Summary
The Gram-negative periodontal pathogen Porphyromonas gingivalis produces a family of outer membrane-anchored proteases, the gingipains, shown to play an essential role in virulence of the organism. The C-terminal domain (CTD) of gingipains and other secreted proteins is known to be the targeting signal for maturation and translocation of the protein through the outer membrane. The CTD is subsequently cleaved during the secretion process. Multiple alignment of various CTDs failed to define a consensus sequence at the putative CTD processing site. Using mutagenesis, we were able to show that cleavage at the site is not dependent on a specific residue and that recognition of the site is independent of local sequence. Interestingly, length of the junction between the CTD and adjacent Ig-like subdomain has a critical influence on post-translational glycan-modification of the protein, whereby insertion of additional residues immediately N-terminal to the cleavage site results in failure of glycan modification and release of soluble protease into the culture medium. Various hypotheses are presented to explain these phenomena. Knowledge of the role CTDs play in maturation of gingipains has broader application for understanding maturation of CTD homologues expressed by bacteria of the Bacteriodetes phylum.
Keywords: glycosylation, gingipains, protease, maturation
Introduction
Porphyromonas gingivalis is an anaerobic, Gram-negative bacterium implicated as one of the primary causative organism of chronic periodontitis (gum disease). The disease is one of the most common inflammatory diseases in humans and is marked by progressive destruction of tissues that support the teeth and in severe cases, results in tooth loss (Williams, 1990). Evidence is accumulating to suggest that periodontal disease, and in particular, P. gingivalis, may also be involved in the initiation and/or progression of cardiovascular disease and rheumatoid arthritis (Gibson et al., 2004, Wegner et al., 2010). Central to the pathogenic potential of the organism is a family of unique cysteine endopeptidases, the gingipains, that are abundantly expressed and translocated across both the inner and outer membranes to be attached to the bacterial surface or secreted into the extracellular milieu (Pike et al., 1994). There are three members of the gingipain family; two termed RgpA and RgpB, are specific for arginyl residues at the carbonyl side of a hydrolysed peptide bond (the P1 position), and one termed Kgp, is specific for lysyl residues at the P1 position. In addition to the catalytic domain, RgpA and Kgp also possess homologous C-terminal extensions of adhesin/haemagglutinin domains which are involved in targeting these proteins to specific host substrates. Together, the gingipains have been shown to contribute significantly to the pathogenicity of the organism (Rapala-Kozik et al., 2011, Fitzpatrick et al., 2009). Collectively, gingipains mediate a wide range of functions essential for survival of the organism in the host environment, from acquisition of essential nutrients to invasion of host tissues and evasion of immune defences (Guo et al., 2010).
As the simplest member of the gingipain family, RgpB has been the focus of structural studies aimed at elucidating post-translational processing and maturation of these enzymes (Eichinger et al., 1999, Nguyen et al., 2007). Newly translated RgpB possesses a number of distinct domains that are modified in the maturation process. These include an N-terminal signal peptide, a pro-peptide domain, a catalytic domain containing caspase-like and immunoglobulin-like subdomains, and a C-terminal domain (CTD) (Fig. 1A) (Potempa et al., 2003, Eichinger et al., 1999). Maturation of the enzyme has been shown to be a complex process involving multiple processing steps, resulting in secretion of passenger protease domain through a new secretory pathway. Briefly, after initial translocation across the inner membrane (IM) via signal peptide targeting to the Sec apparatus, the N-terminal 23 kDa pro-domain of RgpB is sequentially processed to activate the latent proenzyme (Mikolajczyk et al., 2003, Veillard et al., 2013). Concurrently, the C-terminal 22 residues of CTD target the maturing protease to a new secretory system, PorSS, responsible for translocating maturing RgpB through the outer membrane (OM) (Sato et al., 2009, Shoji et al., 2011). During the OM translocation process, CTD is proteolytically removed by a recently described cysteine protease PG0026 (Glew et al., 2012) to produce mature, 48 kDa soluble form of RgpB (Potempa et al., 1998, Eichinger et al., 1999). However, in most strains of P. gingivalis, mature RgpB remains membrane-bound either on the bacterial surface or on outer membrane vesicles. This form is extensively modified by addition of anionic polysaccharides (APS) comprising the sugar component of anionic lipopolysaccharide (A-LPS), to generate membrane-type RgpB (mt-RgpB). Due to extensive but variable APS incorporation, mt-RgpB migrates as a diffuse 70–90 kDa band on SDS-PAGE, some 20–40 kDa higher than for non-modified RgpB (Fig. 1B). Further, APS modification has been proposed as an anchoring mechanism linking mt-RgpB to the outer membrane of the organism. In this regard, defects in sugar biosynthesis pathways imposed by inactivation of the vim locus or porR, result in release of non-APS-modified RgpB into the culture supernatant (Shoji et al., 2002, Vanterpool et al., 2005a, Vanterpool et al., 2005b). In contrast, inactivation of any PorSS secretion system component protein such as the porKLMNP locus (Sato et al., 2009), LptO (Chen et al., 2011) or truncation of the C-terminal signal of CTD invariably results in periplasmic accumulation of partially processed forms of RgpB without APS modification (Nguyen et al., 2007), suggesting the OM translocation step and glycan modification of the protease are intimately linked, although the exact mechanism is presently unknown (Slakeski et al., 2011).
Figure 1. The rgpB translated product.
(A) Nascent RgpB polypeptide is comprised of various domains that direct the maturation of the protein, including the immunoglobulin-like subdomain (IgLD) and the C-terminal domain (CTD). Mature RgpB is the final product after progressive cleavage (arrowed; cleavage occurs after the numbered residues) of the various domains. (B) By Western blot using anti-RgpB, mature, soluble RgpB (lane 1) is approximately 48 kDa whereas the outer membrane anchored APS-modified form, membrane-type RgpB (mt-RgpB; lane 2) appear as a diffuse band migrating at 70–90 kDa.
Apart from the outer membrane translocation signal residing within 22 residues of the C-terminus, very little is known about the function of the remainder of the CTD. Further, cleavage of CTD in a number of proteins has been shown to be mediated by PG0026 (Glew et al., 2012) although the cleavage signal remains enigmatic as multiple alignment of putative CTD cleavage sites from these proteins reveals the absence of a consensus sequence (Fig. 2). In this report, we carried out extensive manipulation of the CTD cleavage region in RgpB to demonstrate cleavage that is independent of local primary sequence but likely mediated by local conformation. Further, that the length of this region has a critical influence on post-translational APS modification of the protein and subsequent attachment to the bacterial surface.
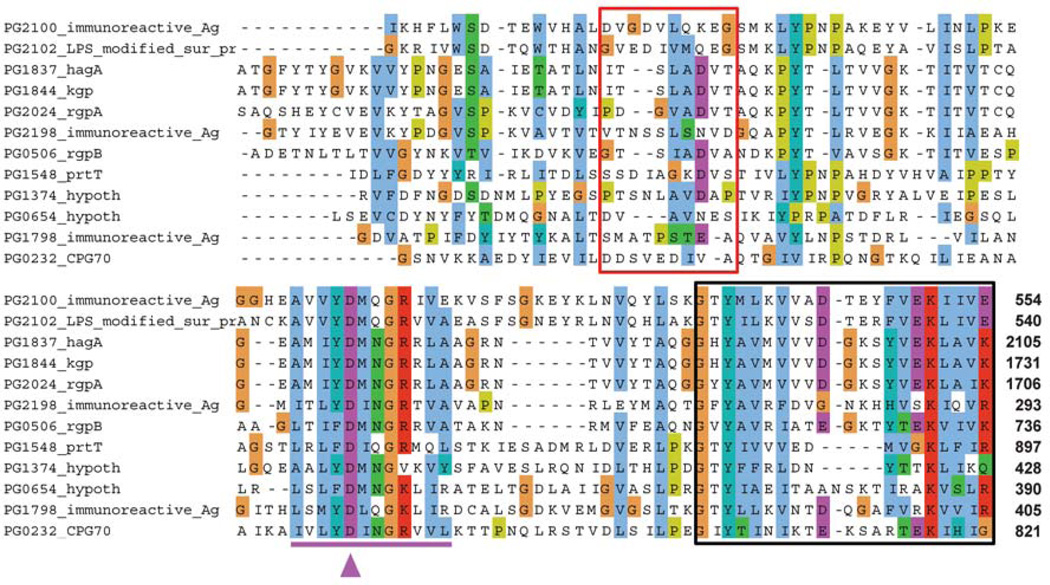
Figure 2. Multiple alignment of the IgLD-CTD junction in proteins from Porphyromonas gingivalis.
C-terminal 100 residues from a number of CTD-class proteins were aligned using ClustalW to show the PorSS outer membrane export signal (black box) and to highlight the variability of the primary sequence at the predicted processing site of the CTD (red box). Residues reported to be essential for the production of RgpB are underlined in purple along with the critical Asp-696 (purple arrowhead). Position of the C-terminal residue is as labelled at the C-terminus.
Results
There is no specific targeted residue for cleavage at the CTD processing site
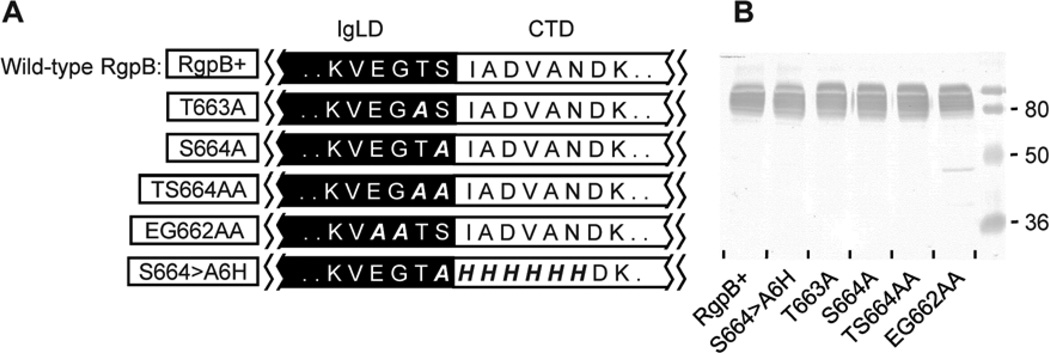
The crystal structure of native, soluble RgpB defined the C-terminal residue of the mature enzyme as Ser664 whereas mass spectrometric data suggest Thr663 as the ultimate residue (Eichinger et al., 1999, Glew et al., 2012). In the present study, a series of mutants were created to replace selected residues at the immunoglobulin-like domain (IgLD)-CTD junction of RgpB to alanine or histidine in an attempt to disrupt cleavage of the CTD and interfere with RgpB maturation and activation (Fig. 3A). To avoid the presence of a highly homologous RgpA gingipain interfering with genetic manipulation and analysis of RgpB maturation, all mutants reported in this paper were created in an rgpA-deficient background as previously described (Nguyen et al., 2007). In combination, 10 residues (Glu661 to Asn670) spanning the junction were mutated to alanine or histidine but none of the mutants showed impairment of RgpB maturation as shown by the presence and similar yield of wild-type APS-modified mt-RgpB on Western blot (Fig. 3B). Also noted was similar quantity and cellular distribution of RgpB enzymatic activity (data not shown). The implication of these findings was the lack of a specific residue at the CTD junction where cleavage occurs and that a specific cleavage recognition sequence is unlikely to exist within this junction region.
Figure 3. Processing site of the CTD is residue independent.
(A) Mutants (boxed) with various residues at the IgLD (black region) and CTD (white region) junction mutated to alanine or histidine were created. (B) Early stationary phase cultures of each mutant and the wild-type RgpB (RgpB+) were adjusted to OD600 1.5 and 50 µL of the cultures were subjected to SDS-PAGE and Western blot against anti-RgpB to assess the status of RgpB maturation (B).
Hexa-histidine scanning of the CTD cleavage site
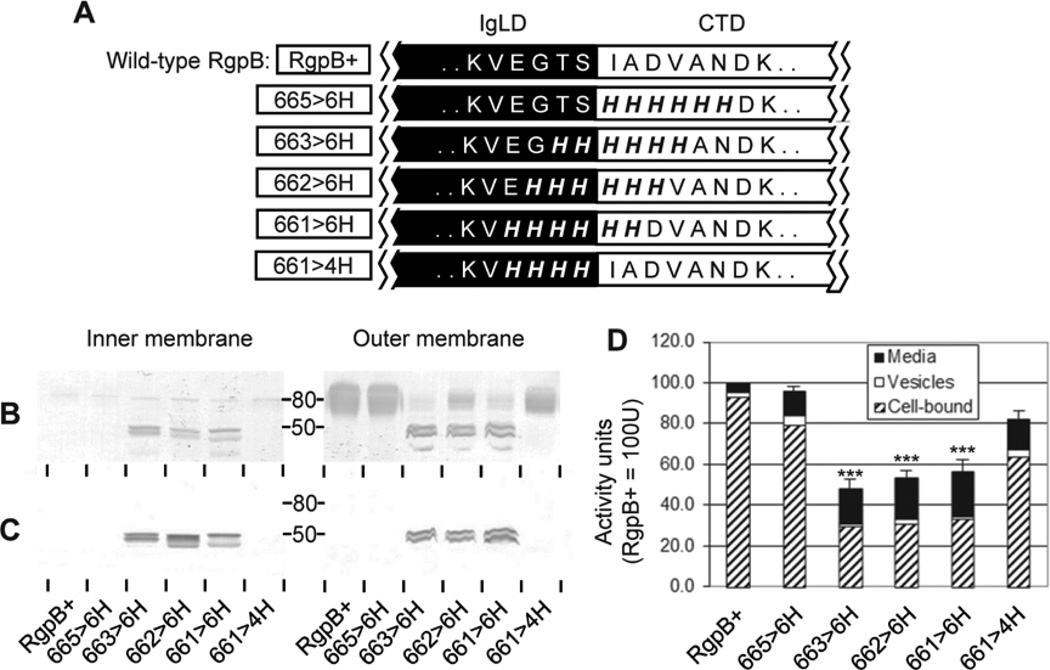
To explain the unusual property of protein processing without a specific residue at the CTD cleavage zone, it was considered conformation of the junction between the immunoglobulin-like domain (IgLD) and the CTD could be important. At present there is no structural information on the region spanning the CTD cleavage zone as the known crystal structure of RgpB terminates at Ser664 (Eichinger et al., 1999). To examine the possibility of CTD processing dependent on local conformation of the junction, a series of hexa-histidine scanning mutations of this region were created using the rationale that bulky imidazole side chains of consecutive histidines would likely disrupt native junction conformation. Indeed, when 6 residues spanning the putative cleavage site were mutated to 6×His (mutants 661>6H, 662>6H and 663>6H), RgpB maturation was impaired as evident by accumulation of partially processed forms containing unprocessed CTD in both the inner membrane (IM) and outer membrane (OM) fractions and reduction of mature, diffuse APS-modified mt-RgpB on the OM by Western blot (Fig. 4B & C). Correlating with data from Western blots, total RgpB enzymatic activity produced by the junction-spanning mutants was significantly depressed (by approximately 50%) as compared to the other mutants and wild-type RgpB (Fig. 4D). Thus, RgpB processing occurred in these mutants at half the efficiency of the wild-type RgpB. In contrast, when 4 residues from Glu661 to Ser664 (mutant 661>4H) in front of the cleavage site or 6 residues from Ile665 to Asn670 (mutant 665>6H) after the cleavage site were changed to histidines, RgpB maturation was similar to the wild-type (Fig. 4B). Although hexa-histidine polypeptides do not self-assemble into an ordered structure (Kaufmann et al., 2002), the presence of consecutive bulky imidazole side chains on these residues could conceivably affect native folding of this site and/or the surrounding region into a wild-type structure for processing. Results suggest that conformation of the junction between the IgLD and CTD may be an important for efficient processing of CTD and subsequent maturation of RgpB.
Figure 4. Hexa-histidine scanning mutagenesis of the IgLD-CTD junction.
(A) Various mutants (boxed) with 4 or 6 consecutive junctional residues changed to 4×His or 6×His, respectively. Early stationary phase cultures of each mutant were normalised to OD600 1.5 and subjected to inner membrane and outer membrane fractionation as per Methods. 50 µL of each membrane fraction was analyzed by Western blot using (B) anti-RgpB and (C) anti-CTDRgpB to determine the maturation status and partitioning of RgpB. (D) 20 µL of normalized cultures were assayed for media, vesicle-bound and cell-associated RgpB activity using synthetic substrates as per Methods. Data represents the mean and SEM from four independent experiments with the total activity in the control RgpB+ mutant equaling 100 U. Significance of total Rgp activity vs. RgpB+ wild-type control: ***, p<0.001.
Elongation of the IgLD-CTD junction disrupts APS-modification of the mature enzyme
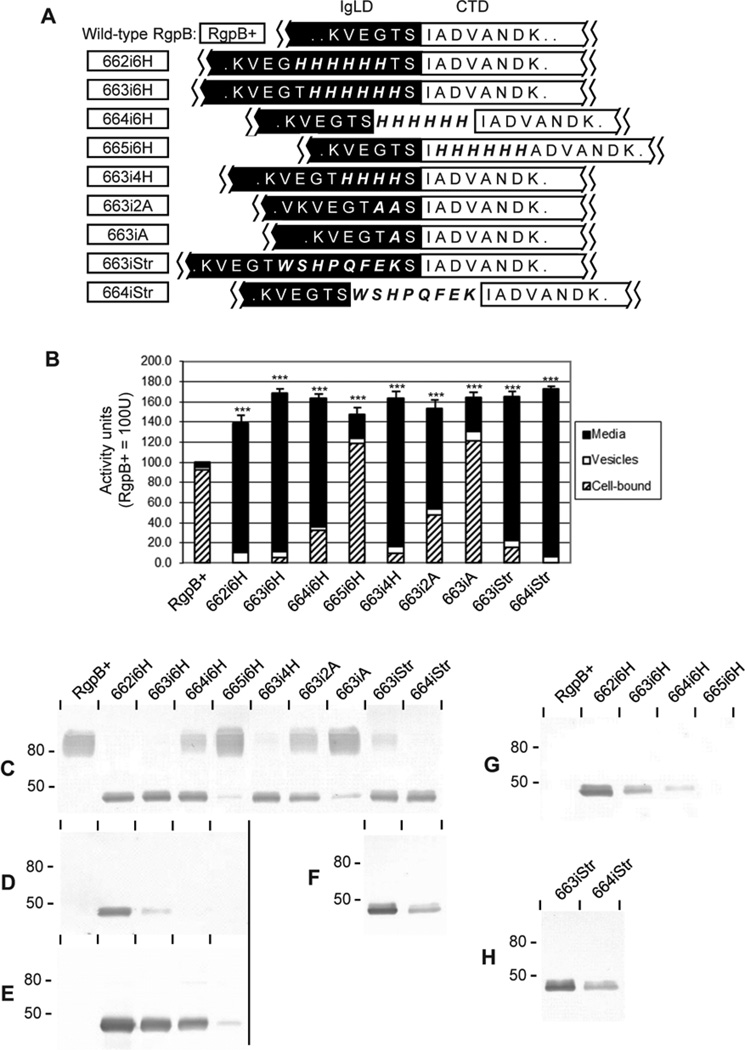
To confirm the role of junctional conformation in directing CTD cleavage, a series of hexa-histidine (6×His) polypeptide insertions into this region was carried out. We hypothesized that extending the length of the junction may disrupt recognition of the cleavage site leading to impairment of CTD processing and subsequently, interfere with RgpB maturation. Unexpectedly, a significant increase in total RgpB activity was detected when 6×His were inserted into the IgLD side of the junction at positions 662 and 663 (mutants 662i6H and 663i6H; Fig. 5A, B). Further, the majority of RgpB activity in these mutants partitioned to the soluble fraction of the growth medium as opposed to cell-bound forms in wild-type RgpB (Fig. 5B). In blots probed with anti-RgpB, the modified enzyme migrated as a sharp band at the predicted molecular mass of the mature protein of 48 kDa. This finding suggested that RgpB was not post-translationally modified with APS in these mutants (Fig. 5C). In addition, these soluble forms of RgpB were found to possess polyhistidine tags as determined by probing with anti-6×His monoclonal antibody (Fig. 5D, G). Purification of the soluble, secreted forms of RgpB over a nickel-chelating column confirmed that the enzyme was not APS-modified as there was no reactivity with anti-APS monoclonal antibody IB5 (Fig. 6) as reported previously for wild-type mt-RgpB (Nguyen et al., 2007).
Figure 5. Peptide insertions into the IgLD-CTD junction.
(A) Mutants (boxed) were created with insertions of various polypeptide lengths into differing sites at the IgLD-CTD junction. (B) Early stationary phase cultures of each mutant were normalised to OD600 1.5 and RgpB activity in 20 µL of the cell-free media, vesicle-bound or cell-associated forms were assayed using synthetic substrates as per Methods. Data represents the mean and SEM from four independent experiments with the total activity in the RgpB+ control equaling 100 U. (C–F) 50 µL of normalised, whole culture of each mutant were subjected to Western blot against (C) anti-RgpB, (D) anti-6×His, (E) anti-4×His or (F) anti-Strep tag® II. Further, 50 µL of cell-free and vesicle-free media fractions of the normalised cultures from various mutants were probed with (G) anti-6×His and (H) anti-Strep tag® II. Significance of total Rgp activity vs. RgpB+ wild-type control was analysed by ANOVA with Bonferroni’s correction: ***, p<0.001.
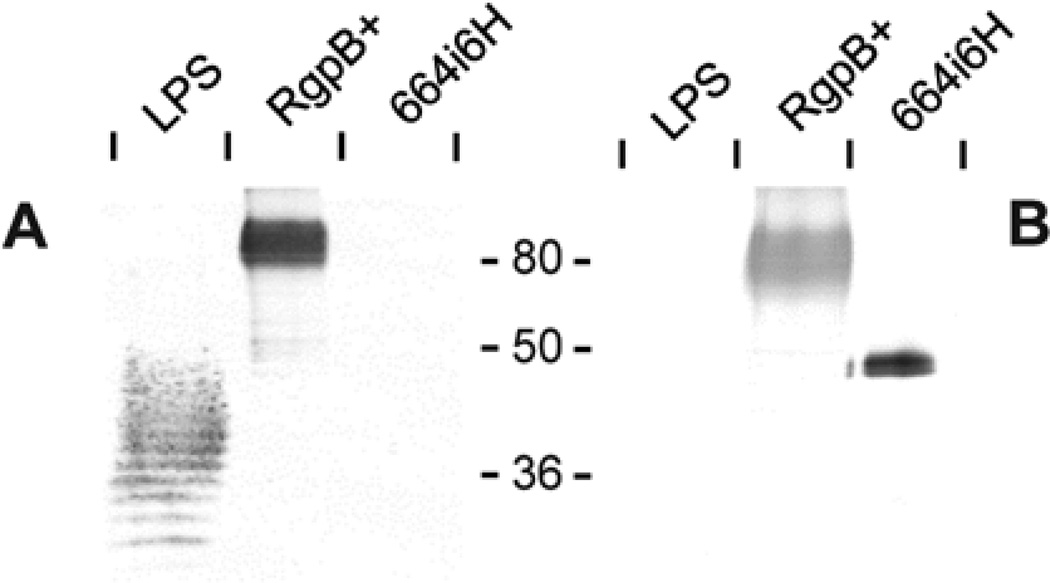
Figure 6. Absence of anionic polysaccharide modification in soluble RgpB from 664i6H mutant.
Cell- and vesicle-free culture media of 664i6H mutant were passed through a nickel-chelating column to purify soluble RgpB as per Methods. 50 µg P. gingivalis LPS and 1 µg purified RgpB from 664i6H mutant or 2 µg of mt-RgpB control (RgpB+) were subjected to Western blot against (A) anti-LPS (monoclonal IB5) and (B) anti-RgpB.
By shifting the 6×His insertion further downstream into the native CTD cleavage site at position 664 (mutant 664i6H), elevated RgpB production of the soluble form predominated although mt-RgpB was also detected (Fig. 5B, C). Further movement of the 6×His insertion past the native cleavage site into the CTD at position 665 (mutant 665i6H), resulted in almost complete recovery of mt-RgpB production, similar to the control RgpB+ strain although total RgpB activity remain elevated (Fig. 5B, C). Significantly, the relative quantity of RgpB enzymatic activity partitioned between soluble or cell-associated forms in all mutants correlated well with the pattern of non-APS-modified or APS-modified forms of RgpB as detected by Western blot, thus providing strong evidence that membrane anchorage of RgpB is mediated by glycan addition to the protein (Fig. 5B, C).
To rule out the possibility that failure of APS-modification of RgpB directly related to the presence of the 6×His polypeptide insertion, substitution with an alternative octapeptide Strep-tag® II (WSHPQFEK) was also carried out. Strep-tag® II insertion at positions 663 and 664 (mutants 663iStr and 664iStr) produced similar effects on RgpB maturation as for 6×His insertion at these locations. This resulted in predominant production of soluble RgpB with detectable Strep-tag® II and partitioning to the medium fraction (Fig. 5B, C, H). Since mutation of the IgLD residues Glu661 to Ser664 into alanine(s) (Fig. 3) or histidines (Fig. 4, mutant 661>4H) did not affect the APS-modification profile of the resultant enzyme, we propose that lengthening of this region results in changes to the conformation at the end of the IgLD resulting in incompatibility with recognition or binding by the APS-modifying enzyme(s).
It was of interest to note that detection of the 6×His epitope within RgpB from the 663i6H mutant by anti-6×His monoclonal antibody was considerably reduced when compared to the 662i6H mutant (Fig. 5D) even though both insertions are within the IgLD and total yield of RgpB in each mutant was similar (Fig. 5C). Further, intact 6×His epitope was undetectable in 664i6H and 665i6H mutants suggesting that cleavage of CTD occurs within the hexahistidine tag. This observation was confirmed by probing with a monoclonal antibody specific for a tetra-histidine (4×His) epitope, showing positive reactivity to modified RgpB from the 664i6H mutant whereas no reactivity was detected with anti-6×His (Fig. 5D, E). Progressive truncation of the 6×His tag within these mutants provided further evidence that cleavage at the junction was not residue-specific but rather a mixed population of differing lengths of poly-histidines was present, with progressively shorter lengths being present as the 6×His tag was moved closer to the CTD junction. Similar results were obtained for the Strep-tagged mutants with considerable loss of detectable Strep-tag® II epitope in the 664iStr mutant as compared to the 663iStr mutant, suggesting that some cleavage also occurred within the Strep-tag® II epitope (Fig. 5F).
Finally, reduction in length of inserted residues to 4×His at position 663 (mutant 663i4H) still resulted in a soluble RgpB phenotype but further reduction to 2×Ala or a single Ala insertion at the same position (mutants 663i2A and 663iA, respectively) resulted in progressive return of RgpB behaviour to the wild-type, cell-associated, heavily APS-modified mt-RgpB form even though total RgpB activity remain elevated (Fig. 5B, C).
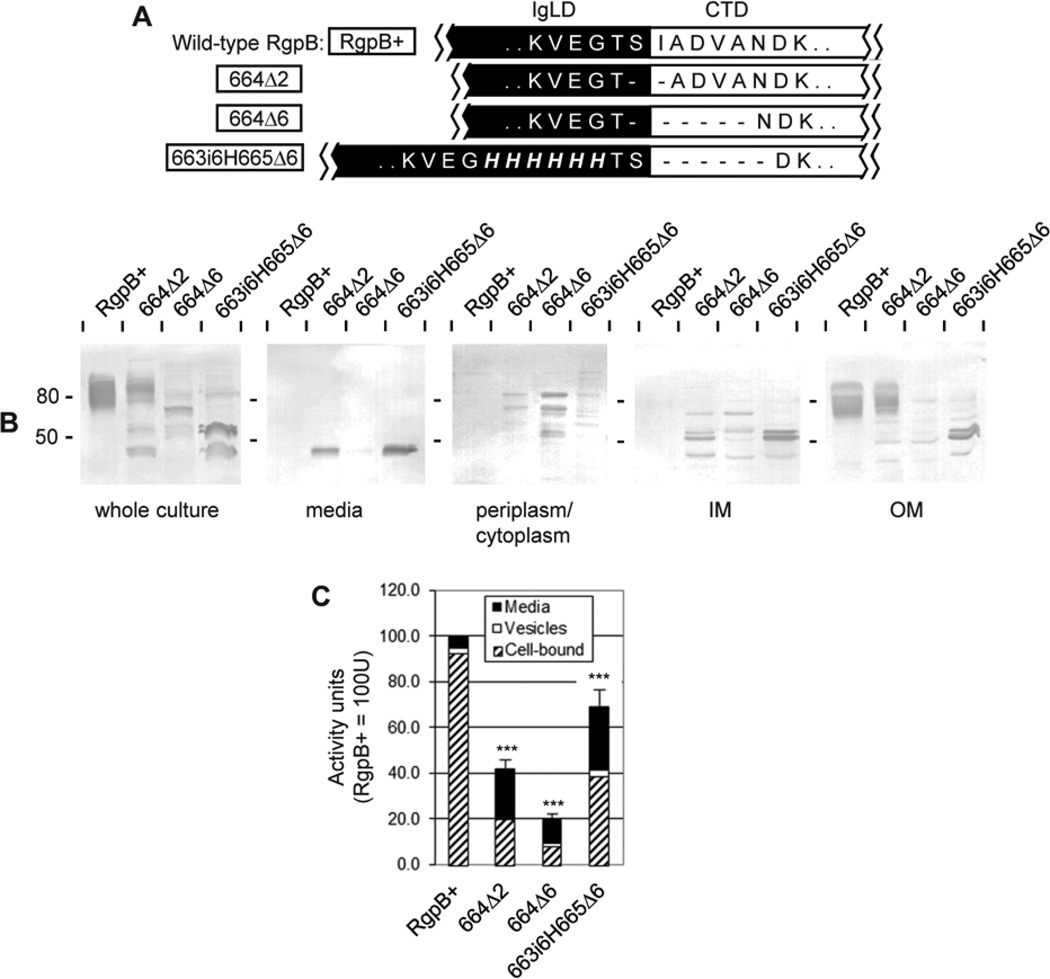
Shortening of the junction interferes with RgpB maturation
The next obvious question was “what would shortening of the junction do to RgpB maturation?”. In this regard, the removal of only two junctional residues spanning the cleavage site Ser664/Ile665 (mutant 664Δ2) resulted in some impairment of RgpB maturation with accumulation of partially processed forms in the inner membrane, traces in the periplasm/cytoplasmic fraction (Fig. 7B) and 60% reduction in the total production of RgpB (Fig. 7C). Moreover, approximately half of the mature RgpB produced was modified with APS and associated with the outer membrane and the remainder unmodified and released into the medium (Fig. 7B). Further, removal of 6 junctional residues from Ser664 to Asn670 (mutant 664Δ6) resulted in 80% reduction of active RgpB production with partially processed forms accumulating within the periplasm/cytoplasm and inner membrane fractions (Fig. 7B, C). It was expected that removal of residues within this region would cause shortening of the backbone polypeptide leading to conformation different than in the native protein and subsequent failure of processing enzyme(s) to recognize the junction.
Figure 7. Deletions at the IgLD-CTD junction.
(A) Mutants (boxed) were created with deletions of various lengths at the IgLD-CTD junction or deletions with compensatory insertions to preserve the length of the junction. (B) Early stationary phase cultures of each mutant were normalised to OD600 1.5 and fractionated into the following fractions: vesicle-free media, periplasm/cytoplasm, inner membrane (IM) and outer membrane (OM) as per Methods. 50 µL of each fraction was subjected to Western blot against anti-RgpB. (C) 20 µL normalised cultures were assayed for media, vesicle-bound and cell-associated RgpB activity using synthetic substrates as per Methods. Data represents the mean and SEM from four independent experiments with the total activity in the RgpB+ control equaling 100 U. Significance of total Rgp activity vs RgpB+ wild-type control was analysed by ANOVA with Bonferroni’s correction: ***, p<0.001.
To confirm these observations, mutant 663i6H665Δ6 was created whereby 6×His was inserted at position 663 within the IgLD while 6 residues of the CTD from Ile665 to Asn670 were removed to maintain the overall length of the peptide backbone (Fig. 7A). Overall, production of RgpB was impaired by 30% in this mutant as compared to the RgpB+ control with some partially processed forms present in the inner and outer membrane fractions as well as mature forms released into the medium fraction (Fig. 5B, C). This result confirmed that a minimum length of junction is required for correct CTD processing during secretion. In addition, a wild-type composition of residues within this region is preferred for optimal folding and processing as deduced from data obtained for His-scanning junctional mutants (Fig. 4).
Maturation and anchorage of Carboxypeptidase-70 (Cpg70) is similar to RgpB
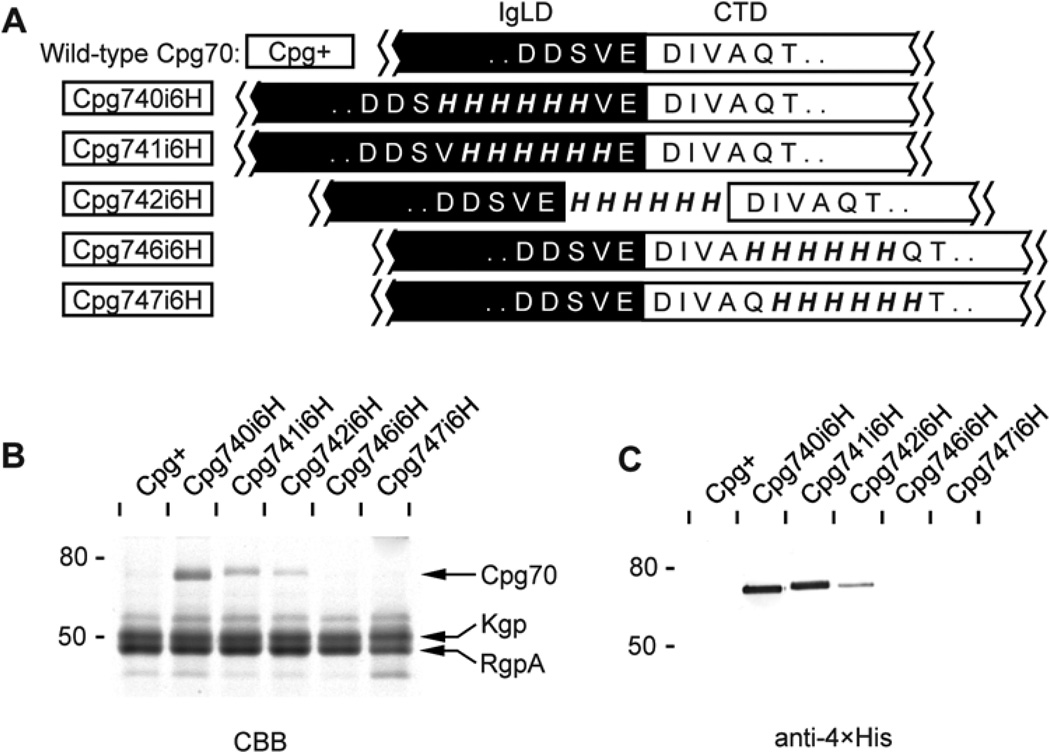
In order to confirm that the observed CTD processing behaviour is not unique to RgpB but is conserved in other proteins secreted via the PorSS system, similar 6×His insertional study was carried out on another CTD-class protein: carboxypeptidase-70 (Cpg70). This enzyme has a similar domain arrangement as RgpB, comprising an N-terminal signal peptide, a pro-domain, a carboxypeptidase domain comprising a metalloprotease catalytic subdomain and three tandem Ig-like subdomains, and a CTD (Chen et al., 2002). The protein is membrane-bound in most strains of P. gingivalis as for RgpB. Multiple alignment predicted that CTD cleavage in Cpg70 occurs between Val740-Glu741 (Fig. 2). Accordingly, 6×His tags were inserted on either side of this site to check the phenotype of the resulting Cpg70. The results were similar to those for RgpB. Following 6×His insertion on the IgLD side and at the putative cleavage site in the Cpg740i6H and Cpg741i6H mutants, respectively, Cpg70 was secreted as a soluble form into the cell-free medium fraction at the predicted mature form of 70 kDa (Fig. 8A, B). Due to the unavailability of specific antibody against Cpg70, the presence of Cpg70 in the cell-free medium fraction was verified by the use of 1D nanoLC ESI MS/MS sequencing (data not shown). Further, secreted Cpg70 from Cpg740i6H and Cpg741i6H possessed poly-histidine tags and reactivity to anti-4×His (Fig. 8C) in a similar manner to the equivalent RgpB mutants 663i6H and 664i6H (Fig. 5). When the 6×His insertion was moved C-terminal to the putative cleavage site in the Cpg742i6H mutant, only a trace amount Cpg70 was secreted with a corresponding decrease in anti-4×His reactivity as observed previously for RgpB in mutant 665i6H. Lastly, 6×His insertions further into the CTD in Cpg746i6H and Cpg747i6H mutants resulted in loss of soluble forms of Cpg70 (Fig. 8). These results suggest that the observed CTD processing and protein anchorage behaviour is conserved across proteins secreted by the PorSS system in P. gingivalis.
Figure 8. Insertions at the IgLD-CTD junction of carboxypeptidase70 (Cpg70).
(A) Mutants (boxed) were created with 6×His insertions at various sites of the IgLD-CTD junction. Early stationary phase cultures of each mutant were normalised to OD600 1.5 and 50 µL of cell-free media fractions were subjected to (B) SDS-PAGE analysis and stained with Coomassie Brilliant Blue (CBB) and (C) Western blot using anti-4×His monoclonal antibody. Protein bands labelled in panel (B) were subjected to 1D nanoLC ESI MS/MS sequencing for identification.
Discussion
Eight types of secretion systems have been described previously for the translocation of proteins and/or peptides through the outer membrane of Gram-negative bacteria (Desvaux et al., 2009). These systems have varied components to secrete proteins either directly from the cytosol through both biological membranes to the exterior of the cell or to translocate proteins via two sequential steps through the inner then outer membranes via distinct pathways. The PorSS protein secretion system, probably representing the ninth secretion system (Type IX Secretion System; T9SS), is a newly described outer membrane translocation pathway devoted to the assembly of the gliding motility apparatus in a range of motile Bacteriodetes phylum species. It also supports secretion of specific virulence proteins in a non-motile group of bacteria within the same phylum (McBride & Zhu, 2013). Presently, this system has been described to be comprised of 11 known components including the porKLMNP locus, sov, porT, porW, lptO, PG0026 and PG0534 (Sato et al., 2009, Chen et al., 2011, Saiki & Konishi, 2010, Glew et al., 2012). Only 2 of these components have been assigned a function: LptO is required for O-deacylation of mono-phosphorylated lipid A in LPS production, while PG26 is the CTD signal peptidase (Chen et al., 2011, Glew et al., 2012). The remaining components have no defined function as yet. It is proposed that after a CTD-class protein has been exported through the inner membrane via the Sec portal, a T9SS component recognises the CTD region of the target protein and mediates its secretion and maturation through the T9SS-created translocon in the outer membrane of the organism.
The CTD has been the focus of recent studies in relation to the role of this domain in the maturation of the gingipains and other virulence proteins from 3 periodontal pathogens belonging the Bacteriodetes phylum: P. gingivalis, Prevotella intermedia and Tannerella forsythia (Nguyen et al., 2007, Karim et al., 2010, Potempa et al., 2009). It acts as a targeting signal for the translocation of these proteins through the T9SS as removal of the last 2 residues at the C-terminus of the CTD was reported to result in failure of RgpB to be correctly processed and secreted from the organism, with partially processed forms remaining trapped within the periplasmic space (Nguyen et al., 2007).Therefore, it was proposed that residues at the C-terminus may act as a recognition signal for targeting RgpB to an outer membrane translocation pathway (Nguyen et al., 2007). This hypothesis was confirmed recently by the heterologous secretion of a green fluorescent protein fusion to CTDs from various CTD-class proteins in P. gingivalis with the outer membrane translocation signal being confined to the C-terminal 20–22 residues of the CTD (Shoji et al., 2011).
Concurrently, Slakeski et al. carried out extensive mutation of various residues within the central region of the CTD from RgpB. They observed that many residues within this central region were redundant except for Asp696 and the residues spanning 692–702 (L692TIFDMNGRRV702) were found to be essential for the production of RgpB (Fig. 2). Mutation of Asp696 to alanine or removal of residues 692–702 was reported to result in complete failure of RgpB production with minimal detection of the nascently translated protein on Western blot (Slakeski et al., 2011). However, in our hands, mutation of the same residue Asp696 to alanine (mutant D696A) resulted in no change in phenotype; with RgpB production at the same quantity, being fully APS-modified and remained cell-bound similar to wild-type mt-RgpB (Fig. S1). Our finding confirmed an earlier communication that mutation of Asp696 had no effect on the production or maturation of RgpB (O'Brien-Simpson et al., 2003), in contrast to the recent observation by Slakeski et al. (Slakeski et al., 2011). Further, numerous truncations of RgpB CTD, including removal in toto resulted only in accumulation of partially processed forms of the protein (Seers et al., 2006, Nguyen et al., 2007), arguing against the premise that a single neutral mutation or removal of only 11 residues within the CTD results in complete loss of the translated product.
While the essential role of the CTD in the maturation of the gingipains is accepted, it has only recently been confirmed to be cleaved off maturing proteins by a newly described gingipain-like protease, PG26, that is associated with the outer membrane of the organism (Glew et al., 2012). However, the cleavage signal for removal of the CTD remains undefined as there appear to be minimal conservation of residues surrounding the cleavage sites identified by MS/MS (Glew et al., 2012) or by multiple alignment (Fig. 2). By using a wide array of RgpB mutants, we have shown in the present study that the cleavage site of the CTD is: (i) sequence-independent as alanine scanning of the cleavage region did not disrupt normal processing (cleavage can also occur within the 6×His and Strep-tag® II); (ii) disruption to the conformation of the IgLD-CTD junction by mutation of spanning residues to 6×His impaired efficient processing of the CTD; (iii) lengthening of the IgLD-side of the junction by insertion of two or more additional residues resulted in progressive failure of APS-modification to the secreted RgpB, and subsequently, the protein is released as a soluble form into the extracellular milieu; (iv) lengthening any part of the junction results in an increase in total RgpB activity production, and (v) shortening of the IgLD-CTD junction results in impairment of RgpB maturation and secretion. Lack of structural data for the CTD and junction region restricts interpretation of the significance of this pattern but as immunoglobulin-like folds have been implicated in protein-protein interactions (Halaby et al., 1999), we propose that during the outer membrane translocation step, the IgLD interacts with CTD to hold the junction in a certain conformation to allow for efficient processing by PG26. Consequently, shortening of the junction by removal of junctional residues impedes proper IgLD-CTD interaction, resulting in impairment of RgpB maturation. Alternatively, both IgLD and CTD may directly interact with the processing enzyme to hold the junctional residues in a conformation compatible with cleavage. Shortening of the junction brings the two domains closer together and prevents proper docking interaction. Further work using purified, recombinant PG26, will be required to confirm this hypothesis.
Conformational recognition for proteolytic enzymes is highly unusual but it has been described previously for the HIV-1 protease that is essential for the processing of the gag and gag-pol polypeptide precursors into viral structural and enzymatic proteins (Hazebrouck et al., 2001). The cleavage sites for HIV-1 protease are highly specific, yet there is limited primary sequence similarity between the various sites. Evidence was presented to highlight the importance of local conformation for efficient processing (Hazebrouck et al., 2001). Further, structural investigations revealed the common structural feature of the natural substrates for HIV-1 protease is the conserved consensus volume that they adopt within the binding site of the enzyme (Prabu-Jeyabalan et al., 2002). Thus, a “dynamic substrate envelope” model was suggested to be the critical determinant for substrate recognition rather than a specific amino acid sequence (Ozen et al., 2011). In this model, the dynamic interaction between the protease-substrate complex is stabilised by conserved hydrogen bonding to the substrate backbone rather than to the side chains, allowing for sequence-independent recognition of the cleavage site. Instead, site specificity was proposed to be mediated by the volume and conformation of the residues surrounding the cleavage site (Ozen et al., 2011). Perhaps a similar mechanism may operate at the CTD cleavage site for PorSS secreted proteins as presented in the present report.
Our observation that APS-modification of RgpB was abolished by the insertion of 4 or more residues into the IgLD segment immediately in front of the CTD cleavage site is significant, as it demonstrates uncoupling of APS modification of RgpB from the outer membrane translocation step. It has been proposed that the two processes are intimately linked based on: (i) absence of an LPS deacylation enzyme LptO prevents maturation and secretion of the gingipains (Chen et al., 2011), (ii) inactivation of other T9SS component proteins resulted in accumulation of non-APS modified and non-secreted precursors of RgpB (Sato et al., 2009) and (iii) removal of the T9SS secretory signal at the C-terminus of RgpB prevented secretion and APS-modification of the protein (Nguyen et al., 2007). Our study showed, however, that APS-modification is a distinct step that is not necessary for secretion of the protein whereby fully-active, non-APS-modified RgpB was readily detected in the cell-free medium of polypeptide insertion mutants such as 662i6H, 663i6H and 663i4H (Fig. 5). This observation is supported by the finding that various P. gingivalis mutants with defects in sugar biosynthesis also produced soluble gingipains that were released into the medium fraction although the proportion of active enzyme varied, posing questions regarding gingipain maturation and secretion in these mutants (Abaibou et al., 2001, Shoji et al., 2002). The release of gingipains in these mutants may have resulted from a “leaky” outer membrane phenotype due to defective outer membrane formation from lack of saccharide components (Ruiz et al., 2006). In our study, secretion of soluble RgpB within mutants with intact sugar biosynthesis pathway strongly suggests a direct relationship between APS-modification of RgpB and its anchorage to the outer membrane; although the exact site and mode of APS attachment is presently unknown. It has been suggested that the APS attachment is via a sortase-like mechanism whereby saccharide linkage is through the C-terminus of the cleaved protein but this hypothesis is yet to be verified experimentally (Slakeski et al., 2011). Presently, our data concur with this hypothesis as polypeptide insertions into the IgLD side of the junction interfere with the APS-modification process, suggesting that the C-terminus created after CTD cleavage is important. We have excluded the possibility that the inserted peptide may disrupt a possible recognition site for the modifying enzyme as conservative mutations of these junctional residues to alanines did not affect the glycosylation profile of the enzyme (Fig. 3). Hence, we hypothesized that lengthening of the IgLD-CTD junction by peptide insertions into the IgLD side should not prevent the translocation-targeting function of the CTD C-terminus but the resultant change in length, and by implication, a conformational change of the junction and/or the adjacent IgLD and CTD structure, is sufficient to interfere with recognition by the APS-transferase enzyme. In this regard, a conformational motif for recognition by glycosyltransferases has been described recently in an E. coli heptosyltransferase Aah, whereby a β-helix is the necessary recognition motif to identify the site for O-glycosylation (Charbonneau et al., 2012). While an Aah homologue could not be found in the genome of P. gingivalis, a similar structural recognition mechanism could be operational in the T9SS glycosyltransferase. Clearly, more investigation will be required to answer these questions.
It is debated whether extracellular proteinase virulence factors such as the gingipains are more effective as cell-bound or soluble forms released into the extracellular milieu during colonization of the host. It has been proposed that soluble forms of the enzymes will be able to penetrate further into the tissues in advance of the bacterial invasion front in order to modulate the recruitment of host immune cells (O'Brien-Simpson et al., 2009). We were able to test this hypothesis using the RgpB-releasing mutant 633i6H for comparison with the mt-RgpB control strain RgpB+ in a mouse subcutaneous chamber model of infection. In a fully controlled situation with the only variable being hexahistidine insertion into RgpB in the 633i6H mutant, it was noteworthy that surface retained mt-RgpB confers greater virulence to P. gingivalis in vivo, particularly in relation to systemic dissemination of the organism (manuscript in preparation). This finding provides impetus for further characterisation of the mechanism of attachment of this class of virulence factors to the bacterial surface.
In conclusion, cumulative findings indicate the importance of CTD-mediated pathways in a new secretory mechanism characteristic of a number of Bacteroidetes phylum organisms (Nguyen et al., 2007, Glew et al. 2012). Understanding the mechanism of recognition and processing of CTD proteins may provide a significant opportunity to impede maturation of these important virulence factors in the treatment of periodontal disease. The present work contributes to that knowledge by characterising the nature of the CTD processing site and its effect on the partitioning of the mature, secreted passenger protein domain.
Experimental Procedures
Bacterial strains and general growth conditions
Porphyromonas gingivalis wild-type strain W83 and mutant derivatives were grown in enriched tryptic soy broth medium (eTSB: 30 g trypticase soy broth, 5 g yeast extract, 5 mg hemin per liter, pH 7.5 and supplemented with 0.5 g L-cysteine and 2 mg menadione) or on blood eTSB agar (eTSB medium + 15 g agar per liter and supplemented with 4% defibrinated sheep’s blood) at 37°C in an anaerobic chamber (Don Whitley Scientific, UK) in an atmosphere of 90% N2, 5% CO2 and 5% H2. Escherichia coli strain DH5α was used for all plasmid construction work and was grown in Luria-Bertani medium and agar. For antibiotic selection in E. coli, ampicillin was used at 100 µg/mL and erythromycin at 250 µg/mL. For P. gingivalis growth selection on solid media, erythromycin was used at 5 µg/mL.
Creation of P. gingivalis RgpB mutants
Creation of suicide plasmids carrying the desired rgpB mutations for homologous recombination into P. gingivalis was as previously described (Nguyen et al., 2007). Briefly, using a modified SLIM method of mutagenesis as describe by Chiu et al. (Chiu et al., 2004), the master pURgpB-E plasmid carrying a 1.8 kb 3′-end fragment of rgpB, an ermF-ermAM cassette and a 1.0 kb 3′ flanking region to rgpB (Nguyen et al., 2007) was mutated using overlap extension PCR and a set of 4 primers (sequence available in Table S1) with an 18-base complementary overhang encoding for the desired mutation. Mutated plasmids were screened for the correct mutation by DNA sequencing of the pertinent region.
Electroporation of the suicide plasmid constructs into P. gingivalis allowed for chromosomal integration of the mutated regions into the P. gingivalis genome via a double homologous recombination event as described previously (Nguyen et al., 2007). Resistant clones were selected for using antibiotic selective media. Mutants were further verified by DNA sequencing of the cloned region of rgpB to confirm only the desired mutation is present in the gene. It should be noted that to avoid the presence of the highly homologous rgpA gene complicating genetic manipulations of rgpB and analysis of RgpB enzymatic reactivity, all work in this study was done in a rgpA-deficient strain as described previously (Table 1) (Nguyen et al., 2007).
Table 1.
P. gingivalis strains and mutants used in this study.
| Strain | Relevant genotype | Source |
|---|---|---|
| W83 | Wild-type | Reference strain (Nelson et al., 2003) |
| RgpB+ | rgpA(Cmr) rgpB+(Emr) | (Nguyen et al., 2007) |
| T663A | rgpA(Cmr) rgpBp.T663A(Emr) | This study |
| S664A | rgpA(Cmr) rgpBp.S664A(Emr) | This study |
| TS664AA | rgpA(Cmr) rgpBp.T663A;S664A(Emr) | This study |
| EG662AA | rgpA(Cmr) rgpBp.E661A;G662A(Emr) | This study |
| S664>A6H | rgpA(Cmr) rgpBp.S664A;I665H;A666H;D667H;V668H;A669H;N670H(Emr) | This study |
| I665>6H | rgpA(Cmr) rgpBp.I665H;A666H;D667H;V668H;A669H;N670H(Emr) | This study |
| T663>6H | rgpA(Cmr) rgpBp.T663H;S664H;I665H;A666H;D667H;V668H(Emr) | This study |
| G662>6H | rgpA(Cmr) rgpBp.G662H;T663H;S664H;I665H;A666H;D667H(Emr) | This study |
| E661>6H | rgpA(Cmr) rgpBp.E661H;G662H;T663H;S664H;I665H;A666H(Emr) | This study |
| E661>4H | rgpA(Cmr) rgpBp.E661H;G662H;T663H;S664H(Emr) | This study |
| 662i6H | rgpA(Cmr) rgpBp.G662_T663insHHHHHH(Emr) | This study |
| 663i6H | rgpA(Cmr) rgpBp.T663_S664insHHHHHH(Emr) | This study |
| 664i6H | rgpA(Cmr) rgpBp.S664_I665insHHHHHH(Emr) | This study |
| 665i6H | rgpA(Cmr) rgpBp.I665_A666insHHHHHH(Emr) | This study |
| 663i4H | rgpA(Cmr) rgpBp.T663_S664insHHHH(Emr) | This study |
| 663i2A | rgpA(Cmr) rgpBp.T663_S664insAA(Emr) | This study |
| 663iA | rgpA(Cmr) rgpBp.T663_S664insA(Emr) | This study |
| 663iStr | rgpA(Cmr) rgpBp.T663_S664insWSHPQFEK(Emr) | This study |
| 664iStr | rgpA(Cmr) rgpBp.S664_I665insWSHPQFEK(Emr) | This study |
| 664d2 | rgpA(Cmr) rgpB∆p.S664_I665(Emr) | This study |
| 664d6 | rgpA(Cmr) rgpB∆p.S664_A669(Emr) | This study |
| 663i6H665d6 | rgpA(Cmr) rgpBp.T663_S664insHHHHHH;∆I665_N670(Emr) | This study |
| D696A | rgpA(Cmr) rgpBp.D696A(Emr) | This study |
| Cpg+ | cpg70+(Emr) | This study |
| Cpg740i6H | cpg70p.S739_V740insHHHHHH(Emr) | This study |
| Cpg741i6H | cpg70p.V740_E741insHHHHHH(Emr) | This study |
| Cpg742i6H | cpg70p.E741_D742insHHHHHH(Emr) | This study |
| Cpg746i6H | cpg70p.A745_Q746insHHHHHH(Emr) | This study |
| Cpg747i6H | cpg70p.Q746_T747insHHHHHH(Emr) | This study |
Creation of Cpg70 mutants
A master suicide plasmid pCpgCeB-B was created in a pUC19 background carrying the following PCR fragments in order: a 1.3-kb PCR fragment spanning the 3′ half of the cpg70 gene, an ermF/AM cassette then a 1-kb fragment 3′ to the cpg70 gene (primer sequences in Table S1). Similar to the creation of RgpB mutants, SLIM mutagenesis was employed to insert a hexahistidine tag at various locations around the predicted CTD cleavage site in Cpg70. Mutated plasmids were screened for the correct mutation by DNA sequencing of the pertinent region before being electroporated into P. gingivalis for homologous recombination as previously described (Nguyen et al., 2007). Mutants were further verified by DNA sequencing of the cloned region of cpg70 to confirm only the desired mutation is present in the gene.
Culture partitioning
Two-day old (early stationary phase) 5 mL culture of each mutant was adjusted to OD600 1.5 and a 0.5 mL sample, designated as the “culture” sample, was collected. Bacteria were removed from the remaining sample by centrifugation at 8,000 × g for 15 min, the supernatant was retained and 0.5 mL sample was collected and designated as the “vesicle/media” sample. The remaining supernatant was subjected to ultracentrifugation at 100,000 × g for 1 h to pellet the vesicles and the supernatant was designated as “media” sample. Subcellular fractionation was carried out as described previously (Nguyen et al., 2007).
Enzyme activity assay
The amidolytic activity of Rgp was assessed by the hydrolysis of the chromogenic substrate benzoyl-L-arginine-p-nitroanilide (BApNA; Sigma-Aldrich Inc., USA). In a 96-well format, samples were pre-incubated in assay buffer (200 mM Tris-HCl, 100 mM NaCl, 5 mM CaCl2, pH 7.6; supplemented with fresh L-cysteine to 10 mM) for 2 min prior to the addition of 0.5 mM substrate in a total volume of 200 µL. The rate of formation of p-nitroanilide was measured using absorbance at 405 nm every 15 s over a 5 min period using a Benchmark Microplate Reader (Bio-Rad Inc., CA, USA). Cell-bound Rgp activity was calculated by subtracting activity of the “vesicle/media” sample from the “culture” sample. Similarly, Rgp activity associated with vesicles was calculated by subtracting activity of the “media” sample from the “vesicle/media” sample. Total Rgp activity in the RgpB+ control mutant was defined as 100 U to allow cross comparison between independent experiments and for statistical analysis.
Western blot analysis
Samples were first boiled in non-reducing SDS-PAGE sample buffer containing 2 mM TLCK for 5 min to inactivate all gingipains prior to the addition of 1% β-mercaptoethanol and boiled for a further 5 min for complete denaturation. Samples were centrifuged briefly at 13,000 × g, 1 min to remove particulates and the supernatant separated on SDS-PAGE. Proteins were subsequently electro-transferred onto 0.45 µm pore-size nitrocellulose membranes and blocked in 2% [w:v] BSA/PBS solution overnight. RgpB was detected using a 1:2,000 dilution of anti-RgpB mouse mAb 18E6 in TTBS (20 mM Tris, 500 mM NaCl, pH 7.5 supplemented with 0.1% Tween 20) for 3 h. Alternatively, rabbit polyclonal anti-CTDRgpB (epitope: 701RVATAKNRM; custom synthesized by Genscript Inc., USA) was used at 1:10,000 dilution; monoclonal anti-LPS IB5 (a kind gift from Dr. Michael Curtis, London) was used at 1:2000 dilution; monoclonal anti-6×His (Roche, Australia) was used at 1:2,000 dilution; monoclonal THE™ anti-4×His (Genscript Inc., USA) was used at 1:8,000 dilution or monoclonal anti-Strep-tag® II (IBA GmbH, Germany) was used at 1:2,000 dilution. Membranes were washed three times with TTBS before being probed for 2 h with a 1:2,000 dilution of an alkaline phosphatase-conjugated anti-mouse or anti-rabbit polyclonal secondary antibody (Dako Cytomation, Denmark). Development was carried out using the AP Conjugate Substrate kit as per manufacturer’s instructions (Bio-Rad Lab., CA, USA).
His-tagged RgpB purification
The media of the 664i6H mutant was clarified by ultracentrifugation at 100,000 × g, 1 h at 4°C to remove all cellular debris and outer membrane vesicles. Proteins were precipitated with 80% [w:v] ammonium sulfate at 4°C overnight and the precipitate collected by centrifugation at 10,000 × g, 20 mins, 4°C. The pellet was resuspended in Binding buffer (20 mM sodium phosphate, 0.5 M NaCl, 10 mM imidazole, pH7.4), clarified by centrifugation and passed through a nickel-chelating column (Qiagen, Australia). Column was washed with Washing buffer (20 mM sodium phosphate, 0.5 M NaCl, 20 mM imidazole, pH7.4) before bound RgpB were eluted with Elution buffer (20 mM sodium phosphate, 0.5 M NaCl, 250 mM imidazole, pH 7.4).
Mass spectrometry
1D nanoLC ESI MS/MS analysis was carried out by the commercial service provider Australian Proteome Analysis Facility (Sydney, Australia). Briefly, bands of interest were excised from the gel and digested with trypsin overnight before mass spectroscopy analysis using a Qstar Elite mass spectrometer (AB Sciex) connected to an Eksigent Tempo nanoLC system and a SGE ProteCol C18 HPLC analytical column. In information dependent acquisition (IDA) mode, a TOF-MS survey scan was acquired (m/z 400–1600, 0.5s), with the three largest multiply charged ions (counts >25) in the survey scan sequentially subjected to MS/MS analysis. MS/MS spectra were accumulated for 2 s (m/z 100–1600). The LC/MS/MS data was searched using Mascot (Matrix Science, London, UK) against the NCBI database to identify proteins from P. gingivalis.
Protein motif search and sequence alignment
The C-terminal 100 residues from proteins harbouring the CTD from P. gingivalis were aligned using the ClustalW2 program at the European Bioinformatics Institute using the ID protein weight matrix with open gap penalty of 10 and gap extension penalty of 1.0. Multi-alignment was displayed using the JalView program (www.jalview.org).
Statistical analysis
Prism v3.03 software (GraphPad Software Inc., CA, USA) was used for all statistical analyses. Enzymatic activities were analysed using one-way ANOVA with Bonferroni’s correction and 95% confidence intervals. P values below 0.05 were considered significant.
Supplementary Material
ACKNOWLEDGEMENTS
KAN acknowledges support from Ramaciotti Foundation [RN38/08] and National Institutes of Health [DE 09761, United States]. JP acknowledges support from Foundation for Polish Science [TEAM project DPS/424-329/10], the National Science Center (NCN) [2012/04/A/NZ1/00051, Krakow, Poland], the European Commission [FP7- HEALTH-2010-261460 “Gums&Joints”, FP7-PEOPLE-2011-ITN-290246 "RAPID" and FP7-HEALTH-F3-2012-306029 "TRIGGER"], Polish Ministry of Science and Higher Education [137/7.PR-EU/2011/2, Warsaw, Poland] and the National Institutes of Health [DE 09761, United States]. The Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University is a beneficiary of the structural funds from the European Union [Grant No: POIG.02.01.00-12-064/08 – “Molecular biotechnology for health”]. We would like to thank Dr. Michael Curtis for the kind gift of the IB5 monoclonal antibody and Dr. Inaki de Diego Martinez for critical reading of the manuscript.
Footnotes
All authors declare that they have no conflict of interest associated with this work.
REFERENCES
- Abaibou H, Chen Z, Olango GJ, Liu Y, Edwards J, Fletcher HM. vimA gene downstream of recA is involved in virulence modulation in Porphyromonas gingivalis W83. Infect Immun. 2001;69:325–335. doi: 10.1128/IAI.69.1.325-335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau ME, Cote JP, Haurat MF, Reiz B, Crepin S, Berthiaume F, Dozois CM, Feldman MF, Mourez M. A structural motif is the recognition site for a new family of bacterial protein O-glycosyltransferases. Mol Microbiol. 2012;83:894–907. doi: 10.1111/j.1365-2958.2012.07973.x. [DOI] [PubMed] [Google Scholar]
- Chen YY, Cross KJ, Paolini RA, Fielding JE, Slakeski N, Reynolds EC. CPG70 is a novel basic metallocarboxypeptidase with C-terminal polycystic kidney disease domains from Porphyromonas gingivalis. J Biol Chem. 2002;277:23433–23440. doi: 10.1074/jbc.M200811200. [DOI] [PubMed] [Google Scholar]
- Chen YY, Peng B, Yang Q, Glew MD, Veith PD, Cross KJ, Goldie KN, Chen D, O'Brien-Simpson N, Dashper SG, Reynolds EC. The outer membrane protein LptO is essential for the O-deacylation of LPS and the co-ordinated secretion and attachment of A-LPS and CTD proteins in Porphyromonas gingivalis. Mol Microbiol. 2011;79:1380–1401. doi: 10.1111/j.1365-2958.2010.07530.x. [DOI] [PubMed] [Google Scholar]
- Chiu J, March PE, Lee R, Tillett D. Site-directed, Ligase-Independent Mutagenesis (SLIM): a single-tube methodology approaching 100% efficiency in 4 h. Nucleic Acids Res. 2004;32:e174. doi: 10.1093/nar/gnh172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvaux M, Hebraud M, Talon R, Henderson IR. Secretion and subcellular localizations of bacterial proteins: a semantic awareness issue. Trends Microbiol. 2009;17:139–145. doi: 10.1016/j.tim.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Eichinger A, Beisel HG, Jacob U, Huber R, Medrano FJ, Banbula A, Potempa J, Travis J, Bode W. Crystal structure of gingipain R: an Arg-specific bacterial cysteine proteinase with a caspase-like fold. EMBO J. 1999;18:5453–5462. doi: 10.1093/emboj/18.20.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick RE, Aprico A, Wijeyewickrema LC, Pagel CN, Wong DM, Potempa J, Mackie EJ, Pike RN. High molecular weight gingipains from Porphyromonas gingivalis induce cytokine responses from human macrophage-like cells via a nonproteolytic mechanism. J Innate Immun. 2009;1:109–117. doi: 10.1159/000181145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson FC, 3rd, Hong C, Chou HH, Yumoto H, Chen J, Lien E, Wong J, Genco CA. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:2801–2806. doi: 10.1161/01.CIR.0000129769.17895.F0. [DOI] [PubMed] [Google Scholar]
- Glew MD, Veith PD, Peng B, Chen YY, Gorasia DG, Yang Q, Slakeski N, Chen D, Moore C, Crawford S, Reynolds EC. PG0026 is the C-terminal signal peptidase of a novel secretion system of Porphyromonas gingivalis. J Biol Chem. 2012;287:24605–24617. doi: 10.1074/jbc.M112.369223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Nguyen KA, Potempa J. Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon's knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000. 2010;54:15–44. doi: 10.1111/j.1600-0757.2010.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaby DM, Poupon A, Mornon J. The immunoglobulin fold family: sequence analysis and 3D structure comparisons. Protein Eng. 1999;12:563–571. doi: 10.1093/protein/12.7.563. [DOI] [PubMed] [Google Scholar]
- Hazebrouck S, Machtelinckx-Delmas V, Kupiec JJ, Sonigo P. Local and spatial factors HIV-determining HIV-1 protease substrate recognition. Biochem J. 2001;358:505–510. doi: 10.1042/0264-6021:3580505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim AY, Kulczycka M, Kantyka T, Dubin G, Jabaiah A, Daugherty PS, Thogersen IB, Enghild JJ, Nguyen KA, Potempa J. A novel matrix metalloprotease-like enzyme (karilysin) of the periodontal pathogen Tannerella forsythia ATCC 43037. Biol Chem. 2010;391:105–117. doi: 10.1515/BC.2010.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann M, Lindner P, Honegger A, Blank K, Tschopp M, Capitani G, Pluckthun A, Grutter MG. Crystal structure of the anti-His tag antibody 3D5 single-chain fragment complexed to its antigen. J Mol Biol. 2002;318:135–147. doi: 10.1016/S0022-2836(02)00038-4. [DOI] [PubMed] [Google Scholar]
- McBride MJ, Zhu Y. Gliding Motility and Por Secretion System Genes Are Widespread among Members of the Phylum Bacteroidetes. J Bacteriol. 2013;195:270–278. doi: 10.1128/JB.01962-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczyk J, Boatright KM, Stennicke HR, Nazif T, Potempa J, Bogyo M, Salvesen GS. Sequential autolytic processing activates the zymogen of Arg-gingipain. J Biol Chem. 2003;278:10458–10464. doi: 10.1074/jbc.M210564200. [DOI] [PubMed] [Google Scholar]
- Nelson KE, Fleischmann RD, DeBoy RT, Paulsen IT, Fouts DE, Eisen JA, Daugherty SC, Dodson RJ, Durkin AS, Gwinn M, Haft DH, Kolonay JF, Nelson WC, Mason T, Tallon L, Gray J, Granger D, Tettelin H, Dong H, Galvin JL, Duncan MJ, Dewhirst FE, Fraser CM. Complete genome sequence of the oral pathogenic bacterium Porphyromonas gingivalis strain W83. J Bacteriol. 2003;185:5591–5601. doi: 10.1128/JB.185.18.5591-5601.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KA, Travis J, Potempa J. Does the importance of the C-terminal residues in the maturation of RgpB from Porphyromonas gingivalis reveal a novel mechanism for protein export in a subgroup of Gram-negative bacteria? J Bacteriol. 2007;189:833–843. doi: 10.1128/JB.01530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien-Simpson NM, Pathirana RD, Walker GD, Reynolds EC. Porphyromonas gingivalis RgpA-Kgp Proteinase-Adhesin Complexes Penetrate Gingival Tissue and Induce Proinflammatory Cytokines or Apoptosis in a Concentration-Dependent Manner. Infect Immun. 2009;77:1246–1261. doi: 10.1128/IAI.01038-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien-Simpson NM, Veith PD, Dashper SG, Reynolds EC. Porphyromonas gingivalis gingipains: the molecular teeth of a microbial vampire. Curr Protein Pept Sci. 2003;4:409–426. doi: 10.2174/1389203033487009. [DOI] [PubMed] [Google Scholar]
- Ozen A, Haliloglu T, Schiffer CA. Dynamics of preferential substrate recognition in HIV-1 protease: redefining the substrate envelope. J Mol Biol. 2011;410:726–744. doi: 10.1016/j.jmb.2011.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike R, McGraw W, Potempa J, Travis J. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Isolation, characterization, and evidence for the existence of complexes with hemagglutinins. J Biol Chem. 1994;269:406–411. [PubMed] [Google Scholar]
- Potempa J, Mikolajczyk-Pawlinska J, Brassell D, Nelson D, Thogersen IB, Enghild JJ, Travis J. Comparative properties of two cysteine proteinases (gingipains R), the products of two related but individual genes of Porphyromonas gingivalis. J Biol Chem. 1998;273:21648–21657. doi: 10.1074/jbc.273.34.21648. [DOI] [PubMed] [Google Scholar]
- Potempa J, Sroka A, Imamura T, Travis J. Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis: structure, function and assembly of multidomain protein complexes. Curr Protein Pept Sci. 2003;4:397–407. doi: 10.2174/1389203033487036. [DOI] [PubMed] [Google Scholar]
- Potempa M, Potempa J, Kantyka T, Nguyen KA, Wawrzonek K, Manandhar SP, Popadiak K, Riesbeck K, Eick S, Blom AM. Interpain A, a Cysteine Proteinase from Prevotella intermedia, Inhibits Complement by Degrading Complement Factor C3. PLoS Pathog. 2009;5:e1000316. doi: 10.1371/journal.ppat.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabu-Jeyabalan M, Nalivaika E, Schiffer CA. Substrate shape determines specificity of recognition for HIV-1 protease: analysis of crystal structures of six substrate complexes. Structure. 2002;10:369–381. doi: 10.1016/s0969-2126(02)00720-7. [DOI] [PubMed] [Google Scholar]
- Rapala-Kozik M, Bras G, Chruscicka B, Karkowska-Kuleta J, Sroka A, Herwald H, Nguyen KA, Eick S, Potempa J, Kozik A. Adsorption of components of the plasma kinin-forming system on the surface of Porphyromonas gingivalis involves gingipains as the major docking platforms. Infect Immun. 2011;79:797–805. doi: 10.1128/IAI.00966-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz N, Kahne D, Silhavy TJ. Advances in understanding bacterial outer-membrane biogenesis. Nat Rev Microbiol. 2006;4:57–66. doi: 10.1038/nrmicro1322. [DOI] [PubMed] [Google Scholar]
- Saiki K, Konishi K. Identification of a novel Porphyromonas gingivalis outer membrane protein, PG534, required for the production of active gingipains. FEMS Microbiol Lett. 2010;310:168–174. doi: 10.1111/j.1574-6968.2010.02059.x. [DOI] [PubMed] [Google Scholar]
- Sato K, Naito M, Yukitake H, Hirakawa H, Shoji M, McBride MJ, Rhodes RG, Nakayama K. A protein secretion system linked to bacteroidete gliding motility and pathogenesis. Proc Natl Acad Sci U S A. 2009;107:276–281. doi: 10.1073/pnas.0912010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seers CA, Slakeski N, Veith PD, Nikolof T, Chen YY, Dashper SG, Reynolds EC. The RgpB C-terminal domain has a role in attachment of RgpB to the outer membrane and belongs to a novel C-terminal-domain family found in Porphyromonas gingivalis. J Bacteriol. 2006;188:6376–6386. doi: 10.1128/JB.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M, Ratnayake DB, Shi Y, Kadowaki T, Yamamoto K, Yoshimura F, Akamine A, Curtis MA, Nakayama K. Construction and characterization of a nonpigmented mutant of Porphyromonas gingivalis: cell surface polysaccharide as an anchorage for gingipains. Microbiology. 2002;148:1183–1191. doi: 10.1099/00221287-148-4-1183. [DOI] [PubMed] [Google Scholar]
- Shoji M, Sato K, Yukitake H, Kondo Y, Narita Y, Kadowaki T, Naito M, Nakayama K. Por Secretion System-Dependent Secretion and Glycosylation of Porphyromonas gingivalis Hemin-Binding Protein 35. PLoS One. 2011;6:e21372. doi: 10.1371/journal.pone.0021372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slakeski N, Seers CA, Ng K, Moore C, Cleal SM, Veith PD, Lo AW, Reynolds EC. The C-terminal domain residues important for the secretion and attachment of RgpB in Porphyromonas gingivalis. J Bacteriol. 2011;193:132–142. doi: 10.1128/JB.00773-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanterpool E, Roy F, Fletcher HM. Inactivation of vimF, a putative glycosyltransferase gene downstream of vimE, alters glycosylation and activation of the gingipains in Porphyromonas gingivalis W83. Infect Immun. 2005a;73:3971–3982. doi: 10.1128/IAI.73.7.3971-3982.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanterpool E, Roy F, Sandberg L, Fletcher HM. Altered gingipain maturation in vimA- and vimE-defective isogenic mutants of Porphyromonas gingivalis. Infect Immun. 2005b;73:1357–1366. doi: 10.1128/IAI.73.3.1357-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillard F, Sztukowska M, Mizgalska D, Ksiazek M, Houston J, Potempa B, Enghild JJ, Thogersen IB, Gomis-Ruth FX, Nguyen KA, Potempa J. Inhibition of gingipains by their profragments as the mechanism protecting Porphyromonas gingivalis against premature activation of secreted proteases. Biochim Biophys Acta. 2013;1830:4218–4228. doi: 10.1016/j.bbagen.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner N, Wait R, Sroka A, Eick S, Nguyen KA, Lundberg K, Kinloch A, Culshaw S, Potempa J, Venables PJ. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: Implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010;62:2662–2672. doi: 10.1002/art.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RC. Periodontal disease. N Engl J Med. 1990;322:373–382. doi: 10.1056/NEJM199002083220606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.