accepted them. I didn’t act because there was nothing really for me to act upon. A lot of stuff that I’m faced with comes with old age and there’s nothing I can do about that. (P17:I3)In contrast, receiving results which were “low-risk” was a motivating factor to make positive health behavior changes for a few participants, the most common being to diet or exercise.Well, I’ve tried to stop frying more and start broiling and eating more vegetables and trying to eat more healthier snacks because that was my problem. One of my conditions was that I was overweight. (P14:I4)Other participants described actions taken in response to testing, such as health and wellness screening, carrying a drug sensitivity list with them, having a particular health condition checked, or opening up communication with extended family related to testing.Well I sort of thought about my, it made me think about more about my health and that I should start maybe being tested more frequent. It made me start thinking about I should exercise, watching my diet, start eating healthier. And it also, another thing that I did was I started getting routine testing. You know they have a program called Lifeline Screening that you can get, and it’s recommended, and you can select the tests you want and then they give you the results. And they have it like once a year. That’s some of the things I started doing. (P14:I4)Receiving perceived “good” results prompted them to make changes they knew would be beneficial, but had not implemented prior to testing. A few participants elaborated on why DTCGT results prompted these specific changes:… I want to stay on that low risk…. I want to remain there. (laughter) So, yeah, that definitely helped me and, you know, persuaded me to move forward with those changes that I needed to do that I have been thinking about and procrastinating and pushing off so many years. The testing just helped me just move forward with it a whole lot quicker. (P13:I4)
-
(c)Participants’ overarching reaction to DTCGT: In evaluating their views of, decisions about, experience of and reactions to DTCGT 1 year after testing, most participants stated they would take the test again and would recommend it to others. Most indicated they thought the testing was helpful regardless of whether they made any changes based on results.Because, like I said, it gives you an excellent feeling. It’s as if you stepped into a time capsule and you went ahead in time and you can see something. I sort of did the same thing because I was able to see that I wasn’t on in a high risk area, which is sort of like going into time saying, “Okay, will I have this problem ahead of me in the future?”… (P17:I4)Only a few expressed reservations about the testing at this point in time.… if it was done through a company rather than through a research project. With that in mind, I don’t think that the test gives enough information to be worth the amount of money that it would cost to do it, and I really think that not being able to interpret the results with a genetic counselor would be one of the reasons I would not recommend it. I think it’s important to be able to go through the information and have somebody be able to explain what the results are. (P15:I4)
Discussion
In this qualitative study of 20 primary care patients who underwent DTCGT and interviews over 12–15 months through a research study, most were women with insurance and a range of income levels. African Americans are represented more highly in our sample than the general US population (12.6 % per US Census website) and participants generally had lower education levels than other studies of DTCGT (Bloss et al. 2011; Kaufman et al. 2012; McGowan et al. 2010; O'Daniel et al. 2010). Recruiting from an urban primary care office may have contributed to a more diverse sample than other early studies of this testing, including those of early users (Kaufman et al. 2012; McGowan et al. 2010). Our participants were not early users or adopters, as none had taken a DTC genetic test prior to the study. This study of participants before, during and after DTCGT offers insight into the views of, decision-making considerations about, experience of and reactions to testing over time. Another primary care population studied in relation to genetic susceptibility testing was similar in demographics, except for education level where our sample was lower. The test offered was not a direct-to-consumer genetic test, but was for eight common health conditions (Kaphingst et al. 2012).
Limited concerns
Most participants did not articulate specific concerns before testing, which is interesting to note. Those that did mentioned confidentiality and privacy concerns and fear of knowing, which have been noted elsewhere (Bloss et al. 2010a; Goldsmith et al. 2012; Hahn et al. 2010; McGuire et al. 2009; Rahm et al. 2012; Wasson et al. 2012). These concerns were limited and not weighty enough to preclude them from undergoing DTCGT and/or their concern to be informed was a higher priority. Since they were a self-selecting group and many had participated in a phase 1 focus group, the time between phases may have allowed them to process their concerns. Phase 1 concerns included questioning accuracy of testing, interpreting results, the implications of revealing results, and ethical issues. These concerns seem to have outweighed the interest in testing for those that declined. Interestingly, the fear of knowing was not evident in the phase 1 data and may have arisen as participants faced the decision to actually take the test rather than simply talk about DTCGT in theory.
Motivations
Curiosity and gaining actionable knowledge were strong motivating factors for many participants, as compatible with other findings (Gollust et al. 2012; McGowan et al. 2010; Su et al. 2011). Participants sought genetic risk information which they could translate into action, in spite of being informed of the uncertain validity and accuracy of results. Participants were strongly motivated by a desire for knowledge about their genetic risks, for themselves and their families, and to contribute altruistically to research knowledge which could impact the health of the public in future. Some wanted to help this research study as well as genetic research more generally.
Our participants expressed a belief that knowledge was an intrinsic good even if the results were “bad” or worrying. A fear of knowing for some was outweighed by the desire to know. They believed learning about their genetic risks would help them prepare for the future, and possibly prevent disease for themselves or others, and that they would act on results. Data remains varied on responses to DTCGT and our study adds in-depth information to the reasons primary care patients may decide to test; what they hope and intend to do and report doing throughout testing and beyond.
Expectations
Tested participants had few, if any, expectations of DTCGT, which is unsurprising given its novelty and the fact that they had no comparable experiences. There was a dichotomy between participants being unsure what to expect from DTCGT and also seeking particular disease risk information. While participants were uncertain what specific results would be returned, some reflected on how they would respond and cope if they received “bad” results. They processed the uncertainty and anxiety surrounding testing and prepared for the possibility of bad news. Some participants expressed ambivalence and conflicting emotions, namely curiosity vs. anxiety about results, often only after receiving results, suggesting they may not have been aware of this conflict or did not wish to articulate it. Some may have sought DTCGT information to soothe their anxiety or reassure them about their health status.
Understanding and recollection of results
Most participants clearly stated that they valued and needed the research team and genetic counselor to understand and interpret their results. This attitude reinforces concerns raised about the need for health professional involvement in DTCGT (American College of Medicine Genetics Board of Directors 2004; Wasson et al. 2006; Wasson 2008). The education level of our participants was notably lower than in other studies where 40–54 % had postgraduate degrees (Gollust et al. 2012; Kaphingst et al. 2012; Kaufman et al. 2012). In general, participant recollection of results diminished over time—half did not recall any specific results at 12 months and half remembered a specific disease risk. This result contrasts with Kaphingst et al. 2012, where 80 % of their participants from a health maintenance organization recalled genetic susceptibility for eight health conditions 3 months after testing. This study was specifically designed to assess participants’ understanding of genetic susceptibility testing and ours was not. In addition, our participants had many more results returned and they were asked to recall them longer after testing.
Impact of testing
Most participants claimed that testing had no impact on them over time. Some expressed keen interest in DTCGT, but were ambivalent after receiving results. This response may be due to multiple factors. First, because of the type of results returned, which included hair/eye color or ear wax type, many of which had no bearing on their health. Second, many participants viewed their disease risk estimates as not worrying or “bad”. Third, they may have understood the uncertainty surrounding the results, i.e., potential lack of validity, accuracy and clinical utility, and not given results significant weight even if they included elevated risk estimates. Fourth, others may have been ambivalent because they were not Caucasian, which was the most common comparison population in the genetic analyses. While being curious and motivated to test, these participants seemed fairly savvy about the impact of results, weighing up different pieces of information in reacting to their results. One surprising longer term impact of DTCGT on participants was that a few participants who received “good” results implemented positive health behavior changes after testing. These individuals saw their positive health status as a key motivating factor for making changes to their behavior, such as healthier dietary options. We might have expected to see those types of changes if a participant had received higher risk results for a particular disease, i.e., diabetes, heart disease, particularly if a healthy lifestyle was known to decrease that risk over time. Instead, our participants articulated that the “good” results were the extra motivation they needed to implement such changes but had not done so. Based on their explanations, it seems the DTCGT process and results prompted them to reflect on their own health and motivated them to change to maintain their perceived healthy status. This response is self-reported from a few participants and must be viewed within those parameters. In contrast, another study found no significant difference in dietary fat intake or anxiety levels in those undergoing DTCGT (Bloss et al. 2011). Our finding may be an anomaly or more evident in smaller numbers of users of DTCGT and warrants further investigation.
Some participants were worried they would receive “bad” results and were greatly relieved when their risk assessments were not perceived as “high” or worrying. In fact, many participants were pleased with or relieved about their results. One concern in the literature is the possibility of inaccurate results and/or interpretation of DTCGT (Leighton et al. 2012; McBride et al. 2010b; Wasson et al. 2006). Consumers of such testing may receive false reassurance based on inaccurate results. For example, the 23andMe panel only includes breast and ovarian cancer-associated mutations that are common in the Ashkenazi Jewish population. A non-Jewish participant may receive a ‘normal’ result, and assume they are not at elevated risk for breast cancer, while ignoring other possible factors, such as age or family history of related cancers. Some of our participants noted that seeing low or decreased risk results for multiple diseases and conditions alleviated their fears. The genetic counselor explained the nature of the results and that a low risk result from this test did not mean that a person would not develop a disease. However, the expression of relief prompts us to consider the psychological boost from receiving a “good report card” even if the source is unproven.
Limitations
This study was an exploratory, qualitative study and should be interpreted within those parameters. The sample was a self-selecting group who expressed a keen interesting in DTCGT and we do not claim it is representative of all primary care patients. The testing was funded and conducted within a research study context, which may have encouraged some to participate who otherwise would not have taken a DTCGT. They may have had more trust in the process or testing because of the health professionals involved, such as a genetic counselor. The investigators viewed testing without genetic counseling as unethical, though this does mean the testing experience was not an exact replica of DTCGT for an individual consumer. Furthermore, participants’ descriptions of their experience, decision-making, and any actions or changes taken are self-reported.
Conclusion
This longitudinal, qualitative study adds more in-depth information to the emerging data on participants’ decision-making process about, experience of and reactions to DTCGT over time. As more studies of DTCGT emerge, the wider impact of such testing remains unclear—for most participants it had no significant effect, though many experienced a positive emotional impact, and nearly all needed help interpreting results. A larger study is needed to confirm or refute the impact of DTCGT found in this study. This study suggests the possibility that DTCGT could raise awareness about a person’s health and prompt him to make changes based on “good” or “bad” results which could affect the individual, families, communities and the wider public if and when DTCGT increases.
Given the exploratory nature of the research and small sample, we are cautious when examining the implications of our findings for other populations. We drew participants from the general population of primary care patients going for care at a medical center, rather than a disease based population, i.e., diabetic clinic. This recruitment decision was made to obtain a more diverse sample than some other early studies in this area. Recruiting from a general population of patients may provide initial insights about why other similar groups who were not early adopters or early users of DTCGT, i.e., African Americans, those with lower education levels, may decide to test, as well as their experience of testing and its impact. It is possible that our findings could be relevant to more general consumers with similar demographics, though further investigation is needed.
Acknowledgments
The authors wish to thank Bryanna Cox and the Primary Care Department at Loyola University Medical Center for their assistance with the research.
Conflicts of interest
The authors have no conflicts of interest to declare.
Statement of research compliance
This research study was conducted in a manner which complies with the current laws in the USA. It was reviewed and approved by the Institutional Review Board at Loyola University Chicago.
Footnotes
P15:I2 is an abbreviation for participant 15: interview 2. The remaining quotations follow this format, indicating for the reader when the interview was conducted.
References
- American College of Medicine Genetics Board of Directors ACMG statement on direct-to-consumer genetic testing. Genet Med. 2004;6(1):60. doi: 10.1097/01.GIM.0000106164.59722.CE. [DOI] [PubMed] [Google Scholar]
- Bloss CS, Ornowski L, Silver E, Cargill M, Vanier V, Schork NJ, et al. Consumer perceptions of direct-to-consumer personalized genomic risk assessments. Genet Med. 2010;12(9):556–566. doi: 10.1097/GIM.0b013e3181eb51c6. [DOI] [PubMed] [Google Scholar]
- Bloss CS, Ornowski L, Silver E, Cargill M, Vanier V, Schork NJ, et al. Consumer perceptions of direct-to-consumer personalized genomic risk assessments. Genet Med. 2010;12(9):556–566. doi: 10.1097/GIM.0b013e3181eb51c6. [DOI] [PubMed] [Google Scholar]
- Bloss CS, Schork NJ, Topol EJ. Effect of direct-to-consumer genomewide profiling to assess disease risk. N Engl J Med. 2011;364(6):524–534. doi: 10.1056/NEJMoa1011893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss CS, Topol EJ, Schork NJ. Association of direct-to-consumer genome-wide disease risk estimates and self-reported disease. Genet Epidemiol. 2012;36(1):66–70. doi: 10.1002/gepi.20664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borry P, Cornel MC, Howard HC. Where are you going, where have you been: a recent history of the direct-to-consumer genetic testing market. J Community Genet. 2010;1(3):101–106. doi: 10.1007/s12687-010-0023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett GR, Metcalfe SA, Amor DJ, Halliday JL. An exploration of genetic health professionals' experience with direct-to-consumer genetic testing in their clinical practice. Eur J Hum Genet. 2012;20(8):825–830. doi: 10.1038/ejhg.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Genetics, American College of Obstetricians and Gynecologists ACOG committee opinion no. 409: direct-to-consumer marketing of genetic testing. Obstet Gynecol. 2008;111(6):1493–1494. doi: 10.1097/AOG.0b013e31817d250e. [DOI] [PubMed] [Google Scholar]
- Edelman E, Eng C. A practical guide to interpretation and clinical application of personal genomic screening. BMJ. 2009;339:b4253. doi: 10.1136/bmj.b4253. [DOI] [PubMed] [Google Scholar]
- Federal Trade Commission. (2006). At-home genetic tests: a healthy dose of skepticism may be the best prescription. http://www.ftc.gov/bcp/edu/pubs/consumer/health/hea02.shtm. Accessed August 11, 2008
- Giovanni MA, Fickie MR, Lehmann LS, Green RC, Meckley LM, Veenstra D, et al. Health-care referrals from direct-to-consumer genetic testing. Genet Test Mol Biomarkers. 2010;14(6):817–819. doi: 10.1089/gtmb.2010.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser BG. The future of grounded theory. Qual Heal Res. 1999;9(6):836. doi: 10.1177/104973299129122199. [DOI] [Google Scholar]
- Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Mill Valley, CA: Sociology Press; 1967. [Google Scholar]
- Goldsmith L, Jackson L, O'Connor A, Skirton H. Direct-to-consumer genomic testing: systematic review of the literature on user perspectives. Eur J Hum Genet. 2012;20(8):811–816. doi: 10.1038/ejhg.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollust SE, Gordon ES, Zayac C, Griffin G, Christman MF, Pyeritz RE, et al. Motivations and perceptions of early adopters of personalized genomics: perspectives from research participants. Public Health Genomics. 2012;15(1):22–30. doi: 10.1159/000327296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon ES, Griffin G, Wawak L, Pang H, Gollust SE, Bernhardt BA. "It's not like judgment day": public understanding of and reactions to personalized genomic risk information. J Genet Couns. 2012;21(3):423–432. doi: 10.1007/s10897-011-9476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S, Letvak S, Powell K, Christianson C, Wallace D, Speer M, et al. A community's awareness and perceptions of genomic medicine. Public Health Genomics. 2010;13(2):63–71. doi: 10.1159/000218712. [DOI] [PubMed] [Google Scholar]
- Hudson K, Javitt G, Burke W, Byers P, American Society of Human Genetics Social Issues,Committee (2007) ASHG statement* on direct-to-consumer genetic testing in the United States. Obstet Gynecol 110(6):1392–1395 [DOI] [PubMed]
- Kaphingst KA, McBride CM, Wade C, Alford SH, Reid R, Larson E, et al. Patients' understanding of and responses to multiplex genetic susceptibility test results. Genet Med. 2012;14(7):681–687. doi: 10.1038/gim.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman DJ, Bollinger JM, Dvoskin RL, Scott JA. Risky business: risk perception and the use of medical services among customers of DTC personal genetic testing. J Genet Couns. 2012;21(3):413–422. doi: 10.1007/s10897-012-9483-0. [DOI] [PubMed] [Google Scholar]
- Khoury MJ, McBride CM, Schully SD, Ioannidis JP, Feero WG, Janssens AC et al (2009) The scientific foundation for personal genomics: recommendations from a National Institutes of Health-Centers for Disease Control and Prevention multidisciplinary workshop. Genet Med 11(8):559–567 [DOI] [PMC free article] [PubMed]
- Leighton JW, Valverde K, Bernhardt BA. The general public's understanding and perception of direct-to-consumer genetic test results. Public Health Genomics. 2012;15(1):11–21. doi: 10.1159/000327159. [DOI] [PubMed] [Google Scholar]
- Madsen SM, Holm S, Riis P. Attitudes towards clinical research among cancer trial participants and non-participants: an interview study using a grounded theory approach. J Med Ethics. 2007;33(4):234–240. doi: 10.1136/jme.2005.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride CM, Koehly LM, Sanderson SC, Kaphingst KA. The behavioral response to personalized genetic information: will genetic risk profiles motivate individuals and families to choose more healthful behaviors? Annu Rev Public Health. 2010;31:89–103. doi: 10.1146/annurev.publhealth.012809.103532. [DOI] [PubMed] [Google Scholar]
- McBride CM, Wade CH, Kaphingst KA. Consumers' views of direct-to-consumer genetic information. Annu Rev Genomics Hum Genet. 2010;11:427–446. doi: 10.1146/annurev-genom-082509-141604. [DOI] [PubMed] [Google Scholar]
- McGowan ML, Fishman JR, Lambrix MA. Personal genomics and individual identities: motivations and moral imperatives of early users. New Genet Soc. 2010;29(3):261–290. doi: 10.1080/14636778.2010.507485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AL, Burke W. An unwelcome side effect of direct-to-consumer personal genome testing: raiding the medical commons. JAMA. 2008;300(22):2669–2671. doi: 10.1001/jama.2008.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AL, Burke W. Health system implications of direct-to-consumer personal genome testing. Public Health Genom. 2011;14(1):53–58. doi: 10.1159/000321962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AL, Diaz CM, Wang T, Hilsenbeck SG. Social networkers' attitudes toward direct-to-consumer personal genome testing. Am J Bioeth. 2009;9(6–7):3–10. doi: 10.1080/15265160902928209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Daniel JM, Haga SB, Willard HF. Considerations for the impact of personal genome information: a study of genomic profiling among genetics and genomics professionals. J Genet Couns. 2010;19(4):387–401. doi: 10.1007/s10897-010-9297-x. [DOI] [PubMed] [Google Scholar]
- Rahm AK, Feigelson HS, Wagner N, Le AQ, Halterman E, Cornish N, et al. Perception of direct-to-consumer genetic testing and direct-to-consumer advertising of genetic tests among members of a large managed care organization. J Genet Couns. 2012;21(3):448–461. doi: 10.1007/s10897-011-9477-3. [DOI] [PubMed] [Google Scholar]
- Rogowski WH, Grosse SD, John J, Kaariainen H, Kent A, Kristofferson U, et al. Points to consider in assessing and appraising predictive genetic tests. J Community Genet. 2010;1(4):185–194. doi: 10.1007/s12687-010-0028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LA. Making sure: registered nurses watching over their patients. Nurs Res. 2010;59(6):400–406. doi: 10.1097/NNR.0b013e3181faa1c9. [DOI] [PubMed] [Google Scholar]
- Stack CB, Gharani N, Gordon ES, Schmidlen T, Christman MF, Keller MA. Genetic risk estimation in the coriell personalized medicine collaborative. Genet Med Off J Am Coll Med Genet. 2011;13(2):131–139. doi: 10.1097/GIM.0b013e318201164c. [DOI] [PubMed] [Google Scholar]
- Su Y, Howard HC, Borry P. Users' motivations to purchase direct-to-consumer genome-wide testing: an exploratory study of personal stories. Journal of Community Genetics. 2011;2:135–146. doi: 10.1007/s12687-011-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasson K. Consumer alert: ethical issues raised by the sale of genetic tests directly to consumers. Am J Bioeth. 2008;8(6):16–18. doi: 10.1080/15265160802248351. [DOI] [PubMed] [Google Scholar]
- Wasson K, Cook ED, Helzlsouer K. Direct-to-consumer online genetic testing and the four principles: an analysis of the ethical issues. Ethics Med Int J Bioeth. 2006;22(2):83–91. [PubMed] [Google Scholar]
- Wasson K, Hogan NS, Sanders TN, Helzlsouer K. Primary care patients' views, attitudes, and decision-making factors regarding direct-to-consumer personal genome testing: results from a qualitative study. Am J Bioeth Prim Res. 2012;3(2):24–35. [Google Scholar]
- White MT, Callif-Daley F, Donnelly J. Genetic testing for disease susceptibility: social, ethical and legal issues for family physicians. Am Fam Physician. 1999;60(3):757–758. [PubMed] [Google Scholar]